Abstract
Background
Respiratory distress syndrome (RDS) and feeding intolerance are common conditions in preterm infants and among the major causes of neonatal mortality and morbidity.
For many years, preterm infants with RDS have been treated with mechanical ventilation, increasing risks of acute lung injury and bronchopulmonary dysplasia.
In recent years non-invasive ventilation techniques have been developed. Showing similar efficacy and risk of bronchopulmonary dysplasia, nasal continuous positive airway pressure (NCPAP) and heated humidified high-flow nasal cannula (HHHFNC) have become the most widespread techniques in neonatal intensive care units. However, their impact on nutrition, particularly on feeding tolerance and risk of complications, is still unknown in preterm infants.
The aim of the study is to evaluate the impact of NCPAP vs HHHFNC on enteral feeding and to identify the most suitable technique for preterm infants with RDS.
Methods
A multicenter randomized single-blind controlled trial was designed. All preterm infants with a gestational age of 25–29 weeks treated with NCPAP or HHHFNC for RDS and demonstrating stability for at least 48 h along with the compliance with inclusion criteria (age less than 7 days, need for non-invasive respiratory support, suitability to start enteral feeding) will be enrolled in the study and randomized to the NCPAP or HHHFNC arm. All patients will be monitored until discharge, and data will be analyzed according to an intention-to-treat model.
The primary outcome is the time to reach full enteral feeding, while parameters of respiratory support, feeding tolerance, and overall health status will be evaluated as secondary outcomes. The sample size was calculated at 141 patients per arm.
Discussion
The identification of the most suitable technique (NCPAP vs HHHFNC) for preterm infants with feeding intolerance could reduce gastrointestinal complications, improve growth, and reduce hospital length of stay, thus improving clinical outcomes and reducing health costs. The evaluation of the timing of oral feeding could be useful in understanding the influence that these techniques could have on the development of sucking-swallow coordination. Moreover, the evaluation of the response to NCPAP and HHHFNC could clarify their efficacy as a treatment for RDS in extremely preterm infants.
Trial registration
ClinicalTrials.gov, NCT03548324. Registered on 7 June 2018.
Similar content being viewed by others
Background
Respiratory distress syndrome (RDS) is a common condition in premature infants and one of the major causes of neonatal mortality [1]. For many years, preterm infants with RDS have been treated with mechanical ventilation, increasing the risks of acute lung injury and long-term morbidity, such as bronchopulmonary dysplasia (BPD) [2,3,4,5]. Offering an appropriate respiratory support in the delivery room, together with early surfactant administration, can allow one to avoid or limit endotracheal ventilation with better outcomes in terms of mortality and short- and long-term complications, above all BPD [6]. Early nasal continuous positive airway pressure (NCPAP) treatment combined with surfactant replacement therapy decreases the need for mechanical ventilation and has been recommended as the first line treatment for RDS [7, 8]. However, NCPAP has significant limitations, mainly related to the type of interface needed. Excessive leaks around the prongs or mask and through the mouth can lead to inadequate support, whereas excessive pressure may result in pneumothorax and damage to the nose and face. Moreover, the bulky fixation devices obscure the infant’s face, interfering with both feeding and positioning [9]. In recent years, heated humidified high-flow nasal cannula (HHHFNC) has been studied as an alternative non-invasive respiratory support (NRS). HHHFNC became popular partially due to some perceived advantages related to the type of interface used. Cannulas are easier to apply than NCPAP prongs or mask, may be more comfortable for infants, and may enable easier access to babies’ faces, thus facilitating feeding and parental bonding [10,11,12]. Whereas the practical advantages seem to be established, there is controversy about HHHFNC efficacy as respiratory support [13,14,15]. Recent studies support that HHHFNC is as effective as NCPAP for the primary treatment of RDS, but evidences are still insufficient and data are still lacking, especially for the extremely preterm population (< 28 weeks’ gestation) [14, 16,17,18]. A recent Cochrane review comparing HHHFNC with other NRS measures showed equivalent rates of treatment failure and similar rates of BPD when used as a post-extubation support in preterm infants [19]. With equivalent effectiveness, the choice of the most adequate NRS should consider the impact on the health status of the premature infant, evaluating above all the effect on nutrition and growth. Along with RDS, feeding intolerance (FI) represents a relevant issue in preterm infants, and the coexistence of the two represents a great challenge for the neonatologist [20]. Because of gastrointestinal immaturity, a considerable proportion of premature infants will develop clinical symptoms of FI, causing interruptions of feeding. This delays the establishment of adequate enteral nutrition and prolongs the need for parenteral nutrition, thus increasing the risk of infections and prolonging hospital stay [21]. Avoiding FI and its complications, such as necrotizing enterocolitis (NEC), is a priority for the neonatologist, who often faces the challenge of interpreting the clinical and prognostic significance of common and aspecific signs of FI. Clear identification of the parameters that should be evaluated to identify FI is still lacking in the literature, although, among controversy, the presence of gastric residuals, vomits and/or regurgitations, and abdominal distension and the onset of crises of apnea/bradycardia are considered the most frequent signs [22, 23]. A correlation between non-invasive ventilation and the occurrence of FI and NEC is plausible, although the mechanisms through which ventilation may induce FI and its incidence in ventilated infants are still unclear [20, 24]. The most common hypothesis is that pressurized gases that are not completely conveyed to the airways could cause bowel distension. Bowel distension in infants on CPAP was described by Jaile et al. [25] as CPAP belly syndrome, but no inferences about feeding tolerance and risk of NEC were drawn. More recent studies evaluated the effect of CPAP on mesenteric flow and gastric emptying, suggesting a role of CPAP as a risk factor for FI [26,27,28]. No specific studies have been designed to evaluate the impact of different types of NRS on FI and the occurrence of NEC, which are generally evaluated as secondary outcomes, susceptible to data analysis and patient selection biases. Our hypothesis is that different techniques of NRS may have different impacts on feeding issues in preterm infants.
We therefore intend to compare the application of NCPAP and HHHFNC in preterm infants with RDS to evaluate their impact on FI.
Methods
Aims
The aims of the study are to evaluate the effects of different NRS techniques (NCPAP vs HHHFNC) on feeding tolerance in preterm infants with RDS and to evaluate their impact on full enteral feeding (FEF) achievement and acquisition of oral feeding. A further aim is to evaluate the response to NCPAP and HHHFNC as treatment for RDS in extremely preterm infants.
Study design and setting
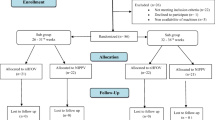
The study has been designed as a multicenter randomized no-mask controlled trial. It will involve the major Italian neonatal intensive care units (NICUs) and will be coordinated by the NICU of the University of Turin.
Inclusion criteria
All infants admitted to the NICUs with a gestational age between 25 and 29 weeks and who will have met the following inclusion criteria will be consecutively enrolled in the study:
-
1.
Presence of RDS
-
2.
Period of stability on HHHFNC or NCPAP for at least 48 h in the first 5 days of life (SatO2 TC 90–95%, pCO2 ≤ 60 mmHg, FiO2 < 40%, Silverman score [29] ≤ 6, ≤ 2 apnea episodes/h with CPAP ≤7 cmH2O if on NCPAP, and flow ≤7 L/min if on HHHFNC)
-
3.
≤ 7 days of life
-
4.
Suitability to start enteral feeding (if already started it should be less than 75 mL/Kg/day)
-
5.
Parental written consent
Exclusion criteria
The following are the study exclusion criteria:
-
1.
Neurological or surgical diseases
-
2.
Sepsis
-
3.
Chromosomal abnormalities
-
4.
Major malformations
Recruitment and randomization
Informed written consent will be signed by both parents, and sufficient time will be allowed for consent. Non-Italian-speaking parents will only be asked for their consent if an adult interpreter is available. Trust interpreter and link worker services will be used to support involvement of participants whose first language is not Italian.
Eligible patients will be allocated to one of the two arms (NCPAP or HHHFNC) by block randomization. A software has been designed to automatically generate a randomization code and to obtain, in each research unit, a balance between patients with gestational age < 28 weeks and ≥ 28 weeks in both arms. The randomization software will be available for all research units, on a password-protected platform on the Enteral Nutrition Tolerance And REspiratory Support (ENTARES) website, and will generate a randomization sequence to which all clinicians are blind.
Monitoring and data collection
Each research unit will adopt its own protocols for clinical management of the patients enrolled in the study while still respecting some minimal standard criteria for respiratory support and enteral nutrition, common for all participating units and defined as follows.
Minimal standard criteria for respiratory support
The suggested initial setup is [30, 31]:
-
CPAP between 5 and 7 cmH2O if on NCPAP and flow between 4 and 7 L/min if on HHHFNC
-
FiO2 set to reach pO2 = 50–60 mmHg (capillary/arterial blood gas test) and SatO2 TC = 90–95%
The criteria to try weaning are [30, 31]:
-
CPAP < 4 cmH2O if on NCPAP and flow < 2 L/min if on HHHFNC
-
FiO2 < 25% to maintain pO2 = 50–60 mmHg (capillary/arterial blood gas test) and SatO2 TC = 90–95%
The failure criteria are [30, 31]:
-
FiO2 > 40%
-
pH < 7.2
-
pCO2 > 65 mmHg
-
≥ 3 episodes of desaturations (SatO2 TC ≤ 80%) per hour
-
≥ 3 episodes of apnea (> 20 s) and/or bradycardia (FC ≤ 80 beats per minute (bpm)) per hour
-
Silverman score [29] > 6
Minimal standard criteria for enteral nutrition
The decision to increase volume of feeds will be up to the clinicians and in accordance with the protocol used in their own NICU; however, a maximum cut-off for feeding progression was set at 30 mL/kg/day [32, 33].
The indications for the interruption of feeding are based on abdominal examination, the occurrence of vomits/regurgitations and cardiorespiratory events, and the evaluation of alvus and gastric residual volumes (evaluated if required by the protocol in use) as detailed in Table 1 [22, 34, 35]. A score system was developed to evaluate abdominal distension (Table 2).
Data on respiratory support, nutrition, growth, and overall clinical status will be collected from enrollment to discharge. According to an intention-to-treat model, each patient will be monitored whatever the occurring clinical events, including the failure of the modality of respiratory support assigned at enrollment. Death or transfer to another hospital before reaching FEF will be the only reasons for a patient to drop the study.
All data to be collected will be obtained from the clinical records. Data will be recorded on a common database available on the ENTARES website and specifically designed for this study. Access to the database will be password protected, and data will be entered by the local principal investigator. Participants will be identified by trial number only.
All data recorded throughout the study period are listed in Table 3.
Outcomes
The primary outcome of the study is the time needed to reach FEF, defined as an enteral intake of 150 mL/Kg/day. Secondary outcomes are listed in Table 4.
The design of the study is outlined in Fig. 1. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure of enrollment, interventions, and assessments is shown in Fig. 2. The SPIRIT checklist is provided as Additional file 1.
Statistical analysis and sample size
Time to reach FEF, the primary outcome, will be analyzed by Kaplan and Meier survival analysis according to the intention-to-treat principle. The two arms will be compared with the log-rank Test [36].
Regarding secondary outcomes, the time to reach half enteral feeding and time to reach full oral feeding will be estimated by Kaplan and Meier analysis, the failure of the respiratory support assigned at randomization will be compared using Fischer’s exact test, and the other secondary outcomes will be estimated using appropriate generalized linear models. This will be a single-blind trial where the blinded person will be the statistician.
Based on a population of infants with a gestational age < 30 weeks who are consecutively admitted to the NICUs of each research unit from January to June 2017 (mean time of FEF 19.6 days) and considering a ratio between the subjects of the two arms of 1:1, a sample size of at least 141 patients per arm has been calculated to observe a difference of 30% between the two arms (5.7 days).
An interim analysis is planned upon reaching the enrollment of half of the patients expected by the sample size calculation.
Quality control and quality assurance procedures
Compliance to protocol
Compliance will be defined as full adherence to protocol. Compliance with the protocol will be ensured by a number of procedures as described below.
Site setup
Local principal investigators participated in preparatory meetings in which details on study protocol, non-invasive ventilation and feeding strategies, and data collection were accurately discussed. All units received detailed written instruction on web-based recording data, and to resolve possible difficulties it will be possible to contact the Clinical Trials Coordinating Unit (Dr. E Maggiora and Dr. SM Borgione).
Safety
Safety endpoints will include incidence, severity, and causality of reported significant adverse events (SAEs). All SAEs will be followed until satisfactory resolution or until the investigator responsible for the care of the participant deems the event to be chronic or the patient to be stable. All expected and unexpected SAEs, whether or not they are attributable to the study intervention, will be reviewed by the local principal investigators to determine if there is a reasonable suspected causal relationship with the intervention. If the relationship is reasonable, SAEs will be reported to the chief investigators, who will then report them to the ethics committee and inform all other investigators to guarantee the safety of the participants.
Discussion
The identification of the most suitable NRS technique for preterm infants with RDS and FI could reduce gastrointestinal complications, improve growth, and reduce hospital stay, thus improving quality of life of infants and their family and reducing health costs.
The evaluation of the timing of oral feeding could be useful in understanding the influence that NRS techniques have on the development of sucking-swallowing coordination.
A standard protocol for the suspension of feeding will be proposed along with a new clinical score to evaluate signs of FI. It may be useful to evaluate the influence, on clinical practice and on the time of achievement of FEF, of the application of a defined and shared method for the evaluation of feeding tolerance. The authors considered a difference of 30% in the time to reach FEF between the groups as the minimum needed to observe a clinically relevant effect. As a consequence, the sample of this study was set at 141 patients per arm.
The evaluation of the response to NCPAP and HHHFNC could clarify their efficacy as treatment for RDS in extremely preterm infants.
Trial status
The protocol is version no. 1, 24 April 2018. The recruitment will begin after approval by the ethics committee of all research units and is expected to begin on 15 September 2018. The time expected to complete the recruitment is about 2 years.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- FI:
-
Feeding intolerance
- HHHFNC:
-
Heated humidified high-flow nasal cannula
- NCPAP:
-
Nasal continuous positive air pressure
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- NRS:
-
Non-invasive respiratory support
- RDS:
-
Respiratory distress syndrome
- SAE:
-
Significant adverse event
- TC:
-
Transcutaneous
References
Mehler K, Grimme J, Abele J, Huenseler C, Roth B, Kribs A. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr Oslo Nor 1992. 2012;101:1232–9.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Fischer HS, Bührer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics. 2013;132:e1351–60.
Ramanathan R, Sekar KC, Rasmussen M, Bhatia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants < 30 weeks’ gestation: a randomized, controlled trial. J Perinatol. 2012;32:336–43.
Attar MA, Donn SM. Mechanisms of ventilator-induced lung injury in premature infants. Semin Neonatol. 2002;7:353–60.
Lista G, Maturana A, Moya FR. Achieving and maintaining lung volume in the preterm infant: from the first breath to the NICU. Eur J Pediatr. 2017;176:1287–93.
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology. 2017;111:107–25.
Committee on Fetus and Newborn, American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–4.
Imbulana DI, Manley BJ, Dawson JA, Davis PG, Owen LS. Nasal injury in preterm infants receiving non-invasive respiratory support: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103:F29–35.
Roberts CT, Manley BJ, Dawson JA, Davis PG. Nursing perceptions of high-flow nasal cannulae treatment for very preterm infants. J Paediatr Child Health. 2014;50:806–10.
Klingenberg C, Pettersen M, Hansen EA, Gustavsen LJ, Dahl IA, Leknessund A, et al. Patient comfort during treatment with heated humidified high flow nasal cannulae versus nasal continuous positive airway pressure: a randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed. 2014;99:F134–7.
Osman M, Elsharkawy A, Abdel-Hady H. Assessment of pain during application of nasal-continuous positive airway pressure and heated, humidified high-flow nasal cannulae in preterm infants. J Perinatol. 2015;35:263–7.
Shin J, Park K, Lee EH, Choi BM. Humidified high flow nasal cannula versus nasal continuous positive airway pressure as an initial respiratory support in preterm infants with respiratory distress: a randomized, controlled non-inferiority trial. J Korean Med Sci. 2017;32:650–5.
Murki S, Singh J, Khant C, Kumar Dash S, Oleti TP, Joy P, et al. High-flow nasal cannula versus nasal continuous positive airway pressure for primary respiratory support in preterm infants with respiratory distress: a randomized controlled trial. Neonatology. 2018;113:235–41.
Soonsawad S, Swatesutipun B, Limrungsikul A, Nuntnarumit P. Heated humidified high-flow nasal cannula for prevention of extubation failure in preterm infants. Indian J Pediatr. 2017;84:262–6.
Roberts CT, Owen LS, Manley BJ, Frøisland DH, Donath SM, Dalziel KM, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. 2016;375:1142–51.
Yoder BA, Stoddard RA, Li M, King J, Dirnberger DR, Abbasi S. Heated, humidified high-flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics. 2013;131:e1482–90.
Kotecha SJ, Adappa R, Gupta N, Watkins WJ, Kotecha S, Chakraborty M. Safety and efficacy of high-flow nasal cannula therapy in preterm infants: a meta-analysis. Pediatrics. 2015;136:542–53.
Wilkinson D, Andersen C, O’Donnell CPF, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2016;2:CD006405.
Bozzetti V, De Angelis C, Tagliabue PE. Nutritional approach to preterm infants on noninvasive ventilation: an update. Nutrition. 2017;37:14–7.
Patel P, Bhatia J. Total parenteral nutrition for the very low birth weight infant. Semin Fetal Neonatal Med. 2017;22:2–7.
Lucchini R, Bizzarri B, Giampietro S, De Curtis M. Feeding intolerance in preterm infants. How to understand the warning signs. J Matern-Fetal Neonatal Med. 2011;24(Suppl 1):72–4.
Gephart SM, Fleiner M, Kijewski A. The conNECtion between abdominal signs and necrotizing enterocolitis in infants 501 to 1500 g. Adv Neonatal Care. 2017;17:53–64.
Amendolia B, Fisher K, Wittmann-Price RA, Bloch JR, Gardner M, Basit M, et al. Feeding tolerance in preterm infants on noninvasive respiratory support. J Perinat Neonatal Nurs. 2014;28:300–4.
Jaile JC, Levin T, Wung JT, Abramson SJ, Ruzal-Shapiro C, Berdon WE. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. AJR Am J Roentgenol. 1992;158:125–7.
Gounaris A, Costalos C, Varchalama L, Kokori P, Kolovou E, Alexiou N. Gastric emptying in very-low-birth-weight infants treated with nasal continuous positive airway pressure. J Pediatr. 2004;145:508–10.
Havranek T, Madramootoo C, Carver JD. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J Perinatol. 2007;27:704–8.
Tyagi P, Gupta N, Jain A, Upadhyay P, Puliyel J. Intra-gastric pressures in neonates receiving bubble CPAP. Indian J Pediatr. 2015;82:131–5.
Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics. 1956;17:1–10.
Roehr CC, Yoder BA, Davis PG, Ives K. Evidence support and guidelines for using heated, humidified, high-flow nasal cannulae in neonatology: Oxford Nasal High-Flow Therapy Meeting, 2015. Clin Perinatol. 2016;43:693–705.
Yoder BA, Manley B, Collins C, Ives K, Kugelman A, Lavizzari A, et al. Consensus approach to nasal high-flow therapy in neonates. J Perinatol. 2017;37(7):809–13.
Maas C, Franz AR, von Krogh S, Arand J, Poets CF. Growth and morbidity of extremely preterm infants after early full enteral nutrition. Arch Dis Child Fetal Neonatal Ed. 2018;103:F79–81.
Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin’s neonatal-perinatal medicine: diseases of the fetus and infant. Amsterdam: Elsevier; 2015.
Li Y-F, Lin H-C, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol. 2014;55:335–40.
Kaur A, Kler N, Saluja S, Modi M, Soni A, Thakur A, et al. Abdominal circumference or gastric residual volume as measure of feed intolerance in VLBW infants. J Pediatr Gastroenterol Nutr. 2015;60:259–63.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457.
Acknowledgements
The authors wish to thank the following for their contribution to the ENTARES Study Research Group.
Investigators:
Fabio Mosca, Anna Orsi, Domenica Mercadante, Stefano Martinelli, Laura Ilardi, Alice Proto, Sara Gatto, Arianna Aceti, Fabrizio Sandri, Roksana Chakrokh, Nicola Laforgia, Antonio Di Mauro, Maria E Baldassarre, Antonio Del Vecchio, Flavia Petrillo, Maria P Spalierno, Francesco Raimondi, Letizia Capasso, Marta Palma, Daniele Farina, Maria F Campagnoli, Tatiana Boetti, Federica Logrippo, Massimo Agosti, Laura Morlacchi, Simona Perniciaro, Carlo Dani, Serena Elia, Giovanni Vento, Luca Maggio, Mauro Stronati, Elisa Civardi, Grappone Lidia, Borrelli Angela.
The authors wish also to thank Eng. Mattia Ferroglio for developing the ENTARES website and database.
Funding
Not applicable.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
FC, EM, and GL conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. SMB and FM reviewed and revised the manuscript and approved the final manuscript as submitted. ES collaborated to design the study and approved the final manuscript as submitted. AC critically reviewed the manuscript and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocol was approved with the approval number 0043331 on 24th March 2018 by the ethics committee of the coordinating unit (Comitato Etico Interaziendale – AOU Città della Salute e della Scienza di Torino; phone: + 39.011.6336547; email: comitatoetico@cittadellasalute.to.it).
The study protocol will be subsequently submitted to the ethics committees of each participating unit.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
SPIRIT 2013 checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 121 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cresi, F., Maggiora, E., Borgione, S.M. et al. Enteral Nutrition Tolerance And REspiratory Support (ENTARES) Study in preterm infants: study protocol for a randomized controlled trial. Trials 20, 67 (2019). https://doi.org/10.1186/s13063-018-3119-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-018-3119-0