Abstract
Background
Henoch-Schönlein purpura (HSP) is the most common vasculitis in childhood; nevertheless, its etiology and pathogenesis remain unknown despite the fact that a variety of factors, mainly infectious agents, drugs and vaccines have been suggested as triggers for the disease. The aim of this study was to estimate the association of HSP with drug and vaccine administration in a pediatric population.
Methods
An active surveillance on drug and vaccine safety in children is ongoing in 11 clinical centers in Italy. All children hospitalized through the local Paediatric Emergency Department for selected acute clinical conditions of interest were enrolled in the study. Data on drug and vaccine use in children before the onset of symptoms leading to hospitalization were collected by parents interview. A case-control design was applied for risk estimates: exposure in children with HSP, included as cases, was compared with similar exposure in children with gastroduodenal lesions, enrolled as controls. HSP cases were validated according to EULAR/PRINTO/PRES criteria. Validation was conducted retrieving data from individual patient clinical record.
Results
During the study period (November 1999–April 2013), 288 cases and 617 controls were included. No increased risk of HSP was estimated for any drug. Among vaccines, measles-mumps-rubella (MMR) vaccine showed an increased risk of HSP (OR 3.4; 95 % CI 1.2–10.0).
Conclusions
This study provides further evidence on the possible role of MMR vaccine in HSP occurrence.
Similar content being viewed by others
Background
Henoch-Schönlein purpura (HSP), recently renamed as IgA vasculitis, is a systemic leukocytoplastic vasculitis characterized by IgA1 dominant immune deposits [1, 2].
HSP is the most common vasculitis in childhood with an incidence of 10–20 cases per 100,000 in children under 17 years with a peak incidence of 70 cases per 100,000 in the 4–6 year age group [1, 2].
According to the last endorsed criteria by the EULAR/PINTO/PRES and American College of Rheumatology (ACR) the HSP diagnosis requires the presence of the palpable purpura and at least one of the following: arthritis or arthralgia, diffuse abdominal pain, renal involvement with haematuria and/or proteinuria or any biopsy showing predominant IgA deposition [2–4].
The HSP has an excellent outcome in the majority of cases with a complete remission in four weeks, about 20–55 % of children have a renal involvement but only <1 % develop an end-stage kidney disease. The pathogenesis of this vasculitis should be attributed to the IgA1 containing immune complexes deposition on the small vessels causing the damage and consequently all clinical manifestations. Although HSP clinical manifestations and prognosis are well-defined, the etiology of the disease remains unknown. It is clear that genetics and an abnormal immune response play a pivotal role in the pathogenesis of HSP [2, 5–7].
However a combination of additional factors has been suggested to trigger the disease, as infectious agents, drugs and vaccinations [8–14].
HSP following drug and vaccine administration has been described in case reports and in a small number of observational studies conducted during vaccination campaigns [15–34].
Since 1999 the Italian National Institute of Health is coordinating an active surveillance on the role of drugs and vaccines in the occurrence of specific clinical conditions responsible for hospitalization of pediatric patients. Non-infectious muco-cutaneous diseases are among the clinical conditions of interest and the present study focused on HSP cases to estimate their association with drug and vaccine use in the pediatric population.
Methods
Setting and study population
The Italian multicenter study on drug and vaccine safety in children involved 11 Italian Pediatric hospitals/wards spread throughout the country (Treviso, Padua, Naples, Genoa, Turin, Florence, Perugia, Palermo, Messina and Rome, with two centers).
Were enrolled in the study all children (age > 1 month and ≤ 18 years) hospitalized through the Emergency Departments (ED) for the following acute conditions: thrombocytopenia (platelet count <100 × 103/L); acute non-infectious, non-febrile neurological disorders; endoscopically confirmed gastroduodenal lesions and/or clinically defined haematemesis and melena and non-infectious muco-cutaneous diseases and vasculitis. Exclusion criteria were represented by a concomitant diagnosis of cancer or immunodeficiency.
Data collection
During hospital admission of the child, a trained pharmacist/physician interviewed parents to collect demographic and clinical information using a structured questionnaire.
Data on drug exposure in a time window of 3 weeks preceding hospitalization, extended to 12 weeks for vaccines, were collected.
For all children the inclusion in the study was based on the diagnosis retrieved from the ED records independently of drug and vaccine exposure.
Ethical approval and consent to participate
According to the Italian regulation, retrospective observational studies are only required to be notified to ethical committees. The study protocol was notified to the ethical committee of each participating Center. Before parents interview, a written informed consent to use data for research purposes was obtained.
Definition of cases and controls
All children hospitalized with a diagnosis of HSP at admission were included as cases. Discharge diagnosis was retrieved from clinical records and validated by clinicians, according to EULAR/PRINTO/PRES criteria for classification of HSP [3, 4]. Validation was conducted retrieving data from individual patient clinical record, blinded with respect to drug and vaccine exposure. Only validated cases were analyzed.
Children hospitalized for gastroduodenal lesions were considered as appropriate controls, since they represent an acute condition admitted through the EDs in the same clinical centers in which cases were identified.
Statistical analysis
Descriptive analyses of demographic characteristics of case and control patients, drug and vaccine exposure were performed. Categorical variables, presented as numbers and percentages, were compared using the Chi Square test, while continuous variables, reported as median and range were compared using the Mann-Whitney U test. All tests were two sided and significance was set at p < 0.05.
A case-control study design was applied to compare drug and vaccine exposure in children with HSP, (cases) and children with gastroduodenal lesions (controls). A multiple logistic regression model was used to estimate adjusted Odds Ratios (ORs) and related 95 % confidence interval (CI). Age and concomitant use of any other drug were considered potential confounding factors. Statistical analysis was performed by means of IBM® SPSS® statistics (version 22).
Results
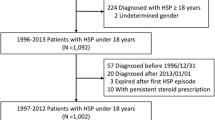
During the period November 1999–April 2013, a total of 2600 patients with a diagnosis of muco-cutaneous disease were enrolled in the study. Among these hospitalized pediatric patients, 366 were diagnosed as HSP at the ED. For the validation phase of HSP cases, clinical records of 298 children (81 % of total), were retrieved. Only 10 cases did not fulfill the diagnostic criteria for the disease as described in the score according to EULAR/PRINTO/PRES criteria (Fig. 1). In all the remaining 288 HSP validated cases, a palpable purpura was present, accompanied by arthralgia/arthritis in 85 %; abdominal involvement with abdominal pain, melena, intussusception in 65 %; renal involvement with haematuria, proteinuria in 35 % and scrotal swelling in 7 %. In the same period 617 children were enrolled with gastroduodenal lesions. Haematemesis and melena accounted for most of gastrointestinal diagnosis. Characteristics of cases and controls are reported in Table 1.
Median duration of hospitalization was similar in both groups, even if the statistical test was statistically significant, indicating difference in the distribution around the median. Controls were significantly younger than cases (6 vs. 3 years). Drug and vaccine exposure resulted significantly higher in controls.
Crude and adjusted ORs were estimated for each drug considering at least five exposed cases. No significant increased risk was estimated for any drug (Table 2).
The risk estimated for HSP within 12 weeks after vaccination resulted higher, more than 3 times, for MMR vaccines with an OR of 3.4 (95 % CI 1.2–10.0) while no significant increased risks were observed for diphtheria, tetanus, acellular pertussis (DTaP) and any vaccine (Table 3).
Discussion
Despite the fact that HSP is the commonest, mainly self-limiting, systemic vasculitis in childhood, its etiology and pathogenesis remain still to be fully understood. Many chemical and infectious triggers have been recognized for the HSP typical vascular IgA deposition, including drugs and vaccines beside the role played by immunological, genetic and environmental factors [13, 14, 35]. Furthermore no risk estimates for HSP and drug or vaccine exposure have been published. This study aimed to investigate the association and the potential role of drugs and vaccines in the occurrence of HSP in a pediatric population.
HSP diagnosis is difficult at admission since there are no disease-specific laboratory abnormalities, neither signs and symptoms, histopathological findings are needed to properly identify HSP cases. Only HSP validated cases, according to the EULAR/PRINTO/PRES criteria, were considered for the statistical analyses. The causality assessment on each HSP case was not performed and was beyond the scope of this study.
The clinical presentation of the 288 validated cases confirmed what previously reported as main clinical features for HSP [2]. In addition to the palpable purpura, hallmark of the disease, articular and abdominal involvement has been described in the majority of the patients whereas renal and genital involvement were less frequent. According to the literature, the median age of HSP cases was in accordance with that reported elsewhere (4-6 years), so as the equal distribution among male and female subjects [1, 2, 5].
The characteristics of the 68 cases in which clinical records were not retrieved were similar for age, gender, previous febrile infections, drug and/or vaccine exposure to the retrieved cases thus excluding a possibility for selection bias.
Children hospitalized for gastroduodenal lesions have been considered as a suitable control group for HSP patients. In this study both cases and controls have been identified through EDs for an acute condition in the same clinical centers and, consequently, drug and vaccine exposure, preceding symptoms causing hospitalization, have been ascertained with the same procedure by interviewing parents during their child hospitalization.
Our results provide no evidence of an increased risk of developing HSP associated with any of the drugs considered.
A three-fold increase risk of developing HSP associated with MMR vaccine was estimated.
Prospective studies conducted during MMR vaccination campaign reported HSP cases following vaccine administration [28–30]. Within the Italian Pharmacovigilance System some spontaneous reports of HSP were reported after MMR vaccine [36]. During the Chinese MMR vaccination campaign, 30 severe adverse reactions out of 14.3 millions of administered doses were reported; among these, 28 cases were diagnosed as HSP with an estimated incidence of 2.1 per million/doses [30]. Our study confirmed these observational data, providing a risk estimate for this association.
Given the case-control design we could not calculate any incidence estimate.
This article confirms that HSP is a rare condition (288 children hospitalized in 14 years). Furthermore, the vaccinated cases were only 8, suggesting a very low absolute risk of the condition in children vaccinated with MMR vaccine. Thus, the benefit/risk profile of MMR vaccine is not affected by our results, being MMR vaccination an effective and safe tool against serious diseases in childhood.
With regard to drugs an association of gastroduodenal lesions with NSAIDs has been previously highlighted [37]. This could have lead to an underestimation of the results obtained in the present study concerning the role of NSAIDs in the development of HSP. It was beyond the objective of the present study to investigate the pathogenetic role of drugs and vaccines in the HSP occurrence.
Conclusions
The association between MMR vaccination and HSP confirms previous published findings and adds a risk estimate. Further studies are needed to increase our understanding of the role of drugs and vaccines in the etiology of HSP, a disease with important effects on health of children for its potential, though rare, chronic outcomes.
Abbreviations
HSP, Henoch-Schönlein purpura; Ig, Immunoglobulin
References
Trnka P. Henoch-Schonlein purpura in children. J Paediatr Child Health. 2013;49(12):995–1003.
McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of Henoch-Schönlein purpura. Eur J Pediatr. 2010;169(6):643–50.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11.
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806.
Saulsubry FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13(1):35–40.
Yang YH, Chuang YH, Wang LC, Huang HY, Gershwin ME, Chiang BL, et al. The immunobiology of Henoch-Schönlein purpura. Autoimmun Rev. 2008;7(3):179–84.
He X, Yu C, Zhao P, Ding Y, Liang X, Zhao Y, et al. The genetics of Henoch–Schonlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33(6):1387–95.
Weiss PF, Klink AJ, Luan X, Feudtner C. Temporal association of Streptococcus, Staphylococcus, and parainfluenza pediatric hospitalizations and hospitalized cases of Henoch-Schönlein purpura. J Rheumatol. 2010;37(12):2587–94.
Gonzalez-Gay MA, Calvino MC, Vazquez-Lopez ME, Garcia-Porrua C, Fernandez-Iglesias JL, Dierssen T, et al. Implications of upper respiratory tract infections and drugs in the clinical spectrum of Henoch-Schönlein purpura in children. Clin Exp Rheumatol. 2004;22(6):781–4.
Ercan G, Kasapcopur O, Akdenizli E, Arisoy N. The role of Streptococcal infection in Henoch-Schonlein purpura. J Trop Pediatr. 2004;50(3):187–8.
Heegaard ED, Taaning EB. Parvovirus B19 and parvovirus V9 are not associated with Henoch-Schönlein purpura in children. Pediatr Infect Dis J. 2002;21(1):31–4.
Vermeulen MJ, Peeters MF, Verbakel H, de Moor RA, Roord JJ, van Dijken PJ. No etiological role for Bartonella henselae infection in Henoch-Schönlein purpura. Pediatr Infect Dis J. 2009;28(12):1142–3.
Rigante D, Castellazzi L, Bosco A, Esposito S. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12(10):1016–21.
Chen JY, Mao JH. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11(1):29-34.
Santoro D, Stella M, Castellino S. Henoch-Schönlein purpura associated with acetaminophen and codeine. Clin Nephrol. 2006;66(2):131–4.
Richards AJ, Lindley DC. Henoch-Schönlein purpura associated with co-dydramol. Br J Rheumatol. 1987;26(1):65.
Dussarat G, Dalger J, Mafart B, Chagnon A. Vascular purpura caused by paracetamol. A case. Presse Med. 1988;17(31):1587.
Michail S, Vaiopoulos G, Nakopoulou L, Revenas C, Aroni K, Karam P, et al. Henoch-Schoenlein purpura and acute interstitial nephritis after intravenous vancomycin administration in a patient with a staphylococcal infection. Scand J Rheumatol. 1998;27(3):233–5.
Bataille S, Daumas A, Tasei AM, Jourde-Chiche N, Dussol B, Burtey S, et al. Vancomycin-induced Henoch-Schönlein purpura:a case report. J Med Case Rep. 2012;6:106.
Gamboa F, Rivera JM, Gomez Mateos JM, Gomez-Gras E. Ciprofloxacin-induced Henoch-Schönlein purpura. Ann Pharmacother. 1995;29(1):84.
Pons R, Escutia B. Ciprofloxacin-induced vasculitis with cutaneous and renal involvement. Nefrologia. 2001;21(2):209–12.
Borràs-Blasco J, Enriquez R, Amoros F, Cabezuelo JB, Navarro-Ruiz A, Pérez M, et al. Henoch-Schönlein purpura associated with clarithromycin. Case report and review of literature. Int J Clin Pharmacol Ther. 2003;41(5):213–6.
De Vega T, Blanco S, Lòpez C, Pascual E, Sánchez M, Zamarrón A. Clarithromycin-induced leukocytoclastic vasculitis. Eur J Clin Microbiol Infect Dis. 1993;12(7):563.
Gavura SR, Nusinowitz S. Leukocytoclastic vasculitis associated with clarithromycin. Ann Pharmacother. 1998;32(5):543–5.
Goldberg EI, Shoji T, Spadin AN. Henoch-Schönlein purpura induced by clarithromycin. Int J Dermatol. 1999;38(9):706–8.
Zink A, Erni S, Fliegner M. Antibiotics, purpura and ulcers: a leukocytoclastic vasculitis after clarithromycin. Dtsch Med Wochenschr. 2006;131(40):2217–20.
Schapira D, Balbir-Gurman A, Nahir AM. Naproxen-induced leukocytoclastic vasculitis. Clin Rheumatol. 2000;19(3):242–4.
Patja A, Davidkin I, Kurki T, Kallio MJ, Valle M, Peltole H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatr Infect Dis J. 2000;19(12):1127–34.
Patjia A, Makinen-Kiljunen S, Davidkin I, Paunio M, Peltola H. Allergic reactions to measles-mumps-rubella vaccination. Pediatrics. 2001;107(2):E27.
Shu M, Liu Q, Wang J, Rui Ao R, Yang C, Fang G, et al. Measles vaccine adverse events reported in the mass vaccination campaign of Sichuan province, China from 2007 to 2008. Vaccine. 2011;29(18):3507–10.
Goodman MJ, Nordin JD, Belongia EA, Mullooly JP, Baggs J. Henoch-Schölein purpura and polysaccharide meningococcal vaccine. Pediatrics. 2010;126(2):325–9.
Sexton K, McNicholas A, Galloway Y, Sexton K, McNicholas A, Galloway Y, et al. Henoch-Schönlein purpura and meningococcal B vaccination. Arch Dis Child. 2009;94(3):224–6.
Watanabe T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1). Pediatr Nephrol. 2011;26(5):795–8.
Jariwala S, Vernon N, Shliozberg J. Henoch-Schönlein purpura after hepatitis A vaccination. Ann Allergy Asthma Immunol. 2011;107(2):180–1.
Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13(4-5):355–8.
Rapporto sulla sorveglianza postmarketing dei vaccini in Italia - Anno 2012 http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_sulla_sorveglianza_postmarketing_dei_vaccin_%20in_Italia_Anno_2012.pdf. Accessed 8 May 2015.
Bianciotto M, Chiappini E, Raffaldi I, Gabiano C, Tovo PA, Sollai S, et al. Drug use and upper gastrointestinal complications in children: a case-control study. Arch Dis Child. 2013;98(3):218–2.
ᅟ
ᅟ
Funding
The study was partially funded by the Italian Medicines Agency (AIFA).
Authors’ contributions
FMI, RDC conceived and designed the study, coordinated and supervised data collection at all sites, analyzed the data, critically reviewed and approved the final manuscript as submitted. LDD conceived and designed the study, coordinated and supervised data collection at two sites, drafted, critically reviewed and approved the final manuscript as submitted. CZ, MSS, NM, AC, UR collected data, validated cases, drafted and reviewed the manuscript. SR, LR, PDP, PB, SS collected and validated data, reviewed and approved the final manuscript as submitted. All components of the Italian Multicenter Study Group for Drug and Vaccine Safety in Children collected and validated the data within the clinical centers, read, commented and approved the final version of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
According to the Italian regulation, retrospective observational studies are only required to be notified to ethical committees (Determinazione AIFA 20 marzo 2008 - http://www.agenziafarmaco.gov.it/allegati/det_20marzo2008.pdf). The study protocol was notified to the ethical committee of each participating Center. Before parents interview, a written informed consent to use data for research purposes was obtained.
Members of the Italian Multicenter Study Group for Drug and Vaccine Safety in Children
Francesca Menniti-Ippolito, Roberto Da Cas, Giuseppe Traversa (National Center for Epidemiology, National Institute of Health, Roma); Carmela Santuccio, Patrizia Felicetti, Loriana Tartaglia, Francesco Trotta (Italian Medicines Agency, Roma); Pasquale Di Pietro, Paola Barabino, Salvatore Renna, Laura Riceputi (Giannina Gaslini Pediatric Hospital, Genova); Pier-Angelo Tovo, Clara Gabiano, Antonio Urbino, Luca Baroero, Daniele Le Serre, Silvia Virano (Department of public health and pediatrics, Torino University, Regina Margherita Pediatric Hospital, Torino); Liviana Da Dalt, Chiara Stefani, Claudia Zerbinati (Department of Pediatrics, Treviso Hospital, Treviso); Giorgio Perilongo, Marco Daverio, Michela Maretti, Beatrice Galeazzo, Giulia Rubin, Stefania Scanferla (Department of Pediatrics, University of Padova); Elena Chiappini, Sara Sollai, Maurizio De Martino, Sabrina Becciani, Martina Giacalone, Simona Montano, Giulia Remaschi, Alessia Stival (Anna Meyer Children’s University Hospital, Firenze); Piera Abate, Ilaria Leonardi (Department of Pediatrics, University of Perugia); Nicola Pirozzi, Umberto Raucci, Antonino Reale, Rossella Rossi (Pediatric Emergency Department, Bambino Gesù Children Hospital, Roma); Nadia Mores, Giulia Bersani, Adele Compagnone, Antonio Chiaretti, Riccardo Riccardi, Costantino Romagnoli (Pharmacology and Pediatrics, Università Cattolica S. Cuore, Roma); Vincenzo Tipo, Michele Dinardo, Fabiana Auricchio, Teodoro Polimeno, Maria Colomba Bonagura (Santobono Pediatric Hospital, Napoli); Alessandra Maccariello (Department of Experimental Medicine, Section of Pharmacology “L. Donatelli”, Second University of Napoli); Fortunata Fucà, Eleonora Di Rosa (Giovanni Di Cristina Pediatric Hospital, Palermo); Domenica Altavilla, Anna Mecchio, Teresa Arrigo (Department of Pediatrics, Gynecologic, Microbiologic and Biomedical Sciences, University Hospital, Messina).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Da Dalt, L., Zerbinati, C., Strafella, M.S. et al. Henoch-Schönlein purpura and drug and vaccine use in childhood: a case-control study. Ital J Pediatr 42, 60 (2016). https://doi.org/10.1186/s13052-016-0267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-016-0267-2