Abstract
Background
In the setting of allogeneic human leukocyte antigen (HLA)-matched bone marrow transplantation, transplanting male patients with grafts from female donors has been associated with a higher incidence of graft-versus-host disease (GVHD) and of nonrelapse mortality (NRM). The aim of the current analysis was to compare transplantation outcomes in male patients given female unrelated cord blood (UCB) versus other gender combinations.
Patients and methods
Data from 552 consecutive patients with acute myeloid leukemia (AML) given a single UCB transplantation between 2000 and 2014 were included.
Results
In comparison with other gender combination, male patients given female UCB (n = 131) had a trend for a higher incidence of grades II–IV acute GVHD (33 versus 25 %, P = 0.08), a trend for a higher incidence of NRM (41 versus 33 %, P = 0.06), and a lower leukemia-free (LFS, 30 versus 41 %, P = 0.01) and overall survival (OS, 33 versus 45 %, P = 0.008). In multivariate analyses, taking into consideration all patients for which data on HLA-matching and cell dose transplanted were fully available (n = 363), male patients transplanted with a female UCB had a trend for a higher incidence of grade III–IV acute GVHD (hazard ratio (HR) = 2.0, P = 0.06), a trend for a higher NRM (HR = 1.5, P = 0.06), and a worse LFS (HR = 1.4, P = 0.04) and OS (HR = 1.3, P = 0.06).
Conclusions
Our data suggest that male patients transplanted with female UCB might have higher risk of acute GVHD and of NRM leading to worse LFS and OS. These results should be confirmed in other large cohorts of patients before used for determining the choice of an UCB unit.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplantation from human leukocyte antigen (HLA)-identical sibling is the treatment of choice for many patients with acute myeloid leukemia (AML) [1, 2]. For patients who lack a suitable HLA-identical sibling, unrelated cord blood transplantation (UCBT) and HLA-haploidentical transplantation have emerged as an adequate alternative to HLA-matched unrelated bone marrow/peripheral blood stem cell transplantation, particularly for patients at high risk of rapid disease relapse who urgently need a transplantation [3–7].
Despite major improvements in the field in the last decades [8], nonrelapse mortality (NRM) has remained the main cause of failure of UCBT for AML [4]. In the setting of allogeneic HLA-matched bone marrow or peripheral blood stem cell transplantation, transplanting male patients with grafts from female donors has been associated with a higher incidence of graft-versus-host disease (GVHD) leading to higher NRM and a lower overall survival (OS) [9, 10]. This is due to recognition by female donor immune cells of minor histocompatibility antigens (HA) encoded by genes on the recipient Y chromosome that are polymorphic to their X chromosome homologue [11–15]. These HA, termed H-Y antigens, are highly immunogenic and are expressed throughout the body [15].
Donor naïve T cells recognizing major or minor histocompatibility antigens expressed on normal host tissues are largely involved in GVHD pathogenesis [16–18]. However, recent findings suggest that effector memory T cells might also play a role in GVHD pathogenesis in humans [19].
Despite it has been considered that cord blood T cells were mostly naïve and dedicated to the development of tolerance, recent findings evidence the presence of CD4+ T cells with effector memory phenotype and function in cord blood, representing 1–3 % of CD4+ T cells and behaving as inflammatory cells with a mixed Th1- and Th2-like function upon activation [20]. Further, a recent study demonstrated that the presence of HLA-allele mismatch(es) increased NRM after UCBT [21], demonstrating that alloreactivity after UCBT was not restricted to HLA-antigen mismatches between recipients and UCB but suggesting a role also for minor HA in GVHD pathogenesis following UCBT. These findings prompted us to assess whether transplanting male AML patients with female UCB has an impact on GVHD and UCBT outcomes.
Results
Patient, disease, and transplant characteristics
Data from 552 consecutive patients with AML given a single unit UCBT between 2000 and 2014 were included. Their characteristics are described in Table 1. Briefly, 131 patients were male patients given female UCB, 119 patients were male patients receiving male UCB, and 302 were female patients. In comparison to other patients, male patients given female UCB were less likely to have high-risk cytogenetic or secondary leukemia (39 versus 62 %, P = 0.005), were less often transplanted following a reduced-intensity conditioning (24 versus 35 %, P = 0.03), received more often cyclosporine A alone as GVHD prophylaxis (39 versus 33 %, P = 0.001), and received less total nucleated cells (2.5 versus 2.8 × 107/kg, P = 0.01). The other characteristics were well-balanced between the two groups of patients.
Engraftment
Overall, cumulative incidence of neutrophil engraftment at day 100 was similar in male patients given female UCB (87 % (95 % confidence interval (CI), 79.5–91.9)) versus in other gender combinations (87.7 % (95 % CI, 84–90.6), P = 0.31). Interestingly, median times for reaching 0.5 × 109/L neutrophils were 23 days (range, 6–68 days) in male patients given female UCB versus 21 days (range, 3–66 days, P = 0.02) in other gender combinations (Fig. 1). This could be possibly due to the fact that male patients given female UCB received less total nucleated cell counts (TNC), or this could be due to the fact that, according previous observations, male CB units include more CD34+ cells and more colony-forming unit than female ones [22].
Given that cytotoxic T cells (CTLs) directed against antigens coded by the Y chromosome have been associated with graft rejection in female patients transplanted with bone marrows from male HLA-identical siblings [23], we also compared engraftment kinetics in female patients transplanted with male UCB (n = 145) versus other patients (n = 407). Overall, the 100-day cumulative incidence of neutrophil engraftment was similar in female patients given male UCB (88.3 % (95 % CI, 81.5–92.7)) and in other gender combinations (87.3 % (95 % CI, 83.5–90.3), P = 0. 68). Median times for reaching 0.5 × 109/L neutrophils were 21 days (6–49) in female patients transplanted with male UCB versus 21 days (3–66 days, P = 0.71) in other patients.
GVHD
Male patients transplanted with female UCB had a trend for a higher incidence of grades II–IV acute GVHD (33 versus 25 %, P = 0.08) than other patients, while rates of grades III–IV acute GVHD were 15 versus 11 % (P = 0.2) in male patients given female UCB and in other patients, respectively (Table 2). In multivariate analyses, male patients transplanted with female UCB had significantly higher incidences of grades II–IV (hazard ratio (HR) 1.7, 95 % CI 1.0–2.7; P = 0.04) and grades III–IV (HR 1.9, 95 % CI 1.0–3.6; P = 0.04) acute GVHD than other patients. Restricting the multivariate analyses to patients for which data on TNC transplanted and HLA compatibility were available (n = 363, including 87 male patients given female UCB), there was still a trend for a higher incidence of grades II–IV (HR 1.7, 95 % CI 1.0–3.0; P = 0.07) and grades III–IV (HR 2.0, 95 % CI 1.0–3.9; P = 0.06) acute GVHD in male patients given female UCB, versus other gender combinations (Table 3). Restricting the analyses to male recipients (n = 250), those given female UCB had an incidence of grades II–IV acute GVHD of 33 %, compared with 27 % (P = 0.32) for those transplanted with male UCB. Finally, the incidence of grades II–IV acute GVHD was 21 % in female recipients given female UCB (P = 0.02 in comparison to male patients given female UCB).
Interestingly, 2-year incidence of chronic GVHD tended to be lower in male patients given female UCB in univariate analysis (16 versus 25 %, P = 0.11) (Table 2), while in multivariate analysis, the incidence of chronic GVHD was not different according to gender combination (HR = 0.8, 95 % CI 0.4–1.4; P = 0.4).
Relapse and NRM
The 2-year incidence of relapse was similar in male patients given female UCB (29.2 %) and in other patients (26.2 %, P = 0.4) (Fig. 2). The figures were 22.8 % in male patients given male UCB (P = 0.35 in comparison to male patients given female UCB) (Fig. 3) and 26.3 % in female patients given female UCB (Table 2). In multivariate analyses including data from all patients, male patients given female UCB had a similar incidence of relapse than other gender combinations (HR = 1.4, 95 % CI 0.9–2.1; P = 0.13). Similar observations were made when the analyses were restricted to patients for whom data on TNC and HLA compatibility were available (HR = 1.2, 95 % CI 0.8–1.9; P = 0.4) (Table 3). Factors associated with increased relapse incidence in multivariate analysis included older recipient age (P = 0.03), CR2 or advanced disease (P < 0.001) versus CR1, reduced-intensity conditioning (RIC) (P = 0.04), and secondary AML (P < 0.001).
Two-year incidences of NRM were 40.8 versus 33.1 % (P = 0.06), respectively, in male patients given female UCB versus in other gender combinations (Fig. 2). The figures were 36.6 % in male patients given male UCB (P = 0.41 in comparison to male patients given female UCB) (Fig. 3) and 28.4 % in female patients given female UCB (Table 2). In multivariate analyses including data from all patients, male patients given female UCB had a significantly higher incidence of NRM than other patients (HR = 1.4, 95 % CI 1.0–2.0; P = 0.04). Restricting the analyses to patients for which data on TNC and HLA compatibility were available, there was still a trend for higher NRM in male patients transplanted with female UCB (HR = 1.5, 95 % CI 1.0–2.2; P = 0.06) (Table 3). Other factors associated with higher NRM in multivariate analyses included CR2 versus CR1 (P = 0.01) and the use of anti-thymocyte globulin (ATG) (P = 0.04).
Overall and leukemia-free survival
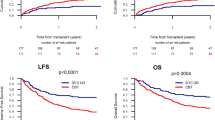
Two-year lower leukemia-free survivals (LFS) were 29.9 versus 40.7 % (P = 0.01), respectively, in male patients given female UCB versus in other patients (Fig. 2). The figures were 40.7 % in male patients given male UCB (P = 0.11 in comparison to male patients given female UCB) (Fig. 3) and 45.3 % in female patients given female UCB (Table 2). In multivariate analyses including data from all patients, male patients given female UCB had a significantly worse LFS than other gender combinations (HR = 1.4, 95 % CI 1.1–1.8; P = 0.01). Restricting the analyses to patients for which data on TNC and HLA compatibility were available, LFS remained significantly worse in male patients transplanted with female UCB (HR = 1.4, 95 % CI 1.0–1.9; P = 0.04) (Table 3). Other factors associated with worse LFS in multivariate analysis included older age (P = 0.03), CR2 (P < 0.001) or advanced disease (P = 0.01) versus CR1, and low number of TNC infused (P = 0.04).
Two-year OS were 33 versus 45 % (P = 0.008), respectively, in male patients given female UCB versus in other gender combinations (Fig. 2). The figures were 42.4 % in male patients given male UCB (P = 0.10) in comparison to male patients given female UCB) (Fig. 3) and 51 % in female patients given female UCB (Table 2). In multivariate analyses including data from all patients, male patients given female UCB had a significantly worse OS than other gender combinations (HR = 1.4, 95 % CI 1.0–1.8; P = 0.02). Similar qualitative observations were made when restricting the analyses to patients for which data on TNC and HLA compatibility were available (HR = 1.3, 95 % CI 1.0–1.8; P = 0.06) (Table 3). Other factors associated with worse OS in multivariate analysis included older age (P = 0.01), CR2 (P < 0.001) or advanced disease (P = 0.01) versus CR1, and low number of TNC infused (P = 0.05). Causes of death were comparable in male patients transplanted with female UCB and in other gender combination. Specifically, main causes of death were disease progression (24 % of transplanted patients), infection (23 % of transplanted patients), and GVHD (8 % of transplanted patients) in male patients transplanted with female UCB versus disease progression (18 % of transplanted patients), infection (17 % of transplanted patients), and GVHD (7 % of transplanted patients) in other gender combinations.
Graft-versus-leukemia effects of chronic GVHD?
Previous studies in the bone marrow or peripheral blood setting have demonstrated a strong link between chronic GVHD occurrence and a lower risk of relapse [24–27]. Since we did not observe a lower risk of relapse in male patients given female UCB, we assessed whether chronic GVHD was the driver of graft-versus-leukemia effects after UCBT. We first performed a landmark analysis selecting patients alive without relapse at 1-year post-transplant and considering chronic GVHD occurring the first year post-transplant (n = 179). As shown in the Fig. 4, 2-year cumulative incidence of relapse was 10.8 % (95 % CI 5.9–17.5 %) in patients without chronic GVHD before 1 year (n = 125), versus 4.5 % (95 % CI 0.8–13.7 %) in patients with chronic GVHD before 1 year (n = 54) (P = 0.9). We confirmed the absence of statistically significant association between chronic GVHD and graft-versus-leukemia effects in a multivariate Cox model that showed that occurrence of chronic GVHD (assessed as a time-dependent covariate) was not associated with a lower risk of relapse (HR = 1.2, 95 % CI 0.7–2.1).
Discussion
Despite cord blood T cells are more tolerant than adult T cells [28, 29], a recent study demonstrated that the presence of HLA-allele mismatch(es) increased NRM after UCBT [21], evidencing that alloreactivity after UCBT was not restricted to HLA-antigen mismatches between recipients and UCB and suggesting a possible role for minor histocompatibility antigens in GVHD pathogenesis following UCBT. These findings prompted us to assess whether alloreactivity against H-Y antigens, a class of well-known highly immunogenic minor HA which are expressed throughout the body [15], played a role in the UCBT setting.
Main observations were that male patients given female UCB had a higher incidence of acute GVHD, leading to increased NRM and worse LFS and OS. These observations are in line with what has been observed in the setting of HLA-identical bone marrow or peripheral blood stem cell (PBSC) transplantation [9]. This suggests that even in the presence of HLA-mismatches, donor cord blood T cells are able to react against H-Y antigens. However, these results are in contrast to those reported by Konuma et al. who observed no impact of sex mismatch on acute GVHD, LFS, nor OS in a cohort of 191 patients who received a single unit UCBT as treatment for various malignancies [30].
Interestingly, in contrast to what was observed in HLA-identical bone marrow or PBSC recipients [9, 31, 32], male recipients of female UCB were not exposed to a higher incidence of chronic GVHD. The reason for this apparent discrepancy is unclear and might be related to differences in the biology of cord blood versus bone marrow transplantation.
Despite having a higher incidence of acute GVHD, male recipients of female UCB were not protected from relapse. While occurrence of acute and/or chronic GVHD has been associated with a lower risk of relapse in AML patients in the setting of HLA-matched bone marrow or peripheral blood stem cells transplantation [26, 33, 34], the impact of GVHD on transplantation outcomes in the UCBT setting remains to be investigated. Our current data suggest that transplanting male patients with female UCB is not associated with increased graft-versus-leukemia effects, in contrast to what has been observed after HLA-identical bone marrow transplantation [9]. These results mirror those observed in a study assessing the impact of HLA-allele mismatches in the UCBT setting where increasing mismatching correlated with acute GVHD and nonrelapse mortality but not with a protection from relapse [21]. These results are also in line with those reported by Konuma et al. who observed no impact of sex mismatch on relapse incidence after single unit UCBT [30].
The current study also confirmed a detrimental impact of ATG on NRM as recently reported in a study including data from patents given UCB after myeloablative conditioning [35], probably due to the negative impact of ATG on immune recovery after allogeneic stem cell transplantation [36]. However, ATG had no impact on relapse incidence, in agreement with recent observations in the peripheral blood stem cell setting [37–39]. Further, as previously observed in the UCB setting [4, 40], older age was associated with worse LFS and OS. Interestingly, while patients given RIC had a higher incidence of relapse, this did not translate to worse LFS or OS due to a trend to lower NRM in patients given RIC (P = 0.09), as previously observed in the setting of UCBT as treatment for ALL [40]. In addition, confirming previous observations, low TNC infused correlated to worse OS [29]. Finally, as expected, advanced disease status at transplantation had a negative impact of on all transplantation outcomes, while patients with secondary (versus primary) AML had a higher risk of relapse in contrast to recent observations in the PBSC transplantation setting [41].
There are some limitation in our study including its design (retrospective registry survey) and the surprising relative imbalance between the two groups. We tried to address these issues by performing multivariate analyses. Nevertheless, current results should be taken with some caution and should be confirmed in other large cohort of patients before recommendations can be made regarding the choice of the gender of the UCB in male patients with AML. Further, translational research looking at the presence (or absence) of CTLs [23] or antibodies [14] directed against antigens coded by the Y chromosome in male patients transplanted with female UCT are needed.
Conclusions
In summary, our data suggest that male AML patients transplanted with female UCB might have higher risk of acute GVHD and of NRM leading to worse LFS and OS. These results should be confirmed in other large cohorts of patients before used for determining the choice of an UCB unit.
Patients and methods
Data Collection
This survey is a retrospective study performed by the Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT) group and by Eurocord. EBMT registry is a voluntary working group of more than 500 transplant centers, participants of which are required once a year to report all consecutive stem cell transplantations and follow-up. Eurocord collects data on UCBT performed in >50 countries worldwide and >500 transplant centers, mainly EBMT centers. Population selection criteria included adult recipients, primary or secondary AML, first allogeneic stem cell transplantation, and single-unit UCBT performed from 2000 to 2014. Grading of acute and chronic GVHD was performed using established criteria [42]. HLA compatibility was based on low-resolution typing for HLA-A and HLA-B and high-resolution typing for HLA-DRB1. For the purpose of this study, all necessary data were prospectively collected according to EBMT and Eurocord guidelines.
Ethics
The scientific boards of the ALWP of EBMT and of Eurocord approved this study.
Statistical analyses
Data from all patients meeting the inclusion/exclusion criteria were included in the analyses(additional file). Start time was date of transplant for all endpoints. Neutrophil engraftment was defined as first of three consecutive days with a neutrophil count of at least 0.5 × 109/L, while platelet engraftment was defined as the first of seven consecutive days of an unsupported platelet count of at least 20 × 109/L.
To evaluate the relapse incidence, patients dying either from direct toxicity of the procedure or from any other cause not related to leukemia were censored. NRM was defined as death while in CR. Patients were censored at the time of relapse or of the last follow-up. Cumulative incidence functions (CIF) were used for relapse incidence and NRM in a competing risk setting, since death and relapse were competing together.
For estimating the cumulative incidence of chronic GVHD, death was considered as a competing event. OS and leukemia-free survival (LFS) were estimated using the Kaplan-Meier estimates. Univariate analyses were done using Gray’s test for CIF and log rank test for OS and LFS. Multivariate analyses adjusted for differences between groups were performed using Cox proportional hazards regression models for OS, LFS, relapse incidence, and NRM and using multivariate logistic regression for acute GVHD. The impact of chronic GVHD on the risk of relapse was assessed by performing a landmark analysis 1 year after transplantation and in a multivariate time-dependent Cox model in which chronic GVHD was modeled as a time-dependent covariate. All tests were two sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes. Statistical analyses were performed with SPSS 19 (SPSS Inc, Chicago, IL) and R 2.13.2 (R Development Core Team, Vienna, Austria) software packages.
References
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. Journal of Hematology and Oncology. 2013;6:15.
Weisdorf D, Eapen M, Ruggeri A, Zhang MJ, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biology of Blood and Marrow Transplantation. 2014;20:816–22.
Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28:779–86.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–82.
Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891–900.
Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. Journal of Hematology and Oncology. 2015;8:84.
Hahn T, McCarthy Jr PL, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. Journal of Clinical Oncology. 2013;31:2437–49.
Randolph SSB, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic cell transplants. Blood. 2004;103:347–52.
Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122:3359–64.
Goulmy E, Termijtelen A, Bradley BA, van Rood JJ. Y-antigen killing by T cells of women is restricted by HLA. Nature. 1977;266:544–5.
Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nature Reviews Cancer. 2004;4:371–80.
Zorn E, Miklos DB, Floyd BH, Mattes-Ritz A, Guo L, Soiffer RJ, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. Journal of Experimental Medicine. 2004;199:1133–42.
Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8.
Popli R, Sahaf B, Nakasone H, Lee JY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol Res. 2014;58:249–58.
Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. NatRevImmunol. 2012;12:443–58.
Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–84.
Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124(3):354–62.
Loschi M, Porcher R, Peffault de Latour R, Vanneaux V, Robin M, Xhaard A, et al. High number of memory T cells is associated with higher risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;21(3):569–74.
Zhang X, Mozeleski B, Lemoine S, Deriaud E, Lim A, Zhivaki D, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Science Translational Medicine. 2014;6:238ra272.
Eapen M, Klein JP, Ruggeri A, Spellman S, Lee SJ, Anasetti C, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–40.
Page KM, Mendizabal A, Betz-Stablein B, Wease S, Shoulars K, Gentry T, et al. Optimizing donor selection for public cord blood banking: influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion. 2014;54:340–52.
Rufer N, Wolpert E, Helg C, Tiercy JM, Gratwohl A, Chapuis B, et al. HA-1 and the SMCY-derived peptide FIDSYICQV (H-Y) are immunodominant minor histocompatibility antigens after bone marrow transplantation. Transplantation. 1998;66:910–6.
Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED, the Seattle Marrow Transplant T. Antileukemic effect of chronic graft-versus-host disease. Contribution to improved survival after allogeneic marrow transplantation. New England Journal of Medicine. 1981;304:1529–33.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Journal of Clinical Oncology. 2005;23:1993–2003.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8.
Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology. 2013;31:1530–8.
Kim YJ, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol. 2011;79:112–26.
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8.
Konuma T, Kato S, Ooi J, Oiwa-Monna M, Ebihara Y, Mochizuki S, et al. Impact of sex incompatibility on the outcome of single-unit cord blood transplantation for adult patients with hematological malignancies. Bone Marrow Transplantation. 2014;49:634–9.
Baron F, Zachee P, Maertens J, Kerre T, Ory A, Seidel L, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society. Journal of Hematology and Oncology. 2015;8:4.
Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). Journal of hematology & oncology. 2014;7:59.
Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28:2235–40.
Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–8.
Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milpied N, Chevallier P, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplantation. 2015;50:45–50.
Hannon M, Beguin Y, Ehx G, Servais S, Seidel L, Graux C, et al. Immune recovery after allogeneic hematopoietic stem cell transplantation following flu-TBI versus TLI-ATG conditioning. Clinical Cancer Research. 2015;21:3131–9.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Baron F, Labopin M, Blaise D, Lopez-Corral L, Vigouroux S, Craddock C, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation. 2014;49:389–96.
Bonifazi F, Solano C, Wolschke C, Patriarca F, Pini M, Nagler A, et al. Prevention of Chronic GvHD after HLA-Identical Sibling Peripheral Hematopoietic Stem Cell Transplantation with or without anti-lymphocyte globulin (ATG). Results from a Prospective, Multicenter Randomized Phase III Trial (ATG family study). Blood. 2014;124:37.
Tucunduva L, Ruggeri A, Sanz G, Furst S, Socie G, Michallet M, et al. Risk factors for outcomes after unrelated cord blood transplantation for adults with acute lymphoblastic leukemia: a report on behalf of Eurocord and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation. 2014;49:887–94.
Michelis FV, Atenafu EG, Gupta V, Kim DD, Kuruvilla J, Lipton JH, et al. Comparable outcomes post allogeneic hematopoietic cell transplant for patients with de novo or secondary acute myeloid leukemia in first remission. Bone Marrow Transplantation. 2015.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Acknowledgements
We thank Emmanuelle Polge and Audrey Mailhol from the office of the ALWP of EBMT. FB is Senior Research Associate at the National Fund for Scientific Research (FNRS) Belgium.
List of institutions
The EBMT registry is a voluntary working group of more than 500 transplant centers, participants of which are required once a year to report all consecutive stem cell transplantations and follow-up. The list of institutions reporting data included in this study is provided in the Additional file 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FBa wrote the manuscript, designed the study, and interpreted the data; ML and AR designed the study, analyzed and interpreted the data, and edited the manuscript; EG and AN designed the study, interpreted the data, and edited the manuscript. MM, GS, NM, AB, AR, FBo, AB, JS, IYA, JMRS, and EG reviewed the manuscript and provided clinical data. All authors approved the final version of the manuscript.
Additional file
Additional file 1:
List of institutions reporting data in this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Baron, F., Labopin, M., Ruggeri, A. et al. Unrelated cord blood transplantation for adult patients with acute myeloid leukemia: higher incidence of acute graft-versus-host disease and lower survival in male patients transplanted with female unrelated cord blood—a report from Eurocord, the Acute Leukemia Working Party, and the Cord Blood Committee of the Cellular Therapy and Immunobiology Working Party of the European Group for Blood and Marrow Transplantation. J Hematol Oncol 8, 107 (2015). https://doi.org/10.1186/s13045-015-0207-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-015-0207-4