Abstract
Background
The feasibility of cord blood transplantation (CBT) in adults is limited by the relatively low number of hematopoietic stem/progenitor cells contained in one single CB unit. The infusion of two CB units from different partially HLA-matched donors (double CBT) is frequently performed in patients who lack a sufficiently rich single CB unit.
Methods
We compared CBT outcomes in patients given single or double CBT following reduced-intensity conditioning (RIC) in a retrospective multicenter registry-based study. Inclusion criteria included adult (≥18 years) patients, acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), complete remission (CR) at the time of transplantation, first single (with a cryopreserved TNC ≥ 2.5 × 107/kg) or double CBT between 2004 and 2014, and RIC conditioning.
Results
Data from 534 patients with AML (n = 408) or ALL (n = 126) receiving a first single (n = 172) or double (n = 362) CBT were included in the analyses. In univariate analysis, in comparison to patients transplanted with a single CB, double CB recipients had a similar incidence of neutrophil engraftment but a suggestion for a higher incidence of grade II–IV acute GVHD (36 versus 28%, P = 0.08). In multivariate analyses, in comparison to single CBT recipients, double CBT patients had a comparable incidence of relapse (HR = 0.9, P = 0.5) and of nonrelapse mortality (HR = 0.8, P = 0.3), as well as comparable overall (HR = 0.8, P = 0.17), leukemia-free (HR = 0.8, P = 0.2) and GVHD-free, relapse-free (HR = 1.0, P = 0.3) survival.
Conclusions
These data failed to demonstrate better transplantation outcomes in adult patients receiving double CBT in comparison to those receiving single CBT with adequate TNC after RIC.
Similar content being viewed by others
Background
Allogeneic umbilical cord blood transplantation (CBT) is a treatment option for many patients with acute myeloid (AML) or acute lymphoblastic (ALL) leukemia who lack an HLA-matched donor [1,2,3,4]. In the last two decades, the development of reduced-intensity conditioning (RIC) regimens for CBT has allowed extending its use to patients who were deemed ineligible for myeloablative (MAC) conditioning because of older age or medical comorbidities [5,6,7,8,9,10,11]. We recently compared outcomes of AML or ALL patients given CBT after RIC (n = 415) versus MAC (n = 479) regimens. We observed that, in comparison to MAC patients, RIC recipients had a higher incidence of disease relapse and a lower nonrelapse mortality (NRM), translating to comparable leukemia-free (LFS), GVHD-free, relapse-free survival (GRFS), and overall (OS) survival [11].
Previous studies have demonstrated poor outcomes in patients receiving CB graft containing <2.5 × 107 total nucleated cells (TNC) per kilogram at cryopreservation, particularly in the presence of human leukocyte antigen (HLA)-mismatches [12]. Unfortunately, many adult patients lack a sufficiently rich CB unit to allow safe CBT. Based on these observations, the Minnesota group pioneered the infusion of two CB units from different partially HLA-matched donors (dCBT) for patients who lack a sufficiently rich single CB unit [13]. Based on preliminary encouraging results, this approach has been extended to patients who had a single CB unit containing >2.5 × 107 total nucleated cells (TNC) per kilogram at cryopreservation [14]. This has been particularly the case in the setting of RIC-CBT since it was hypothesized that in comparison with single CBT, double CBT might promote engraftment and increase graft-versus-leukemia effects [15]. The later might be due at least in part via graft-versus-graft alloreactivity as recently demonstrated [16].
In a previous study, we compared transplantation outcomes of adult AML or ALL patients transplanted with one single CB or two CB units after myeloablative conditioning regimen (n = 239) [17]. Among patients transplanted with one single CB unit (sCBT), those receiving a thiotepa, busulfan, and fludarabine (TBF) regimen had better LFS than those transplanted with busulfan- or total body irradiation (TBI)-based regimens. When the sCBT group was restricted to patients given TBF-based conditioning, transplantation outcomes were comparable between patients receiving sCBT or dCBT, with the exception for a higher incidence of grade II–IV acute GVHD in dCBT recipients. Similarly, two recent prospective randomized studies demonstrated that dCBT following myeloablative conditioning failed to improve transplantation outcomes in comparison to sCBT in children and/or young adult patients who had a sufficiently rich single CB unit [18, 19].
In the current registry study, we investigated whether these observations remained true in the setting of adults after RIC CBT, which depends primarily on engraftment of donor immune cells and on graft-versus-leukemia effects for disease eradication.
Methods
Data collection
This survey is a retrospective, multicenter registry-based study performed by the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) and by Eurocord. EBMT registry is a voluntary working group of more than 500 transplant centers, participants of which are required once a year to report all consecutive stem cell transplantations and follow-up. Audits are routinely performed to determine the accuracy of the data. Eurocord collects data on CBT performed in >50 countries worldwide and >500 transplant centers, mainly EBMT centers. Inclusion criteria were adult (≥18 years) patients, AML or ALL, complete remission (CR) at the time of transplantation, first single (with a cryopreserved TNC ≥2.5 × 107/kg) or double CBT between 2004 and 2014, and RIC conditioning. RIC was defined as use of fludarabine associated with <6 Gy TBI, or busulfan ≤8 mg/kg, melphalan ≤140 mg/m2 or other nonmyeloablative drugs, as previously reported [11, 20, 21]. HLA-compatibility requirements followed the current practice of antigen level typing for HLA-A and -B and allele level typing of HLA-DRB1. CB units were 4–6/6 HLA-A, -B, and -DRB1 matched to the recipient and to the other unit in case of dCBT in most patients. However, more recently, some centers are no longer matching the CB units between them with regard to HLA based on the study by Avery et al. [22]. HLA disparities between each unit and the recipient and between the two units were not necessarily at the same loci. Grading of acute and chronic GVHD was performed using established criteria [23].
For the purpose of this study, all necessary data were collected according to EBMT and Eurocord guidelines.
Statistical analyses
Data from all patients meeting the inclusion/exclusion criteria were included in the analyses. Start time was date of transplant for all endpoints. Neutrophil engraftment was defined as first of three consecutive days with a neutrophil count of at least 0.5 × 109/L. Platelet engraftment was defined as the first of seven consecutive days of an unsupported platelet count of at least 20 × 109/L [2].
To evaluate the relapse incidence, patients dying either from direct toxicity of the procedure or from any other cause not related to leukemia were censored. NRM was defined as death without experiencing disease recurrence. Patients were censored at the time of relapse or of the last follow-up. Cumulative incidence functions were used for relapse incidence and NRM in a competing risk setting since death and relapse were competing together.
For estimating the cumulative incidence of chronic GVHD, death was considered as a competing event. OS and LFS were estimated using the Kaplan-Meier estimates. GRFS was defined as being alive with neither grade III–IV acute GVHD, severe chronic GVHD nor disease relapse [24]. Univariate analyses were done using Gray’s test for cumulative incidence function and log rank test for OS and LFS. Associations between single or double CBT and transplantation outcomes (chronic GVHD, relapse, NRM, LFS, and OS) were evaluated in multivariable analyses, using Cox proportional hazards. Variables introduced in the Cox models included recipient age (in decades), disease type (AML versus ALL), disease status at CBT, type of conditioning regimen (TBI, fludarabine, and cyclophosphamide (TCF) versus other), cytogenetic risk, and the use of ATG or not. Exploratory analyses of the heterogeneity of sCBT versus dCBT among pre-transplant subgroups for relapse, NRM, OS, LFS, and GRFS were performed using Cox models. The results of these Cox models were presented graphically using forest plots [25].
All tests were two sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes. Statistical analyses were performed with SPSS 19 (SPSS Inc., Chicago, IL), and R 2.13.2 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient, disease, and transplant characteristics
Patients and disease characteristics are described in Table 1. Briefly, data from 534 patients with AML (n = 408) or ALL (n = 126) receiving a first single (n = 172) or double (n = 362) CBT were included in the analyses. Among dCBT recipients, 47 received two CB units containing less than 2.5 × 107 TNC/kg each. In comparison to sCBT patients, dCBT recipients had a shorter follow-up (34 versus 54 months, P = 0.0005), were more frequently male (56 versus 41%, P = 0.002), received a conditioning combining TBI, cyclophosphamide and Flu (TCF regimen, 83 versus 66%, P < 0.001) more frequently, and received ATG less frequently (16 versus 37%, P < 0.001). The two groups were not different for recipient age at transplantation (52 versus 50 years, P = 0.17) as well as for other important factors such as disease type (76% of the patients with AML in both group), disease status at transplantation (CR1 in 57 versus 53% of the patients, P = 0.6), time from diagnosis to transplantation (9.4 versus 9.5 months, P = 0.8) and cytogenetic risks (P = 0.85). Finally, as expected, TNC (median 5.1 versus 3.8 × 107 TNC/kg, P < 0.001) and CD34+ cell (median 4.0 versus 3.1 × 105 cell/kg, P = 0.003) doses at cryopreservation were significantly higher in dCBT than in sCBT recipients (Table 2).
Engraftment and GVHD
Overall, the cumulative incidence of neutrophil engraftment at day 60 was not different in sCBT (median 77%; 95% CI 70–83%) and dCBT (median 83%; 95% CI 78–86%) recipients (P = 0.4). The median time to neutrophil engraftment was 19 and 24 days, for sCBT and dCBT, respectively, (P < 0.001) (Additional file 1: Figure S1). Similarly, the cumulative incidence of platelet engraftment at 6 months was not different in sCBT (median 65%; 95% CI 57–73%) and dCBT (median 71%; 95% CI 65–76%) recipients (P = 0.9).
There was a trend for a lower incidence of grade II–IV acute GVHD (28 versus 36%, P = 0.08) in sCBT recipients, but this was no longer the case after adjusting for confounding factors (HR = 1.1, P = 0.22). In contrast, incidences of grade III–IV acute (11 versus 13%, P = 0.6), chronic (28 versus 36% at 2 years, P = 0.2) and extensive chronic (10.6 versus 12% at 2 years, P = 0.69) GVHD were comparable in sCBT and dCBT recipients.
Relapse, NRM, GRFS LFS, and OS
At 2 year, in comparison to sCBT recipients, dCBT had a similar cumulative incidence of relapse (32 versus 35%, P = 0.5) and of NRM (22 versus 29%, P = 0.2). GRFS (37 versus 31%, P = 0.13), and LFS (46 versus 36%, P = 0.06) were similar according to the type of graft. DCBT showed a significantly better OS (51 versus 41%, P = 0.03) (Additional file 1: Table S1 and Figure S2). Of note, outcomes of the 47 patients given 2 CB units containing <2.5 × 107 TNC/kg each were at least as good as those observed in sCBT recipients with 2-year OS and LFS of 56.4 and 42.9%, respectively, (Additional file 1: Figure S3).
After adjusting for potential confounding factors in multivariate analyses, dCBT and sCBT recipients had a similar risk of relapse (HR = 0.9; 95% CI 0.6–1.3, P = 0.5) and NRM (HR = 0.8; 95% CI 0.5–1.2, P = 0.3), and similar GRFS (HR = 1.0; 95% CI 0.9–1.0, P = 0.3), LFS (HR = 0.8; 95% CI 0.7–1.1, P = 0.2), and OS (HR = 0.8; 95% CI, 0.6–1.1 P = 0.17) (Fig. 1) (Table 3). The only factor associated with lower OS in multivariate analysis was the use of ATG (HR = 1.8; 95% CI 1.2–2.8, P = 0.01). This was due to a significantly higher NRM in ATG in comparison with non-ATG recipients (HR = 2.4; 95% CI 1.3–4.3, P < 0.001) while relapse incidence was not affected by ATG (HR = 1.0, 95% CI 0.6–1.9, P = 1.0).
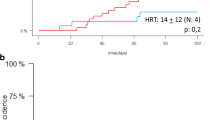
CBT outcomes in acute leukemia patients transplanted following RIC with one (sCBT, n = 172) or a two (dCBT, 362) CB unit(s). The figures show the unadjusted curves for sCBT patients and the adjusted curves for dCBT recipients. Curves were adjusted for age at transplantation (in decades), CR2 versus CR1, AML versus ALL, TCF conditioning versus other, ATG, cytogenetic poor versus good/intermediate, and cytogenetic missing versus good/intermediate. GRFS GVHD-free relapse-free survival, OS overall survival, RI relapse incidence, and NRM nonrelapse mortality
As shown in the Table 4 and in the Additional file 1: Table S2, causes of death were not statistically different between sCBT and dCBT recipients. However, there was a suggestion for more deaths from GVHD (13/362 (3.5%) versus 3/172 (1.7%)) in dCBT than in sCBT recipients in the first 100 days after transplantation, while the incidence of death from infection in the first 100 days after CBT was comparable between the 2 groups (22/362 (6.1%) in dCBT versus 13/172 (7.6%) in sCBT recipients, respectively).
Subgroup analyses
To further dissect the impact of sCBT versus dCBT, we performed additional (univariate) Cox analyses separately for various pre-transplant/transplant variables. The results of these analyses are presented graphically using Forest plots in Figs. 2 and 3. There were no interactions between patient age at transplantation, patient gender, number of cells infused, disease type, disease status, HLA-matching and conditioning type (TCF versus other), and the association between sCBT versus dCBT and GRFS or OS. Further multivariate Cox models assessing possible interactions between ATG and sCBT versus dCBT demonstrated the absence of statistically significant interactions for relapse incidence (P = 0.27), NRM (P = 0.37), LFS (P = 0.97), and OS (P = 0.59). Similarly, there was no interaction between disease status (CR1 versus other) and the impact of sCBT versus dCBT on GRFS (P = 0.61).
Impact of cell dose
We finally assessed what was the combined impact of cell dose and sCBT versus dCBT. In order to address this issue, we performed multivariate Cox models including four graft type groups: sCBT and TNC above median, dCBT and TNC above median, sCBT and TNC below median, and dCBT and TNC below median. As observed in the Table 5, in comparison to the reference group (sCBT and TNC above median), patients given sCBT with TNC below the median had a higher risk of relapse (HR = 2.0, 95% CI 1.0–3.9, P = 0.04) and a suggestion for a worse LFS (HR = 1.5, 95% CI 0.9–2.3, P = 0.11), while outcomes were comparable between patients receiving sCBT and TNC above median and those given dCBT (irrespective of the cell dose received).
Discussion
Umbilical CB units contain a limited number of hematopoietic cells. This is unfortunate given that cell dose is one of the main predictive factors for CBT outcomes [26,27,28]. Transplantation of two CB units has been introduced by investigators from the university of Minnesota to increase the cell dose infused [13, 29]. Preliminary studies have demonstrated that this strategy allowed safe CBT in adult patients who lacked a sufficiently rich CB unit [30]. Further studies observed that dCBT induced graft-versus-graft reactions that could increase alloreactivity and perhaps graft-versus-leukemia effects [15]. This prompted us to compare post-transplantation outcomes in patients with acute leukemia receiving sCBT or dCBT after RIC, a transplantation approach that depends mainly on graft-versus-leukemia effects for tumor eradication [31, 32]. Several observations were made.
A first observation was that indeed, dCBT allowed safe CBT in adult patients who lacked a CB unit containing at least 2.5 × 107 TNC/kg since OS and LFS were at least as good in these patients than in those transplanted with a single CB unit containing ≥2.5 × 107 TNC/kg. This is in concordance with the observations reported by the University of Minnesota [30].
A second observation was that patients who received dCBT had a similar incidence of relapse than those given sCBT. This was also true when comparing the relapse incidence in patients receiving sCBT with TNC > median to those receiving dCBT with TNC > median. These observations suggest that graft-versus-leukemia effects are comparable after sCBT or dCBT. A comparable incidence of relapse in patients receiving sCBT or dCBT has also been observed in recent registry [17, 30, 33] or prospective randomized [18, 19] studies including patients given CBT after myeloablative conditioning. Other approaches to decrease relapse incidence after CBT might include post-transplant administration of disease-targeted medications [34,35,36] or of chimeric antigen receptor T cells [37].
In multivariate analyses, sCBT and dCBT patients had comparable NRM, LFS, GRFS, and OS. These observations are also in accordance with those made in patients receiving CBT after myeloablative conditioning [17,18,19, 30, 33, 38]. Subgroup analyses revealed no interaction between patient age at transplantation, patient gender, number of cells infused, disease type, disease status, HLA-matching, use of ATG and conditioning type (TCF versus other), and the associations between sCBT versus dCBT and GRFS or OS.
The current study also confirmed a detrimental impact of ATG on NRM (leading to a significantly inferior OS) as recently reported in a study including data from patents given CBT after myeloablative conditioning [39] or RIC dCBT [40]. Further, despite ATG not only induces in vivo T cell depletion of the graft but also promotes the generation of regulatory T cells [41, 42], ATG failed to prevent chronic GVHD in the current study, in contrast to what has been observed in peripheral blood stem cell recipients [43,44,45]. These results are also in accordance with those reported by Admiraal et al. who demonstrated that reducing the exposure of ATG after CBT (allowing early CD4+ T cell recovery) improved outcomes in pediatric CBT [46].
There are some limitations in our study including its design (retrospective registry survey) and the relative imbalance in the two groups such as more frequent use of the TCF conditioning regimen but less frequent use of ATG in dCBT patients. These differences were carefully adjusted for in multivariate analyses. Another potential limitation of the study is a potential lack of statistical power to detect small advantages of one group to another. However, the number of patients included in the current study (n = 534) is higher than the number of patients included in prior registry studies in adults (n = 409 in the CIBMTR study [30] and n = 239 in the Eurocord/EBMT study [17]) or in recent prospective randomized studies in children (n = 224 in the study reported by Wagner et al. [18] and n = 151 in the study reported by Michel et al. [19]). Nevertheless, further prospective randomized studies in the RIC setting are needed to draw definitive conclusions. Finally, further studies should compare outcomes after CBT or HLA-haploidentical transplantation following RIC regimens [47,48,49].
Conclusions
In summary, we observed comparable outcomes in patients given dCBT or sufficiently rich sCBT with a TNC dose at cryopreservation >2.5 × 10e7/Kg. Recent advances in the field of CBT expansion are likely to improve outcomes of RIC sCBT [50].
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- ATG:
-
Anti-thymocyte globulin
- CB:
-
Umbilical cord blood
- CBT:
-
Umbilical cord blood transplantation
- CI:
-
Confidence interval
- CR:
-
Complete remission
- dCBT:
-
Double-unit cord blood transplantation
- EBMT:
-
European Society for Blood and Marrow Transplantation
- GRFS:
-
GVHD-free, relapse-free survival
- GVHD:
-
Graft-versus-host disease
- HLA:
-
Human leukocyte antigen
- HR:
-
Hazard ratio
- LFS:
-
Leukemia-free survival
- NRM:
-
Nonrelapse mortality
- OS:
-
Overall survival
- RIC:
-
Reduced-intensity conditioning
- sCBT:
-
Single-unit cord blood transplantation
- TBF:
-
Conditioning regimen combining thiotepa, busulfan and fludarabine
- TBI:
-
Total body irradiation
- TCF:
-
Conditioning regimen combining TBI, cyclophosphamide and fludarabine
- TNC:
-
Total nucleated cells
References
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, Sirvent A, Champlin RE, Chao N, Gee AP, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–60.
Baron F, Labopin M, Ruggeri A, Mohty M, Sanz G, Milpied N, Bacigalupo A, Rambaldi A, Bonifazi F, Bosi A, et al. Unrelated cord blood transplantation for adult patients with acute myeloid leukemia: higher incidence of acute graft-versus-host disease and lower survival in male patients transplanted with female unrelated cord blood––a report from Eurocord, the Acute Leukemia Working Party, and the Cord Blood Committee of the Cellular Therapy and Immunobiology Working Party of the European Group for Blood and Marrow Transplantation. J Hematol Oncol. 2015;8:107.
Ballen KK, Lazarus H. Cord blood transplant for acute myeloid leukaemia. Br J Haematol. 2016;173:25–36.
Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, Witherspoon R, Mielcarek M, Deeg JH, Sorror M, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–53.
Barker JN, Weisdorf DJ, Defor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–9.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8.
Somers JA, Braakman E, van der Holt B, Petersen EJ, Marijt EW, Huisman C, Sintnicolaas K, Oudshoorn M, Groenendijk-Sijnke ME, Brand A, Cornelissen JJ. Rapid induction of single donor chimerism after double umbilical cord blood transplantation preceded by reduced intensity conditioning: results of the HOVON 106 phase II study. Haematol. 2014;99:1753–61.
Tucunduva L, Ruggeri A, Sanz G, Furst S, Socie G, Michallet M, Arcese W, Milpied N, Yakoub-Agha I, Linkesch W, et al. Risk factors for outcomes after unrelated cord blood transplantation for adults with acute lymphoblastic leukemia: a report on behalf of Eurocord and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49:887–94.
Rio B, Chevret S, Vigouroux S, Chevallier P, Furst S, Sirvent A, Bay JO, Socie G, Ceballos P, Huynh A, et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid leukemia using reduced-intensity conditioning: a prospective phase II multicenter trial. Biol Blood Marrow Transplant. 2015;21:445–53.
Devillier R, Harbi S, Furst S, Crocchiolo R, El-Cheikh J, Castagna L, Etienne A, Calmels B, Lemarie C, Prebet T, et al. Poor outcome with nonmyeloablative conditioning regimen before cord blood transplantation for patients with high-risk acute myeloid leukemia compared with matched related or unrelated donor transplantation. Biol Blood Marrow Transplant. 2014;20:1560–5.
Baron F, Ruggeri A, Beohou E, Labopin M, Sanz G, Milpied N, Michallet M, Bacigalupo A, Blaise D, Sierra J, et al. RIC versus MAC UCBT in adults with AML: a report from Eurocord, the ALWP and the CTIWP of the EBMT. Oncotarget. 2016;7:43027–38.
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8.
Barker JN, Weisdorf DJ, Defor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7.
Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, Verneris MR, Appelbaum FR, Wagner JE, Delaney C. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9.
Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, Burke MJ, Blazar BR, Miller JS, McGlave PB, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9.
Lamers CH, Wijers R, van Bergen CA, Somers JA, Braakman E, Gratama JW, Debets R, Falkenburg JH, Cornelissen JJ. CD4+ T-cell alloreactivity towards mismatched HLA-class II alleles early after double umbilical cord blood transplantation (dUCBT). Blood. 2016;128:2165-74.
Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, Yakoub-Agha I, Ribera JM, Mannone L, Sierra J, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28:779–86.
Wagner Jr JE, Eapen M, Carter S, Wang Y, Schultz KR, Wall DA, Bunin N, Delaney C, Haut P, Margolis D, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–94.
Michel G, Galambrun C, Sirvent A, Pochon C, Bruno B, Jubert C, Loundou A, Yakoub-Agha I, Milpied N, Lutz P, et al. Single versus double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127:3450-7.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, Finke J, Schwerdtfeger R, Eder M, Bunjes D, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–7.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, Vindelov LL, Blaise D, Janssen JJ, Petersen E, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8.
Avery S, Shi W, Lubin M, Gonzales AM, Heller G, Castro-Malaspina H, Giralt S, Kernan NA, Scaradavou A, Barker JN. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–85.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplant. 1974;18:295–304.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplantation. 2016;51:610-1.
Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–80.
Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, Sender L, Cairo MS. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, et al. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–81.
Wagner JE, Barker JN, Defor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8.
Stanevsky A, Shimoni A, Yerushalmi R, Nagler A. Double umbilical cord blood transplant: more than a cell dose? Leuk Lymphoma. 2010;51:975–82.
Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, Cutler C, Delaney C, Kan F, Isola L, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–8.
Baron F, Storb R. Hematopoietic cell transplantation after reduced-intensity conditioning for older adults with acute myeloid leukemia in complete remission. Curr Opin Hematol. 2007;14:145–51.
Shimoni A, Labopin M, Savani B, Volin L, Ehninger G, Kuball J, Bunjes D, Schaap N, Vigouroux S, Bacigalupo A, et al. Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol. 2016;9:118.
Sanz J, Wagner JE, Sanz MA, DeFor T, Montesinos P, Bachanova V, Lorenzo I, Warlick E, Sanz GF, Brunstein C. Myeloablative cord blood transplantation in adults with acute leukemia: comparison of two different transplant platforms. Biol Blood Marrow Transplant. 2013;19:1725–30.
Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. J Hematol Oncol. 2017;10:93.
Ehx G, Fransolet G, De leval L, D'Hondt S, Lucas S, Hannon M, Delens L, Dubois S, Drion P, Beguin Y, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2017;6:e1314425.
Fransolet G, Ehx G, Somja J, Delens L, Hannon M, Muller J, Dubois S, Drion P, Caers J, Humblet-Baron S, et al. Azacytidine mitigates experimental sclerodermic chronic graft-versus-host disease. J Hematol Oncol. 2016;9:53.
Liu J, Zhong JF, Zhang X, Zhang C. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol. 2017;10:35.
Sanz J, Gale RP. One or two umbilical cord blood cell units? Caveat emptor. Bone Marrow Transplant. 2017;52:341–3.
Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milpied N, Chevallier P, Tabrizi R, Labalette M, Gluckman E, Labopin M, Yakoub-Agha I. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50:45–50.
Pascal L, Tucunduva L, Ruggeri A, Blaise D, Ceballos P, Chevallier P, Cornelissen J, Maillard N, Tabrizi R, Petersen E, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126:1027–32.
Shimony O, Nagler A, Gellman YN, Refaeli E, Rosenblum N, Eshkar-Sebban L, Yerushalmi R, Shimoni A, Lytton SD, Stanevsky A, et al. Anti-T lymphocyte globulin (ATG) induces generation of regulatory T cells, at least part of them express activated CD44. J Clin Immunol. 2012;32:173–88.
Ehx G, Hannon M, Beguin Y, Humblet-Baron S, Baron F. Validation of a multicolor staining to monitor phosphoSTAT5 levels in regulatory T-cell subsets. Oncotarget. 2015;6:43255–66.
Baron F, Zachee P, Maertens J, Kerre T, Ory A, Seidel L, Graux C, Lewalle P, Van Gelder M, Theunissen K, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society. J Hematol Oncol. 2015;8:4.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Rubio MT, D'Aveni-Piney M, Labopin M, Hamladji RM, Sanz MA, Blaise D, Ozdogu H, Daguindeau E, Richard C, Santarone S, et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party. J Hematol Oncol. 2017;10:31.
Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, Boelens JJ. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016;128:2734–41.
Rubio MT, Savani BN, Labopin M, Piemontese S, Polge E, Ciceri F, Bacigalupo A, Arcese W, Koc Y, Beelen D, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25.
Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, Huang H, Beelen D, Gorin NC, Craddock C, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10:24.
Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol. 2016;9:35.
Baron F, Ruggeri A, Nagler A: Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev Hematology. 2016;9:297-314.
Acknowledgements
We thank Emmanuelle Polge and Audrey Mailhol from the office of the ALWP of EBMT and Chantal Kenzey and Fernanda Volt from the Eurocord study office. FB is a Senior Research Associate at the National Fund for Scientific Research (FNRS), Belgium.
Funding
Not applicable.
Availability of data materials
AR, EB, ML, MM, and AN had full access to all the data in the study (available upon data specific request).
Authors’ contributions
FBa wrote the manuscript, designed the study, and interpreted the data. AR, EB, and ML designed the study, analyzed and interpreted the data, and edited the manuscript. EG and AN designed the study, interpreted the data, and edited the manuscript. MM and BS helped in the study design and edited the manuscript. DB, JC, PC, GS, and EP reviewed the manuscript and provided clinical data. All authors approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The scientific boards of the ALWP of EBMT and of Eurocord approved this study.
Institutions
The EBMT registry is a voluntary working group of more than 500 transplant centers, participants of which are required once a year to report all consecutive stem cell transplantations and follow-up. The list of institutions reporting data included in this study is provided in the supplemental data.
Supplementary information is available at the Journal’s website.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Supplemental figures 1–3 and Tables 1–2. (DOCX 670 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Baron, F., Ruggeri, A., Beohou, E. et al. Single- or double-unit UCBT following RIC in adults with AL: a report from Eurocord, the ALWP and the CTIWP of the EBMT. J Hematol Oncol 10, 128 (2017). https://doi.org/10.1186/s13045-017-0497-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-017-0497-9