Abstract
Objective
To study intra-gastric pressures in neonates receiving bubble continuous positive airway pressure (BCPAP) by nasopharyngeal prong.
Methods
Twenty seven neonates were recruited for the study. BCPAP pressure of 6 cm water was used in all the neonates. A pressure sensor attached to orogastric tube, measured the intra-gastric pressure prior to starting BCPAP and again between 30 and 90 min of BCPAP. The clinical variables like Downe’s score, oxygen saturation, venous blood gas pH, pCO2 and abdominal girth were recorded alongside with pressure readings.
Results
BCPAP resulted in improvement (p < 0.05) in parameters of respiratory distress such as Downe’s score (DS), oxygen saturation (SpO2) and venous blood gas parameters (pH, pCO2). There was no statistical significant increase in intra-gastric pressures (p = 0.834). There were no gastrointestinal complications; abdominal distention, necrotising enterocolitis or gastric perforation during the study.
Conclusions
Nasopharyngeal BCPAP at 6 cm of water pressure is an effective modality of treating babies with respiratory distress and the present study shows that it is not associated with a significant rise in intra-gastric pressures.
Similar content being viewed by others
Introduction
Early use of continuous positive airway pressure (CPAP) in newborns is associated with lower incidence of chronic lung disease [1]. This has led to increase in the use of CPAP as an alternative to intubation and ventilation, in some units [2, 3].
CPAP has also been established as an effective method for weaning from mechanical ventilation, in preventing extubation failure and is used in the management of apnea of prematurity [4, 5].
Although bubble CPAP (BCPAP) is seen to be superior to continuous steady pressure CPAP [6–11], its pressure delivery system can be highly variable and unpredictable as reported by Kahn et al. [12].
CPAP has been associated with gastrointestinal adverse effects – although rarely. In 1992 Jaile et al. published a case and coined the term CPAP belly syndrome [13]. They used this term to denote the gaseous bowel-distension in infants treated with nasal CPAP. These infants do not have abdominal distension at birth, but after treatment with nasal CPAP for a short period develop soft, strikingly distended abdomens and visibly dilated loops. The continuous flow of air in the nasopharynx causes an increase in swallowing of air. The distended abdomen causes increase in pressure on the diaphragm, which may result in a compromised respiratory state [14].
Rare case reports of gastric insufflations potentially leading to aspiration, abdominal distention and perforation in neonates on CPAP have been reported [15, 16]. Reporting of 2 deaths in the BCPAP study group of Gupta et al. [17] due to necrotizing enterocolitis (NEC) are disconcerting and raises question marks on serious gastrointestinal side effects of BCPAP and safety of this potentially good, cost effective intervention for saving newborn lives.
There are no studies that have looked at intra-gastric pressures during CPAP. The pressures transmitted from oropharynx to stomach during BCPAP are not known.
This study was designed to measure and to look for any change in the intra-gastric pressures after starting BCPAP. The authors also looked out for gastrointestinal complications such as increased abdominal distention, CPAP Belly syndrome, NEC or gastric perforation in babies receiving nasopharyngeal BCPAP.
Material and Methods
This prospective study measuring intra-gastric pressures in neonates requiring BCPAP was done from July 2011 through July 2012 at Neonatal Intensive Care Unit (NICU) of St. Stephen’s hospital, New Delhi. This study was approved by St. Stephen’s hospital Ethical Committee in June 2011.
The simple circuit nasopharyngeal BCPAP that has been described earlier by Kaur et al. [18] was used for the present study. All babies with gestational age 28 to 42 wk requiring BCPAP for mild to moderate respiratory distress based on Downe’s score [19] ≥ 4, post extubation and apnea were included. A chest radiograph was done in all cases to rule out congenital anomalies like diaphragmatic hernia, tracheoesophageal fistula or other causes of respiratory distress. Newborns requiring intubation at birth, respiratory distress secondary to birth asphyxia, congenital anomalies like cleft lip, cleft palate, choanal atresia and congenital heart disease diagnosed after starting of BCPAP were excluded from the study.
Written informed consent was obtained from parents to record pressures during BCPAP. Data recorded included anthropometry and post conceptual age. Downe’s score was used to monitor improvement in respiratory distress of babies every 30 min on BCPAP in all cases by principal investigator of this study.
Oxygen saturation was recorded by Phillips AvantTM 9600 pulse oximeter. Venous blood gas pH and pCO2 were recorded before starting CPAP and when the intra-gastric pressures were re-measured. Downe’s score, oxygen saturation, and abdominal girth were recorded alongside. All observations were recorded in supine position.
Before starting BCPAP, All babies were kept supine, nil per oral and feeds were withheld for entire duration of 90 minutes to record intragastric pressures.
Flow was maintained such that there was a continuous flow of bubbles from the blow off valve to generate a pressure of 6 cm of H2O mixed (Air + Oxygen) BCPAP without agitating water violently.
The pressure sensor probe by Sensoromedic ® was attached to a 5 V regulated power supply and the voltage drop across appropriate leads of the sensor, which was proportional to the pressure difference, was measured by a milli-voltmeter. This probe shows 1 mV of change in milli-voltmeter for a pressure difference of 2.91 cm of H2O. As the milli voltmeter could read with changes up to 0.01 mV, therefore minimum sensitivity of the sensor was 0.291 mm or simply 0.3 mm of water height. We checked the calibration routinely prior to, employing the probe at different depths of water and found it accurate with an error of less than 2 %. For continuous monitoring of the data the serial port of the milli-voltmeter was logged to a computer by its RS 232 port. The software for the data logging was provided by the milli-voltmeter manufacturer - MECO® 81 K -TRMS
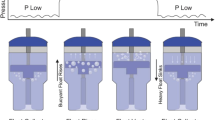
In order to prevent dissipation of intra-gastric pressures through orogastric (OG) tube end and to get accurate pressure changes induced by BCPAP OG tube end was attached to pressure sensor probe which was passed through oropharynx into the stomach. The sensor probe was devised such that OG tube number 8 was best fit to the circuit, without any leaks, for the study (Fig. 1). The mean of 10 readings was taken as the initial pressure (IG0). Without removing the sensor probe attached to OG tube lying in stomach, intra-gastric (IG1) readings were taken after 30 min but within 90 min of BCPAP.
In the absence of data of intra-gastric pressure from neonates, accurate sample size calculations to avoid Type 2 errors could not be done and this study proposed to use a sample size of 25 neonates as a pilot to look at the variance in pressures which can allow accurate sample size calculations in the future.
A paired samples t test with 95 % confidence limits was used to compare difference in pressures, and clinical variables before and after BCPAP.
Results
Total 31 neonates were monitored for pressures; three neonates had to be excluded because of diagnosis of congenital heart disease and one due to diagnosis of diaphragmatic hernia which were made after starting of BCPAP. Results of the remaining 27 infants were analyzed.
BCPAP was used in 17 neonates with respiratory distress, in 6 neonates post-extubation and in 2 neonates with meconium aspiration and apnea respectively.
The mean post conceptual age at the time of measuring pressures was 35.2 wk (SD 3.1) and mean weight was 2,084 g (SD 773).
There was statistical improvement (p < 0.05) in parameters of respiratory distress like Downe’s score (DS), oxygen saturation (SpO2), venous blood gas parameters (pH, pCO2). Table 1 gives the details of change in clinical variables after BCPAP.
The mean intra-gastric pressure before starting BCPAP was 12.422 cm H2O, (95 % CI 8.65 to 16.18) and during BCPAP it was 12.88 cm H2O (95 % CI 10.48 to 15.29). The intra-gastric pressure always remained positive and the overall change in intra-gastric pressure recordings (paired t test) was 0.464 cm H2O (95 % CI −5.11 to + 4.18,) (p = 0.838). Table 1 shows the intra-gastric pressures monitored.
Out of total 27 neonates 2 babies did not improve on BCPAP and required mechanical ventilation due to recurrent apnea of prematurity. There was no increase in abdominal girth, gastric distention leading to aspiration pneumonia, incidence of NEC or any other abdominal complications during nasopharyngeal BCPAP in the study.
Discussion
In the present study authors found that intra-gastric pressures changed only marginally by 0.464 cm H2O which was statistically insignificant (p = 0.834). Omari et al. suggested that the tone at upper end of esophagus and at gastro esophageal junction is higher than CPAP pressures so the air goes preferentially to the airways rather than the esophagus [20]. However, he noted that ‘CPAP belly’ can occur occasionally. He suggested the babies on CPAP, should have an orogastric (OG) tube left in situ with the outer end open to atmosphere. The authors routinely take this precaution in babies on BCPAP but during the duration of study they had the upper end of the OG tube closed so as to measure the pressures generated by CPAP.
Jackson et al. have observed that excessive abdominal distension usually occurred with use of incorrect nasopharyngeal prong position and after switching to shorter nasopharyngeal tube, the incidence of abdominal distention was reduced remarkably [21]. In the present study the authors used soft single nasopharyngeal tube to deliver BCPAP [18]. None of neonates in the index study developed abdominal complication, increase in abdominal girth or NEC.
In a study by Ellina et al. BCPAP was associated with greater breathing asynchrony and increased work of breathing compared to ventilator derived CPAP in preterm infants with mild respiratory distress [22]. The results of index study showed decrease in work of breathing and improvement in Downe’s score (p-0.0001) which further upholds the results by Prashanth et al. [10] in which 80 % of newborns with respiratory distress syndrome (RDS) given BCPAP had marked improvement in Downe’s score.
Arguably the present study is the first attempt to measure intra-gastric pressures, while monitoring for gastrointestinal complications in babies receiving nasopharyngeal BCPAP. This study has good internal validity as pressures were measured and compared in same neonates, individually such that each child acts as its own control.
There are certain limitations of the present study. The authors measured pressures after 30 to 90 min post BCPAP; it is not clear whether monitoring continuous pressure for 24 h would have altered the findings. However the authors felt it would be unethical to monitor the child with the orogastric tube closed for such a long time.
In the present study, authors used nasopharyngeal tube as the interface, other side effects such as pneumonia and infections were not studied and comparison arm was not present. Also it would be difficult to conclusively comment on efficacy or safety with a short duration single centered study.
The index study relatively had stable near term and term newborns. These findings cannot be generalized on very preterm babies with RDS and co-morbidities. Finally the sample size was perhaps too small to pick up rare cases of intra abdominal complications following BCPAP.
Conclusions
It was found that nasopharyngeal BCPAP at pressure of 6 cm water, decreased work of breathing, improved gas exchange and improved Downe’s score. The authors found no significant increase in intra-gastric pressures and gastrointestinal complications such as abdominal distention, NEC, perforation during their study.
Further multi centric studies with larger number of cases, are required for predicting accurate changes in intra-gastric pressures.
References
Birenbaum HJ, Dentry A, Cirelli J, Helou S, Pane MA, Starr K, et al. Reduction in the incidence of chronic lung disease in very low birth weight infants: results of a quality improvement process in a tertiary level neonatal intensive care unit. Pediatrics. 2009;123:44–50.
Aly H, Milner JD, Patel K, El-Mohandes AA. Does the experience with the use of nasal continuous positive airway pressure improve over time in extremely low birth weight infants? Pediatrics. 2004;114:697–702.
Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New Engl J Med. 2008;358:700–8.
Lemyre B, Davis PG, De Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database Syst Rev. 2002;1:CD002272.
Davis PG, Henderson-Smart DJ. Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2003;2:CD000143.
Mazzella M, Bellini C, Calevo MG, Campone F, Massocco D, Mezzano P, et al. A randomized control study comparing the infant flow driver with nasal continuous positive airway pressure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F86–90.
Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387–92.
Chan KM, Chan HB. The use of bubble CPAP in premature infants: local experience. HK J Paediatr (new series). 2007;12:86–92.
Pillow J, Hillman N, Moss T, Polglase G, Bold G, Beaumont C, et al. Bubble continuous positive airway pressure enhances lung volume and gas exchange in preterm lambs. Am J Respir Crit Care Med. 2007;176:63–9.
Urs PS, Khan F, Maiya PP. Bubble CPAP- a primary respiratory support for respiratory distress syndrome in newborns. Indian Pediatr. 2009;46:409–11.
Tagare A, Kadam S, Vaitya U, Pandit A, Patole S. A pilot study of BCPAP vs VCPAP in preterm infants with early onset respiratory distress. J Trop Pediatr. 2010;56:191–4.
Kahn DJ, Courtney SE, Steele AM, Habib RH. Unpredictability of delivered bubble nasal continuous positive airway pressure role of bias flow magnitude and nares-prong air leaks. Pediatr Res. 2007;62:343–7.
Jaile JC, Levin T, Wung JT, Abramson ST, Ruzal-Shapiro C, Berdon WE. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. Am J Roentgenol. 1992;158:125–7.
Han VK, Beverley DW, Clarson C, Sumabat WO, Shaheed WA, Brabyn DG, et al. Randomized controlled trial of very early continuous distending pressure in the management of preterm infants. Early Hum Dev. 1987;15:21–32.
Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics. 1985;76:406–10.
Kiciman NM, Andreasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatr Pulmonol. 1998;25:175–81.
Gupta S, Sinha SK, Donn SM. A randomized controlled trial of post extubation bubble CPAP or infant flow driver CPAP in preterm infants with respiratory distress syndrome. J Pediatr. 2009;154:645–50.
Kaur C, Sema A, Beri RS, Puliyel JM. A simple circuit to deliver bubbling CPAP. Indian Pediatr. 2008;45:312–4.
Rusmawati A, Haksari EL, Naning R. Downes score as a clinical assessment for hypoxemia in neonates with respiratory distress. Paediatr Indones. 2008;48:342–5.
Omari TI, Benninga MA, Barnett CP, Haslam RR, Davidson GP, Dent J. Characterization of esophageal body and lower esophageal sphincter motor function in the very premature neonate. J Pediatr. 1999;135:517–21.
Jackson JK, Vellucci J, Johnson P, Kilbride HW. Evidence-based approach to change in clinical practice: introduction of expanded nasal continuous positive airway pressure use in an intensive care nursery. Pediatrics. 2003;111:e542–7.
Liptsen E, Aghai ZH, Pyon KH, Saslow JG, Nakhla T, Long J, et al. Work of breathing during nasal continuous positive airway pressure in preterm infants: a comparison of bubble vs variable-flow devices. J Perinatol. 2005;25:453–8.
Acknowledgments
The author would like to express his deep gratitude to Dr. Jacob M. Puliyel, Consultant and Head, Department of Pediatrics and Neonatology, St. Stephen’s Hospital & Dr. Pramod Upadhayaya, Senior Researcher, Faculty at National Institute of Immunology, New Delhi. They both were instrumental in transforming this project from paper into reality by helping to set up portable pressure transducer connectable to computer and to interpret its data for this study. The author expresses his sincere thanks to Dr. V. Sreenivas, Department of Biostatistics, AIIMS, New Delhi for his kind help in statistical analysis of this study.
Contributions
JP, PU and NG: Conceived the project; PU: Designed the interface for recording pressure; PT and AJ: Did the measurements. All authors contributed to the manuscript preparation. JP will act as guarantor for the study.
Conflict of Interest
None.
Source of Funding
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tyagi, P., Gupta, N., Jain, A. et al. Intra-gastric Pressures in Neonates Receiving Bubble CPAP. Indian J Pediatr 82, 131–135 (2015). https://doi.org/10.1007/s12098-014-1545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-014-1545-x