Abstract
Background
Malaria vector control using insecticide-based approaches has proven to be an effective strategy. However, widespread insecticide resistance among malaria vector populations across sub-Saharan Africa threatens to derail control efforts. This study was conducted in Chikwawa district, an area in rural southern Malawi characterised by persistent malaria transmission and reports of insecticide resistance in the local mosquito population. The aim of the was to characterise the intensity of insecticide resistance within a population of Anopheles funestus sensu lato (s.l.), a major vector of malaria in this district.
Methods
Live adult females belonging to the An. funestus group were collected from households by indoor aspiration. The CDC bottle assay was used for phenotypic quantification of resistance to deltamethrin, permethrin and alpha-cypermethrin at 1×, 2.5×, 5× and 10× the recommended diagnostic dose for each of these insecticides. WHO tube assays were used to determine susceptibility to bendiocarb, dichlorodiphenyltrichloroethane (DDT) and pirimiphos-methyl insecticides at diagnostic concentrations.
Results
Anopheles funestus s.l. exposed to 10× the recommended diagnostic dose was highly resistant to alpha-cypermethrin (mortality 95.4%); in contrast, mortality was 100% when exposed to both deltamethrin and permethrin at the same dose. Despite showing susceptibility to deltamethrin and permethrin at the 10× concentration, mortality at the 5× concentration was 96.7% and 97.1%, respectively, indicating moderate resistance to these two insecticides. WHO susceptibility assays indicated strong resistance against bendiocarb (mortality 33.8%, n = 93), whereas there was full susceptibility to DDT (mortality 98.9%, n = 103) and pirimiphos-methyl (mortality 100%, n = 103).
Conclusions
Strategies for managing resistance to insecticides, particularly against pyrethroids, must be urgently implemented to maintain the effectiveness of insecticide-based vector control interventions in the area. Such strategies include the wide-scale introduction of third-generation synergist insecticide-treated bed nets (ITNs) and next-generation dual active ingredient ITNs. The use of effective non-pyrethroids, such as pirimiphos-methyl, clothianidin and potentially DDT, could provide a window of opportunity for indoor residual spraying across the district. This strategy would support the current Malawi Insecticide Resistance Management Plan which aims at rotating insecticides to minimise selection pressure and slow down the evolution of resistance to approved insecticides. These actions will help to prevent malaria vector control failure and improve progress towards malaria elimination.
Graphical Abstract

Similar content being viewed by others
Background
Good progress had been made in reducing the burden of malaria in the African region over the past two decades, despite stalls in progress and an increase in cases between 2020 and 2021 associated with disruptions in the implementation of control programs due to the COVID-19 pandemic [1]. In the African region, it was estimated that Plasmodium falciparum prevalence declined by 50% while incidence decreased by 42% between 2000 and 2019 [2]. In Malawi, the implementation of evidence-based policies, including the provision of indoor residual spraying (IRS), insecticide-treated bed nets (ITNs) and intermittent preventive therapy for pregnant women (iPTp), has contributed to a decline in malaria prevalence from 44% in 2010 to 22% by 2017 [3]. Mean modelled prevalence rates of P. falciparum declined from 29% in 2010 to 15% in 2017, with the percent decline ranging from 3 to 79% in some areas [4]. However, these gains are threatened by increases of insecticide resistance in malaria vector populations, both locally and across sub-Saharan Africa [5,6,7,8,9].
In Malawi, resistance to at least one insecticide has been reported in many areas of the country, including resistance to multiple insecticides in Chikwawa district. Chikwawa is an area along the Lower Shire Valley. Malaria transmission is persistent in this area, and it is known to be among the highest transmission risk areas, with P. falciparum prevalence rates as high as 15% (n = 9646) among participants, as reported in a recent study [10]. Anopheles funestus sensu stricto (s.s.), the primary malaria vector in this district, has shown reduced susceptibility to pyrethroids and carbamates, with studies indicating that this resistance is enzyme-mediated [11,12,13]. Anopheles arabiensis, a secondary vector in the district, has also shown reduced levels of susceptibility to pyrethroids [11, 12, 14]. However, the extent to which the rise of insecticide resistance might be hampering malaria control efforts in the district remains under-explored.
The level of pyrethroid resistance in Malawian An. funestus sensu lato (s.l.) has slowly risen since it was first reported in 2008 by Hunt et al. [15]. In 2015, reports from Chikwawa district indicated an increase in multiple resistance mechanisms in the local An. funestus population, namely over-expression of the pyrethroid resistance genes CYP6Pa/b and CYP7M7 and presence of the dieldrin resistance mutation (A296S-RDL) [13, 16, 17]. Such reports suggest that insecticide-based vector control tools (such as ITNs and IRS) may slowly lose their effectiveness over time, especially in the wake of multiple resistance, as recently reported in neighbouring Mozambique where the same resistance mechanisms in the local An. funestus population have induced a loss of efficacy of piperonyl-butoxide (PBO)-based ITNs [18], indicating other uncharacterised mechanisms at play. In Cote d’Ivoire, PBO pre-exposure of resistant An. gambiae s.l. did not yield full restoration of susceptibility to pyrethroids and neither was there full susceptibility to chlorfenapyr, a novel insecticide used for public health purposes in combination with pyrethroids in the newer generation ITNs [19].
There is a lack of compelling evidence-based data in Malawi on the nature of the relationship—if any−between insecticide resistance and the effect of ITNs on mosquito mortality in the field. Specifically, it is unknown whether malaria transmission control is compromised in people using ITNs and whether there is any relationship between mosquito resistance and epidemiological profiles of clinical malaria in the district. These factors are important in establishing the real impact of resistance to operational malaria control [20,21,22]. In this context, the aim of this study focused on determining the intensity of resistance of mosquitoes to deltamethrin, permethrin and alpha-cypermethrin (pyrethroids) and alternative non-pyrethroid insecticides. The study was part of a larger study which aimed at investigating the impact of insecticide resistance on vector control efforts in the district. The main driver of this study was the need to determine the operationally significant level of resistance that may serve as an early warning signal of potential loss of effectiveness in order to guide the local vector control intervention policy and minimise or prevent control failure.
Methods
Study design
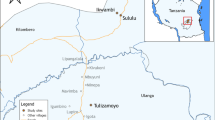
This entomological study was conducted after the rainy season, between April and July 2018, across two malaria sentinel sites, Chakanira (15°58′54.1″ S, 34°48′09.1″ E) and Sisewu (16°04′39.9″ S, 34°49′39.9″ E) villages in Chikwawa district, southern Malawi (Fig. 1). Malaria transmission is perennial in this area, but cases peak at the height of the rainy season, usually between November and April [23].
Mosquito collections
Collections of live adult mosquitoes were carried out inside houses using a battery-powered Prokopak aspirator (John W Hock Co., Gainesville, FL, USA)). As this method collects resting mosquitoes, there was a chance that some of the captured mosquitoes would had a blood meal or a partial blood meal. Hence, following capture, all mosquitoes were held for 1–2 days to allow them to rest and also digest any existing blood meals, if any [24], ensuring that the mosquitoes used in bioassays were free from any blood meals. The absence of any blood meal was also verified visually prior to the bioassay. Following capture in the field, mosquitoes were placed in paper cups which were placed in cooler boxes and provided with a sugar solution prior to transport to the laboratory in Blantyre. The local vectors are An. funestus s.s. (most abundant) and An. arabiensis [25]. All mosquitoes used in this study were wild-caught female An. funestus group mosquitoes of unknown age; no specimens were reared in the laboratory or insectary for the insecticide susceptibility tests. The WHO protocol allows for the direct use of wild-caught mosquitoes for bioassays as they are operationally relevant [26].
CDC bottle assays
Tests were performed on three replicates of 20–25 wild-caught adult females of unknown age. These mosquitoes were exposed for 1 h in 250-ml Wheaton glass bottles coated with a pyrethroid insecticide (permethrin, deltamethrin or alpha-cypermethrin) at a concentration of 1×, 2.5× DC, 5× DC and 10× of the respective diagnostic concentration (DC). Final concentrations were: (i) deltamethrin: DC = 12.5 µg/ml, 2.5× DC = 31.3 µg/ml, 5× DC = 62.5 µg/ml, 10× DC = 125 µg/ml; (ii) permethrin: DC = 21.5 µg/ml, 2.5× DC = 53.8 µg/ml, 5× DC = 107.5 µg/l, 10× DC = 215 µg/ml; and (iii) alpha-cypermethrin: DC = 12.5 µg/ml, 2.5× DC = 31.3 µg/ml, 5× DC = 62.5 µg/ml, 10× DC = 125 µg/ml. Negative controls were bottles coated with acetone, the solvent used to dilute the test insecticides. Knockdown was recorded at 10-min intervals from the time of exposure until 1 h post-exposure, at which time mosquitoes were then transferred to holding cages. A small piece of cotton wool moistened with 10% sugar solution was provided to the mosquitoes during the recovery period after the 1-h exposure, until 24 h post-exposure [26]. Mortality was recorded at 24 h post-exposure. The strength of resistance, also called intensity, was characterised by comparing the mortality at 24 h for each of the insecticides at the various multiples of the diagnostic dose (Table 1). Resistance intensity was classified as low, moderate or high using a threshold of 90% for determining a resistant population at the diagnostic dose (1× DC) [27, 28]. The CDC bottle assays were used to determine resistance intensity as they allow for evaluation of customised multiple concentrations of an insecticide [24].
WHO susceptibility tests
Wild-caught live female An. funestus mosquitoes were tested for phenotypic resistance to permethrin (0.75%), dichlorodiphenyltrichloroethane (DDT) (4%), bendiocarb (0.1%) and pirimiphos-methyl (0.25%) using standard WHO insecticide susceptibility assays [26]. The mosquitoes were exposed to papers treated with the respective insecticide for 1 h, knockdown was then assessed and the mosquitoes were transferred to holding tubes and provided with 10% sugar solution. Knockdown was assessed every 10 min from exposure to 1 h post-exposure, then mortality was recorded at 24 h post-exposure
Species identification by PCR
Following exposure the insecticides in the bioassay, a sample of the An. funestus population was subjected to species identification using PCR. Samples were analysed using standard operating procedures for identifying An. funestus group members [29, 30]. The PCR cycling conditions were: 94 °C for 2 min, 30 cycles at 94 °C for 30 s, 45 °C for 30 s, 72 °C for 40 s, with a final extension at 72 °C for 5 min.
Data analysis
Minitab statistical software 20 was used to analyse data. Most data were summarised using descriptive statistics, including mosquito counts and mean mortalities. Mosquito mortality was calculated by dividing the number of dead/moribund mosquitoes over the total of mosquitoes exposed to a given insecticide/dose, then expressed as a percentage. Group means of mortality rates for different insecticides and doses were compared using analysis of variance (ANOVA). F-test P-values were reported from the ANOVA tests. The Tukey’s post-hoc test was then used for pairwise comparisons of group means at a confidence level of 95%, with the aim to look for differences in mean mortality rates among the insecticides and given doses, and determine by how much they differed.
Results
A total of 1332 live wild An. funestus s.l. females were collected, of which 1153 came from Chakanira village and 179 from Sisewu village. These were used for the insecticide susceptibility tests (CDC bottle assays and WHO tube assays). A subsample of 400 (75 from Sisewu and 325 from Chakanira) was then selected for molecular identification. A small number of An. gambiae s.l. was also collected (1 specimen from Chakanira and 12 from Sisewu), but these were not processed further.
CDC bottle assays
The results of the pyrethroid intensity tests for the two villages are summarised in Table 1, which shows the numbers of mosquitoes tested per insecticide concentration (dose), percent mortality rates and adjusted mortality rates using Abbott’s formula [26].
Figures 2, 3, 4 show the percentage knockdown of An. funestus s.l. from Chakanira village to the four concentrations of each of the three pyrethroids. Mortalities were adjusted using Abbott’s correction formula as per the WHO test procedures [26].
Resistance intensity
Observed mortalities at the diagnostic dose (1× DC) were 82.6% (n = 38) for deltamethrin, 92.5% (n = 73) for permethrin and 83.1% (n = 52) for alpha-cypermethrin. These results confirm resistance to both deltamethrin and alpha-cypermethrin and suspected resistance to permethrin (Table 1; Fig. 5). For deltamethrin and alpha-cypermethrin, mortalities were still under the 90% threshold at 2.5× DC while they were in the range 90–98% at 5× DC in the Chakanira population [28].
For the given doses, ANOVA test results were as follows: 1× DC (ANOVA, F = 0.71, df = 2, P = 0.543); 2.5× DC (ANOVA, F = 0.49, df = 2, P = 0.636); 5× DC (ANOVA, F = 1.83, df = 2, P = 0.240; 10× DC (ANOVA, F = 4.77, df = 2, P = 0.087. Tukey’s post-hoc test was then used for pairwise comparisons of group means at a confidence level of 95% (Fig. 6). These test probabilities were all above the significance threshold (alpha = 0.05), which indicated that the test could not find an instance where the group mean for each insecticide at a given dose was statistically different from the others. In other words, the mean mortalities between the insecticides at each given dose belonged to the same population (were similar) and there was no evidence that any one of the insecticides performed better than the other at a given dose. This result was also indicated in the interval plots by the overlapping 95% confidence intervals of the means (Fig. 7).
WHO susceptibility tests
From the total live collections, 408 specimens were used for WHO susceptibility tests against DDT, bendiocarb, pirimiphos methyl and permethrin (Table 2). Only a single WHO susceptibility test was done for Sisewu village on permethrin, due to low numbers of mosquitoes collected.
Molecular species identification
The molecular study for species identification on 400 wild females revealed that 98% (n = 392) were An. funestus s.s., followed by Anopheles parensis (1.5%, n = 6) and Anopheles rivulorum (0.5%, n = 2). While the majority of these specimens were from Chakanira village (81.2%, n = 325), 18.8% (n = 75) were from Sisewu village and were identified as follows: 72 An. funestus s.s., two An. rivulorum and one An. parensis.
Discussion
The results of the present study highlight moderate to high resistance intensities in An. funestus from Chikwawa village against pyrethroid insecticides. Resistance to deltamethrin was confirmed in Anopheles funestus s.l. mosquitoes exposed to this insecticide at 1× DC, while in assays of these mosquitoes exposed to 2.5× DC and 5× DC, the mortality was < 98%. Susceptibility to deltamethrin was only restored when a 10× dose was used; hence, the classification of moderate intensity [28]. Similar results were observed for permethrin, with the only difference being that mortality at 1× DC was 92.5%, indicating possible resistance, which was confirmed when higher doses were used: at 2.5× and 5× DC, mortalities ranged from 90 to 98%. Susceptibility was also restored at 10× DC, similar to deltamethrin, indicating moderate resistance. Mortality to alpha-cypermethrin was < 90% at 1× DC and 2.5× DC, while it fell to 90–98% at 5× and 10× DC, indicative of a high intensity of resistance to this pyrethroid.
In addition to observing resistance against all three pyrethroids, we observed a low mortality (33.8%, n = 93) of An. funestus to the carbamate bendiocarb. However, intensity assays were not carried out in this study, and this observation warrants further study. Previous studies carried out in southern Malawi reported that An. funestus mortality to bendiocarb dropped from 60% in 2009 to 30.1% in 2014, then fell further to 19% in 2015 [16]. Unlike previous studies which reported moderate resistance or variable responses to DDT in the An. funestus s.s. population [13, 16], complete susceptibility to DDT (mortality 98.9%, n = 103) was observed in the current study. The present study also corroborates previous observations of full susceptibility to organophosphates, with an observed mortality of 100% (n = 103) to pirimiphos-methyl [11,12,13,14, 16].
One of the limitations of using wild mosquitoes is the possibility that they could have been exposed to various field conditions, including varying degrees of insecticide doses (e.g. on nets, wall surfaces or in agricultural fields), which potentially increases the risk of over-estimating resistance in the given mosquito population [26]. Older mosquitoes may tend to be weaker and hence more susceptible, risking an under-estimation of resistance [26]. However, this scenario more closely represents the actual conditions that any given intervention would encounter in the field; in addition, that wild-collected specimens are also the epidemiologically relevant cohort of vectors [23]. Changes in insecticide susceptibility in wild-caught vectors will thus more closely reflect changes in intervention efficacy [26, 31]. Another limitation of the current study is that we could not test many insecticides at all doses due to low sample numbers. However, even given the low numbers, we were still able to document the problem and its severity. Advanced molecular or biochemical tests for resistance mechanisms were performed as these were previously described in the study area by Riveron et al. [15].
The Malawi National Malaria Control Programme (NMCP) recently adopted an Integrated Vector Control Strategy (IVCS) 2020–2024 and the Insecticide Resistance Management Plan (IRMP) 2019–2022; these two programmes currently form the core of the malaria control programme in the country. For vector control, universal coverage of all at-risk populations with ITNs is the main approach together with complementary IRS in selected districts. In Malawi, ITNs are distributed via mass campaigns every 3 years, as well as through routine distribution in clinics to pregnant mothers and children under the age of 5 years, and IRS is implemented yearly in four districts [11, 32]. However, the implementation of IRS over the years has not been consistent in all the selected districts due to logistical and financial constraints and the rise in pyrethroid resistance. It was this increase in pyrethroid resistance that prompted the development of the strategic plan for monitoring and managing resistance (the IRMP) to counter the potential detrimental impact of resistance [11, 33]. The current study highlights the extent of the problem of resistance in Chikwawa village, warranting immediate action to safeguard progress in malaria control.
Although DDT is still effective against local mosquitoes and is recommended in the current vector control strategy, the potential use of this insecticide is hampered by concerns surrounding its use [34,35,36,37]. In 2006, the WHO made a call for the use of DDT for vector control and provided clear and strict guidelines which it adopted from the Stockholm Convention, stipulating how countries can incorporate DDT for malaria control in their malaria management programmes while strongly mitigating against any potential risks. Following the 2006 WHO recommendation for the use of DDT in highly endemic settings, Malawi announced that it would pilot a DDT IRS programme [36, 38]. Evidence shows that countries that continue both timely and correct use of DDT for IRS can reduce malaria transmission by up to 90% [39]. When South Africa reintroduced DDT spraying in 2000, they reduced the number of malaria cases and deaths drastically, managed to keep levels under control and, by 2012, set the goal to eliminate the disease entirely [40, 41]. The number of countries in Africa adopting DDT spraying for malaria control has risen, and since 2005, the list includes Malawi’s eastern and southern neighbour Mozambique, where An. funestus s.s. is a also key vector [42].
However, it has been argued that because Malawi has largely an agricultural economy, the benefits of using DDT for the fight against malaria may be outweighed by the potential detrimental effects on the agricultural industry and the economy at large [43]. This debate is also common in other African countries, and some studies have even suggested that the potential benefits of DDT for malaria vector control would be in the same order of magnitude as its detrimental effects on infant mortality [44]. Compounds such as clothianidin and deltamethrin/clothianidin combinations thus offer a good option for a rotation strategy with organophosphates in the IRS programme. The current vector control strategy also recommends PBO-nets and combination ITNs for areas with high levels of pyrethroid resistance [11, 33]. The present study supports and highlights the critical importance of this strategy as resistance intensity data have predictive value for making informed insecticide choices, to prevent compromised transmission control [27, 28, 31].
Clearly, a vector control strategy such as IRS that incorporates DDT and pirimiphos-methyl or other organophosphates may be a preferred means to manage the current situation of resistance. Such a strategy would serve as an interim solution as novel compounds are being developed or await registration and WHO approval and recommendation. Also, strategies that incorporate insecticides with new generation modes of action, or those that do not involve the use of insecticides at all, should complement ongoing interventions to avoid further risks that resistance may pose to the control programme. These findings also highlight the importance of making new generation ITNs (such as PBO-nets) readily and widely accessible to communities as they have demonstrated to have greater entomological and epidemiological impact than standard long-lasting insecticidal nets (LLINs) in settings of high insecticide resistance [31]. Such a multi-pronged approach will aid the country as it transitions towards elimination.
In this study, An. funestus s.s. was found to be the most abundant mosquito species, as also previously observed [12]. Anopheles parensis and An. rivulorum were also detected, and continuous surveillance and monitoring of these species needs to be routinely incorporated into control programmes, as these two species have been shown elsewhere to be contributing to transmission and also found biting humans indoors [45, 46]. Very few An. gambiae s.l. were collected (n = 13) in this study and no further investigations were done on these specimens.
Conclusion
The level of resistance in Chikwawa village has grown over the years, and current intensity determined in the present study warrants an urgent solution. Our results highlight the importance of a resistance management strategy to cushion against the potential negative impact of resistance. The use of third-generation synergists or dual active ingredient ITNs, and pirimiphos-methyl- or DDT-based IRS would help to control the current resistance intensity in An. funestus. Such steps will prevent control failure and safeguard the control gains that have been achieved so far. More research needs to be done to further validate the intensity of resistance against carbamates.
Availability of data and materials
Datasets used for the analysis of findings of this study are available upon request via the authors’ email addresses.
References
WHO. World malaria report 2021. Switzerland: World Health Organization; 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 31 May 2022 .
WHO. World malaria report. 20 years of global progress and challenges. Switzerland: World Health Organization; 2020. https://www.who.int/publications/i/item/9789240015791. Accessed 31 May 2022.
Mwendera CA, De Jager C, Longwe H, Kumwenda S, Hongoro C, Phiri K, et al. Challenges to the implementation of malaria policies in Malawi. BMC Health Serv Res. 2019;19:194. https://doi.org/10.1186/s12913-019-4032-2.
Chipeta MG, Giorgi E, Mategula D, Macharia PM, Ligomba C, Munyenyembe A, et al. Geostatistical analysis of Malawi’s changing malaria transmission from 2010 to 2017. Wellcome Open Res. 2019;4:57. https://doi.org/10.12688/wellcomeopenres.15193.2.
Tizifa TA, Kabaghe AN, McCann RS, van den Berg H, Van Vugt M, Phiri KS. Prevention efforts for malaria. Curr Trop Med Rep. 2018;5:41–50. https://doi.org/10.1007/s40475-018-0133-y.
Choi KS, Christian R, Nardini L, Wood OR, Agubuzo E, Muleba M, et al. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:464.
Moyes CL, Athinya DK, Seethaler T, Battle KE, Sinka M, Hadi MP, et al. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc Natl Acad Sci USA. 2020;117:22042–50. https://doi.org/10.1073/pnas.2006781117.
Owuor KO, Machani MG, Mukabana WR, Munga SO, Yan G, Ochomo E, et al. Insecticide resistance status of indoor and outdoor resting malaria vectors in a highland and lowland site in Western Kenya. PLoS ONE. 2021;16:0240771. https://doi.org/10.1371/journal.pone.0240771.
Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020;19:408. https://doi.org/10.1186/s12936-020-03483-3.
Andronescu LR, Buchwald AG, Coalson JE, Cohee L, Bauleni A, Walldorf JA, et al. Net age, but not integrity, may be associated with decreased protection against Plasmodium falciparum infection in southern Malawi. Malar J. 2019;18:329. https://doi.org/10.1186/s12936-019-2930-8.
Abeku T, Mzilahowa T, Beard J, Yihdego Y, Dandalo L, Mokuena M, et al. Insecticide resistance management plan, Malawi, 2019 – 2022. Lilongwe: Malaria Consortium. 2019.
PMI VectorLink Project. Malawi entomological monitoring annual report. Blantyre: Malaria Alert Centre. 2019.
Mzilahowa T, Chiumia M, Mbewe RB, Uzalili VT, Luka-Banda M, Kutengule A, et al. Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar J. 2016. https://doi.org/10.1186/s12936-016-1610-1.
Abeku T, Mzilahowa T, Yihdego Y, Dandalo L, Burnett J, Mokuena M. Integrated vector control strategy for malaria control, Malawi, 2020–2024. Lilongwe: Malaria Consortium; 2020.
Hunt R, Edwardes M, Coetzee M. Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasit Vectors. 2010;3:1.
Riveron JM, Chiumia M, Menze BD, Barnes KG, Irving H, Ibrahim SS, et al. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: a major concern for malaria vector control. Malar J. 2015;14:1–9.
Riveron JM, Ibrahim SS, Chanda E, Mzilahowa T, Cuamba N, Irving H, et al. The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genom. 2014;15:1.
Riveron JM, Huijben S, Tchapga W, Tchouakui M, Wondji MJ, Tchoupo M, et al. Escalation of pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of piperonyl butoxide–based insecticide-treated nets in Mozambique. J Infect Dis. 2019;220:467–75. https://doi.org/10.1093/infdis/jiz139.
Kouassi BL, Edi C, Tia E, Konan LY, Akré MA, Koffi AA, et al. Susceptibility of Anopheles gambiae from Côte d’Ivoire to insecticides used on insecticide-treated nets: evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar J. 2020;9(1):454. https://doi.org/10.1186/s12936-020-03523-y.
Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–9. https://doi.org/10.1016/s1473-3099(18)30172-5.
Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, Bigoga J, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:282. https://doi.org/10.1186/s12936-015-0782-4.
Wondji CS, Coleman M, Kleinschmidt I, Mzilahowa T, Irving H, Ndula M, et al. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc Natl Acad Sci USA. 2012; 109:19063–70. http://www.pnas.org/content/109/47/19063.full.pdf.
Kabaghe AN, Chipeta MG, Gowelo S, Mburu M, Truwah Z, McCann RS, et al. Fine-scale spatial and temporal variation of clinical malaria incidence and associated factors in children in rural Malawi: a longitudinal study. Parasit Vectors. 2018;11:1–11.
Brogdon W, Chan A. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. Atlanta:Centers for Disease Control and Prevention; 2010.
Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa district. Southern Malawi Malar J. 2012;11:1–9.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, 2nd ed; Geneva, Switzerland: World Health Organisation; 2018. https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf.Accessed 31 May 2022.
Bagi J, Grisales N, Corkill R, Morgan JC, N’Falé S, Brogdon WG, et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malar J. 2015;14:1–9.
Venter N, Oliver SV, Muleba M, Davies C, Hunt RH, Koekemoer LL, et al. Benchmarking insecticide resistance intensity bioassays for Anopheles malaria vector species against resistance phenotypes of known epidemiological significance. Parasit Vectors. 2017;10. https://doi.org/10.1186/s13071-017-2134-4. https://parasitesandvectors.biomedcentral.com/track/pdf/10.1186/s13071-017-2134-4.
Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69:200–5.
Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: culicidae) group. Am J Trop Med Hyg. 2002;66:804–11.
Gleave K, Lissenden N, Chaplin M, Choi L, Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst Rev. 2021;5:012776. https://doi.org/10.1002/14651858.CD012776.pub3.
Ye Y, Andrada A. Estimating malaria incidence through modeling is a good academic exercise, but how practical is it in high-burden settings? Am J Trop Med Hyg. 2020;102:701–2. https://doi.org/10.4269/ajtmh.20-0120.
WHO. Insecticide-treated nets for malaria transmission control in areas with insecticide-resistant mosquito populations: preferred product characteristics. Geneva: World Health Organization; 2021. https://www.who.int/publications/i/item/9789240018730. Accessed 31 May 2022.
News24: Malawian MPs in bizzare debate over use of DDT to fight malaria. 2010. https://www.news24.com/news24/Archives/City-Press/Malawian-MPs-in-bizarre-debate-over-use-of-DDT-to-fight-malaria-20150430. Accessed 31 Jul 2021.
Kabange SC. African countries debate using DDT in anti-malaria efforts. 2011. https://www.voanews.com/africa/african-countries-debate-using-ddt-anti-malaria-efforts. Accessed 31 Jul 2021.
van den Berg H. Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect. 2009;117:1656–63. https://doi.org/10.1289/ehp.0900785.
Coetzee M, Koekemoer LL. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu Rev Entomol. 2013;58:393–412. https://doi.org/10.1146/annurev-ento-120811-153628.
Lancet T. DDT for malaria control: the issue of trade. Lancet. 2007;369:248. https://doi.org/10.1016/s0140-6736(07)60119-6.
Rehwagen C. WHO recommends DDT to control malaria. BMJ. 2006;333:622. https://doi.org/10.1136/bmj.333.7569.622-b.
Hargreaves K, Koekemoer L, Brooke B, Hunt R, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–9. http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2915.2000.00234.x/abstract.
Maharaj R, Morris N, Seocharan I, Kruger P, Moonasar D, Mabuza A, et al. The feasibility of malaria elimination in South Africa. Malar J. 2012;11:423.
Coleman M, Casimiro S, Hemingway J, Sharp B. Operational impact of DDT reintroduction for malaria control on Anopheles arabiensis in Mozambique. J Med Entomol. 2008;45:885–90.
Chanda E, Mzilahowa T, Chipwanya J, Mulenga S, Ali D, Troell P, et al. Preventing malaria transmission by indoor residual spraying in Malawi: grappling with the challenge of uncertain sustainability. Malar J. 2015;14:1. https://doi.org/10.1186/s12936-015-0759-3.
Chen A, Rogan WJ. Nonmalarial infant deaths and DDT use for malaria control. Emerg Infect Dis. 2003;9:960–4. https://doi.org/10.3201/eid0908.030082.
Burke A, Dahan-Moss Y, Duncan F, Qwabe B, Coetzee M, Koekemoer L, et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar J. 2019. https://doi.org/10.1186/s12936-019-2889-5.
Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit Vectors. 2012;5:230. https://doi.org/10.1186/1756-3305-5-230.
Acknowledgements
We would like to thank all study participants, Health Surveillance Assistants (HSAs), village chiefs and all people who supported us during the study. We thank the Wits Research Institute for Malaria, Centre for Emerging Zoonotic & Parasitic Diseases at the National Institute for Communicable Diseases, and MAC Communicable Diseases Action Center for institutional space and support.
Funding
This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand, and funded by the Carnegie Corporation of New York (Grant No–B 8606.R02), SIDA (Grant No:54100029) and the DELTAS Africa Initiative (Grant No: 107768/Z/15/Z). The statements made and the views expressed are solely the responsibility of the researchers. LLK is supported by the DST/NRF South African Research Chairs Initiative (Grant No. 64763).
Author information
Authors and Affiliations
Contributions
JK, MC, and TM designed the study. JK conducted the field collection of specimen and bioassays. LK led the molecular laboratory work, JK drafted the original manuscript. MC, TM and LK led the interpretation of findings. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance for the study was obtained from the Human Research Ethics Committee at the University of the Witwatersrand in South Africa (Clearance number: M170662) and from the College of Medicine Research Ethics Committee (CoMREC) in Malawi (Clearance number: P.12/17/2324). Study participants were recruited as volunteers from the study area and informed consent was obtained before participation.
Consent for publication
All authors read and approved the final manuscript, and consented for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kumala, J., Koekemoer, L.L., Coetzee, M. et al. Intensity of insecticide resistance in the major malaria vector Anopheles funestus from Chikwawa, rural Southern Malawi. Parasites Vectors 15, 220 (2022). https://doi.org/10.1186/s13071-022-05299-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05299-3