Abstract
Background
Mindfulness-based interventions have been shown to improve psychological outcomes including stress, anxiety, and depression in general population studies. However, effectiveness has not been sufficiently examined in racially and ethnically diverse community-based settings. We will evaluate the effectiveness and implementation of a mindfulness-based intervention on depressive symptoms among predominantly Black women at a Federally Qualified Health Center in a metropolitan city.
Methods
In this 2-armed, stratified, individually randomized group-treated controlled trial, 274 English-speaking participants with depressive symptoms ages 18–65 years old will be randomly assigned to (1) eight weekly, 90-min group sessions of a mindfulness-based intervention (M-Body), or (2) enhanced usual care. Exclusion criteria include suicidal ideation in 30 days prior to enrollment and regular (>4x/week) meditation practice. Study metrics will be assessed at baseline and 2, 4, and 6 months after baseline, through clinical interviews, self-report surveys, and stress biomarker data including blood pressure, heart rate, and stress related biomarkers. The primary study outcome is depressive symptom score after 6 months.
Discussion
If M-Body is found to be an effective intervention for adults with depressive symptoms, this accessible, scalable treatment will widely increase access to mental health treatment in underserved, racial/ethnic minority communities.
Trial registration
ClinicalTrials.gov NCT03620721. Registered on 8 August 2018.
Similar content being viewed by others
Introduction
Background
Socio-economically disadvantaged adults, including racial/ethnic minorities, are at greater risk for a depressive episode in the last year [1,2,3,4,5]. Stress is an important factor in the development and chronicity of depressive symptoms [6, 7]. Psychosocial stress is associated with increased concentrations of C-reactive protein (CRP), IL-6, and IL-1β [8,9,10], inflammatory cytokines that have been consistently linked to depression [11, 12]. Black adults are disproportionally exposed to psychosocial stressors such as economic deprivation, unemployment, racism, and exposure to violence [6, 7, 13,14,15]. The disproportionate burden of stress experienced by Black adults places them at greater risk for depression.
Depression among socio-economically disadvantaged, Black adults is not adequately treated. Black adults are 40–60% less likely than those who are White to receive mental health treatment [16,17,18] and less likely to receive adequate treatment [19, 20]. Negative attitudes toward depression treatment [21], stigmatizing beliefs about mental illness and treatment [22,23,24], shame and self-blame associated with treatment-seeking [25], and higher levels of medical mistrust [26] are barriers to receiving treatment among Black adults.
Those who do receive depression treatment are more likely to find it in primary care versus specialty mental health clinics [27,28,29]. Federally Qualified Health Centers (FQHC) are safety net providers of primary, preventive, and specialty outpatient healthcare, including mental health/substance abuse, to individuals in underserved communities. Integration of evidence-based depression treatments in FQHCs is economically justified, likely to reduce systemic and psychological barriers, and may facilitate access to and engagement in mental health care [30,31,32].
The US Department of Health and Human Services Agency for Healthcare and Research Quality recommends mindfulness-based interventions in their guidelines for non-pharmaceutical management of depression in adults [33]. Mindfulness-based Stress Reduction (MBSR), an 8-week group intervention that teaches mindfulness skills through a range of formal and informal practices including mindfulness of breath, bodily sensations, sounds, thoughts, and everyday activities, is one of the most widely used mindfulness interventions [34]. Mindfulness-based interventions have collectively been shown to improve physical and mental health [35,36,37,38] and reduce depression symptom severity (Cohen’s d ~.59–.69) [37, 39]. MBSR specifically has been associated with improved stress biomarkers including reduced blood pressure [40,41,42,43,44,45], reduced inflammatory response [46, 47], and increased immune response [48].
There is a paucity of research examining the effectiveness of mindfulness-based interventions among socio-economically disadvantaged, racial/ethnic minority adults. A recent systematic review examining the socio-economic and demographic characteristics of participants in mindfulness-based randomized controlled trials found an over-representation of participants who were White, held a college degree, were employed, and had an annual income of over $40,000 [49]. Only one randomized controlled trial has been conducted in the USA to test the effectiveness of MBSR or Mindfulness-Based Cognitive Therapy (MBCT) in improving psychological outcomes among Black adults in a community-based setting [50]; however, effectiveness data from this study have not been published [50, 51].
In a meta-analysis of acceptance and mindfulness-based treatments with underserved populations (N= 35) [52], only two studies evaluated MBSR or MBCT with majority racial/ethnic minority adults [53, 54]. One of the two studies found that participation in MBSR among socio-economically disadvantaged, racial/ethnic minority adults was associated with significant changes in reported psychological outcomes compared to a no-contact control (Hedge’s g = .67) [53]. More recently, another study evaluated the effectiveness of MBCT in improving psychological outcomes of racial/ethnic and sexual minority women and demonstrated improvement in depressive and stress symptoms in addition to satisfactory feasibility and acceptability of the intervention [55].
The minority stress theory [56], based on stress and coping theory, frames our proposed research. Stress and coping theory states that stress occurs when the demands of a situation are perceived as exceeding the resources available and coping is the cognitive and behavioral efforts put forth to deal with the stressful situation [57]. The minority stress theory distinguishes the excess stress that individuals belonging to stigmatized social categories, including categories related to socioeconomic status, race/ethnicity and gender, experience that likely have physical and mental effects. This theory accounts for the stress associated with personal events (i.e., trauma) and environmental and social conditions.
Appropriately tailored interventions and service delivery systems are urgently needed to increase access to and engagement in evidence-based mental health treatments among individuals who are at greater risk for depression but have less access to treatment. In this paper, we will describe the rationale behind, design, and implementation of a randomized controlled trial to evaluate the comparative effectiveness of a mindfulness-based intervention on depressive symptoms among predominantly Black women in a Federally Qualified Health Center network.
Prior work
The PI led a series of pilot studies that demonstrated the acceptability, feasibility, and impact on primary outcomes of a mindfulness-based intervention (M-Body) among Black women with depressive symptoms in an FQHC. M-Body is a version of the evidence-based Mindfulness-based Stress Reduction (MBSR) program that has been tailored for the FQHC patient population and healthcare service delivery setting [58].
Women ages 18–65 with a positive depression screen were recruited from a FQHC on the South Side of Chicago. Interested, eligible participants were invited to participate in the 8-week, 90-min-per-session, M-Body group intervention. Depression (Inventory of Depressive Symptomatology – Clinician Rated and Self-Report and Mini International Neuropsychiatric Interview 6.0, Quick Inventory of Depressive Symptomatology – Clinician Rated), well-being (Ryff Psychological Well-Being Scale – 42 item), mindfulness (Five Facet Mindfulness Questionnaire), stigma (Depression Self Stigma Scale), functioning (World Health Organization Disability Adjustment Scale 2.0 – 12 item), and stress (Perceived Stress Scale) were assessed as primary outcomes at baseline and 8 and 16 weeks.
In total, 161 women were referred, 114 screened, 102 eligible, and 74 enrolled in the single-arm M-Body intervention. All participants except one were of Black race. The mean age was 49 years, the majority were single (58%), 61% had a personal income of $19,999 or less, 28% were unemployed, 43% had Medicaid, and 21% had Medicare. The majority of participants self-referred.
The first two groups (N=11, N=13) were led by the PI, who has extensive experience with mindfulness-based interventions. Participants attended an average of 6.1 sessions and completed 156 min of home practice of formal mindfulness skills (meditation, body scan, yoga) per week; retention rate at 16 weeks was 90%. Participants demonstrated a significant decrease in stress (t = 2.65, p=.01, d=1.06) and increase in mindfulness (t=−2.21, p=.04, d=−0.88) from baseline to 8 weeks. From baseline to 16 weeks, participants demonstrated a significant decrease in depression (t=2.14, p=.04, d=.84) and stress (t=2.61, p=.02, d=1.02) and significant increase in mindfulness (t=−2.71, p=.01, d=−1.06) [58].
Participants from these first two groups were invited to participate in follow-up focus groups. In the focus groups, participants reported that the group helped them to feel calm and relaxed, manage anger, and gain control of thoughts, emotions, behavior, and ability to be in the present moment. They noted that the group being in a familiar location, bus transportation support, shorter length of classes, course materials (manual and audio CD), and the group format were factors that facilitated participation. One participant stated “We, black women, always got to be ‘superwomen’ we have to be able to do everything and that brings about a lot of stress…I came because I had put so much stress on myself and this helped me understand that I have an opportunity to learn how to calm myself down and recognize what’s going on.” The focus groups confirmed that the modifications that were made to the MBSR format and surface content were acceptable [59].
The third and fourth groups (N=19, N=22) were led by a staff health educator at the FQHC who received streamlined training in the intervention. The PI, in conjunction with another experienced mindfulness teacher, developed the training protocol and manual and trained the staff. Participants attended an average of 5.9 sessions and completed 123 min of home practice of formal mindfulness skills (meditation, body scan, yoga) per week. The retention rate at 16 weeks was 85%. Participants demonstrated a significant decrease in depression (t =8.46, p<.001, d=.69) and stress (t=4.83, p<.001, d=.69) and increase in mindfulness (t=−4.5, p<.001, d=−.56), functioning (t=3.4, p<.001, d=.43), and well-being (t=4.48, p<.001, d=−.56) from baseline to 8 weeks. From baseline to 16 weeks, participants demonstrated a significant decrease in depression (t=5.51, p<.001, d=.83) and stress (t=5.90, p<.001, d=.95) and increase in mindfulness (t=−4.71, p<.001, d=.68), functioning (t=3.51, p<.001, d=.53), and well-being (t=−4.17, p<.001, d=−.69).
Overall, outcomes among participants in the experienced instructor group were comparable to those in the novice instructor group. These preliminary data provide evidence of the acceptability, feasibility, and improved depression and stress outcomes over time from the M-Body intervention led by a FQHC staff member with streamlined teacher training [60].
Study aims
This study is a two-arm group-treated individually randomized controlled trial to test the clinical effectiveness of a mindfulness-based intervention (M-Body) on reducing depressive symptoms among socio-economically disadvantaged, predominantly Black women in an FQHC. The M-Body intervention will be led by trained FQHC staff; fidelity to the intervention, adherence, and competence will be continuously assessed. Feedback on factors relevant to the implementation of the intervention in the FQHC, including attitudes toward implementation of intervention, implementation leadership, and attitudes toward adoption and clinical effectiveness of evidence-based practices, will be obtained by convening quarterly workgroups and individual semi-structured interviews with FQHC staff and leadership.
Our specific aims and corresponding hypotheses are to:
-
1.
Examine the effectiveness of a mindfulness-based intervention (M-Body) compared to enhanced usual care on reducing depressive symptoms over a 6-month period among racial/ethnic minority adults at a Federally Qualified Health Center (FQHC).
-
H1a: We hypothesize that participants in the M-Body arm will experience a significantly greater reduction in depressive symptoms than those in the enhanced usual care arm.
-
H1b: We hypothesize that greater class attendance in the M-Body intervention will be associated with a greater reduction in depressive symptoms.
-
-
2.
Conduct a broad assessment of organization and individual agency factors related to the implementation of the M-Body intervention at a FQHC using a mixed methods approach.
Methods
Overview
Adults who demonstrate a minimum threshold of depressive symptoms will be randomly assigned to either an eight-session mindfulness group intervention or enhanced usual care (“control”). Participants will be followed for 6 months after randomization, with assessments at baseline and 2, 4, and 6 months post-baseline (Table 1). During an in-person visit at baseline, a trained research assistant will administer the clinical instruments, collect stress-related biomarker data, and oversee the completion of the self-report surveys. At follow-up, participants will complete self-report surveys and stress-related biomarker data collection during an in-person visit and an independent evaluator (trained research assistant) will contact them by phone to assess the primary outcome. The primary outcome will be depressive symptoms at 6 months post-baseline. To take advantage of the repeated outcome assessments, secondary outcomes will compare 2-, 4-, and 6-month measurements of depression, anxiety, trauma symptoms, and anger. Northwestern University’s Institutional Review Board (IRB) and the research committee of the FQHC approved the study protocol.
Study setting
This study will be conducted in partnership with the Near North Health Services Corporation, a group of eight FQHC’s in Chicago, IL, USA. The two primary sites for recruitment are accessible via public transportation. They are centrally located on a bus route. The FQHC network has approximately 46,130 patient visits per year; the majority of patients are racial/ethnic minority (71% Black, 23% Hispanic), uninsured (43.2%), and living at or below the poverty line (74%).
Recruitment
Patients at the FQHC who had a positive Patient Health Questionnaire-9 (PHQ-9) screen (>5) at a recent primary care visit will be recruited to participate in the study. Initial contact will be made via postal mail, followed by phone calls and text messages describing the study and inviting participation. Additionally, the FQHC healthcare providers will provide a brochure and overview of the study to all patients who exhibit depressive symptoms and can directly refer their patients to the study team. Research staff will visit the clinic at least weekly to encourage recruitment and talk to interested participants. Self-referral will be encouraged via posted flyers and brochures within the FQHC, advertisement on social media (Facebook, Twitter, and Instagram), posts on the Near North website and Facebook page, and brochure distribution at neighborhood business.
Interested persons will be offered the opportunity to participate in the study. For self-referrals and FQHC staff referrals, verbal consent to conduct the screening and eligibility assessments will be obtained by phone; for onsite referrals, this consent will be obtained in writing. Written informed consent for all eligible study participants will be obtained in person at the first study visit (baseline assessment) by a research assistant.
Participant eligibility
Participants will be eligible to enroll in the study if they are between the ages of 18 and 65 years, fluent in English, and enrolled in care at a FQHC and have score greater or equal to 5 on the PHQ-9, which indicates mild depressive symptoms. Exclusion criteria will include a suicide attempt in the past 30 days; endorsement of severe suicidal thoughts on the PHQ-9 (item 9); a current meditation practice defined as four or more sessions per week; or employment at the FQHC. Mental health treatment (individual or group psychotherapy and psychiatric medication) is permitted for the duration of the trial.
Study design
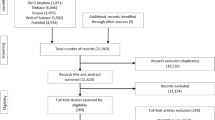
This study will be a block-randomized controlled trial with two arms: the M-Body intervention arm and a control arm consisting of enhanced usual care. Participants will be recruited in cohorts and randomized to conditions within cohorts; this is equivalent to a block randomized experiment with cohorts corresponding to blocks. Outcomes will be assessed at baseline and at 2, 4, and 6 months after baseline. Measurements will be obtained through a clinical interviews and completion of self-report surveys at baseline. At follow-up assessments, measures will be obtained through self-reported surveys and a phone evaluation with an independent evaluator. Figure 1 describes the intended study timeline and randomization numbers by group.
M-Body intervention arm
The M-Body intervention will consist of 8 weekly, 90-min group sessions led by trained FQHC staff. The M-Body curriculum will include the core content from Mindfulness-based Stress Reduction (MBSR [34];) and has been tailored to fit within the context of the FQHC setting and patient population (see Table 2 for a list of didactics). The course curriculum will include a manual and audio (CD or digital electronic file), in session and at home components. Each session will include a brief check-in, didactic, skill practice, inquiry, and review of homework. Following a skill practice, participants will engage in inquiry, a dialogue about what was noticed during the practice. Sessions will conclude by reviewing homework for the upcoming week. Participants will be asked to engage in formal home practice “as much as they can” and informal mindfulness practices. Formal practices will include sitting meditation, body scan, and hatha yoga. Informal practices will include cultivating awareness in everyday activities such as eating, walking, and communicating, noticing pleasant and unpleasant events and practicing STOP (Stop, Take Stock, Observe, Proceed). Participants will record the type and minutes of formal practice they complete and will record their informal practice on worksheets and turn it into the instructor weekly.
Control arm
To compare the effectiveness of the M-Body intervention against the existing real-world standard practice for depression treatment in the FQHC setting, usual care was selected as an appropriate control. The usual care to be offered here may be considered minimally enhanced because it will likely include more systematic evaluation and monitoring of depressed adults than may be typical of a community health center. Participants in this arm will receive feedback about their depression score, as well as an opportunity to ask questions of a sensitive and knowledgeable clinician. Participants will be able to contact the study team about increasing symptoms or urgent situations at any point in the study period. Participants will be provided with a brief one-page brochure on stress management skills. After their participation in the study ends, they will be invited to participate in ongoing M-Body group classes with no assessments or added incentives.
Intervention training
The PI will work with the FQHC leadership to identify staff who are qualified to lead the M-Body intervention. The PI and an experienced mindfulness teacher will train FQHC staff in the M-Body intervention using the same training framework established during the pilot work [60]. The instructor training was informed by the Mindfulness-based Teacher Training Program outlined by Crane et al. [61]. The instructor training includes the following: (1) Foundational training includes (a) attending an 8-week, 2.5-h-per-session MBSR course with an experienced teacher, and (b) developing a personal mindfulness practice. (2) Basic teacher training includes (a) participation in an 8-h professional training workshop led by the PI and another experienced mindfulness instructor on mindfulness theory, science, principles, pragmatics, and techniques; (b) continuing a personal mindfulness practice; (c) teaching a course; and (d) regular supervision with the PI.
All M-Body classes will be audio/video recorded. The PI will review all audio/videotapes from the previous week’s session and provide feedback to the instructor during weekly 1-h supervision. Preferred qualifications for the instructor are a minimum of a masters’ level education in psychology, social work, nursing, or other related fields. To date, two instructors were health educators, one was a licensed clinical professional counselor, one a licensed clinical psychologist, and one a licensed clinical social worker.
Participation incentives
All participants will be provided with single-ride public transportation cards to attend classes and study visits as needed. All study participants will receive a $20 gift card after completing the baseline assessment, and a $30 gift card after completing each of the 2-, 4-, and 6-month assessments for a total of $110 in gift cards for full participation. All M-Body participants will receive an instructional manual and audio CD, yoga mat, tote bag, and pen for use inside and outside of group sessions. Light meals will be provided at each of the in-person sessions.
Randomization
Following consent but prior to randomization, participants will complete a clinical interview and baseline surveys, and stress biomarker data will be collected. We will then implement a block randomization procedure, with each participant in a cohort randomly assigned to 1 of the 2 arms (overall allocation ratio, 1:1). Block randomization with randomly varying block sizes was chosen to prevent prediction of assignments and thereby protect from selection bias. Randomization will be stratified by gender, initial severity of depression (Inventory of Depressive Symptomatology- Clinician Administered (IDS-C) ≥38), and receipt of treatment for depressive symptoms (antidepressants and/or psychotherapy versus none) [62]. To avoid all possibility of interference from the investigators, randomization will be fully automated, with the randomization sequence prespecified in R [63] and implemented through Research Electronic Data Capture (REDCap) [64, 65]. After randomization, research staff will provide participants with a sealed envelope containing their condition assignment. Neither the participants, study coordinators, statistical analysis team, nor the treatment providers will be blinded to treatment assignments. Independent evaluators and study co-investigators will remain blinded. To maintain the overall quality and legitimacy of the clinical trial, unblinding should occur only in exceptional circumstances when knowledge of the actual treatment is absolutely essential for further management of the participant. The Principal Investigator will discuss with the co-investigators and DSMB if she believes that unblinding is necessary.
Retention and adherence strategies
To enhance recruitment and recognize the contributions of participants in the UC condition, these individuals will be invited to participate in the M-Body group at the end of 6 months after all data have been collected. To improve engagement in the intervention, the research staff will phone, email, or text subjects whenever they miss a session to encourage participation. Reminders will be sent to all participants to remind them of upcoming data collection and the incentives they will receive. The study team will make every effort to be flexible and accommodate participant schedules.
Measures
For a brief overview of all measures and assessment times, see Table 3.
Primary outcome
Depressive symptoms, measured 6 months after baseline, will be the primary outcome of this study, assessed using the Inventory of Depressive Symptomatology- Clinician Administered (IDS-C) [87]. The IDS-C is a 30-item scale that was administered by an independent evaluator who is blind to study condition at baseline and 2, 4, and 6 months. The IDS-C has high internal consistency with a Cronbach’s alpha of .92 [87]. The Longitudinal Interval Follow-up Evaluation – II (LIFE) will be used to assess symptoms at follow-up periods [72].
Secondary outcomes
Major depressive disorder and persistent depressive disorder will also be assessed as a secondary outcome using The Mini International Neuropsychiatric Interview (MINI 6.0) at baseline [71]. The MINI is a short clinician-administered diagnostic interview that assesses current and lifetime psychiatric disorders based on the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders – 4th edition (DSM-IV) and International Classification of Diseases (ICD-10) [71]. Study team members administering the MINI were trained by an expert clinician and rated mock interviews until they reached acceptable reliability with the expert clinician.
Longitudinal measures of depressive symptoms, generalized anxiety, anger, and trauma symptoms will also be secondary outcomes (Table 3). Generalized anxiety will be measured using the Generalized Anxiety Disorder-7 Scale (GAD-7) [66]. The GAD-7 is a 7-item self-report measure of generalized anxiety symptoms. Scores of 10 or greater are predictive of a likely GAD diagnosis. Anger will be measured using the Anger Self-Report Questionnaire, which is a single-factor, 30-item self-report measure of anger [67]. Both anxiety and anger will be assessed by self-report at baseline and 2, 4, and 6 months. Post-traumatic stress will be measured using the PTSD Checklist for DSM-5 (PCL-5) [68]. The PCL-5 is a 20-item self-report measure of PTSD symptom severity, administered at baseline and 6 months.
Process outcomes
M-Body group audio/video recordings will be used in intervention fidelity assessments. Coders will review the audio/video recordings of the M-Body sessions and assess fidelity, adherence, and competence using the Mindfulness-based Interventions – Teaching and Assessment Criteria scale [88]. Fidelity assessments will be completed for each instructor, each session, for every group for the duration of the study. These data will be used to monitor fidelity to intervention delivery and improve staff training.
Over the course of the M-Body study, all stakeholders will complete measures to assess implementation readiness, feasibility, and fidelity. Stakeholders will include the FQHC Medical Director, Director of Behavioral Health, Director of Quality Assurance Improvement, Instructors, and any other FQHC staff who will be directly involved in the research study. A complete list of implementation measures can be found in Table 3.
Covariates, moderators, and mediators
A secondary aim of this study is to investigate M-Body effects on specified biological, psychological, and socio-environmental mechanisms. To this end, we will collect data in three realms: social and environmental factors, psychological outcomes, and stress-related biomarkers.
We will consider age, socioeconomic characteristics, and social and environmental factors as potential moderators of the intervention effects. Socioeconomic characteristics will include education (high school diploma or less versus some college or more), employment (unemployed, part-time, full-time), and income (below versus above the federal poverty line). Social and environmental factors will be assessed at study baseline using (1) the Social Problems Questionnaire (SPQ) as a proxy for environmental stress; (2) spirituality; (3) social support; and (4) a summary of traumatic life events via the PTSD Checklist for DSM-5 with Life Events Checklist for DSM-5 (LEC-5) and Extended Criterion A as a proxy for long-term stress.
Potential mediators will include the following: (1) mindfulness measured via Five Facet Mindfulness Questionnaire (FFMQ); (2) stress, measured via Perceived Stress Scale; (3) Cognitive Regulation measured via Cognitive and Emotion Regulation Questionnaire (CERQ-SF); (4) Emotion Regulation measured via Difficulties in Emotion Regulation Scale (DERS-SF); (5) Reflection and Rumination measured via Reflection and Rumination Questionnaire; (6) Self-Compassion via Self Compassion Scale; and (7) stress-related inflammatory biomarkers, including c-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and IL-10.
Stress-related inflammatory biomarkers will be analyzed using dried blood spot (DBS) samples. Trained study personnel will collect one to five drops of free-flowing capillary blood on standardized filter paper of the same grade used for neonatal screening using a disposable and sterile micro-lancet. Samples will be covered and left to dry overnight and then frozen at −30 °C until analyzed. Samples will be analyzed for CRP using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) previously validated for DBS. IL-6, TNF-α, and IL-10 will be quantified using a highly sensitive multiplex electrochemiluminescent immunoassay that allows for the simultaneous quantification of each cytokine in DBS. The protocol has excellent lower limits of detection (0.2 to 0.7 pg/mL), precision (intra-assay CVs<7.5%), and reliability (inter-assay CV<9%) and high correlations between matched plasma and DBS samples across the entire assay range. We will be applying standard laboratory quality control procedures, including running samples in duplicate, inspecting calibration curves for fit and day-to-day variation, and measuring quality control samples with each assay.
Data collection, quality control, and confidentiality
Data handling procedures for data transfer, entry, and maintenance will be performed by trained study personnel under the supervision of Northwestern Information Technology Services. Self-reported data will be collected via online assessment directly into a Northwestern University-hosted REDCap database. We will collect information at every stage of recruitment, randomization, and intervention so that we can report patient flow according to the CONSORT (CONsolidated Standards Of Reporting Trials) guidelines [89].
Protection of human participants and assessment of safety
This trial will be monitored for safety by an independent Data and Safety Monitoring Board (DSMB), composed of two professors of psychology and one psychiatrist with expertise in randomized controlled trials, mood disorders, women’s mental health, and mindfulness interventions. Protocol violations, including inadequate or delinquent informed consent, failure to satisfy inclusion criteria, improper unblinding, incorrect or missing baseline assessments, mishandled samples, materially inadequate record keeping, missed primary outcome data collection, intentional deviation from protocol, Good Clinical Practice (GCP), or regulations by study personnel, and repeated protocol non-compliance will be reported to the Northwestern IRB immediately. Serious adverse events (SAEs), including psychiatric hospitalization determined to be study related, major adverse cardiac events (stroke, myocardial infarction), and death, will be reported to the IRB by filing a report on the Northwestern IRB website. The trial will be stopped if the DSMB determines that there is a risk of SAEs in the intervention arm; in this case, the DSMB has power to recommend study continuation, modification, or termination the study.
Spirit reporting guidelines are being followed in the conduct of this trial [90].
Statistical plans
Sample size
Our sample size was chosen to ensure adequate power to detect significant differences in the primary study outcome, IDS-C, between the intervention arm and enhanced usual care. We plan to enroll both sexes, but we anticipate 85% of participants to be female; thus, power considerations are stratified by sex. The project will enroll 274 adults (226 women, 48 men; ages 18–65 years) who meet previously described eligibility criteria.
To determine study sample size, we assumed a two-sided 5% type-I error rate, standard deviation of up to 14 units for the IDS-C (according to preliminary data on the IDS-self report), and a meaningful difference across arms of six points on average on the IDS-C [58]. Analyses will use linear mixed effects models with random effects for blocks and fixed effects for treatment assignment. Since participants will be enrolled in groups of approximately 15 at a time and intervention will be delivered in a group setting, there is a possibility of an intra-cluster correlation (ICC). Previous literature has suggested that ICC tends to be low in group intervention studies, so here we considered ICC values 0.001–0.01 [91]. Under these assumptions, eight groups of women per arm (each of size 12; i.e., 96 women per arm) or 192 women total would ensure 84% power to detect a meaningful difference across arms. To combat anticipated 15% study attrition, we plan to enroll 226 women [59]. Power analyses were conducted via simulation in the R programming language (version 4.1.1) and verified using PASS (version 15) [92]. Note that our initial design and power analyses involved cluster randomization rather than block randomization. Because cluster randomized trials tend to have lower power, our original power analyses suggested 240 women would be required for 80% power, and thus, opting for block randomization reduces the number of participants required.
Additionally, we plan to enroll two groups of men, each of size approximately 12 (i.e., 48 men). We do not anticipate that we will have adequate power to detect meaningful differences across arms in men, and thus, analyses in men will be hypothesis-generating and assess feasibility in this population.
Though our power is greater than 80% for our primary aim, we anticipate an ultimate sample size of 192 women provides 80% power to detect small to moderate standardized effect sizes (d≥0.4) in secondary outcomes under the same assumptions. With respect to correlation coefficients (since partial correlation coefficients will play a role in mediation analyses), we will have 80% power to detect a correlation coefficient of 0.2 at the 5% level in either the overall sample or just in women, and a correlation coefficient of 0.39 or larger in men.
Statistical analyses
Proportions, means, medians, and ranges will be used to summarize baseline distributions of demographic factors and clinical mental health presentation (i.e., baseline depression, anxiety, trauma, stress) at baseline stratified by study arm. Exploratory data analyses will be conducted wherein individual trajectories of each primary and secondary study outcome over 6 months by study arm are visualized.
Primary outcome analyses
We intend to conduct all primary statistical analyses according to the intention-to-treat (ITT) principle. We will estimate the average treatment effect for participants classified by study arm, independent of participation in any M-Body classes. This approach is conservative because it protects the effect of randomization from confounding introduced by subject dropout and crossover. We will employ both a classic ITT analysis as well as a modified ITT analysis, wherein all participants completing at least one follow-up visit will be included, regardless of adherence. This will allow us to understand the bias introduced by study dropout.
The primary outcome analysis will consist of a linear mixed model (LMM) in which mean 6-month IDS-C will be compared across study arms (fixed effect), controlling for gender, baseline IDS-C scores, and baseline receipt of psychotherapy, the variables upon which randomization is stratified. We will include a random group effect as this is an individually randomized group-treated design. This approach will account for within-group associations to more precisely estimate intervention effects. The coefficient for study arm will address the question: “for two patients with the same baseline depressive symptoms, gender, and baseline receipt of psychotherapy, if one is given the M-Body intervention and the other enhanced usual care, will the patients have different depressive symptoms after 6 months?”
As a sensitivity analysis, we will estimate the average treatment effect among the treated. This analysis will allow us to estimate among individuals randomized to the intervention whether there is a dose-effect relationship between the number of classes attended and depressive symptoms. This analysis, done only in the intervention arm, will consist of a LMM in which 6-month IDS-C will be regressed upon the number of M-Body sessions attended (fixed effect), controlling for gender, baseline IDS-C scores, and baseline receipt of psychotherapy, and as before, including a random group effect. The coefficient on number of sessions attended will address the question: “on average, what is the difference in six-month IDS-C that attending one more M-Body session affords, holding gender, baseline IDS-C score, and baseline receipt of psychotherapy constant?”
Secondary outcome analyses
We will conduct four exploratory secondary outcome analyses, one each for depression, anxiety, anger, and trauma, designed to leverage the longitudinal nature of outcome assessment in this study. The first will consist of a LMM in which IDS-C will be compared across study arms at each follow-up visit (three fixed effects), controlling for gender, baseline IDS-C scores, and baseline receipt of psychotherapy. Here we will include random effects for group participation and individual, as we will need to account for within-person correlation. We will consider a simpler model for parsimony including follow-up visit as a continuous variable, as in a linear growth mixed effects model, and select the model that minimizes the Bayesian Information Criterion. The visit-specific coefficients on study arm will address the question: “for two patients with the same baseline depressive symptoms, gender, and baseline receipt of psychotherapy, if one is given the M-Body intervention and the other enhanced usual care, will the patients have different depressive symptoms after 2, 4, and/or 6 months?” The secondary outcome analyses for GAD and anger will use similar models to those described here for IDS-C, this time additionally including baseline GAD or anger scores as appropriate. The secondary outcome analysis for trauma will consist of a linear mixed model (LMM) in which mean 6-month trauma will be compared across study arms (fixed effect), controlling for gender, baseline IDS-C scores, and baseline receipt of psychotherapy, with a random group effect. If issues with model convergence are encountered, we will consider simpler models, such as a linear model with fixed effects for groups, or a linear model with no group effects specified. No interim analysis is planned or will take place; we do not anticipate study developments to require interim analyses.
Protocol amendments
Modifications to the protocol which may impact the conduct of the study, including changes of study objectives, study design, patient population, sample size, and study procedures, will require a formal amendment to the protocol. Such amendment will be agreed upon by the investigative team, discussed with the NIMHD program officer, and approved by the Northwestern University IRB.
COVID-19 modifications
Setting
The COVID-19 pandemic began during active recruitment, enrollment, and follow-up of study participants. All in-person research activities including recruitment, screening, baseline and follow-up assessments, and M-Body classes have been conducted virtually since March 2020 using text, email, phone calls, and virtual tele-conference. For the foreseeable future, all study activities will be held virtually, in accordance with social distancing guidelines put in place by local governing officials. If state and city ordinances indicate that it is again safe to meet in person, we will re-evaluate the feasibility of returning to in-person study activities.
Recruitment
All recruitment activities from group 5 onwards were conducted virtually, using the electronic health records obtained via Alliance Chicago (https://alliancechicago.org/mission-history/), who maintains the electronic health record for the Near North Health Service Centers (Fig. 1). Using a curated patient list, potential participants were contacted by the research team via text, phone call, and email. As a part of receiving care at Near North, patients consent to their data being used for research purposes. Information about the study was placed on the health center’s website as well as social media outlets including Facebook and Twitter. Potential participants were also referred by their providers and word of mouth.
Measurements
Baseline clinical interviews that were previously administered in person (MINI and IDS-C) are now being administered via phone by a member of the research team (Table 1). Follow-up assessments that were previously conducted with an independent evaluator by phone are being administered in the same manner. Self-report surveys are distributed via a REDCap link by email/text depending on participant preference to be completed on their own within a two-week time frame. Collection of dried blood spot samples, blood pressure, and body mass index (BMI) have been temporarily paused until it is safe to resume in-person data collection.
Intervention
All M-Body group sessions are being conducted using Zoom. The research staff contacts all participants before the first session to assist them with the set up and functionality of Zoom. Additionally, a staff member is available to assist participants with the operationality of Zoom during group sessions, if needed. Participants are given the option to have their camera on or off. All Zoom sessions are recorded and reviewed by the PI for adherence to the protocol.
Study materials
Study materials including the manual and tote bag are mailed to the participants before the first session. Participants in the UC condition also receive a mailing containing key study dates and a stress reduction tip sheet.
Payments
Participants receive their stipend for participation using an electronic gift card. They are emailed instructions on how to access the gift card and also provided with a YouTube tutorial. If a participant is unable to access the gift card or has a preference not to have an electronic gift card, a physical gift card is sent to them in the mail.
Statistical analysis
The statistical analysis plan will remain largely unchanged. In principle, using group effects in our models will sufficiently account for the differences between groups, including changes in the delivery of the intervention due to COVID-19. That said, if there are any analyses where including group effects is not feasible or plausible, we will consider adding a fixed effect to our modeling indicating whether or not the group was conducted during COVID-19. Additionally, any analysis using dried blood spot data will be considered exploratory as our sample size is limited because DBS collection was paused during this time.
Dissemination
Throughout the duration of the study, newsletters will be disseminated to health center partners and patients providing updates on study activities. Upon completion of data collection and data analyses, study results will be disseminated to participants, collaborators, and the community at large via newsletters, scientific publications, and presentations at local and national conferences. Topics for presentation or publications, contributing authors, and authorship order will be discussed among the investigative team, research team, and community health center partners. We plan to make our study protocol public and have registered all hypotheses and analyses on ClinicalTrials.gov. In addition, we will make patient-level data and analytic code available upon reasonable request and in accordance with all local, state, federal, and institutional restrictions.
Trial status
This comparative effectiveness trial is ongoing and began recruiting participants on February 18, 2019, according to the protocol available at ClinicalTrials.gov. As of October 21, 2022, 232 individuals had completed all baseline assessments and were randomized to either M-Body or enhanced usual care. By the conclusion of data collection in June 2023, we anticipate that we will have held 16 M-Body courses, and enrolled and randomized 274 individuals (226 women and 48 men).
Discussion
The proposed research offers an innovative integrative approach to depression treatment that is likely to be more accessible and acceptable among socio-economically disadvantaged, racial/ethnic minority adults. We are examining the effectiveness of a mindfulness-based intervention, which has demonstrated efficacy for improving psychological outcomes in the general population but has not been sufficiently examined in racial/ethnic minority groups. Additionally, are conducting a broad assessment of organizational and individual agency factors related to preparation and implementation of the M-Body intervention in a Federally Qualified Health Center (FQHC) that serves low-income, predominantly racial/ethnic minority adults. This research will be used to develop a generalizable model for delivery of streamlined mental health interventions in community settings that will be broadly disseminated and scalable to wider populations.
This study has some limitations that should be acknowledged. We are collaborating with a FQHC network in Chicago that may not be generalizable to FQHCs nationwide. To follow, it is possible that the effectiveness of the implementation of the intervention within this specific FQHC will be impacted by the unique characteristics of our partnering organization. Additionally, we are recruiting a population that experiences a greater burden of psychosocial stressors. While we are collecting some data on social problems and traumatic stress events, this does not cover in totality the stressors (i.e., exposure to violence, neighborhood chaos) experienced by our participants. These factors are likely to impact not only their engagement in the study but also psychological outcomes. Finally, the study is likely to be influenced by organizational changes that are occurring within the system. However, the strong relationship we have with our FQHC site will allow us to anticipate and account for potential changes, for example, in federal and state health care and mental health service policies that potentially, health center funding, and staff turnover.
Despite these limitations, we hypothesize the M-Body intervention will be effective in reducing depression symptoms among low-income Black women. Additionally, we anticipate receiving strong buy-in from our collaborators at the FQHC to recruit and patients for participation and train health center staff to deliver the intervention. Should we find that the intervention is effective in improving symptoms of stress, and in turn depression, and can effectively be implemented within the FQHC context, it will be a scalable model for broad implementation of culturally tailored mental health interventions in settings where the need has been extreme and access to services severely limited.
Availability of data and materials
The statistical analysis team (study biostatistician JMS, statistical analyst KBZ) will have access to the final trial dataset, as well as the study principal investigator IBZ and the project coordinator EZ. There are no contractual agreements that limit access for investigators.
Abbreviations
- M-body:
-
Mind-Body
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor Necrosis Factor-α
- FQHC:
-
Federally Qualified Health Centers
- MBSR:
-
Mindfulness-Based Stress Reduction
- MBCT:
-
Mindfulness-Based Cognitive Therapy
- PHQ-9:
-
Patient Health Questionnaire-9
- PI:
-
Principal Investigator
- STOP:
-
Stop, Take Stock, Observe, Proceed
- REDCap:
-
Research Electronic Data Capture
- CONSORT:
-
Consolidated Standards of Reporting Trials
- IDS-C:
-
Inventory of Depressive Symptomatology—Clinician Administered
- MINI:
-
Mini International Neuropsychiatric Interview
- GAD-7:
-
Generalized Anxiety Disorder-7 Scale
- PCL-5:
-
PTSD Checklist for DSM-5
- SPQ:
-
Social Problems Questionnaire
- LEC-5:
-
Life Events Checklist for DSM-5
- FFMQ:
-
Five Facet Mindfulness Questionnaire
- CERQ-SF:
-
Cognitive and Emotion Regulation Questionnaire—Short Form
- DERS-SF:
-
Difficulties in Emotion Regulation Scale—Short Form
- DBS:
-
Dried blood spot
- ELISA:
-
Enzyme-linked immunosorbent assay
- DSMB:
-
Data and Safety Monitoring Board
- GCP:
-
Good Clinical Practice
- SAEs:
-
Serious adverse events
- ICC:
-
Intra-cluster correlation
- ITT:
-
Intention-to-treat
- LMM:
-
Linear mixed model
- BMI:
-
Body mass index
- LIFE:
-
Longitudinal Interview Follow-Up Evaluation
- LEC-5:
-
Life Events Checklist for DSM-5
- No.:
-
Number of
- HIV:
-
Human immunodeficiency virus
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
References
Gwynn RC, McQuistion HL, McVeigh KH, Garg RK, Frieden TR, Thorpe LE. Prevalence, diagnosis, and treatment of depression and generalized anxiety disorder in a diverse urban community. Psychiatr Serv. 2008;59(6):641–7. https://doi.org/10.1176/ps.2008.59.6.641.
Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097–106. https://doi.org/10.1001/archpsyc.62.10.1097.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. https://doi.org/10.1001/jama.289.23.3095.
Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. https://doi.org/10.1001/archpsyc.1994.03950010008002.
Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: mental health findings. Rockville: Substance Abuse and Mental Health Services Administration; 2014.
Grote NK, Bledsoe SE, Wellman J, Brown C. Depression in African American and White women with low incomes: the role of chronic stress. Soc. 2007;23(2-3):59–88. https://doi.org/10.1080/19371910802148511.
Israel B, Farquhar SA, Schulz AJ, James SA, Parker EA. The relationship between social support, stress, and health among women on Detroit's east side. Health Educ Behav. 2002;29(3):342–60. https://doi.org/10.1177/109019810202900306.
Djuric Z, Bird CE, Furumoto-Dawson A, Rauscher GH, Ruffin MT, Stowe RP, et al. Biomarkers of psychological stress in health disparities research. Open Biomark J. 2008;1:7–19. https://doi.org/10.2174/1875318300801010007.
McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68(3):376–81. https://doi.org/10.1097/01.psy.0000221371.43607.64.
Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–12. https://doi.org/10.1016/j.bbi.2007.03.011.
Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–11. https://doi.org/10.1016/j.pnpbp.2013.09.017.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of Major Depression. Biol. 2009;65(9):732–41. https://doi.org/10.1016/j.biopsych.2008.11.029.
Matthews DD, Hammond WP, Nuru-Jeter A, Cole-Lewis Y, Melvin T. Racial discrimination and depressive symptoms among African-American men: the mediating and moderating roles of masculine self-reliance and John Henryism. Psychol Men Masc. 2013;14(1):35–46. https://doi.org/10.1037/a0028436.
Pieterse AL, Todd NR, Neville HA, Carter RT. Perceived racism and mental health among Black American adults: a meta-analytic review. J Couns Psychol. 2012;59(1):1–9. https://doi.org/10.1037/a0026208.
Ward E, Mengesha M. Depression in African American men: a review of what we know and where we need to go from here. Am J Orthopsychiatry. 2013;83(2pt3):386–97. https://doi.org/10.1111/ajop.12015.
Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–23.
Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA. 2002;287(2):203–9. https://doi.org/10.1001/jama.287.2.203.
Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States. Arch Gen Psychiatry. 2005;62:629–40. https://doi.org/10.1001/archpsyc.62.6.629.
Alegria M, Chatterji P, Wells K, Cao Z, Chen CN, Takeuchi D, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv. 2008;59(11):1264–72. https://doi.org/10.1176/appi.ps.59.11.1264.
Neighbors HW, Caldwell C, Williams DR, Nesse R, Taylor RJ, Bullard KM, et al. Race, ethnicity, and the use of services for mental disorders: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64(4):485–94. https://doi.org/10.1001/archpsyc.64.4.485.
Brown C, Conner KO, Copeland VC, Grote N, Beach S, Battista D, et al. Depression stigma, race, and treatment seeking behavior and attitudes. J Community Psychol. 2010;38(3):350–68. https://doi.org/10.1002/jcop.20368.
Conner KO, Lee B, Mayers V, Robinson D, Reynolds CF III, Albert S, et al. Attitudes and beliefs about mental health among African American older adults suffering from depression. J Aging Stud. 2010;24(4):266–77. https://doi.org/10.1016/j.jaging.2010.05.007.
Menke R, Flynn H. Relationships between stigma, depression, and treatment in White and African American primary care patients. J Nerv Ment Dis. 2009;197(6):407–11. https://doi.org/10.1097/NMD.0b013e3181a6162e.
Rusch LC, Kanter JW, Manos RC, Weeks CE. Depression stigma in a predominantly low income African American sample with elevated depressive symptoms. J Nerv Ment Dis. 2008;196(12):919–22. https://doi.org/10.1097/NMD.0b013e31818ec5d9.
Tucker JR, Hammer JH, Vogel DL, Bitman RL, Wade NG, Maier EJ. Disentangling self-stigma: are mental illness and help-seeking self-stigmas different? J Couns Psychol. 2013;60(4):520–31. https://doi.org/10.1037/a0033555.
Brandon DT, Isaac LA, Laveist TA. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl. 2005;97(7):951–6.
Alegria M, Canino G, Rios R, Vera M, Calderon J, Rusch D, et al. Mental health care for Latinos: inequalities in use of specialty mental health services among Latinos, African-Americans, and non-Latino Whites. Psychiatr Serv. 2002;53:1547–55. https://doi.org/10.1176/appi.ps.53.12.1547.
Pingitore D, Snowden L, Sansone RA, Klinkman M. Persons with depressive symptoms and the treatments they receive: a comparison of primary care physicians and psychiatrists. Int J Psychiatry Med. 2001;31(1):41–60. https://doi.org/10.2190/6BUL-MWTQ-0M18-30GL.
US Department of Health and Human Services. Mental health: culture, race and ethnicity, a supplement to mental health, a report of the Surgeon General, vol. 2. Rockville: Substance Abuse and Mental Health Services Administration (US); 2002.
World Health Organization. Integrated health services – what and why? WHO technical brief No. 1. Geneva: World Health Organization; 2008
World Health Organization. World Organization of Family Doctors. Integrating mental health into primary care: a global perspective. Geneva: World Health Organization; 2008.
Lardiere MR, Jones E, Perez M. NACHC 2010 Assessment of Behavioral health services in federally qualified health centers. Washington, DC: National Organization of Community Health Centers; 2011.
National Guideline Clearinghouse, Non-pharmaceutical management of depression in adults. A national clinical guideline. Rockville: Agency for Healthcare Research and Quality (AHRQ); Content last reviewed May 2021. https://www.ahrq.gov/prevention/guidelines/index.html.
Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. 15th anniversary ed. New York: Delta Trade Paperback/Bantam Dell; 2005.
Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol. 2003;10(2):125–43. https://doi.org/10.1093/clipsy.bpg015.
Gotink RA, Chu P, Busschbach JJ, Benson H, Fricchione GL, Hunink MG. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. https://doi.org/10.1371/journal.pone.0124344.
Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev. 2013;33(6):763–71. https://doi.org/10.1016/j.cpr.2013.05.005.
Park C. Mind-body CAM interventions: current status and considerations for integration into clinical health psychology. J Clin Psychol. 2013;69(1):45–63. https://doi.org/10.1002/jclp.21910.
Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–83. https://doi.org/10.1037/a0018555.
Amutio A, Martinez-Taboada C, Delgado LC, Hermosilla D, Mozaz MJ. Acceptability and effectiveness of a long-term educational intervention to reduce physicians’ stress-related conditions. J Contin Educ Health Prof. 2015;35(4):255–60. https://doi.org/10.1097/CEH.0000000000000002.
Campbell TS, Labelle LE, Bacon SL, Faris P, Carlson LE. Impact of mindfulness-based stress reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: a waitlist-controlled study. J Behav Med. 2012;35(3):262–71. https://doi.org/10.1007/s10865-011-9357-1.
Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21(8):1038–49. https://doi.org/10.1016/j.bbi.2007.04.002.
Momeni J, Omidi A, Raygan F, Akbari H. The effects of mindfulness-based stress reduction on cardiac patients’ blood pressure, perceived stress, and anger: a single-blind randomized controlled trial. J Am Soc Hypertens. 2016;10(10):763–71. https://doi.org/10.1016/j.jash.2016.07.007.
Nejati S, Zahiroddin A, Afrookhteh G, Rahmani S, Hoveida S. Effect of group mindfulness-based stress-reduction program and conscious yoga on lifestyle, coping strategies, and systolic and diastolic blood pressures in patients with hypertension. J Tehran Heart Cent. 2015;10(3):140–8.
Nyklicek I, Mommersteeg PM, Van Beugen S, Ramakers C, Van Boxtel GJ. Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychol. 2013;32(10):1110–3. https://doi.org/10.1037/a0032200.
Gallegos AM, Lytle MC, Moynihan JA, Talbot NL. Mindfulness-based stress reduction to enhance psychological functioning and improve inflammatory biomarkers in trauma-exposed women: a pilot study. Psychol. Trauma. 2015;7(6). https://doi.org/10.1037/tra0000053.
Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun. 2013;27(1):174–84. https://doi.org/10.1016/j.bbi.2012.10.013.
Fang CY, Reibel DK, Longacre ML, Rosenzweig S, Campbell DE, Douglas SD. Enhanced psychosocial well-being following participation in a mindfulness-based stress reduction program is associated with increased natural killer cell activity. J Altern Complement Med. 2010;16(5). https://doi.org/10.1089/acm.2009.0018.
Waldron EM, Hong S, Moskowitz JT, Burnett-Zeigler I. A systematic review of the demographic characteristics of articipants in US-based randomized controlled trials of mindfulness-based interventions. Mindfulness. 2018;9:1671–92. https://doi.org/10.1007/s12671-018-0920-5.
Dutton MA, Bermudez D, Matas A, Majid H, Myers NL. Mindfulness-based stress reduction for low-income, predominantly African American women with PTSD and a history of intimate partner violence. Cogn Behav Pract. 2013;20(1):23–32. https://doi.org/10.1016/j.cbpra.2011.08.003.
Dutton MA. In: Follette JBVM, Rozelle D, Hopper JW, Rome DI, editors. Mindfulness-based stress reduction for underserved populations, in Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York: Guilford Publications; 2015.
Fuchs C, Lee JK, Roemer L, Orsillo SM. Using mindfulness- and acceptance-based treatments with clients from nondominant cultural and/or marginalized backgrounds: clinical considerations, meta-analysis findings, and introduction to the special series. Cogn Behav Pract. 2013;20(1):1–12. https://doi.org/10.1016/j.cbpra.2011.12.004.
Roth B, Robbins D. Mindfulness-based stress reduction and health-related quality of life: findings from a bilingual inner-city patient population. Psychom Med. 2004;66(1):113–23. https://doi.org/10.1097/01.psy.0000097337.00754.09.
Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. (Report). J Psychoactive Drugs. 2010;42(2):177. https://doi.org/10.1080/02791072.2010.10400690.
Hunter-Jones J, Gilliam S, Davis C, Brown D, Green D, Hunter C, et al. Process and outcome evaluation of a mindfulness-based cognitive therapy intervention for cisgender and transgender African American women living with HIV/AIDS. AIDS Behav. 2021;25(2):592–603. https://doi.org/10.1007/s10461-020-03017-7.
Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674–97. https://doi.org/10.1037/0033-2909.129.5.674.
Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company; 1984.
Burnett-Zeigler IE, Satyshur MD, Hong S, Yang A, Moskowitz JT, Wisner KL. Mindfulness based stress reduction adapted for depressed disadvantaged women in an urban federally qualified health center. Complement Ther Clin Pract. 2016;25:59–67. https://doi.org/10.1016/j.ctcp.2016.08.007.
Burnett-Zeigler I, Satyshur MD, Hong S, Wisner KL, Moskowitz J. Acceptability of a mindfulness intervention for depressive symptoms among African-American women in a community health center: a qualitative study. Complement Ther Med. 2019;45:19–24. https://doi.org/10.1016/j.ctim.2019.05.012.
Burnett-Zeigler I, Hong S, Waldron EM, Maletich C, Yang A, Moskowitz J. A mindfulness-based intervention for low-income African American women with depressive symptoms delivered by an experienced instructor versus a novice instructor. J Altern Complement Med. 2019;25(7):699–708. https://doi.org/10.1089/acm.2018.0393.
Crane RS, Kuyken W, Hastings RP, Rothwell N, Williams JM. Training teachers to deliver mindfulness-based interventions: learning from the UK experience. Mindfulness. 2010;1(2):74–86. https://doi.org/10.1007/s12671-010-0010-9.
Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87. https://doi.org/10.1016/0165-1781(86)90060-0.
R Core Team. R: a language and environment for statistical computing. Vienna; 2016. Available from: https://www.R-project.org/
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95. https://doi.org/10.1016/j.jbi.2019.103208. Accessed 2017.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) –a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. https://doi.org/10.1001/archinte.166.10.1092.
Reynolds NS, Walkey FH, Green DE. The anger self report: a psychometrically sound (30 item) version. N Z J Psychol. 1994;23(2):64–70.
Weathers F, Litz B, Keane T, Palmieri T, Marx BP, Schnurr P. (2013). The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov.
Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–98. https://doi.org/10.1002/jts.22059.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33 quiz 34-57.
Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–8. https://doi.org/10.1001/archpsyc.1987.01800180050009.
Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281(24):2305–15. https://doi.org/10.1001/jama.281.24.2305.
Corney RH, Clare AW. The construction, development and testing of a self-report questionnaire to identify social problems. Psychol Med. 1985;15(3):637–49. https://doi.org/10.1017/s0033291700031494.
Cohen S, Kamarck T, Mermelstein R. Perceived Stress Scale. APA PsycTests; 1983. https://doi.org/10.1037/t02889-000.
Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM. (2013). The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD at www.ptsd.va.gov.
Gray M, Litz B, Hsu J, Lombardo T. Psychometric properties of the Life Events Checklist. (PDF). Assessment. 2004;11:330–41. https://doi.org/10.1177/1073191104269954.
Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. https://doi.org/10.1177/1073191105283504.
Trapnell P, Campbell J. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. J Pers Soc Psychol. 1999;76:284–304. https://doi.org/10.1037//0022-3514.76.2.284.
Kaufman EA, Xia M, Fosco G, Yaptangco M, Skidmore CR, Crowell S. The Difficulties in Emotion Regulation Scale Short Form (DERS-SF): validation and replication in adolescent and adult samples. J Psychopathol Behav Assess. 2016;38(443). https://doi.org/10.1007/s10862-015-9529-3.
Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Pers Individ. 2001;30(8):1311–27. https://doi.org/10.1016/S0191-8869(00)00113-6.
Raes F, Pommier E, Neff KD, Van Gucht D. Construction and factorial validation of a short form of the Self-Compassion Scale. Clin Psychol Psychother. 2011;18:250–5. https://doi.org/10.1002/cpp.702.
Delaney C. The spirituality scale: development, refinement and psychometric testing of an instrument to assess the human spiritual dimension [dissertation on the Internet]. Doctoral Dissertations. Mansfield: University of Connecticut; 2003. [cited 2021 October 1]. Available from: https://opencommons.uconn.edu/dissertations/AAI3118943
Aarons GA, Ehrhart MG, Farahnak LR. The Implementation Leadership Scale (ILS): Development of a brief measure of unit level implementation leadership. Implement Sci. 2014;9(1):45. https://doi.org/10.1186/1748-5908-9-45.
Aarons GA. Mental health provider attitudes toward adoption of evidence-based practice: the Evidence-Based Practice Attitude Scale (EBPAS). Ment Health Serv Res. 2004;6(2):61–74. https://doi.org/10.1023/b:mhsr.0000024351.12294.65.
Upton D, Lewis BK. Evidence based practice: a questionnaire to assess knowledge, attitudes and practice. Br J Psychother. 1998;5:647–50. https://doi.org/10.18060/897.
Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9(2):45–59. https://doi.org/10.1002/mpr.79.
Crane RS, Eames C, Kuyken W, Hastings RP, Williams JMG, Bartley T, et al. Development and validation of the Mindfulness-Based Interventions – Teaching Assessment Criteria (MBI:TAC). Assessment. 2013;20(6):681–8. https://doi.org/10.1177/1073191113490790.
Schulz KF, Altman DG, Moher D, & CONSORT Group. CONSORT. statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed). 2010;2010(340):c332. https://doi.org/10.1136/bmj.c332.
Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586. https://doi.org/10.1136/bmj.e7586.
Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and statistics in medicine. Stat Med. 2007;26(1):2–19. https://doi.org/10.1002/sim.2731.
PASS 15 Power Analysis and Sample Size Software (2017). NCSS, LLC. Kaysville. https://ncss.com/software/pass. Accessed 2017.
Roles and responsibilities: sponsorship
Program officer:
Michelle Doose, PhD Social and Behavioral Sciences Administrator (Program Official) Division of Clinical and Health Services Research Michelle.Doose@nih.gov
310-402-4620
This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results
Roles and responsibilities: committees
The Principal Investigator oversees all aspects of the trial. She is responsible of execution of the study design, protocol, data collection and analysis plan, preparation of manuscripts, and presentations as well as other dissemination of results. The research staff, including the research coordinator and research assistants, are responsible for the day to day management of the study under supervision by the PI. They are responsible for recruitment, screening, enrollment and follow-up of participants, data management, adherence to the protocol, and maintaining study materials. They visit to the community health center(s) regularly. They coordinate meetings between the PI, co-investigators, and FQHC. The research coordinator is responsible, jointly with the PI, for coordination of all study activities including IRB approval, development of the manual of operations (MOO), training the research team, and coordinating meetings with the community health center stakeholders, co-investigators, and the Data Safety and Monitoring Board. S/he will also oversee recruitment, screening, and enrollment of participants; protocol adherence; REDCap database development; data management; quality monitoring; safety monitoring; prompt review; and reporting of adverse events. The study biostatistician and data analyst is responsible for maintenance of trial IT system, data management, randomization, data analysis, and reporting to the DSMB. The CHC collaborators include the medical director, director of behavioral health, director of research, and study interventionists. The CHC is responsible for identifying staff to participate in the training and deliver the intervention, referring potential participants to the study, and participating in quarterly stakeholder meetings to assess the process of implementation. The DSMB will be responsible for safeguarding the interests of study participants, assessing the safety and efficacy of study procedures, and for monitoring the overall conduct of the study. The DSMB is an independent group advisory to the Director of the National Institute for Minority Health and Disparities (NIMHD) and will be required to provide recommendations about starting, continuing, and stopping the study. In addition, the DSMB is asked to make recommendations, as appropriate, to the NIMHD.
Funding
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities under award number 1R01MD012236-01A1. All project costs were financed by the National Institutes of Health. Total amount awarded was $2,127,268. The Asher Center for the Study and Treatment of Depressive Disorders provided funding to support the publication of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
IBZ is the principal investigator; she conceived the study, led the project design and protocol development, and oversees the execution of the protocol. LCP, JDC, and JS were the lead trial methodologists. JM, KW, TM, CHB, and JG were study co-investigators and contributed their scientific expertise to the study design and trial oversight. EZ and LL were centrally involved in the execution of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Northwestern University Institutional Review Board #STU00207126. Written, informed consent to participate will be obtained from all participants.
Consent for publication
Not applicable.
Competing interests
LCP receives research funding for unrelated work from Omron Healthcare Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Burnett-Zeigler, I., Zhou, E., Martinez, J.H. et al. Comparative effectiveness of a mindfulness-based intervention (M-Body) on depressive symptoms: study protocol of a randomized controlled trial in a Federally Qualified Health Center (FQHC). Trials 24, 115 (2023). https://doi.org/10.1186/s13063-022-07012-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-07012-2