Abstract
Purpose
To investigate the global burden of sepsis in hospitalized adults by updating and expanding a systematic review and meta-analysis and to compare findings with recent Institute for Health Metrics and Evaluation (IHME) sepsis estimates.
Methods
Thirteen electronic databases were searched for studies on population-level sepsis incidence defined according to clinical criteria (Sepsis-1, -2: severe sepsis criteria, or sepsis-3: sepsis criteria) or relevant ICD-codes. The search of the original systematic review was updated for studies published 05/2015–02/2019 and complemented by a search targeting low- or middle-income-country (LMIC) studies published 01/1979–02/2019. We performed a random-effects meta-analysis with incidence of hospital- and ICU-treated sepsis and proportion of deaths among these sepsis cases as outcomes.
Results
Of 4746 results, 28 met the inclusion criteria. 21 studies contributed data for the meta-analysis and were pooled with 30 studies from the original meta-analysis. Pooled incidence was 189 [95% CI 133, 267] hospital-treated sepsis cases per 100,000 person-years. An estimated 26.7% [22.9, 30.7] of sepsis patients died. Estimated incidence of ICU-treated sepsis was 58 [42, 81] per 100,000 person-years, of which 41.9% [95% CI 36.2, 47.7] died prior to hospital discharge. There was a considerably higher incidence of hospital-treated sepsis observed after 2008 (+ 46% compared to the overall time frame).
Conclusions
Compared to results from the IHME study, we found an approximately 50% lower incidence of hospital-treated sepsis. The majority of studies included were based on administrative data, thus limiting our ability to assess temporal trends and regional differences. The incidence of sepsis remains unknown for the vast majority of LMICs, highlighting the urgent need for improved epidemiological sepsis surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this updated and extended systematic review and meta-analysis, we found a high incidence of sepsis across all regions; one out of four sepsis patients did not survive their hospital stay. Substantial gaps remain regarding data availability from low- and middle-income countries, the lack of community-based studies and inherent limitations of epidemiological research based on administrative data, highlighting the urgent need for improved epidemiological sepsis surveillance. |
Introduction
Sepsis is the dysregulated immune response to infection that leads to life-threatening organ dysfunction [1]. It is a medical emergency associated with high mortality and long-term disability in survivors [2]. In the USA (US), sepsis was identified as the most expensive condition in the hospital setting in 2011, responsible for 6.2% of the aggregate costs for all hospitalizations, or 23.7 billion USD, annually [3]. In 2017, the World Health Organization (WHO) member states declared that the improvement of sepsis prevention, recognition, and treatment is a global health priority [4]. In a systematic review and meta-analysis published in 2016, we estimated that more than 19 million annual severe sepsis cases occur worldwide, with at least five million deaths [5]. A recent study from the Institute for Health Metrics and Evaluation (IHME) on the global burden of sepsis [6] estimated 48.9 million incident sepsis cases and 11 million sepsis-related deaths worldwide in 2017. These estimates primarily used the Global Burden of Disease (GBD) 2017 Study cause of death estimates, with additional sepsis-specific death certificate data from four countries, and employed complex modelling to produce global estimates. Compared to previous estimates from cohort studies [5], the incidence estimated by IHME is considerably higher. IHME data also suggest a decrease in sepsis incidence by approximately 37% between 1990 and 2017, contrary to the results from several individual studies that observed an increase in sepsis incidence over time [5]. To better understand these conflicting data, we aimed to compile recent evidence on population-level sepsis incidence and mortality, particularly in LMICs, and to compare incidence estimates with respect to WHO region, observation years and sepsis case definition.
Methods
The review followed a predesigned protocol registered in PROSPERO (CRD42019136286).
Search strategy and eligibly criteria
The literature search included (1) an updated search of the 2016 review [5], (2) an extended search aiming to increase the number of studies from LMICs, and (3) hand search and expert queries for regions with limited data availability. We searched 13 databases for studies on population-level sepsis incidence with no language or publication restrictions: PubMed, EMBASE, LILACS, African Journals Online, OpenGREY, MedCarib, Index Medicus for the WHO Eastern Mediterranean Region, South East Asia Region, Western Pacific Region and African Region, IndMed, Web of Science, and WHOLIS. The full list of search terms is provided in the Supplement M1. In short, we (1) used the same search terms as in our previous review [5] and updated it for the time frame between 05/2015 and 02/2019. We (2) added a search covering studies published between 01/1979 and 02/2019 that combined a list of sepsis terms with individual LMIC country names, according to the World Bank classification of countries, and specific search terms for LMICs as suggested by the EPOC group LMIC filters applied to the title and abstract of the studies (https://epoc.cochrane.org/lmic-filters).
We included studies that reported on population-level sepsis incidence or prevalence in adults, including studies that reported observed sepsis cases in hospitals, ICUs, Emergency Departments (ED), or a community. The term population-level refers to studies that report sepsis incidence rates for a defined population. Sepsis had to be defined according to sepsis-1/2 criteria for severe sepsis [7, 8], sepsis-3 criteria [1], sepsis-relevant ICD-9/ICD-10 codes [9], or implicit ICD-9/10-case identification (combined infection and organ dysfunction codes, known as Angus implementation [10]). We accepted minor modification of the clinical criteria, e.g. in the limits of physiological parameters or if laboratory testing was unavailable. We excluded studies on sepsis incidence among hospital admission, studies on sepsis without organ dysfunction according to sepsis-1/2 criteria, and studies limited to sub-groups of sepsis, causative pathogens, selected patient groups, or treatment units (e.g. surgical ICUs). Studies with insufficient reporting on methodology were excluded.
Data compilation
Abstracts were reviewed by two independent investigators (LM, CFS) and those considered to fulfil the inclusion criteria underwent full-text review by the same two investigators. Discrepancies were resolved by a third reviewer (KER). Non-English articles were assessed by native speakers with medical backgrounds. Extracted data included study location and methodology, number and age of patients, cases of sepsis observed, number of deaths from sepsis, sepsis in-hospital mortality rate, population denominator, and sepsis incidence, prevalence, and mortality per 100,000 population. Missing population data were requested from the authors or identified in national census databases. If there was a distinction between sepsis patients and observed sepsis episodes, the number of episodes was included for analysis. In this systematic review and meta-analysis update, sepsis is defined as an infection complicated by organ dysfunction, in accordance with sepsis-3, formerly known as severe sepsis. This includes cases of septic shock. We did not investigate the epidemiology of sepsis without organ failure. Studies that met the inclusion criteria were assessed for risk of bias using the tool suggested by Hoy et al. by two independent investigators [11].
Statistical analyses
Two outcomes were considered for meta-analysis: (a) Population-level incidence rates, and (b) mortality rates of sepsis. Different calculations and standardizations used in the original studies may lead to a method-dependent overestimation of between-study variance. Therefore, our analyses are based on the reported numbers of observed hospital sepsis cases and population data. We included the most recent years or time frames reported in the studies in the meta-analysis. From studies that applied alternative definitions to the same data source, we prioritized sepsis-3 over sepsis-2, explicit over implicit ICD-based case definitions, and the most recent years of observation reported. Details of this selection process are described in the Supplement M2. We utilized meta-analytic random-effects models to perform the meta-analyses. A random intercept Poisson regression model was used for hospital- and ICU-treated sepsis incidence rates. For the meta-analyses of the mortality of hospital- and ICU-treated sepsis, we used a logistic random intercept model. Between-study heterogeneity was expressed by the standard deviation of the random intercepts (denoted by τ). Model parameters were back transformed to present the results as number of cases per 100,000 person-years (incidence rates) or the per cent of patients who died (mortality). For better interpretation, we additionally report the estimated averages across the studies obtained by numerical integration (for detailed explanations see Supplement M3).
The Poisson and the logistic random-effects models were extended to meta-regression models to investigate differences in incidence and mortality rates (a) across WHO regions (African Region (AFR), European region (EURO), Pan-American Region (PAR), Western Pacific Region (WPR)), and (b) arising from different sepsis case definitions {clinical criteria (sepsis-1 [7], -2 [8] or -3 [1]) vs. ICD-case identification (implicit [10] or explicit case identification [9])}. The multi-parameter F-test [12] as well as pairwise post hoc tests with p values adjusted for multiple testing [13] were used for statistical inference in the meta-regression models. The specifications of all models are described in the Supplement. We present 95% Poisson-confidence intervals (CIs) for estimated incidence rates of the single studies and 95% Wilson score intervals for mortality rates. For the meta-analytic estimates of the overall incidence and mortality rate, we provide 95% CIs as well as 95% prediction intervals.
All statistical analyses were conducted within the R (Version 3.6.0; R core team, 2019) software environment. We used the metarate, metaprop, and metareg functions from the R package meta [14] and the glht function from the multcomp package [13]. The R code is provided in Supplement 2.
Results
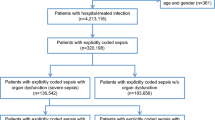
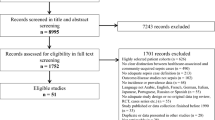
Our search yielded 4746 results, of which 28 met the inclusion criteria [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Fig. 1, Tables E1-E3). Interrater agreement on the study inclusion was 0.9 [95% CI 0.8, 1.0]. Risk of bias was judged as moderate for most studies, mainly due to missing national representativeness of single-centre studies or studies limited to certain geographical regions, or the use of administrative data and ICD-based sepsis case identification, which has known inaccuracies (Table E4). 21/28 studies were included in the meta-analyses; reasons for exclusion from meta-analysis are provided in Fig. 1. We pooled these 21 studies together with 30 studies included in the previous meta-analysis. Countries covered by the resulting 51 studies are shown in Fig. 2; 46/51 studies were from high-income countries. The distribution of the studies among WHO regions was as follows: PAR 21.5% (n = 11/51), WPR 15.7% (n = 8/51), EUR 60.8% (n = 31/51), AFR 2.0% (n = 1/51), Eastern Mediterranean Region (EMR)/Southeast Asia Region (SEAR)—no studies included. The data sources of the individual studies and their population coverage are provided in Supplement Table E5. We did not identify any community-based studies, and thus stratified studies according to the inpatient setting (ICU/hospital/ED) for further analyses and report the incidence and mortality of treated sepsis cases in the respective setting. Given the small number of studies from LMICs in the strata, no meaningful subgroup analyses by income level were possible.
Hospital-treated sepsis
Twenty-eight studies were analysed. The majority were retrospective, based on ICD-coded hospital discharge databases (22/28), chart review (2/28), and case identification in electronic health records (2/28). Two studies used prospective observations to assess sepsis incidence and case fatality. We found a random-effects estimator of 189 [95% CI 133, 267] hospital-treated sepsis cases per 100,000 person-years (τ = 0.936, Fig. 3a, last decade estimator: 276 [95% CI 189, 403] per 100,000 person-years, τ = 0.698, Figure E2A). Differences between WHO regions were not significant (p = 0.068, Table E6 and E7). Comparing incidence estimates according to sepsis case definition, we found no significant differences comparing implicit and explicit case identification strategies in ICD-based studies (p = 0.169) and between clinical sepsis definitions (p = 0.214, Table E8 and E9). The estimated hospital mortality rate was 26.7% [95% CI 22.9, 30.7] (τ = 0.475, Fig. 4A) and did not differ significantly between WHO regions (p = 0.158, Table E6 and E7) nor between implicit and explicit (p = 0.240), or sepsis-1 or sepsis-3 criteria (p = 0.769, Table E8 and E9).
ICU-treated sepsis
We meta-analysed the data of 34 studies. One study originated from Rwanda, which differs substantially from other countries in the number of ICUs. Only two ICUs existed in Rwanda at the time of the publication (2013) [16], representing a complete nationwide survey of ICU-treated sepsis cases. The study found a low estimated incidence rate (2 per 100,000 population) and acts as an outlier with a high leverage in the meta-analysis. We therefore report two random-effects meta-analysis estimators, with and without the data from Rwanda. The random-effects estimator of the ICU-treated sepsis was 58 [95% CI 42, 81] per 100,000 person-years (τ = 0.998, Fig. 3B) including all studies and 65 [95% CI 50, 85] per 100,000 person-years (τ = 0.790) without the Rwanda data. A statistically significant difference was found between the WHO regions (p < .001, Table E6 and E7), with the highest sepsis incidence in PAR . Incidence rates of ICU-treated sepsis did not significantly differ depending on the sepsis case identification (p = 0.126, Table E8 and E9). The mortality rate estimate of ICU-treated sepsis patients was 41.9% [95% CI 36.2, 47.7] (τ = 0.517, Fig. 4B) (39.9% [95% CI 35.4; 44.6], τ = 0.405 without Rwanda data), with significant differences between WHO regions (p = 0.013 ) (Table E6 and E7). Highest mortality rates were found in the AFR. There were no significant differences between sepsis case identification strategies based on ICD-codes or clinical criteria (p = 0.211, Table E8 and E9).
ED-treated sepsis
Only four studies reported the incidence of ED-treated sepsis; thus, we presented the data in Table E3 and did not perform a meta-analysis.
Discussion
This updated and extended systematic review identified 28 new studies, among which 21 provided data to be meta-analysed. It increases our understanding of sepsis epidemiology by synthesizing population-level data from around the world, and to our knowledge, this study represents the largest review of published literature on the population-level burden of sepsis among hospitalized adults. Using a specific search strategy that was designed to increase the number of results from LMICs, we were able to add five studies from two upper middle- and one low-income country to the original systematic review and meta-analyses, which formerly relied exclusively on studies from high-income countries (HICs). Based on a total of 51 studies from 22 countries and 4 WHO regions, we found a pooled incidence of 189 [95% CI 133, 267] hospital-treated adult sepsis cases per 100,000 person-years and a mortality rate of 26.7% [95% CI 22.9, 30.7]. There was a considerably higher incidence of sepsis observed in more recent studies (from 2008 onwards, +46% compared to the overall time frame). This estimate is mainly based on high-income-country data; it is likely that sepsis epidemiology differs considerably in countries with low and middle income due to a higher burden of infectious diseases [43], varying patterns of underlying comorbidities such as HIV or Mycobacterium tuberculosis infection [44], limited infection prevention, and fewer resources for sepsis treatment and intensive care [45]. 85.0% [95% UI 82.2, 87.4%] of the incident sepsis cases worldwide occurred in low- and middle-income countries in 2017 according to the IHME sepsis estimates [6]. This may be one explanation for the considerably lower global sepsis incidence estimate we found compared to the IHME sepsis data (276 [95% CI 189, 403] in the past decade vs. 678 [95% UI 536–876] cases per 100,000 in 2017 [6]). However, methodological differences hamper the comparability of the estimates in the IHME study and our meta-analysis. The IHME estimates were based on death certificate data, rather than hospital-based data, and thus capture sepsis cases that contributed to death outside the hospital. Furthermore, IHME estimates included children, which were excluded in most of our underlying studies; notably, the IHME study found very high incidence and mortality among children under 1 year of age.
An interesting finding of the IHME study is that global sepsis cases decreased by 37% between 1990 and 2017. This is contrary to the results from most individual studies included in our review, which found an increase in sepsis incidence over time (see Supplement Tables E1–3). There may be objective reasons for an increase in sepsis rates especially in HICs, likely an ageing population and a high number of elderly with comorbidities and increasing invasive and complex treatments, which may lead to more health care-associated infections and sepsis [46]. Higher rates of antimicrobial resistance may result in the progression of more infections to sepsis [47]. Decrease in sepsis incidence found in the IHME study may be driven by a decreasing number of deaths from infectious diseases in LMICs. However, modelling assumptions and imputation steps in the IHME study can introduce considerable bias, as the model inputs were derived from the multiple cause of death (MCOD) data from four countries and hospital data from ten countries. These countries were high- and middle-income countries and data were subsequently extrapolated to low-income countries [6, 48]. Therefore, longitudinal trends might be unreliable: for example, improvements in burden of sepsis in one country that was used as primary data source would project these benefits to other settings [48]. This approach leads to lower incidence estimates for high-income countries such as the USA or Sweden compared to data from individual observational epidemiological studies [15, 39]. This may be due to large variances of the coding of sepsis differences even among high-income countries, and from the undercoding of sepsis in comparison with patient hospital records [15, 39, 49]. To date, the validity of sepsis coding from MCOD data is unknown.
Likewise, the most likely reason for the observed increase in sepsis incidence in our study may result from the fact that most studies included relied on administrative data, with several inherent methodological limitations. Improved awareness, capacity for diagnosis, and external incentives may have led to a Will Rogers phenomenon (increase in detection of cases and coding) [50]. Rhee and colleagues found in a recent US study that sepsis incidence rates using clinical criteria in electronic health records were relatively stable (+ 0.6% increase per year), whereas sepsis incidence per claims data increased by + 10.3% per year [15]. Interestingly, we found no significant differences in meta-analytic estimates comparing different case definitions used to identify sepsis cases in administrative or clinical data. These comparisons, however, are hampered by the low number of studies in each stratum and may also reflect variations in country, settings, and observation years rather than differences arising from case definitions.
To our knowledge, we present the first comprehensive systematic review providing global estimates for the incidence and mortality rates of ICU-treated sepsis among hospitalized adults worldwide. Interestingly, ICU-based studies were mostly prospective and performed in selected ICUs or larger ICU networks, whereas studies on hospital-based sepsis nearly completely relied on administrative data. There was an up to 80-fold difference between the individual estimates of ICU-treated sepsis in the included studies, which may be a reflection of the structural differences in availability and access to intensive care [51], differences in health care systems, or different underlying study designs. Additionally, ICU capacities have stronger variations than hospital capacities between countries [52]. Beyond the scarcity of ICUs in many LMICs, access to existing (often private) ICUs may not be affordable for patients or logistically hampered or delayed [53]. This might contribute to the higher mortality rates in these countries, such as reported from Rwanda with 71.2% among sepsis patients [16]. In the range of estimates contributed by the other included studies, the Rwanda study appears to be an outlier. However, it might be representative for many LMICs, which implies that it should not be considered as such. It also suggests that there are much larger variances in incidence and mortality rates across countries than reflected in our meta-analyses relying mainly on HIC data. Much of this variance might be explainable by factors on the level of the health care systems that differ across countries or regions.
Despite the strengths of our systematic review, which include data inputs from 51 studies of which several were based on nationally representative datasets, the study has several important limitations. First, we present data on hospital- and ICU-treated sepsis cases rather than population-based estimates. Availability of and access to hospital care is likely to differ between countries. Consequently, a varying proportion of sepsis cases may not receive hospital treatment. In the USA, this proportion was found to be 12% based on an analysis of death certificates [54]. Second, we observed considerable between-study heterogeneity that may be caused by differences in sepsis definitions, study designs, sampling strategies and study settings. There also remains between-study variance due to variation in the considered time periods and the potential changes in incidence rates and mortality rates over time. Differences in the case mix of the population under observation are also likely to influence sepsis incidence and case fatality estimates. Furthermore, a number of studies used extrapolations to derive nationwide incidence estimates with potential assumptions and modelling. These factors hamper comparability of results within and across WHO regions and between different sepsis case identification methods and limit the generalizability of results. Third, the limitations of retrospective studies reporting on sepsis incidence using administrative data apply to the majority of the included studies. This has driven the overall moderate to high risk of bias assigned to nearly all included studies (94%). Bias may also be introduced by limited national representativeness of studies undertaken in single hospitals. Furthermore, publication bias may have impacted our results. We tried to minimize the risk of publication bias by including databases for grey, unpublished literature in our search. Fourth, not all data sources had complete population coverage, which can lead to an underestimation of the true sepsis incidence if not corrected for. This applies primarily for some nationwide hospital discharge databases and representative hospital samples, which excluded military hospitals, veteran admissions, or psychiatric facilities. Fifth, to avoid dependence of estimates, we selected incidence and case fatality estimates from studies with overlapping or identical data source. This selection was grounded on the available evidence such as the validity of case identification; however, it may also have introduced a certain selection bias. Sixth, the majority of hospital-based studies used administrative data as the data source, thus information on causative pathogens, as well as antimicrobial susceptibility and resistance, are unavailable for most studies. Finally, we were unable to identify studies reporting sepsis-attributable mortality, limiting our understanding of the role of sepsis in the pathway towards death.
In conclusion, in this updated and extended systematic review and meta-analysis, we compiled a comprehensive number of studies with improved geographical representation compared to our previous review. We found high incidence of sepsis across all regions; one out of four sepsis patients did not survive their hospital stay. Substantial gaps remain regarding data availability from low- and middle-income countries, the lack of community-based studies and inherent limitations of epidemiological research based on administrative data, limiting our understanding of temporal trends and geographical disparities. There is an urgent need to improve sepsis surveillance in all countries at the facility and health care provider level, but also to conduct community-based studies that improve our understanding of sepsis occurring outside the hospital setting. An improved documentation and coding of sepsis is needed. In addition, reference standards for sepsis epidemiology research and reporting are crucial for a better understanding of the problem and comparability of estimates, and ultimately to improve our understanding of the medical and economic burden of sepsis.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). J Am Med Assoc 315:801–810
Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM (2012) Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 60:1070–1077
Torio CM, Moore BJ (2016) National inpatient hospital costs: the most expensive conditions by payer, 2013: statistical Brief #204Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Rockville (MD)
World Health Organization (2017) World Health Assembly 70, Resolution 70.7: improving the prevention, diagnosis and clinical management of sepsis. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R7-en.pdf
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K (2016) Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med 193:259–272
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M (2020) Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R (2012) Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65:934–939
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22:2693–2710
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Schwarzer G (2007) Meta: an R package for meta-analysis. R News 7:40–45
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, Program CDCPE (2017) Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. J Am Med Assoc 318:1241–1249
Nzarora J, Beach ML, Riviello ED, Twagirumugabe T (2016) Epidemiology and outcomes of sepsis in two intensive care units in Rwanda. Am J Respiratory Crit Care Med 193
Marques AC, Janiszewski M, Houlis D (2007) Analysis of incidence, resource use and costs of severe sepsis in Brazil and the economic impact of drotrecogin-alfa activated. Value Health 10:A162–A162
Lorencio C, Yébenes JC, Gonzalez Londoño J, Cleriès M, Vela E, Espinosa L, Ruiz JC, Rodriguez A, Esteban E, Ferrer R, Artigas A (2018) Incidence and mortality of multiple organ failure (MOF) in septic patients. An 11 year review in Catalonia. Intensive Care Med Exp 6(Suppl2):40
Lee CC, Yo CH, Lee MG, Tsai KC, Lee SH, Chen YS, Lee WC, Hsu TC, Lee SH, Chang SS (2017) Adult sepsis—A nationwide study of trends and outcomes in a population of 23 million people. J Infect 75:409–419
Kim J, Kim K, Lee H, Ahn S (2019) Epidemiology of sepsis in Korea: a population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin Exp Emerg Med 6:49–63
Dupuis C, Bouadma L, Ruckly S, Perozziello A, Mourvillier B, Bailly S, Sonneville R, Timsit J-F (2017) Septic shock in France from 2009 to 2014: incidence, outcome, and associated costs of care. Ann Intensive Care 7:32–33
Yebenes JC, Ruiz JC, Ferrer R, Artigas A, Lorencio C, Rodriguez A, Nuvials X, Martin-Loeches I, Bordeje L, Bosch A, Cleries M (2014) Trends in incidence and hospital outcomes among patients with severe sepsis in catalonia during the 2008–2012 period. Intensive Care Med 40(1):S152
Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Heublein S, Dennler U, Reinhart K (2016) Hospital Incidence and Mortality Rates of Sepsis. Deutsches Arzteblatt Int 113:159–166
Zhou J, Tian H, Du X, Xi X, An Y, Duan M, Weng L, Du B, for China Critical Care Clinical Trials G (2017) Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit Care Med 45:1168–1176
Vakkalanka JP, Harland KK, Swanson MB, Mohr NM (2018) Clinical and epidemiological variability in severe sepsis: an ecological study. J Epidemiol Community Health 72:741–745
Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K (2017) Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth 119:626–636
Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R, Angus DC, Pontes Azevedo LC, Investigators S, Latin American Sepsis Institute N (2017) The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 17:1180–1189
Bertullo M, Carbone N, Brandes M, Silva M, Meiss H, Tejera D, Deicas A, Buroni M, Gerez J, Limongi G, Cancela M, Hurtado J (2016) Epidemiología, diagnóstico y tratamiento de la sepsis severa en Uruguay: un estudio multicéntrico prospectivo. Rev méd Urug 32:178–189
Yu CW, Chang SS, Lai CC, Wu JY, Yen DW, Lee MG, Yeh CC, Chung JY, Lin YJ, Lee CC (2019) Epidemiology of Emergency Department Sepsis: a National Cohort Study Between 2001 and 2012. Shock 51:619–624
Herran-Monge R, Muriel-Bombin A, Garcia-Garcia MM, Merino-Garcia PA, Martinez-Barrios M, Andaluz D, Ballesteros JC, Dominguez-Berrot AM, Moradillo-Gonzalez S, Macias S, Alvarez-Martinez B, Fernandez-Calavia MJ, Tarancon C, Villar J, Blanco J (2019) Epidemiology and changes in mortality of sepsis after the implementation of surviving sepsis campaign guidelines. J Intensive Care Med 34:740–750
Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW (2016) Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest 150:829–836
Cowan SL, Holland JA, Kane AD, Frost I, Boyle AA (2015) The burden of sepsis in the emergency department: an observational snapshot. Eur J Emerg Med 22:363–365
de Miguel-Yanes JM, Mendez-Bailon M, Jimenez-Garcia R, Hernandez-Barrera V, Perez-Farinos N, Lopez-de-Andres A (2015) Trends in sepsis incidence and outcomes among people with or without type 2 diabetes mellitus in Spain (2008–2012). Diabetes Res Clin Pract 110:266–275
Azkarate I, Choperena G, Salas E, Sebastian R, Lara G, Elosegui I, Barrutia L, Eguibar I, Salaberria R (2016) Epidemiology and prognostic factors in severe sepsis/septic shock. Evolution over six years. Medicina Intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias 40:18–25
Knoop ST, Skrede S, Langeland N, Flaatten HK (2017) Epidemiology and impact on all-cause mortality of sepsis in Norwegian hospitals: a national retrospective study. PLoS ONE 12:e0187990
Stoller J, Halpin L, Weis M, Aplin B, Qu W, Georgescu C, Nazzal M (2016) Epidemiology of severe sepsis: 2008-2012. J Crit Care 31:58–62
Kubler A, Adamik B, Ciszewicz-Adamiczka B, Ostrowska E (2015) Severe sepsis in intensive care units in Poland—a point prevalence study in 2012 and 2013. Anaesthesiol Intensive Ther 47:315–319
Huggan PJ, Bell A, Waetford J, Obertova Z, Lawrenson R (2017) Evidence of high mortality and increasing burden of Sepsis in a regional sample of the New Zealand Population. Open forum Infect Dis 4:ofx106
Mellhammar L, Wullt S, Lindberg A, Lanbeck P, Christensson B, Linder A (2016) sepsis incidence: a population-based study. Open Forum Infectious Dis 3:ofw207
Bouza C, Lopez-Cuadrado T, Amate-Blanco JM (2016) Use of explicit ICD9-CM codes to identify adult severe sepsis: impacts on epidemiological estimates. Crit Care 20:313
Alvaro-Meca A, Jimenez-Sousa MA, Micheloud D, Sanchez-Lopez A, Heredia-Rodriguez M, Tamayo E, Resino S, Group of Biomedical Research in Critical Care M (2018) Epidemiological trends of sepsis in the twenty-first century (2000–2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr 16:4
Fleischmann-Struzek C, Mikolajetz A, Schwarzkopf D, Cohen J, Hartog C, Pletz M, Gastmeier P, Reinhart K (2018) Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between national health systems—secular trends in sepsis and infection incidence and mortality in Germany. Intensive Care Med 44:1826–1835
Cheng AC, West TE, Limmathurotsakul D, Peacock SJ (2008) Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med 5:e175
Lewis JM, Feasey NA, Rylance J (2019) Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Crit Care 23:212
Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, Baker T, Dunser MW (2011) Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care 15:R10
Walkey AJ, Lagu T, Lindenauer PK (2015) Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thor Soc 12:216–220
Goldstein E, MacFadden DR, Karaca Z, Steiner CA, Viboud C, Lipsitch M (2019) Antimicrobial resistance prevalence, rates of hospitalization with septicemia and rates of mortality with sepsis in adults in different US states. Int J Antimicrob Agents 54:23–34
Kempker JA, Martin GS (2020) A global accounting of sepsis. Lancet 395:168–170
Fleischmann-Struzek C, Thomas-Ruddel DO, Schettler A, Schwarzkopf D, Stacke A, Seymour CW, Haas C, Dennler U, Reinhart K (2018) Comparing the validity of different ICD coding abstraction strategies for sepsis case identification in German claims data. PLoS ONE 13:e0198847
Feinstein AR, Sosin DM, Wells CK (1985) The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. New Engl J Med 312:1604–1608
Rudd KE, Kissoon N, Limmathurotsakul D, Bory S, Mutahunga B, Seymour CW, Angus DC, West TE (2018) The global burden of sepsis: barriers and potential solutions. Crit Care 22:232
Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, Rowan KM (2008) Variation in critical care services across North America and Western Europe. Crit Care Med 36(2787–2793):e2781–e2789
Turner HC, Hao NV, Yacoub S, Hoang VMT, Clifton DA, Thwaites GE, Dondorp AM, Thwaites CL, Chau NVV (2019) Achieving affordable critical care in low-income and middle-income countries. BMJ Glob Health 4:e001675
Melamed A, Sorvillo FJ (2009) The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care 13:R28
Acknowledgements
Open Access funding provided by Projekt DEAL. The authors would like to thank Liuqing Tong and Olga Morath for assessment of a Chinese and Russian publications, and Tomas Allen and Safiah Mai for supporting the extension of the search strategy. We also thank Salvador Resino García, Manu Shankar Hari, Marija Todorovic Markovic, Priyanka Vakkalanka, Claire Dupuis, José M. de Miguel-Yanes and Flavia Machado, who provided additional data or information on their studies.
Funding
This study was funded by the World Health Organization.
Author information
Authors and Affiliations
Contributions
CFS, AC, BA and KR designed the study. CFS, LM and KER did the literature review, data extraction and risk of bias assessment. NR performed the statistical analysis. PS provided methodological advice. CFS, NR, LM and KR drafted the manuscript. All authors revised the review for important intellectual content and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
KR reports grants from the Innovation Funds of the German Government, personal fees from Adrenomed Berlin, and is unpaid President for the Global Sepsis Alliance, outside of the submitted work. CFS and NR received funding from the Innovation Funds of the German Government (FKZ 01VSF17010). The other authors declare no competing interests.
Ethics approval
No ethical approval was obtained because data from previous published studies were compiled and analysed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code)
The software codes used during the current study are available in the Supplement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fleischmann-Struzek, C., Mellhammar, L., Rose, N. et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med 46, 1552–1562 (2020). https://doi.org/10.1007/s00134-020-06151-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06151-x