Abstract
Although the etiology of Henoch-Schönlein purpura (HSP) remains unclear, influenza vaccinations have been implicated as possible triggers for HSP. We describe four patients with HSP following influenza vaccinations which developed during the pandemic of influenza A (H1N1) 2009 and review the literature concerning HSP associated with this vaccine. HSP in patients developed in October and November, 2009. Four patients exhibited purpura, three patients complained of arthralgias, and one patient had both abdominal pains and renal involvement. Reviewing the literature, 11 patients with HSP following influenza vaccinations have been reported. Eight patients were children and five patients had past histories of immunologically mediated diseases including HSP, drug eruptions, and food allergy. While a favorable outcome was noted in most patients, one patient developed end-stage renal failure and another patient exhibited chronic glomerulonephritis. Although the precise reason for clustering of our patients with HSP following influenza vaccination was unclear, increasing use of the recent influenza vaccine associated with the pandemic of influenza A (H1N1) 2009 might explain this phenomenon. Because the incidence of HSP caused by influenza vaccination was very low, influenza vaccination should not be limited for this reason. However, caution may be required with its use in children with immunologically mediated diseases such as HSP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Henoch-Schönlein purpura (HSP) is the most common systemic vasculitis of children [1]. Although the etiology of HSP remains unclear, infectious agents, drugs, and vaccinations have been implicated as possible triggers for HSP [1]. Patients with HSP following influenza vaccinations have been described in case reports [2–8]. However, a case series of HSP after influenza vaccination has never been reported.
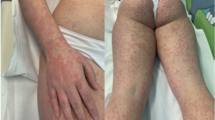
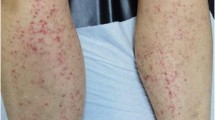
We describe here four patients with HSP following influenza vaccinations, which developed in October and November, 2009 during the pandemic of influenza A (H1N1) 2009 and review the literature concerning HSP associated with this vaccine. HSP was defined by the EULAR/PRINTO/PRES criteria for HSP, which consists of the finding of palpable purpura with lower limb predominance in the presence of one of either diffuse abdominal pain, a biopsy showing predominant IgA deposition, arthritis or arthralgia and/or renal involvement (any hematuria and/or proteinuria) [9].
Case reports
Case 1
A 7-year-old girl was admitted to our hospital on October 15, 2009, because of abdominal pain 2 days following seasonal influenza vaccination (lot number: L23A, BIKEN, Japan). She had a history of drug eruptions caused by amoxicillin (AMPC). She had had uneventful seasonal influenza vaccinations in each of the three preceding years. Physical examination revealed diffuse abdominal pain and tenderness. Three days later, she exhibited palpable purpura on the lower extremities and laboratory studies revealed normal values for the following: complete blood cell count, prothrombin time (PT), partial thromboplastin time (PTT), C-reactive protein, liver enzymes, blood urea nitrogen (BUN), creatinine, IgG, IgA, IgM, C3, C4, and urinalysis. She was then diagnosed with HSP. Oral prednisolone therapy (1 mg/kg) improved her condition gradually and she was discharged 40 days after admission. Two months later, the patient developed hematuria (red blood cell counts of >100/high-power field) and proteinuria (0.8 g/g creatinine). Because the abnormal urinalysis of the patient continued, the patient was hospitalized and underwent a renal biopsy. The renal biopsy specimen showed mild focal and segmental mesangial proliferative glomerulonephritis with IgA and fibrinogen deposition on the mesangium and along with the capillary walls. The patient was diagnosed with Henoch-Schönlein purpura nephritis (HSPN, Class IIa according to Emancipator classification [10]) and oral administration of dipyridamole (5 mg/kg/day) was started. Three months later, the proteinuria completely disappeared with improvement of hematuria (red blood cell counts of 10 ∼ 15/high-power field).
Case 2
A four-year-old girl developed palpable purpura on the bilateral lower extremities on November 5, 2009. The patient had received two doses of influenza vaccinations 15 days (lot number: L23C, KAKETSUKEN, Japan) and 1 day (lot number: 381-A, DENKA, Japan) before the development of purpura. Ten days later, she noted right ankle joint pain and swelling and was admitted to our hospital because she was unable to walk. She had a past history of exanthema following an ingestion of soba, which is Japanese noodles made from buckwheat. Physical examination revealed purpura in the legs and buttocks, and right ankle joint pain and swelling. Laboratory studies revealed normal values for the following: complete blood cell count, PT, PTT, liver enzymes, BUN, creatinine, and urinalysis. She was diagnosed with HSP. Bed rest and oral administration of acetaminophen rapidly improved the purpura and arthritis and the patient was discharged 7 days after admission.
Case 3
A previously healthy, 6-year-old boy received two doses of seasonal influenza vaccines (lot numbers: L25C and L30A, KAKETSUKEN, Japan) and one pandemic influenza H1N1 vaccine (lot number: HP01A, BIKEN, Japan) on October 26, November 13, and November 18, 2009, respectively. One day following pandemic influenza H1N1 vaccination, the patient suffered from right ankle pain and swelling. Three days later, the patient developed palpable purpura in the legs and was referred to our hospital. He had had uneventful seasonal influenza vaccinations in each of the four preceding years. Physical examination revealed purpura in the legs and buttocks, and right ankle joint pain and swelling. Laboratory studies revealed normal values for the following: complete blood cell count, PT, PTT, liver enzymes, BUN, creatinine, and urinalysis. The patient was diagnosed with HSP. Purpura and arthritis subsided 1 week later without specific therapy.
Case 4
A 5-year-old girl was referred to our hospital because of palpable purpura and pain and swelling of the left ankle joint on November 10, 2009. The patient received two doses of seasonal influenza vaccinations (lot numbers: 388-B, DENKA, Japan and HA096B, BIKEN, Japan) on October 23 and November 9, 2009. Physical examination revealed purpura in the legs, and left ankle joint pain and swelling. Laboratory studies revealed normal values for the following: complete blood cell count, PT, PTT, and urinalysis. The patient was diagnosed with HSP. Purpura and arthritis disappeared 1 month later without specific therapy.
Discussion
We presented four patients with HSP following influenza vaccinations, which developed during the pandemic of influenza A (H1N1) 2009. Since Damjanov described a patient with HSP, whose mild renal disease progressed irreversibly after influenza vaccination, 11 patients with HSP following influenza vaccination including our patients have been reported (Table 1) [2–8]. Eight of 11 patients were children. Past medical histories of HSP were present in three patients, as well as a history of drug eruptions by AMPC in one patient and allergic reactions to soba in another patient. HSP developed after receiving one dose of vaccination in seven patients, two doses of vaccination in three patients, and three doses of vaccination (two seasonal vaccines and one pandemic vaccine) in one patient. The time from vaccination to the onset of the symptoms ranged from 1 to 22 days with an average of 11.7 days. All patients exhibited purpura, nine showed arthralgias, six developed abdominal pains, and three had renal involvement. While favorable outcome was noted in most patients, one patient developed end-stage renal failure and another patient exhibited chronic glomerulonephritis. A causal relationship between HSP and influenza vaccination was suspected from the timing of HSP in many cases; however, direct proof of linkage between them has never been obtained [6].
Although the mechanisms underlying the development of HSP induced by influenza vaccine remain unknown, a possible link between influenza vaccination and autoimmunity has been suggested [11, 12]. The association between vaccines and autoimmunity may be mediated through several mechanisms including immune-mediated responses to the infectious antigen or other components of vaccine such as gelatin, ovalbumin, and phosphate buffers [11].
All our patients suffered from HSP in October and November, 2009. Because the lot numbers of all vaccines used for our patients were different, HSP in our patients did not result from a specific batch of influenza vaccine. A novel influenza A (H1N1) virus of swine origin caused human infection during the spring of 2009 in Mexico [13]. Thereafter, the virus spread globally, resulting in the influenza pandemic including in Japan [13, 14]. Because vaccination is the most effective means of preventing influenza-associated morbidity and mortality, vaccination against influenza is recommended [13]. Although the precise reason for clustering of our patients with HSP following influenza vaccination was unclear, increasing use of the recent influenza vaccine associated with the pandemic of influenza A (H1N1) 2009 might explain this phenomenon.
Because the incidence of HSP caused by influenza vaccination was very low, influenza vaccination should not be limited for this reason. However, caution may be required with its use in children with immunologically mediated diseases such as HSP, drug eruptions or food allergy; patients with a history of vaccine-induced vasculitis should not be revaccinated [6, 12].
References
Tizard EJ, Hamilton-Ayres MJ (2008) Henoch-Schönlein purpura. Arch Dis Child Educ Pract Ed 93:1–8
Damjanov I, Amato JA (1979) Progression of renal disease in Henoch-Schönlein purpura after influenza vaccination. JAMA 242:2555–2556
Blumberg S, Bienfang D, Kantrowitz FG (1980) A possible association between influenza vaccination and small-vessel vasculitis. Arch Intern Med 140:847–848
Yoshimoto T, Matsuda T, Kamada M (1987) A case of Schönlein-Henoch purpura and subsequently occurred purpuric nephritis after influenza vaccination (in Japanese). Hako Ishi 11:77–80
Patel U, Bradley JR, Hamilton DV (1988) Henoch-Schönlein purpura after influenza vaccination. BMJ 296:1800
Watanabe T, Onda H (2001) Henoch-Schönlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr Nephrol 16:458–459
Hattori K, Furuse A (2004) Two cases of Henoch-Schönlein purpura after DPT and influenza vaccinations (in Japanese). Shyoni Kansen Men-eki 16:377–381
Mormile R, D’Alterio V, Treccagnoli G, Sorrentino P (2004) Henoch-Schönlein purpura with antiphospholipid antibodies after influenza vaccination: how fearful is it in children? Vaccine 23:567–568
Ozen S, Pistorio A, Iusan SM, Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, Rigante D, Cantarini L, Hilario MO, Silva CA, Alegria M, Norambuena X, Belot A, Berkun Y, Estrella AI, Olivieri AN, Alpigiani MG, Rumba I, Sztajnbok F, Tambic-Bukovac L, Breda L, Al-Mayouf S, Mihaylova D, Chasnyk V, Sengler C, Klein-Gitelman M, Djeddi D, Nuno L, Pruunsild C, Brunner J, Kondi A, Pagava K, Pederzoli S, Martini A, Ruperto N, Paediatric Rheumatology International Trials Organisation (PRINTO) (2010) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis 69:798–806
Emancipator SN (1998) IgA nephropathy and Henoch-Schönlein purpura. In: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds) Heptinstall’s pathology of the kidney, 5th edn. Lippincott-Raven Publishers, Philadelphia, pp 479–540
Zafrir Y, Agmon-Levin N, Shoenfeld Y (2009) Post-influenza vaccination vasculitides. J Clin Rheumatol 15:269–270
Lohse A, Michel F, Auge B, Toussirot E, Wendling D (1999) Vascular purpura and cryoglobulinemia after influenza vaccination. Rev Rhum Engl Ed 66:359–362
Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA (2010) 2009 H1N1 influenza. Mayo Clin Proc 85:64–76
Kamigaki T, Oshitani H (2009) Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr RRN1139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1). Pediatr Nephrol 26, 795–798 (2011). https://doi.org/10.1007/s00467-010-1722-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1722-8