Abstract
Among the factors affecting the effectiveness of malaria control is poor knowledge of the entomologic drivers of the disease. We investigated anopheline populations as part of a baseline study to implement house screening of windows and doors as a supplementary malaria control tool towards elimination in Jabi Tehnan district, Amhara Regional State of Ethiopia. The samples were surveyed monthly using CDC light traps between June 2020 and May 2021. Mosquito trap density (< 3 mosquitoes/trap) was low, however, with a high overall Plasmodium sporozoite rate (9%; indoor = 4.3%, outdoor = 13.1%) comprising P. falciparum (88.9%) and P. vivax (11.1%). Anopheles gambiae s.l., mostly An. arabiensis, comprised > 80% of total anopheline captures and contributed ~ 42% of Plasmodium-infected mosquitoes. On the other hand, morphologically scored Anopheles funestus s.l., constituting about 6% of anopheline collections, accounted for 50% of sporozoite-infected mosquitoes. Most of the infected An. funestus s.l. specimens (86.7%) were grouped with previously unknown or undescribed Anopheles species previously implicated as a cryptic malaria vector in the western Kenyan highlands, confirming its wider geographic distribution in eastern Africa. Other species with Plasmodium infection included An. longipalpis C, An. theileri, An. demillioni, and An. nili. Cumulatively, 77.8% of the infected mosquitoes occurred outdoors. These results suggest efficient malaria parasite transmission despite the low vector densities, which has implications for effective endpoint indicators to monitor malaria control progress. Additionally, the largely outdoor infection and discovery of previously unknown and cryptic vectors suggest an increased risk of residual malaria transmission and, thus, a constraint on effective malaria prevention and control.
Similar content being viewed by others
Introduction
Malaria remains a public health menace in Ethiopia1. While the disease in humans is caused by five different Plasmodium species globally, Plasmodium falciparum and P. vivax are the two major protozoan parasites responsible for the documented burden in Ethiopia1,2. Parasite transmission in the country is largely driven by Anopheles arabiensis as the primary vector while other species including An. pharoensis, An. coustani s.l., An. nili, and An. funestus, and An. demeilloni play minor roles2. Like most malaria-endemic countries worldwide, implementation of a suite of strategies over the past two decades (i.e., long-lasting insecticidal nets (LLINs), and indoor residual spraying (IRS), prompt case diagnosis and treatment) led to significant declines in malaria cases and consequent mortalities in most parts of Ethiopia. For instance, a 31% reduction in malaria cases and accompanying decline in deaths (58%) were observed between 2016 and 20203.
Amidst the decline, compared to the instances in 2021, a 32% higher incidence was reported nationwide in 20224. In addition, malaria transmission remains highly heterogenous across the country in different risk areas, partly influenced by climatic factors such as rainfall, altitude, and human population movements. A review of clinical data in health facilities in the Oromia Special Zone, Amhara Regional State of Ethiopia (2014–2019) revealed a 12.5% malaria parasite positivity rate with changes in the age-associated burden being highest in populations aged > 15 years5. Their findings highlight the need for active vector and parasite surveillance to track changes in malaria dynamics as interventional efforts are implemented6. As a critical component of vector control programs, surveillance includes measures such as assessing changes in vector composition and density, behaviour changes, pathogen infection rates and risk of transmission; and guiding timely decisions regarding interventions and allocation of resources efficiently.
Effective malaria control towards attainment of elimination, requires monitoring of critical entomological endpoints such as mosquito survival, host preference, species and infection status, and susceptibility to interventions7,8. Improved surveillance is essential to guide targeted or new strategies in response to changes in local transmission dynamics6,9. In Ethiopia, surveillance efforts have revealed emerging threats to malaria control including widespread pyrethroid resistance as in An. arabiensis, and likewise in the recently introduced vector An. stephensi10,11. In fact, this latter species is fast spreading in the country10,12 and already implicated in the spread of drug-resistant malaria parasites13. Recently, An. pretoriensis and An. cinereus were incriminated as novel vectors found to harbour the malaria parasite P. falciparum in the highlands of the Northwestern part of the country14.
Lately, house screening was evaluated as a supplementary intervention towards achieving malaria elimination in the Jabi Tehnan district, West Gojjam Zone, Amhara Regional State, Northwestern Ethiopia15. An important requirement for evaluating the efficacy of interventions entails characterization of the baseline malaria epidemiology including information on the driving vectors, genetic structure and associated bionomic features. Thus, the objective of the present study was to describe malaria entomologic risk factors pertaining to anopheline composition, abundance trends and Plasmodium infection rates. Additionally, we analysed the genetic diversity of anopheline populations found to harbour the malaria parasites.
Materials and methods
Study site
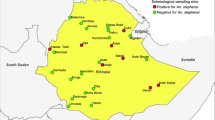
This study was conducted in Jabi Tehnan district (37.074°–37.508°E; 10.405°–10.945°N), located in the West Gojjam Zone, Amhara Regional State of Northwestern Ethiopia (Fig. 1). The area has an altitude that ranges between 1300 and 2330 m above sea level (asl), and receives an annual precipitation of about 1356–1720 mm. Mean daily temperatures range from 15 to 20 °C. The district is endemic for malaria16 and encompasses 38 rural villages and 3 smaller towns with an estimated population of > 200,000 inhabitants residing in 45,827 rural and 7,927 urban households17. Malaria transmission is bi-modal with the peak occurring after the heavy rainy season (June to September) and another during the short rainy season (March to May). Recent data in the district indicate about 75% reduction in malaria cases between 2015 (24,427) and 2019 (6119)18.
Study design, field mosquito sampling and identification
Surveys of adult host seeking anophelines were conducted in village clusters in three agro-ecological settings (June 2020–May 2021) with heterogeneous transmission and unknown entomological drivers. These settings include: (i) ‘subtropical dry mountainous highland’ (dry mountain) found > 2000 m asl, (ii) ‘subtropical plateau mid-highlands’ (plateau highlands) (1800–2000 m asl), and (iii) ‘subtropical semi-arid/semi-desert lowlands’ (semi-arid) (< 1800 m asl) (Fig. 1). We trapped mosquitoes using Centre for Disease Prevention and Control (CDC) light traps (Model 512, John W. Hock, Gainesville, FL, USA), deployed both indoors and outdoors in selected households each day. Each sampling month focused on four randomly selected households in a village representative of each agroecological setting. Two traps were placed in each household (1-indoor, 1-outdoor) making a total of eight traps set in a selected village per zone. Thus, a total of 24 randomly selected households were targeted across the agroecological area each sampling month. To ensure a spatial coverage, the same plan was enforced targeting different sets of randomly selected households. Traps were operated from 18:00 h to 06:00 h (local time) the following day. Mosquitoes captured were knocked down using chloroform, sorted and then identified morphologically to species level using published keys19,20. The physiological status of each female was classed as either blood-fed, gravid, half-gravid or unfed through observation of the abdomen21.
DNA extraction
Extraction of genomic DNA (gDNA) from head/thorax from individual adult females was achieved using the ISOLATE II Genomic DNA Kit (Bioline, Meridian Bioscience, Germany) as per the protocol of the manufacturer. The DNA solution was used for malaria parasite detection via PCR (Polymerase Chain Reaction) assays as well as to identify sibling species in species complexes (described below).
Mosquito screening for malaria parasite infection
A combination of methods was used for screening of Plasmodium infection in the samples. These included PCR-HRM (high resolution melting) analysis of the non-coding mitochondrial sequence (ncMS) followed by sequencing. Details of the PCR conditions are described in Kinya et al.6. Also, nested PCR targeting the 18S rRNA22 that detects and discriminates Plasmodium sporozoite of the species P. falciparum. P. vivax, P. malariae and P. ovale, was employed. The simian malaria parasite P. knowlesi is not known to infect humans in Africa. The first round of amplification in a 20 µL reaction volume comprised 10 µM of genus-specific primers rPLU-5 and rPLU-6, 4 µL of 2 × MyTaq Mix (Bioline, Meridian Bioscience, Germany), 0.2 U MyTaq polymerase, 10.58 µL of PCR water and ~ 20 ng of gDNA. The second round of amplification entailed same constituents except template being 4 µL of the first PCR product and species-specific primer pairs each in separate PCRs (rFAL1 + rFAL2, rVIV1 + rVIV2, rMAL1 + rMAL2, and rOVAL1 + rOVAL2)23. In both first and second round amplification, the cycling conditions were 5 min at 95 °C; 25 cycles (first round, 30 cycles for second round) of 30 s at 94 °C, 2 min at 58 °C and 2 min at 72 °C and final extension for 5 min at 72 °C. The nested PCR products were separated on a 2% agarose gel to identify the Plasmodium species. The third method specifically detected sporozoite of P. falciparum by amplifying the single copy gene glutathione reductase (PfGR) using the primers PfGR_F 5′-GCTGCCTCAGTTCATGATATTT-3′ and PfGR_R 5′-CTTATCCCTTCTCTCTACCAACAG-3′24. Each PCR reaction contained, 4 µL of 2 × MyTaq Mix, 0.2 U MyTaq polymerase, 11.6 µL of PCR water, 20 ng of gDNA and 10 µM of each primer. The thermal profile used for PCR was: 5 min at 95 °C, 30 cycles of 30 s at 94 °C, 30 s at 60 °C and 30 s at 72 °C and final extension for 5 min at 72 °C. Amplicons were verified on 2% agarose gel electrophoresis against a 100 bp DNA HyperLadder (Bioline, Meridian Bioscience, Tennessee, USA). Negative (uninfected An. arabiensis DNA) and positive control (DNA from each of the Plasmodium species from National Institute for Biological Standards and Control (NIBSC; London, UK) were included in PCR runs.
Identification of the sibling species of An. gambiae complex
The HRM-PCR protocol by Zianni et al.25 that distinguishes An. arabiensis from An. gambiae was used, the former species being the main primary vector in the An. gambiae s.l. in Ethiopia2. The PCR used primers that targeted the ribosomal internal transcribed spacer subunit-2 (ITS2) region. The DNA amplification with the Solis Biodyne kit (Solis BioDyne, Tartu, Estonia), in a 10 μL reaction mix contained 2 μl of 5X Hot Firepol EvaGreen HRM Mix, 0.5 μM of each primer, ~ 20 ng of DNA template and PCR water. Positive (DNA of lab-reared An. arabiensis maintained at the Insectary Unit, icipe Duduville Campus), Nairobi and negative (PCR grade water) controls were included. The thermal conditions entailed an initial denaturation (1 min at 95 °C) followed by 40 cycles of denaturation (95 °C for 30 s), annealing (57 °C for 30 s), and extension (72 °C for 45 s) with a further extension at 72 °C for 7 min. Generated HRM profiles were analysed using HRM analysis tools in the RGQ software (Qiagen). Plasmodium sporozoite-positive An. gambiae s.l. specimens were similarly processed to identify the sibling species as described above.
Molecular analysis of morphologically scored An. funestus mosquitoes
This was limited only to morphologically scored An. funestus group specimens that tested positive for Plasmodium sporozoite infection. They were analysed by PCR amplification of the internal transcribed spacer 2 (ITS2) region of the ribosomal DNA (rDNA)26 using established protocols6. This genetic marker has been widely used to discriminate closely related anophelines and other mosquito species with abundant sequences available in public databases (e.g., GenBank). Briefly, PCRs performed in a 15 μL reaction volume comprised 0.5 μM of the forward and reverse primers, 3 μL of 5X HOT FIREPol Blend Master Mix (Solis BioDyne, Estonia) and ~ 20 ng of DNA template. Thermal cycling conditions were 95 °C for 15 min followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 46 °C for 30 s and extension at 72 °C for 40 s followed by final extension at 72 °C for 10 min. Amplicons were confirmed by gel electrophoresis and the PCR products similarly purified and sequenced as explained earlier.
Sequence and phylogenetic analyses
Mosquito sequences were viewed and edited in Chromas, embedded in MEGA v.6.027 and queried in GenBank using Basic Local Alignment Search Tool (BLastn). Multiple sequence alignments of the resulting contiguous sequences were performed using MUSCLE in MEGA v.6.0 with default parameters. A neighbor-joining (NJ) tree was inferred for ITS2 sequences of infected An. funestus mosquitoes using the p-distance method with nodal support for different groupings evaluated through 1000 bootstrap replications. Indels were excluded from analysis.
Ethical approval
Review of the study protocol and ethical approval were given by the Regional Public Health Research Ethics Review Committee /RERC/ (Ref. No: H/R/T/T/D/5/3) from Amhara Public Health Research Institute, Ethiopia. The research activity was started by providing information about the whole objective of the study to the community and consensus obtained from household owners for the project related activities done with the directions given by local stakeholders.
Statistical analyses
Relative abundance was used to estimate the composition of the anopheline mosquitoes indoors and outdoors. Daily counts of female mosquito/trap for An. gambiae s.l. were compared between the agro-ecological zones using generalised linear models (GLM) with negative binomial error structure based on best-fit model residuals. Replicate trap catches were pooled for the village clusters in each agro-ecological area viz: plateau highlands, dry mountains and semi-arid. The main predictor variables were agro-ecology and location (indoor/outdoor). The mean catches/trap/night was computed for each of the four species that comprised > 90% of the total catches: An. gambiae s.l., An. funestus s.l., An. coustani s.l. and An. squamosus. Pair-wise mean comparisons in the abundance of these species indoors versus outdoors for each agroecology was ensured using the package emmeans with a p-value adjustment equivalent to Tukey test. The P. falciparum sporozoite infection rates (Pfsp) were expressed as the number of positive specimens of the total number examined for each species and the proportion compared between indoors and outdoors using Pearson χ2 test. All analysis was implemented in R v. 4.2.128 including GLMs with the MASS package, and results considered significant at p ≤ 0.05.
Results
Anopheline mosquito composition
A total trapping effort of 436 trap nights (194 indoors, 242 outdoors) yielded 1318 anopheline females comprising 19 species. The differences in the replicates were attributed to malfunctioned traps that resulted in zero catches and were excluded from the experiment. The species trapped and classified based on morphology were Anopheles gambiae s.l., An. funestus s.l., An. coustani s.l., An. squamosus, An. pharoensis, An. cinereus, An. natalensis, An. pretoriensis, An. christyi, An. nili, An. maculipalpis, An. marshalli, An. demeilloni, An. rupicolus, An. wellcomei, An. kingi, An. obscurus, An. seydeli, and An. harperi (Fig. 2).
Anopheline mosquito composition based on morphology, collected in Jabi Tehnan district, Amhara Regional State, Northwestern Ethiopia. The number of trap nights (n) were indoors: plateau highland = 69, dry mountain = 61, semi-arid = 64; outdoors: plateau highland = 82, dry mountain = 70, semi-arid = 90.
The species occurrences were non-uniform across the different agro-ecological zones. An. gambiae s.l. was relatively predominant accounting for 62.8% of the total anopheline collections. This was followed by An. coustani s.l. (12.1%), An. funestus s.l. (6.3%) and An. squamosus (6.1%). Sparse occurrence of the other anophelines was observed with individual proportions ranging from 0.08 to 2.4% of the total anopheline catches.
Abundance trends were compared for An. gambiae s.l. and found not to differ by agroecologic areas (χ22,433 = 318.10, p = 0.20) nor between indoors and outdoors (χ21,432 = 317.20, p = 0.35). In contrast, captures of An. funestus s.l. mosquitoes varied by agroecology (χ22,433 = 136.08, p = 0.005) being significantly higher both in dry mountain (Z value = 2.89, p = 0.004) and plateau highland (Z value 1.98, p = 0.048) than semi-arid. Also, the species were significantly encountered outdoors than indoors (Z value = 2.90, p = 0.004).
Variation in mosquito densities
Mosquito trap densities were generally low (< 3 mosquitoes/trap) and varied between the three agro-ecological zones and indoors versus outdoors for certain species. This was compared for the four dominant species (An. gambiae s.l., An. coustani s.l., An. funestus s.l., and An. squamosus (comprised ~ 90% of total anopheline collections). Mean An. gambiae s.l. densities ranged from 1.38 to 2.68/trap-night and differed indoors and outdoors in the dry mountain highland areas. The generally low An. funestus s.l. densities (< 1/trap-night) were not significantly different between indoors and outdoors except in the dry mountain highland (Fig. 3). There was no significant difference in captures of An. coustani s.l. and An. squamosus between indoor and outdoor locations in any of the agroecological setting, although there was a tendency for more captures outdoors.
Mean captures (± se) of dominant anopheline species encountered in Jabi Tehnan district, Amhara Regional State, Northwestern Ethiopia. Comparisons are made between indoor and outdoor captures in each agroecology zone for each species, * significant difference at p < 0.05; ns, non-significant difference; se = standard error.
Physiological status of mosquitoes
The mosquitoes were classified as blood-fed, gravid, half-gravid or unfed. Three hundred and one (301), 80 and 18 mosquitoes were blood-fed, gravid and half-gravid, respectively. This translated into a cumulative engorged (blood-fed + gravid + half-gravid) rate of 30.3% (399/1318) of the trap catches. Overall engorged rates were 37.7 and 27.7% for An. gambiae s.l. and An. funestus s.l., respectively. Higher rates were evident for other species, but they occurred in very low levels (Table 1).
Plasmodium sporozoite infection rates in anopheline mosquitoes
Head/thorax of only engorged cohorts was tested for Plasmodium infection, of which five anopheline species tested positive: An. gambiae s.l., An. funestus s.l., An. coustani s.l., An. nili, and An. rupicolus (Table 2). Engorged cohort has had biting encounters at least once with a host (including humans) and more likely to be infected. Plasmodium infection prevalence in An. gambiae s.l. (all found to be An. arabiensis) was 4.8% (15/312) and higher outdoors than indoors but not significantly different (9/151 vs 6/161; χ2 = 0.43, df = 1, p = 0.51). Of the infection in An. arabiensis, P. falciparum was predominant (12/15) followed by P. vivax (3/15) (Table 2). A high proportion of An. funestus s.l. tested positive for P. falciparum (18/23, 78.3%) and infection was encountered across the agro-ecologic areas. Higher numbers of infectious An. funestus s.l. mosquitoes occurred outdoors compared to indoors but with comparable rates (16/20 vs 2/3; Table 2). One specimen each of An. coustani s.l., and An. rupicolus was infected with P. falciparum encountered outdoors in the plateau highland area. Also, one An. nili trapped outdoor tested positive for P. vivax in the semi-arid zone. Overall, infection prevalence was significantly higher outdoors relative to indoors (28/213 vs 8/186; χ2 = 8.42, df = 1, p = 0.004). In fact, 77.8% of the infectious mosquitoes were captured outdoors.
Molecular analysis of mosquitoes
The 52 An. gambiae s.l. samples including Plasmodium infected and non-infected, were found to be An. arabiensis.
To confirm the species identity of the An. funestus s.l. infected specimens, PCR of the ITS2 region and then sequencing and phylogenetic analyses were performed. Phylogenetic analysis of the successfully generated sequences (13/16) based on NJ trees showed the samples clustered in diverse clades that were well supported (Bootstrap (BS) values > 99%). Of the samples, one clustered with An. longipalpis C; one with An. theileri, 3 with a previously reported by uncharacterised species in Ethiopia (accession number OP950369), 1 with An. demeilloni, and the majority (n = 7) with yet-to-be described species recently detected in Ethiopia (accession number OP950370) and previously reported in Kenya as a malaria vector (accession no. KJ522813). Two positive specimens did not cluster with any known species (Fig. 4).
Neighbour-joining tree for sequences of mosquitoes (259–496 bp) morphologically scored as An. funestus group infected with P. falciparum sporozoites. Bootstrap support values are indicated at relevant nodes. Filled circles are sequences generated in this study from P. falciparum infected specimens; the novel cryptic species undescribed but previously reported in literature (pink/black), clustering with described species (blue/grey/green) or unreported species (red). Sequences of specimens harbouring Plasmodium sporozoite infection were deposited in GenBank (PP419038- PP419052).
Discussion
This study investigated and established baseline entomological determinants of malaria in Jabi Tehnan district in view of implementation of house screening for malaria control. The findings of the study depict an overall low density of anophelines (estimated by trap catches), but unexpected high infection rates in An. arabiensis and mosquitoes morphologically scored as belonging to the An. funestus group. The data on An. arabiensis is consistent with its primary vectoring role in the country1,2,29. Few parasite detections were associated with An. coustani and An. nili previously implicated as secondary vectors in parts of the country and elsewhere in Africa30,31,32. However, the association of wild An. rupicolus with the malaria parasite represents novel data for the country. Further, the high infection rate for the supposed An. funestus mosquitoes were unexpected which were found to not be any of the commonly known sibling species in the Funestus group. Instead, the results based on sequencing and phylogeny showed the specimens grouped in diverse well supported clades indicative of presence of different species. Two issues arise from the observed data. First is the likely case of misidentification. Some of the sequenced samples clustered with referenced samples of the species An. theileri, and An. demeilloni. Infection in An. demeilloni supports literature1,2 and its known role as secondary malaria vector in Ethiopia while infection in An. theileri represents a novel malaria parasite relationship in the country. Second is the presence of cryptic species, novel or previously reported that cannot be unravelled without molecular analysis33,34. Most of the infected specimens grouped with yet-to-be described Anopheles species previously implicated as a cryptic malaria vector in western Kenya highlands (accession no. KJ522813). This species was also recently reported in Ethiopia (accession number OP950370); however, we provide new data about its infection with P. falciparum in Ethiopia and its potential wider geographic distribution in eastern Africa. Likewise, An. longipalpis C incriminated in the dryland ecosystem of Kenya6,9 was observed for the first time harbouring Plasmodium parasite infection in Ethiopia. Together, the findings suggest possible evolution and shifting vectorial drivers of malaria transmission and importance of novel and perhaps cryptic vectors, in the malaria epidemiologic landscape as previously reported in East and southern Africa6,32,33,34. Whether this trend is a consequence of existing control measures requires close monitoring.
The high proportion of infected mosquitoes could indicate efficient transmission despite low overall mosquito densities. This was particularly obvious for the morphologically scored An. funestus mosquitoes which could be highly competent vectors and contributing to residual malaria transmission (RMT), i.e., persistence of malaria transmission following the implementation in time and space of a widely effective malaria programme35. This is particularly relevant given that a higher proportion of these mosquitoes occurred outdoors including those infected with Plasmodium parasites. The finding is indicative of outdoor malaria transmission that is less susceptible to traditional vector control methods (i.e., IRS, LLINs), and an important contributor to RMT6,35. Previous studies have argued for consideration of infection status over abundance or occurrence as a better risk indicator of dominance of a species in residual malaria6,35. While densities of An. arabiensis exceeded those of the morphologically scored An. funestus by higher order of magnitude (16:1 ratio), the latter still contributed more than 50% of malaria infections. The results have implications for effective endpoint indicators to monitor control progress; the proportion of infectious mosquitoes than routine monitoring of adult mosquito densities could represent the primary, most direct indicator of programme performance.
The extremely low densities yet high mosquito infectiousness remains paradoxical. Whether related to the competence of the populations as malaria is being controlled (i.e., hyper-endemic regions becoming meso-endemic) remains unknown. It has been postulated that efficiency of malaria parasite transmission (human-mosquito) increases as malaria is being controlled, likely attributed to human host factors relating to higher host gametocyte density36. Mosquito factors related to competence cannot be discounted if these species exhibit high survival abilities; these require further assessments. On the other hand, these mosquitoes could be poorly captured as adults in CDC light traps known to bias certain anopheline species37 likely explaining the observed low densities. Inclusion of human landing collections and animal or odour-baited tools should be explored in future studies37.
The uncovering of several cryptic species mostly undescribed via PCR and phylogeny highlights the limitation of morphologic identification. The high infection rates of these species suggest a substantial contribution to sustaining malaria in the study areas. As the threat of invasive species like An. stephensi13 gains traction, the role played by cryptic species in malaria transmission cannot be overlooked. Increasingly, reports of novel as well as cryptic vectors have been observed in malarious areas in Kenya and southern Africa6,32,33,34, indicating a growing menace that requires consideration. In the present study, only a subset of infected cohorts was processed for identification by PCR; thus, the species richness could even be much higher. Genetic characterisation of the vector populations using a suite of markers will aid in revealing the species richness and diversity in the studied ecologies and discriminate between vector and non-vector species that can mislead vector control strategies. A substantial proportion of Plasmodium infected mosquitoes were sampled outdoors (> 80%) representing a significant challenge beyond the reach of LLINs which are the primary commodities for malaria transmission control indoors. Thus, for malaria elimination to be achieved, outdoor biting fractions of anopheline vectors must also be effectively tackled6. Furthermore, despite the presence of bednets in households, mosquitoes were still captured indoors some of which were infected with malaria parasites. The factors which underlie this observation are unclear but could be associated with humans (e.g. net use patterns) or mosquitoes (e.g., insecticide resistance), that require further investigations.
Conclusions
The results from this study show that in the Jabi Tehnan district of northwestern Ethiopia, there was an overall low densities of Anopheles mosquitoes evident in the low trap catches, both indoors and outdoors. While An. arabiensis was the predominant vector, undescribed but previously reported or unknown species seem to support transmission on account of their high infection rate yet low abundance. Uncovering of these species, highlights the need to integrate molecular techniques into routine malaria entomological surveillance. The presence of highly infectious mosquitoes is indicative of efficient transmission despite low densities and has implications for effective endpoint indicators to monitor malaria control progress. Most of the infectious mosquitoes occurred outdoors (~ 80%) raising the urgent need to tackle such fractions out of reach of existing interventions mainly deployed indoors. The present data provide strong evidence of possible associated changes in the vectorial landscape. Thus, monitoring for changing and perhaps novel threats should be a prerequisite as malaria control towards elimination is being pursued.
Data availability
Sequences of mosquito specimens harbouring Plasmodium sporozoite infection were deposited in GenBank (PP419038- PP419052).
References
Girum, T., Shumbej, T. & Shewangizaw, M. Burden of malaria in Ethiopia, 2000–2016: Findings from the Global Health Estimates 2016. Trop. Dis. Travel Med. Vaccines. 5, 11 (2019).
Aschale, Y. et al. Systematic review of sporozoite infection rate of Anopheles mosquitoes in Ethiopia, 2001–2021. Parasit Vectors. 16, 437 (2023).
World Malaria Report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
World malaria report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO.
Tefera, S., Bekele, T., Getahun, K., Negash, A. & Ketema, T. The changing malaria trend and control efforts in Oromia Special zone, Amhara Regional State. North-East Ethiopia. Malar. J. 21, 128 (2022).
Kinya, F. et al. Outdoor malaria vector species profile in dryland ecosystems of Kenya. Sci. Rep. 12, 7131 (2022).
Tchouassi, D. P. et al. Characterization of malaria transmission by vector populations for improved interventions during the dry season in the Kpone-on-Sea area of coastal Ghana. Parasit Vectors. 5, 212 (2012).
Chaumeau, V. et al. Entomological determinants of malaria transmission in Kayin state, Eastern Myanmar: A 24-month longitudinal study in four villages. Wellcome Open Res. 3, 109 (2019).
Ogola, E. O. et al. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasit Vectors. 11, 577 (2018).
Carter, T. E. et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 188, 180–186 (2018).
Yared, S. et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malaria J. 19, 180 (2020).
Balkew, M. et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 13, 35 (2020).
Emiru, T. et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat. Med. 29, 3203–3211 (2023).
Lemma, W. et al. Anopheles cinereus implicated as a vector of malaria transmission in the highlands of north-west Ethiopia. Parasit Vectors. 12, 557 (2019).
Asale, A. et al. The combined impact of LLINs, house screening, and pull-push technology for improved malaria control and livelihoods in rural Ethiopia: Study protocol for household randomised controlled trial. BMC Public Health. 22, 930 (2022).
Aschale, Y. et al. Anopheles gambiae s.l (Diptera: Culicidae) seasonal abundance, abdominal status and parity rates in Metema-Armachiho lowland, Northwest Ethiopia. BMC Infect. Dis. 20, 333 (2020).
Tibebu, T. Evaluation The Impact Of World Vision Ethiopia, Water, Sanitation And Hygiene Project On The Community: The Case Of Amhara Region, West Gojam Zone, Jabi Tehnane Woreda (Doctoral dissertation, St. Mary's University, 2016).
Asale, A., Kussa, D., Girma, M., Mbogo, C. & Mutero, C. M. Community based integrated vector management for malaria control: lessons from three years’ experience (2016–2018) in Botor-Tolay district, southwestern Ethiopia. BMC Public Health. 19, 1318 (2019).
Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malaria J. 19, 70 (2020).
O’connor, C. T. The distribution of anopheline mosquitoes in Ethiopia. Mosqu. News. 27, 42–54 (1967).
World Health Organization. Manual on Practical Entomology in Malaria. Part 2 Methods and Techniques (Geneva, World Health Organization, H.M.S.O., 1975)
Snounou, G. et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61, 315–320 (1993).
Natchema, S. et al. Entomological longitudinal surveys in two contrasted eco-climatic settings in Cameroon reveal a high malaria transmission from Anopheles funestus associated with GSTe2 metabolic resistance. BMC Infect. Dis. 23, 738 (2023).
Nyasembe, V. O. et al. Adipokinetic hormone signaling in the malaria vector Anopheles gambiae facilitates Plasmodium falciparum sporogony. Commun. Biol. 6, 171 (2023).
Zianni, M. R., Nikbakhtzadeh, M. R., Jackson, B. T., Panescu, J. & Foster, W. A. Rapid discrimination between Anopheles gambiae s.s. and Anopheles arabiensis by High-Resolution Melt (HRM) analysis. J. Biomol. Tech. 24, 1–7 (2013).
Beebe, N. W. & Saul, A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction-restriction fragment length polymorphism analysis. Am. J. Trop. Med. Hvg. 53, 478–481 (1995).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
R. Version 4.3.2. The Foundation for Statistical Computing Platform (2023).
Getachew, D., Gebre-Michael, T., Balkew, M. & Tekie, H. Species composition, blood meal hosts and Plasmodium infection rates of Anopheles mosquitoes in Ghibe River Basin, southwestern Ethiopia. Parasit Vectors. 12, 257 (2019).
Goupeyou-Youmsi, J. et al. Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar. Parasit Vectors. 13, 430 (2020).
Fornadel, C. M., Norris, L. C., Franco, V. & Norris, D. E. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonot. Dis. 11, 1173–1179 (2011).
Stevenson, J. C. & Norris, D. E. Implicating cryptic and novel anophelines as malaria vectors in Africa. Insects. 8(1), 1 (2016).
Ogola, E. O., Chepkorir, E., Sang, R. & Tchouassi, D. P. A previously unreported potential malaria vector in a dry ecology of Kenya. Parasit Vectors. 12, 80 (2019).
Zhong, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci. Rep. 10, 16139 (2020).
Okumu, F. & Finda, M. Key characteristics of residual malaria transmission in two districts in South-Eastern Tanzania—Implications for improved control. J. Infect. Dis. 223(Suppl 2), S143–S154 (2021).
Churcher, T. S., Trape, J. F. & Cohuet, A. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat. Commun. 6, 6054 (2015).
Lima, J. B. P., Rosa-Freitas, M. G., Rodovalho, C. M., Santos, F. & Lourenço-de-Oliveira, R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? - A Review. Mem. Inst. Oswaldo Cruz. 109(5), 685–705 (2014).
Acknowledgements
The authors would like to acknowledge all personnel including the study household owners, district health office team and the health extension workers who participate in field sampling and facilitating the study. We are also grateful to Janet Kosgei and Masese Shadrack, at icipe Duduville Campus, Nairobi, for technical support. Special thanks to Dr Saliou Niassy for additional support for subsistence of the student (AKB).
Funding
AKB was supported by the German Academic Exchange Service (DAAD) In-Region Postgraduate Scholarship under the African Regional Postgraduate Programme in Insect Science (ARPPIS) tenable at the International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya, and a bursary award from the University of Pretoria as well. This study, conducted under the project Combatting Arthropod Pests for Better Health, Food, and Climate Resilience (CAP-Africa), was funded by the Norwegian Agency for Development Cooperation (Norad) (Grant number: RAF-3058 KEN-18/0005). Additional operational support for the study was provided by the Wellcome Trust International Intermediate Fellowship awarded to DPT (222005/Z/20/Z). We gratefully acknowledge the financial support for this research by the following organizations and agencies: the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Norwegian Agency for Development Cooperation (Norad); the German Federal Ministry for Economic Cooperation and Development (BMZ); and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors.
Author information
Authors and Affiliations
Contributions
A.K.B., A.A. C.M.M., D.P.T. conceived and designed the experiments. A.K.B., A.A. implemented mosquito surveys. A.K.B. conducted the experiments. A.K.B., F.K., D.P.T. analysed the data. C.M.M., B.T., D.P.T. contributed to resources and funding acquisition. A.A., C.L.S., A.A.Y., B.T., C.M.M., D.P.T., supervised the student (A.K.B.). A.K.B., D.P.T. wrote the draft manuscript. All authors reviewed subsequent versions of the manuscript and approved the final submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Belay, A.K., Asale, A., Sole, C.L. et al. Vectorial drivers of malaria transmission in Jabi Tehnan district, Amhara Regional State, Ethiopia. Sci Rep 14, 13669 (2024). https://doi.org/10.1038/s41598-024-64436-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64436-3
- Springer Nature Limited