Abstract
Background
Post-traumatic stress has been identified as a frequent long-term complication in survivors of critical illnesses after sepsis. Little is known about long-term trajectories of post-traumatic stress and potentially modifiable risk factors following the ICU stay. Study objective was to explore and compare different clinical trajectories of post-traumatic stress symptoms in sepsis survivors up to two years after discharge from ICU.
Methods
Data on post-traumatic stress symptoms by means of the Post-traumatic Symptom Scale (PTSS-10) were collected in sepsis survivors at one, six, 12 and 24 months after discharge from ICU. Data on chronic psychiatric diagnoses prior ICU were derived from the primary care provider’s health records, and data on intensive care treatment from ICU documentation. Trajectories of post-traumatic symptoms were identified ex post, discriminating patterns of change and k-means clustering. Assignment to the trajectories was predicted in multinomial log-linear models.
Results
At 24 months, all follow-up measurements of the PTSS-10 were completed in N = 175 patients. Three clusters could be identified regarding clinical trajectories of PTSS levels: stable low symptoms (N = 104 patients [59%]), increasing symptoms (N = 45 patients [26%]), and recovering from symptoms (N = 26 patients [15%]). Patients with initially high post-traumatic symptoms were more likely to show a decrease (OR with 95% CI: 1.1 [1.05, 1.16]). Females (OR = 2.45 [1.11, 5.41]) and patients reporting early traumatic memories of the ICU (OR = 4.04 [1.63, 10]) were at higher risk for increasing PTSS levels.
Conclusion
Post-traumatic stress is a relevant long-term burden for sepsis patients after ICU stay. Identification of three different trajectories within two years after ICU discharge highlights the importance of long-term observation, as a quarter of patients reports few symptoms at discharge yet an increase in symptoms in the two years following. Regular screening of ICU survivors on post-traumatic stress should be considered even in patients with few symptoms and in particular in females and patients reporting traumatic memories of the ICU.
Similar content being viewed by others
Introduction
With advances in intensive care, the survival rate of critical illnesses as sepsis has increased [1, 2]. As a result, there is growing concern about the long-term impact on health-related quality of life after discharge from the intensive care unit (ICU) [3]. Survivors of critical illness often suffer from cognitive, mental and physical impairments, summarized as the Post-Intensive Care Syndrome (PICS) [4]. Within this, a large body of literature has found that more than one in five critical illness survivors may show clinical symptoms of depression and/ or Post-traumatic Stress Disorder (PTSD) [5,6,7]. Health-related quality of life (HRQOL) may be reduced for months and years [3, 8]. Therefore, the “International Guidelines for Management of Sepsis” [9] recommend continuous follow-up. However, there is still a lack of specific aftercare programs [10], while existing post-ICU clinics were not clearly found to be effective [11]. Additionally, mental health care often is hampered by structural capacity deficits in the provision of psychotherapy [12].
A recent meta-analysis [13] showed that mental trauma in medical populations is significantly more relevant than previously thought. In particular, sepsis survivors, who are affected by invasive medical care, show high rates of clinically significant PTSD symptoms [14, 15]. However, most existing studies are limited by small sample sizes [16,17,18], cross-sectional design [16] or short duration of follow-up [18]. Furthermore, only few of these studies specifically examined potential risk factors for post-sepsis PTSD [14, 16, 19].
In light of the growing evidence of adverse health and functional outcomes associated with post-traumatic symptoms [20, 21], and the high annual incidence of sepsis [2] describing the trajectories and potentially modifiable risk factors for adverse mental health outcomes after sepsis has important implications for population health, for example to identify patient groups at particular risk [22].
Aim of this study was to identify and predict trajectories of post-traumatic stress symptoms over two years in a cohort of sepsis survivors.
Methods
Study design and context
A retrospective observational cohort study on post-traumatic stress symptoms after sepsis was performed as a secondary analysis. Data were gathered as part of the SMOOTH-study (Sepsis survivors MOnitoring and coordination in Outpatient healTH care) [23], a multicenter, non-blinded, two-armed randomized clinical trial. Core components of the SMOOTH-trial’s intervention included post-ICU-discharge case management focusing on proactive patient symptom monitoring, clinical decision support for the primary care physicians by a consulting physician and training for both patients and their primary care physicians in evidence-based post-sepsis care. The trial was approved by the institutional review board of the Jena University Hospital (No.3001/111). Detailed methods and results of the SMOOTH-trial are described elsewhere [12, 23,24,25]. This secondary analysis identified different clusters ex post in the trajectories of post-traumatic stress symptoms among the SMOOTH patients. Predictors of these trajectory clusters were assessed by regression analysis.
Sample
Patients were recruited in nine ICU study centers across Germany between February 2011 and December 2013. Follow-up assessments were completed in December 2015. Patients were eligible for inclusion if they were adult (≥ 18 years) survivors of severe sepsis or septic shock, (now defined as “sepsis”) [26] and fluent in the German language. Clinical diagnoses of sepsis were made by intensivists according to American College of Chest Physicians/ Society of Critical Care Medicine consensus criteria [27]. The key exclusion criterion was cognitive impairment that would prevent participation in the intervention, being assessed by the modified Telephone Interview of Cognitive Status (TICS-M; score ≤ 27) within one month after discharge [28].
Procedure
Baseline data were collected through standardized face-to-face interviews with patients within one month of ICU discharge. Further clinical data were obtained from their ICU records. Follow-up data were collected by standardized telephone interviews at 6, 12 and 24 months after enrolment. All interviews were conducted by trained study nurses using a standardized interview guide.
Measures
The primary outcome was post-traumatic stress which was assessed by telephone interviews using the Post-traumatic Symptom Scale (PTSS-10). This questionnaire assesses 10 major post-traumatic symptoms on a 7-point Likert scale, such as nightmares, irritability or fear of places and situations. It has been shown to be a responsive, valid and reliable instrument in screening for post-traumatic symptoms. The total sum score of the 10 items shows good internal consistency and test–retest reliability (range, 10–70; higher scores indicate greater impairment, scores above 35 are considered to indicate a PTSD diagnosis, scores above 23 to be clinically relevant) [29, 30]. However, the PTSS-10 does not replace a clinical interview to make a clinically confirmed diagnosis of a PTSD.
Based on the four assessments of the PTSS-10, different groups of longitudinal trajectories of post-traumatic symptoms were identified, see statistical analysis. To predict these trajectory groups, a set of 15 possible predictor variables was derived from literature, clinical reasoning, and availability of measures collected during the SMOOTH-trial. Only measures collected one month after ICU discharge were considered, to achieve a temporal sequence of predictors and trajectories. Predictors considered were:
-
a)
Patient demographics: age, sex, education, marital status [21, 31,32,33,34].
-
b)
Pre-existing comorbidities: presence of a diagnosis from chapter F of ICD-10 (mental and behavioral disorders) [32, 35,36,37], Charlson Comorbidity Index (range of possible scores, 0–37; high score indicates high impairment) [38].
-
c)
Extend of intensive care and severity of critical illness: mechanical ventilation, renal replacement therapy, ICU length-of-stay, number of ICD-10 diagnoses at discharge [6, 33, 39, 40].
-
d)
Symptoms at one-month follow-up: pain intensity as assessed by the Graded Chronic Pain Scale (GCPS, range of possible scores, 0–100; high score indicates high impairment) [41, 42], cognitive function [43] assessed by the TICS-M (range of possible scores, 0–50; includes only scores above 27 by inclusion criterion; high score indicates low impairment) [28] presence of at least 2 of 4 types of traumatic memories of the ICU experience as measured by the corresponding additional items of the PTSS-10 [44]
-
e)
Randomization status (control vs. intervention) was added as a control variable, as the intervention had an effect on the PTSS level at the 24-month follow-up [25].
-
f)
The first measurement of the PTSS-10 at the one-month follow-up was included as a control variable, as trajectories over time are influenced by their initial levels (ceiling and floor effects, regression to the mean).
Statistical analysis
All analyses were performed using R version 4.1.2 statistical software [45]. Each patient's four follow-up PTSS-10 sum scores defined the patient's individual longitudinal trajectory of post-traumatic stress symptoms. We conducted an exploratory analysis to identify different clusters of individual trajectories. In order to do this, we followed the three-step procedure proposed by Leffondre et al. [46, 47] which makes no assumptions about specific trajectory shapes. This procedure involves (1) calculating 24 measures describing the characteristics of the trajectories; (2) using factor analysis to select the most important subset of the 24 measures and (3) using cluster analysis based on these measures to identify clusters of trajectories, and classify each individual trajectory into one of the clusters. Steps 1 and 2 were performed using the traj package (functions step1measures and step2factors). The number of clusters (step 3) was determined using the NbClust package (function NbClust with k-means clustering), which provides 26 fit indices for the number of clusters. The number of clusters, for which the best fit was indicated by a relative majority of the indices, was selected. Finally, the step3clusters function from the traj package was used to assign each individual trajectory to one of the clusters by k-means clustering. To describe the clusters, the individual trajectories as well as the median and interquartile range of the PTSS score per cluster were visualized for the whole sample as well as stratified by intervention and control group, see Additional file 3.
Identified clusters were compared descriptively with respect to the predictor variables given above. The significance of the predictor and control variables was assessed in two steps: (1) calculation of a multinomial log-linear model for each predictor, controlling for the PTSS-10 sum score measured at the one-month follow-up; (2) inclusion of all predictors significant at P ≤ 0.05 in step 1 in a multiple multinomial log-linear model, also controlling for the effect of the PTSS-10 sum score at the one-month follow-up. Multinomial log-linear models were calculated using the multinom function of the nnet package.
As the trajectory clustering analysis required at least four measurements for each trajectory, only those patients were included for whom data on post-traumatic stress were available for all four measurements. Analyses were performed on complete data. Patterns of missing data were analyzed descriptively to assess possible effects of missing data, see Additional File 2: Fig. 2. All statistical tests were performed at a two-sided alpha level of 0.05.
Results
Longitudinal trajectories of post-traumatic stress symptoms
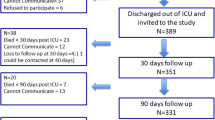
At 24 months after discharge from ICU, N = 186 (64%) of initially N = 291 enrolled patients completed the 24-month follow-up, N = 64 patients have died and N = 41 patients were excluded or dropped out for other reasons. N = 175 of these N = 186 patients (94%) provided all four follow-up PTSS-10 assessments and were included in the analysis, see Additional file 1: Fig. 1.
Of the 175 included patients, 94 were in the intervention group and 81 in the control group. The 116 excluded patients were older and had comorbidity with lower cognitive function, see Additional File 1: Fig. 1). The pattern of missing values for PTSS-10 was mostly monotonic due to loss to follow-up, see Additional File 2: Fig. 2.
Out of 26 fit indices, a relative majority of 12 indices suggested the number of three trajectory clusters as optimal solution, see Fig. 1. The three trajectories can be interpreted as stable low (panel A, N = 104 patients [59%]), increasing (panel B, N = 45 patients [26%]), and recovering to normal level (panel C, N = 26 patients [15%]) with respect to the development of post-traumatic symptoms, see Fig. 2. The recovering cluster had a higher initial PTSS-10 sum score than the stable low and increasing clusters (median 31.5 vs. 20 and 19, respectively). The increasing cluster showed a median PTSS-10 sum score of 35 at 24 months, which indicate a high likelihood of PTSD. The three trajectory clusters were comparable between the intervention and control groups, see Additional file 3.
Three main trajectories of post-traumatic symptoms up to 24 months after ICU care were identified in sepsis survivors (means). The recovering cluster, on average, started with clinically relevant symptoms that decreased clearly over time. In contrast, the increasing cluster started with mild symptoms and had a high likelihood of manifest PTSD at 24 months
Clusters of trajectories of post-traumatic symptoms. Panel A: cluster is interpreted as stable low severity of symptoms, N = 104 patients. Panel B: cluster is interpreted as increasing severity of symptoms, N = 45 patients. Panel C: cluster is interpreted as recovering from symptoms, N = 26 patients. Sum scores above 35 are considered to indicate PTSD, above 23 to be clinically relevant
Prediction of trajectories
Table 1 shows the descriptive comparison of predictor variables between the three clusters without adjustment for the initial severity of post-traumatic symptoms. Table 2 provides results of the multinomial log-linear models: Controlling for the effect of the initial symptom severity (i.e., PTSS-10 at one month), randomization to the intervention group, female gender and the presence of more than two traumatic ICU memories at one-month follow-up, showed significant individual effects (p ≤ 0.036). These effects were confirmed after inclusion in the multivariate model, see Table 3: Patients in the intervention group and those with higher initial post-traumatic symptoms were more likely to be in the recovering group (OR with 95% CI = 1.44 [0.54, 3.86] and 1.1 [1.05, 1.16], respectively) and less likely to be in the increasing group (OR = 0.33 [0.15, 0.75], and 0.93 [0.89, 0.98], respectively). In contrast, female patients (OR = 2.45 [1.11, 5.41]) and patients reporting more than two traumatic memories (OR = 4.04 [1.63, 10]) were more likely to show increasing than stable low symptoms.
Discussion
The aim of this secondary analysis of a randomized clinical trial was to explore trajectories of post-traumatic stress symptoms in sepsis survivors. Our sample was similar to other cohorts of sepsis survivors in terms of age, sex and comorbidities, but had longer duration of mechanical ventilation and ICU stay [24]. Excluded patients were older and had more comorbidities and lower cognitive function, which may be because most patients dropped out due to mortality.
We identified three clusters with a distinct trajectory of symptoms over two years of follow-up. The majority of patients showed rather stable and mostly low levels of post-traumatic symptoms. Other patients recovered from initially severe symptoms, and a third group showed a trajectory of increasing post-traumatic stress over time. In particular, female patients and patients who reported traumatic memories shortly after ICU discharge appeared to be at higher risk of increasing clinically relevant symptoms in the long-term.
Outside the ICU context, it has been widely shown, that post-traumatic symptoms and a clinically confirmed PTSD can follow different trajectories, which have been investigated in numerous studies following traumatic events such as war, military deployment, accidents, or violence. In a study of tsunami survivors (one, three and six years after the traumatic event), Johannesson et al. showed four long-term PTSD trajectories: resilient (72.3%), severe chronic (4.6%), moderate chronic (11.2%) and recovering (11.9%) [48]. In a meta-analysis of 54 studies, Galatzer-Levy et al. identified 12 different trajectory groups across studies, of which four were most commonly reported: chronic (12%, high symptoms over time), resilient (65%), delayed onset and recovering [49]. Across studies, they found a mean prevalence of 65% for resilient trajectories, which match the stable low group found in our sample (59%). In the meta-analysis, recovery had a mean prevalence of 23%, roughly consistent with our results (15%), and delayed onset occurred in 18%, consistent with our increasing pattern (26%). Our results are therefore broadly consistent with previous research on PTSD outside the ICU.
There is a paucity of research on the long-term course of PTSD in ICU survivors, as most previous studies included only one follow-up assessment [50]. Only two ICU related studies have described the longitudinal development of PTSD at group level [19, 51]. Both studies used predefined clinical criteria to define trajectory groups, whereas we—like most studies outside the ICU context—took an exploratory, data-driven approach to cluster trajectories [49, 51]: Bienvenu et al. followed ICU survivors for two years with four follow-up measurements: Based on the presence of clinically relevant symptoms they found four subgroups: no symptoms, maintainers, remitters and relapsers [51]. Most patients either reported no relevant or maintained clinically relevant PTSD symptoms over time, which corresponds to our stable low trajectory group. Remission was observed in approximately 14% of patients, which corresponds to the 15% of patients in our recovering trajectory. In contrast, the relapse group was small (4.8%) and showed little remission of symptoms over time. For a five-year follow-up, Bienvenu et al. only distinguished between maintaining/ recurring symptoms and no symptoms/ remitting symptoms [52] which makes comparisons with our data difficult. Wintermann et al. [19] measured PTSD symptoms at three and six months after discharge, corresponding roughly to the period between our first and second assessment. They found that delayed onset PTSD symptoms occurred in a quarter of patients—defined as the first occurrence of clinically relevant symptoms six months after discharge. This corresponds to our trajectory group of increasing symptoms. Our results also suggest that symptoms may continue to worsen in the second year after discharge. Taken together, these results may indicate that ICU treatment is associated with a higher risk of late-onset PTSD symptoms compared with other traumatic events.
Consistent with the meta-analysis by Parker et al. [6], which included follow-up data up to 12 months, we did not find that the duration or invasiveness of the ICU treatment predicted post-traumatic symptoms. The authors discussed that other factors, such as the type of sedation, may be more important in the development of traumatic memories in the ICU. Unlike other traumatic events, ICU patients usually do not experience a sudden, single trauma, rather than a cumulative traumatization due to the experienced helplessness, exposure to invasive medical interventions, severity of illness and medically induced sedation or altered states of consciousness, including delirium [53]. Continuity of experience may prevent emotional processing and limit the ability to integrate early traumatic memories. As a result, these memories are often implicit and fragmentary [54]. Similar to being awake under anesthesia, snippets of conversations, sounds, pain and other impressions are recalled which, cannot be placed in time and space and are later re-experienced as real [55]. In line with this, early memories of the ICU emerged as a significant predictor of increasing post-traumatic symptoms in our sample, possibly indicating the intensity of traumatization, consistent with literature [6, 30, 56]. In particular, also Wintermann et al. identified the number of traumatic memories as a predictor of late-onset PTSD [19]. Even if PTSD severity cannot be predicted by a single factor, early traumatic memories should be given more attention in screening procedures. This may be supported by the fact, that ICU diaries or documentation by ICU nurses have been shown to help ICU survivors integrate and emotionally process traumatic experiences [57].
Although women have been shown to be at increased risk of PTSD [58, 59], long-term PTSD trajectories were rarely studied from a gender perspective [60]. In contrast to our findings, van Zuiden et al. demonstrated that women were more likely to recover from PTSD symptoms one year after a serious injury, whereas men were more likely to show a delayed symptom onset [31]. Consistent with our findings, Lowe et al. [32] showed in their pooled analysis of six longitudinal studies in adult survivors of civilian injuries that women were at higher risk of both initial post-traumatic symptoms and late onset. The authors suggest that both the nature of the trauma and the intensity of the acute emotional response may account for the disproportionate risk of PTSD in women. Other authors discuss sex differences in brain neurocircuitry, anatomy and neurobiological processes involved in memory consolidation [61, 62]. As (1) the consolidation of traumatic experiences depends on stress hormone levels [56, 63, 64] and (2) sepsis patients often show high inflammatory and neuroendocrine stress responses [15] or alterations in the endocannabinoid system anyway [40], sex-specific neurobiological interactions may also be relevant for our sample [64].
Our findings support both the need for timely screening for early traumatic memories after discharge from the ICU and for regular monitoring of post-traumatic stress symptoms in the long-term. As our results show that women have higher rates of post-traumatic stress symptoms and are also reported to respond better to treatment [65], female patients may receive particular attention. Particularly, GPs need to be aware of this issue, as they provide the most continuity of care for patients [66].
This study has several strengths: Long-term trajectories of post-traumatic symptoms in ICU survivors are still poorly described, even less so with multiple follow-ups and in sepsis survivors. In addition, the identification of individual risk factors has been barely studied. Our secondary analysis includes symptom assessment at four time points over a total period of two years, which even allows the identification of undulating trajectories. In addition, a wide range of individual risk factors for post-traumatic symptoms after sepsis could be examined, such as age, gender, socioeconomic status, pre-existing physical and psychological morbidity and intensive care parameters.
This study has limitations: It was an exploratory secondary analysis of trial data. In addition, patients in the intervention group received additional care that affected the outcome studied. Thus, we used randomization status as a covariate in the identification of predictors of outcome groups. At a descriptive level, the same clusters were found in both the intervention and control groups, see Additional file 3.
Grouping of longitudinal trajectories is a complex exploratory analysis that is susceptible to method bias, with different methods leading to different trajectory groups [67]. Therefore, findings need to be replicated in larger prospective cohorts with more measurement points, which would allow comparison of the results of different grouping methods [67, 68].
Finally, the use of the PTSS-10 for screening [30] does not allow to define a clinical diagnosis of a Post-Traumatic Stress Disorder (PTSD), which requires a detailed diagnostic interview by a psychiatrist. Consequently, patients with high PTSS-10 scores need to be referred for further psychiatric assessment.
Although our data are almost a decade old, they still appear to reflect the state of the art when compared in terms of clinical characteristics or intensive care procedures with current studies, such as an RCT by our research group on PTSD in critical illness survivors [69, 70].
Conclusion
Post-traumatic stress is a relevant long-term burden for sepsis patients after ICU stay. This analysis of predictive trajectories supports both the identification of patients at risk for PTSD after sepsis and the importance of their long-term observation. Women in particular may be at risk of increasing symptom severity, and the presence of traumatic ICU memories could be used as an early warning sign for the development of PTSD.
Regular screening of sepsis survivors for post-traumatic stress symptoms should be considered, even in patients with few initial symptoms and beyond 12 months, as future worsening is possible. Given the high continuity of care in general practice, screening for symptoms may be best implemented in this setting.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence Interval
- HRQOL:
-
Health-Related Quality of Life
- ICU:
-
Intensive Care Unit
- OR:
-
Odds ratio
- PTSD:
-
Post-traumatic Stress Disorder
- PTSS:
-
Post-traumatic Symptom Scale
References
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–62.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11.
Hofhuis JGM, Schrijvers AJP, Schermer T, Spronk PE. Health-related quality of life in ICU survivors-10 years later. Sci Rep. 2021;11(1):15189.
Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–9.
Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–53.
Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121–9.
Hatch R, Young D, Barber VS, Griffiths J, Harrison DA, Watkinson PJ. Anxiety, depression and post-traumatic stress disorder management after critical illness: a UK multi-centre prospective cohort study. Crit Care. 2020;24(1):633.
Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–83.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–82.
Ea S. Die Betreuung des Intensivpatienten ist mit der Entlassung nicht zu Ende: Post-ICU-Care. Intensiv-News. 2016;1:26–8.
Schofield-Robinson OJ, Lewis SR, Smith AF, McPeake J, Alderson P. Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Database Syst Rev. 2018;11:cd012701.
Schmidt KFR, Huelle K, Reinhold T, Prescott HC, Gehringer R, Hartmann M, et al. Healthcare utilization and costs in sepsis survivors in Germany-secondary analysis of a prospective cohort study. J Clin Med. 2022;11(4):1142.
Cyr S, Guo X, Marcil MJ, Dupont P, Jobidon L, Benrimoh D, et al. Posttraumatic stress disorder prevalence in medical populations: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2021;69:81–93.
Calsavara AJ, Costa PA, Nobre V, Teixeira AL. Prevalence and risk factors for post-traumatic stress, anxiety, and depression in sepsis survivors after ICU discharge. Braz J Psychiatry. 2021;43(3):269–76.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62–75.
Boer KR, van Ruler O, van Emmerik AA, Sprangers MA, de Rooij SE, Vroom MB, et al. Factors associated with posttraumatic stress symptoms in a prospective cohort of patients after abdominal sepsis: a nomogram. Intensive Care Med. 2008;34(4):664–74.
Rosendahl J, Brunkhorst FM, Jaenichen D, Strauss B. Physical and mental health in patients and spouses after intensive care of severe sepsis: a dyadic perspective on long-term sequelae testing the Actor-Partner Interdependence Model. Crit Care Med. 2013;41(1):69–75.
Wintermann GB, Brunkhorst FM, Petrowski K, Strauss B, Oehmichen F, Pohl M, et al. Stress disorders following prolonged critical illness in survivors of severe sepsis. Crit Care Med. 2015;43(6):1213–22.
Wintermann GB, Rosendahl J, Weidner K, Strauss B, Petrowski K. Risk factors of delayed onset posttraumatic stress disorder in chronically critically Ill patients. J Nerv Ment Dis. 2017;205(10):780–7.
Schlenger WE, Corry NH, Williams CS, Kulka RA, Mulvaney-Day N, DeBakey S, et al. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. Am J Epidemiol. 2015;182(12):980–90.
Zatzick DF, Rivara FP, Nathens AB, Jurkovich GJ, Wang J, Fan MY, et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med. 2007;37(10):1469–80.
Apitzsch S, Larsson L, Larsson AK, Linder A. The physical and mental impact of surviving sepsis - a qualitative study of experiences and perceptions among a Swedish sample. Arch Public Health. 2021;79(1):66.
Schmidt K, Thiel P, Mueller F, Schmuecker K, Worrack S, Mehlhorn J, et al. Sepsis survivors monitoring and coordination in outpatient health care (SMOOTH): study protocol for a randomized controlled trial. Trials. 2014;15(1):283.
Schmidt K, Worrack S, Von Korff M, Davydow D, Brunkhorst F, Ehlert U, et al. Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial. JAMA. 2016;315(24):2703–11.
Schmidt KFR, Schwarzkopf D, Baldwin L-M, Brunkhorst FM, Freytag A, Heintze C, et al. Long-term courses of sepsis survivors: effects of a primary care management intervention. Am J Med. 2020;133(3):381-5.e5.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA-J Am Med Assoc. 2016;315(8):801–10.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. American-college of chest physicians society of critical care medicine consensus conference - definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74.
de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18(4):318–24.
Eid J, Thayer JF, Johnsen BH. Measuring post-traumatic stress: a psychometric evaluation of symptom–and coping questionnaires based on a Norwegian sample. Scand J Psychol. 1999;40(2):101–8.
Stoll C, Kapfhammer HP, Rothenhausler HB, Haller M, Briegel J, Schmidt M, et al. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999;25(7):697–704.
van Zuiden M, Engel S, Karchoud JF, Wise TJ, Sijbrandij M, Mouthaan J, et al. Sex-differential PTSD symptom trajectories across one year following suspected serious injury. Eur J Psychotraumatol. 2022;13(1):2031593.
Lowe SR, Ratanatharathorn A, Lai BS, van der Mei W, Barbano AC, Bryant RA, et al. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: pooled results from the International Consortium to predict PTSD. Psychol Med. 2021;51(7):1129–39.
Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44(5):954–65.
Khitab A, Reid J, Bennett V, Adams GC, Balbuena L. Late onset and persistence of post-traumatic stress disorder symptoms in survivors of critical care. Can Respir J. 2013;20(6):429–33.
Dickstein BD, Suvak M, Litz BT, Adler AB. Heterogeneity in the course of posttraumatic stress disorder: trajectories of symptomatology. J Trauma Stress. 2010;23(3):331–9.
Gros DF, Price M, Magruder KM, Frueh BC. Symptom overlap in posttraumatic stress disorder and major depression. Psychiatry Res. 2012;196(2–3):267–70.
Outcalt SD, Kroenke K, Krebs EE, Chumbler NR, Wu J, Yu Z, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38(3):535–43.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–9.
Hauer D, Kaufmann I, Strewe C, Briegel I, Campolongo P, Schelling G. The role of glucocorticoids, catecholamines and endocannabinoids in the development of traumatic memories and posttraumatic stress symptoms in survivors of critical illness. Neurobiol Learn Mem. 2014;112:68–74.
von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–49.
Ravn SL, Hartvigsen J, Hansen M, Sterling M, Andersen TE. Do post-traumatic pain and post-traumatic stress symptomatology mutually maintain each other? A systematic review of cross-lagged studies. Pain. 2018;159(11):2159–69.
Calsavara AJC, Nobre V, Barichello T, Teixeira AL. Post-sepsis cognitive impairment and associated risk factors: a systematic review. Aust Crit Care. 2018;31(4):242–53.
Puntillo KA, Max A, Chaize M, Chanques G, Azoulay E. Patient Recollection of ICU Procedural Pain and Post ICU Burden: The Memory Study. Crit Care Med. 2016;44(11):1988–95.
Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2021.
Leffondre K, Abrahamowicz M, Regeasse A, Hawker GA, Badley EM, McCusker J, et al. Statistical measures were proposed for identifying longitudinal patterns of change in quantitative health indicators. J Clin Epidemiol. 2004;57(10):1049–62.
Sylvestre MP, McCusker J, Cole M, Regeasse A, Belzile E, Abrahamowicz M. Classification of patterns of delirium severity scores over time in an elderly population. Int Psychogeriatr. 2006;18(4):667–80.
Johannesson KB, Arinell H, Arnberg FK. Six years after the wave. Trajectories of posttraumatic stress following a natural disaster. J Anxiety Disord. 2015;36:15–24.
Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: a review and statistical evaluation. Clin Psychol Rev. 2018;63:41–55.
Righy C, Rosa RG, da Silva RTA, Kochhann R, Migliavaca CB, Robinson CC, et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care. 2019;23(1):213.
Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Shanholtz C, Dennison-Himmelfarb CR, Pronovost PJ, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3):642–53.
Bienvenu OJ, Friedman LA, Colantuoni E, Dinglas VD, Sepulveda KA, Mendez-Tellez P, et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. 2018;44(1):38–47.
Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–79.
Shaffer KM, Riklin E, Jacobs JM, Rosand J, Vranceanu AM. Mindfulness and coping are inversely related to psychiatric symptoms in patients and informal caregivers in the neuroscience ICU: implications for clinical care. Crit Care Med. 2016;44(11):2028–36.
Elbert T, Maggie Schauer, and Frank Neuner. Narrative exposure therapy (NET): Reorganizing memories of traumatic stress, fear, and violence." Evidence based treatments for trauma-related psychological disorders: A practical guide for clinicians: Springer International Publishing; 2022.
Schelling G. Post-traumatic stress disorder in somatic disease: lessons from critically ill patients. Prog Brain Res. 2008;167:229–37.
McIlroy PA, King RS, Garrouste-Orgeas M, Tabah A, Ramanan M. The effect of ICU diaries on psychological outcomes and quality of life of survivors of critical illness and their relatives: a systematic review and meta-analysis. Crit Care Med. 2019;47(2):273–9.
Christiansen DM, Berke ET. Gender- and Sex-Based Contributors to Sex Differences in PTSD. Curr Psychiatry Rep. 2020;22(4):19.
Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry. 2017;4(1):73–82.
Kornfield SL, Hantsoo L, Epperson CN. What does sex have to do with it? the role of sex as a biological variable in the development of posttraumatic stress disorder. Curr Psychiatry Rep. 2018;20(6):39.
Ishikawa R, Uchida C, Kitaoka S, Furuyashiki T, Kida S. Improvement of PTSD-like behavior by the forgetting effect of hippocampal neurogenesis enhancer memantine in a social defeat stress paradigm. Mol Brain. 2019;12(1):68.
Kida S. Reconsolidation/destabilization, extinction and forgetting of fear memory as therapeutic targets for PTSD. Psychopharmacology. 2019;236(1):49–57.
Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: are gonadal hormones the link? Br J Pharmacol. 2019;176(21):4119–35.
Ney LJ, Matthews A, Bruno R, Felmingham KL. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci Biobehav Rev. 2018;94:302–20.
Wade D, Varker T, Kartal D, Hetrick S, O’Donnell M, Forbes D. Gender difference in outcomes following trauma-focused interventions for posttraumatic stress disorder: systematic review and meta-analysis. Psychol Trauma. 2016;8(3):356–64.
Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502.
Mesidor M, Rousseau MC, O’Loughlin J, Sylvestre MP. Does group-based trajectory modeling estimate spurious trajectories? BMC Med Res Methodol. 2022;22(1):194.
Twisk J, Hoekstra T. Classifying developmental trajectories over time should be done with great caution: a comparison between methods. J Clin Epidemiol. 2012;65(10):1078–87.
Gensichen J, Schultz S, Adrion C, Schmidt K, Schauer M, Lindemann D, et al. Effect of a combined brief narrative exposure therapy with case management versus treatment as usual in primary care for patients with traumatic stress sequelae following intensive care medicine: study protocol for a multicenter randomized controlled trial (PICTURE). Trials. 2018;19(1):480.
Sanftenberg L, Dreischulte T, Hardtlein A, Kosub H, Gagyor I, Kurotschka PK, et al. Process evaluation in practice based research networks: a study protocol for a mixed-methods implementation study. BMJ Open. 2023;13(7): e065947.
Acknowledgements
We thank the SMOOTH-Study group for patient recruitment, data collection and analysis: Dimitry Davydow (Comprehensive Life Resources Tacoma); Anne Bindara-Klippel; Frank-Martin Brunkhorst, MD, Carolin Fleischmann, MD; Antje Freytag ScD, Ursula Jakobi, MD; Susan Kerth; Heike Kuhnsch; Konrad Reinhart, MD; Mercedes Schelle; André Scherag, MSc PhD; Nico Schneider, MSc; Paul Thiel, MD (Jena University Hospital); Susanne Ullmann (German Sepsis Aid); Andrea Geist and Torsten Schreiber, MD (Zentralklinik Bad Berka); Christian Berhold, MD; Marcel Corea, MD; Adrian Freytag, MD; Herwig Gerlach, MD, MBA; Rainer Kuehnemund, MD; Josefa Lehmke, MD; Peter Lehmkuhl, MD; Lorenz Reil, MD; Guenter Tiedemann, MD and Susanne Toussaint, MD (Vivantes Clinics Berlin); Anton Goldmann, MD; Michael Oppert, MD; Didier Keh, MD; Sybille Rademacher, MD; Claudia Spies, MD; and Lars Toepfer, MD (Charité University Medicine Berlin); Frank Klefisch, MD (Paulinen Hospital Berlin); Armin Sablotzki, MD (St Georgs-Hospital Leipzig); Frank Oehmichen, MD and Marcus Pohl, MD (Bavaria Clinic Kreischa); Andreas Meier-Hellmann, MD (Helios Clinic Erfurt); Farsin Hamzei, MD (Moritz-Clinic Bad Klosterlausnitz); Christoph Engel, MD and Christine Pausch, PhD (University of Leipzig) and Michael von Korff, ScD (Kaiser Permanente Washington Health Research Institute).
Funding
Open Access funding enabled and organized by Projekt DEAL. The Smooth-Study has been funded by the German Ministry of Education and Research (BMBF), grant no. 01 E0 1002.
Author information
Authors and Affiliations
Contributions
K.S., M.S., M.K. and D.S. developed study concept and design. K.S., J.G. and Chr.H. acquired the data, K.S. and D.S. analyzed the data, K.S., M.S., M.K., F.M. and D.S. interpreted the data and drafted the manuscript, and K.S., J.G., M.S., M.K., F.M., G.S., S.G.B., M.B., Chr.H., M.W. and D.S. critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol of the SMOOTH-Study was approved by the institutional review board of the University of Jena, January 26th, 2011 (No.3001/111). The SMOOTH-Study was registered in the International Traditional Medicine Clinical Trial Registry (ISRCTN) on April 19th, 2011: https://www.isrctn.com/ISRCTN61744782 All enrolled patients were informed about the use of their anonymized data and provided written consent.
Consent for publication
Not applicable.
Competing interests
None conflicts of interest are declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
: Flow chart of the study population
Additional file2
: Measures of post-traumatic stress over time: Patterns of missing values In total, there were 116 patients with missing values on at least one measurement of the Post-traumatic Symptom Scale (PTSS-10). Horizontal bars present the number of patients with missing measurement for each time point, vertical bars present the number of patients with a specific pattern of missing values across all time points. For example, the largest pattern includes N=59 patients with missing values at the six months follow-up and all subsequent follow-ups as marked by the connected points below. The vast majority of missing values are monotonic, meaning that after a missing follow-up measurement, all subsequent follow-up measurements are also missing.
Additional file3
: Comparison of cluster-results between control and intervention group. Panels A, C, and E show stable low, increasing, and recovering clusters for the control group. Panels B, D, and F present same clusters for the intervention group. Sum scores above 35 are considered to indicate PTSD, above 23 to be clinically relevant.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schmidt, K.F.R., Gensichen, J.S., Schroevers, M. et al. Trajectories of post-traumatic stress in sepsis survivors two years after ICU discharge: a secondary analysis of a randomized controlled trial. Crit Care 28, 35 (2024). https://doi.org/10.1186/s13054-024-04815-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04815-4