Abstract
Background
Patients with colorectal cancer (CRC) are more likely to develop severe course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and experience increased risk of mortality compared to SARS-CoV-2 patients without CRC.
Objectives
To estimate the prevalence of SARS-CoV-2 infection in CRC patients and analyse the demographic parameters, clinical characteristics and treatment outcomes in CRC patients with COVID-19 illness.
Methods
For this systematic review and meta-analysis, we searched Proquest, Medline, Embase, Pubmed, CINAHL, Wiley online library, Scopus and Nature for studies on the incidence of SARS-CoV-2 infection in CRC patients, published from December 1, 2019 to December 31, 2021, with English language restriction. Effect sizes of prevalence were pooled with 95% confidence intervals (CIs). Sub-group analyses were performed to minimize heterogeneity. Binary logistic regression model was used to explore the effect of various demographic and clinical characteristics on patient’s final treatment outcome (survival or death).
Results
Of the 472 papers that were identified, 69 articles were included in the systematic review and meta-analysis (41 cohort, 16 case-report, 9 case-series, 2 cross-sectional, and 1 case-control studies). Studies involving 3362 CRC patients with confirmed SARS-CoV-2 (all patients were adults) were analyzed. The overall pooled proportions of CRC patients who had laboratory-confirmed community-acquired and hospital-acquired SARS-CoV-2 infections were 8.1% (95% CI 6.1 to 10.1, n = 1308, 24 studies, I2 98%, p = 0.66), and 1.5% (95% CI 1.1 to 1.9, n = 472, 27 studies, I2 94%, p < 0.01). The median patient age ranged from 51.6 years to 80 years across studies. The majority of the patients were male (n = 2243, 66.7%) and belonged to White (Caucasian) (n = 262, 7.8%), Hispanic (n = 156, 4.6%) and Asian (n = 153, 4.4%) ethnicity. The main source of SARS-CoV-2 infection in CRC patients was community-acquired (n = 2882, 85.7%; p = 0.014). Most of those SARS-CoV-2 patients had stage III CRC (n = 725, 21.6%; p = 0.036) and were treated mainly with surgical resections (n = 304, 9%) and chemotherapies (n = 187, 5.6%), p = 0.008. The odd ratios of death were significantly high in patients with old age (≥ 60 years) (OR 1.96, 95% CI 0.94–0.96; p < 0.001), male gender (OR 1.44, 95% CI 0.41–0.47; p < 0.001) CRC stage III (OR 1.54, 95% CI 0.02–1.05; p = 0.041), CRC stage IV (OR 1.69, 95% CI 0.17–1.2; p = 0.009), recent active treatment with chemotherapies (OR 1.35, 95% CI 0.5–0.66; p = 0.023) or surgical resections (OR 1.4, 95% CI 0.8–0.73; p = 0.016) and admission to ICU (OR 1.88, 95% CI 0.85–1.12; p < 0.001) compared to those who survived.
Conclusion
SARS-CoV-2 infection in CRC patient is not uncommon and results in a mortality rate of 26.2%. Key determinants that lead to increased mortality in CRC patients infected with COVID-19 include older age (≥ 60 years old); male gender; Asian and Hispanic ethnicity; if SARS-CoV-2 was acquired from hospital source; advanced CRC (stage III and IV); if patient received chemotherapies or surgical treatment; and if patient was admitted to ICU, ventilated or experienced ARDS.
Similar content being viewed by others
Background
Since its outbreak in China in December 2019, corona virus disease 2019 (COVID-19) has spread across the world to become a global pandemic. According to the World Health Organization (WHO), as of July 21, 2022, 562,672,324 confirmed cases of COVID-19 have been recorded worldwide, with 6,367,793 deaths [1]. Established, probable, and possible comorbidities that have been associated with severe COVID-19 in at least 1 meta-analysis or systematic review, in observational studies, or in case series were: age ≥ 65 years, asthma, cancer, cerebrovascular disease, chronic kidney disease, chronic lung disease (interstitial lung disease, pulmonary embolism, pulmonary hypertension, bronchiectasis, chronic obstructive pulmonary disease), chronic liver disease (cirrhosis, non-alcoholic fatty liver disease, alcoholic liver disease, autoimmune hepatitis), diabetes mellitus, type 1 and type 2, heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies), human immunodeficiency virus (HIV), obesity (BMI ≥ 30 kg/m2) and overweight (BMI 25 to 29 kg/m2), pregnancy or recent pregnancy, primary immunodeficiencies, smoking (current and former), sickle cell disease or thalassemia, solid organ or blood stem cell transplantation, tuberculosis, use of corticosteroids or other immunosuppressive medications [2,3,4]. In a systematic analysis that calculated the total number of community infections through seroprevalence surveys from 53 countries prior to vaccine availability, the COVID-19 infection mortality rate was 0.005 percent at 1 year, decreased to 0.002 percent by age 7, and increased exponentially after that: 0.006 percent at age 15, 0.06 percent at age 30, 0.4 percent at age 50, 2.9 percent at age 70, and 20 percent at age 90 [5].
Colorectal cancer (CRC) is common and deadly disease, and globally, CRC still remains the third most commonly diagnosed cancer in males and the second in females [6]. CRC is the most common gastrointestinal malignancy and disproportionately affects medically underserved populations [7]. Patients with CRC are more likely to develop severe course of severe acute respiratorcy syndrome coronavirus 2 (SARS-CoV-2) infection and experience increased risk of mortality compared to SARS-CoV-2 patients without CRC [8,9,10,11,12,13,14,15,16]. Higher mortality rates in CRC patients infected with COVID-19 case-series and cohort studies were reported; for instance, in two small Chinese case-series, rates of death reached up to 61.5 to 70% [11, 15], and in a large French cohort (a total of 376 CRC patients infected with COVID-19 cases), mortality rate was 37.8% [8]; and there was a lower proportion of death of all hospitalized CRC patients infected with COVID-19 based on two different studies in China (5.9%) and Turkey (6.4%) [14, 17]. The recent UK Coronavirus Cancer Monitoring Project (UKCCMP) prospective cohort study of 2,515 patients conducted at 69 UK cancer hospitals among adult patients (≥ 18 years) with an active cancer and COVID-19 reported a 38% (966 patients) mortality rate with an association between higher mortality in patients with haematological malignant neoplasms, particularly in those with acute leukaemias or myelodysplastic syndrome (OR, 2.16; 95% CI, 1.30–3.60) and myeloma or plasmacytoma (OR, 1.53; 95% CI, 1.04–2.26) [18]. Lung cancer was also significantly associated with higher COVID-19-related mortality (OR, 1.58; 95% CI, 1.11–2.25) [18]. A possible reason for increased mortality due to SARS-CoV-2 in patients with CRC is because most health care systems have been required to reorganize their infrastructure and staffing to manage the COVID-19 pandemic [19]. The pandemic has called for a review of healthcare workers daily medical practices, including our approach to CRC management where treatment puts patients at high risk of virus exposure. Given their higher median age, CRC patients are at an increased risk for severe symptoms and complications in cases of infection, especially in the setting of immunosuppression. Considering that the reduction in CRC screening following SARS-CoV-2 pandemic is due to the restrictions imposed for the high prevalence of COVID-19 illness and the lack of referrals due to the fear of developing SARS-CoV-2 infection [20,21,22] (see Fig. 1).
To date, some studies have been performed to evaluate the SARS-CoV-2 infection in CRC patients, but the results of these studies were inconsistent because most of these are single-centre studies with limited sample sizes [23,24,25,26,27,28,29,30,31,32,33,34,35,36]. In light of newer case reports, case-series and cohort studies that were done to re-evaluate the development of COVID-19 disease in CRC patients, we aimed to estimate the prevalence of SARS-CoV-2 infection in CRC patients and analyse the demographic parameters, clinical characteristics and treatment outcomes in CRC patients with COVID-19 illness with larger and better-quality data.
Methods
Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) in conducting this systematic review and meta-analysis [37]. The following electronic databases were searched: PROQUEST, MEDLINE, EMBASE, PUBMED, CINAHL, WILEY ONLINE LIBRARY, SCOPUS and NATURE with Full Text. We used the following keywords: COVID-19 OR SARS-CoV-2 OR Severe acute Respiratory Syndrome Coronavirus 2 OR Coronavirus Disease 2019 OR 2019 novel coronavirus AND colorectal cancer OR colon OR rectal OR rectum OR CRC OR bowel cancer OR tumor OR cancer OR neoplasm. The search was limited to papers published in English between 1 December 2019 and 31 December 2021. Based on the title and abstract of each selected article, we selected those discussing and reporting occurrence of CRC in COVID-19 patients.
Inclusion–exclusion criteria
Inclusion criteria are as follows: (1) published case reports, case series and cohort studies that focused on COVID-19 in CRC patients that included children and adults as our population of interest; (2) studies of experimental or observational design reporting the prevalence of SARS-CoV-2 infection in patients with CRC; (3) the language was restricted to English.
The exclusion criteria are as follows: (1) editorials, commentaries, case and animal studies, discussion papers, preprints, news analyses, reviews and meta-analyses; (2) studies that did not report data on CRC and SARS-CoV-2; (3) studies that never reported details on SARS-CoV-2 identified cases with CRC; (4) studies that reported CRC in patients with negative COIVD-19 PCR tests; (5) duplicate publications.
Data extraction
Six authors critically reviewed all of the studies retrieved and selected those judged to be the most relevant. Data were carefully extracted from the relevant research studies independently. Articles were categorized as case report, case series, cross-sectional, case–control or cohort studies. The following data were extracted from selected studies: authors; publication year; study location; study design and setting; age; proportion of male patients; patient ethnicity; methods used for CRC diagnosis; total number of patients and number of CRC patients with positive PCR SARS-CoV-2; source of SARS-CoV-2 infection [community-acquired or hospital-acquired]; CRC staging; treatments received; symptoms from tumor; comorbidities; if patient was admitted to intensive care unit (ICU), placed on mechanical ventilation, and/or suffered acute respiratory distress syndrome (ARDS); assessment of study risk of bias; and treatment outcome (survived or died); which are noted in Table 1.
Quality assessment
The quality assessment of the studies was undertaken based on the Newcastle–Ottawa Scale (NOS) to assess the quality of the selected studies [38]. This assessment scale has two different tools for evaluating case–control and cohort studies. Each tool measures quality in the three parameters of selection, comparability, and exposure/ outcome, and allocates a maximum of 4, 2, and 3 points, respectively [38]. High-quality studies are scored greater than 7 on this scale, and moderate-quality studies, between 5 and 7 [38]. Quality assessment was performed by six authors independently, with any disagreement to be resolved by consensus.
Data analysis
We examined primarily the proportion of confirmed SARS-CoV-2 infection in patients with CRC. This proportion was further classified based on source of SARS-CoV-2 infection (if CRC patient contracted SARS-CoV-2 from the community or hospital). Community-acquired SARS-CoV-2 infection is the infection that CRC patients contracted outside the hospital (i.e., SARS-CoV-2 infection that become clinically apparent within 48 h of the hospital admission or CRC patients have had the infection when admitted to the hospital for some other reason) [39]. Hospital-acquired SARS-CoV-2 infection is the infection that CRC patients contracted within the hospital, the SARS-CoV-2 infections contracted within the hospital but not become clinically apparent until after the discharge of the CRC patient, or SARS-CoV-2 infections contracted by the healthcare workers as a result of their direct or indirect contact with the CRC patients [39]. Taking a conservative approach, a random effects with the DerSimoniane-Laird model was used [40], which produces wider confidence intervals (CIs) than a fixed effect model. Results were illustrated using forest plots. The Cochran’s chi-square (χ2) and the I2 statistic provided the tools of examining statistical heterogeneity [41]. An I2 value of > 50% suggested significant heterogeneity [42]. Examining the source of heterogeneity, a subgroup analysis was conducted based on study location (if continent of Asia, America, Europe or multi-countries).
Individual CRC patient data on demographic parameters and clinical variables and associated treatment outcomes (survived or died) were extracted from the included studies. Univariate and multivariable logistic regression analysis were used to estimate odds ratio (OR) and 95% CIs of the association of each variable with the treatment outcomes of CRC patients with SARS-CoV-2 infection. All p-values were based on two-sided tests and significance was set at a p-value less than 0.05. R version 4.1.0 with the packages finalfit and forestplot was used for all statistical analyses.
Results
Study characteristics and quality
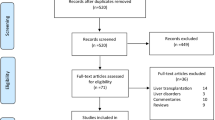
A total of 1076 publications were identified (Fig. 2). After scanning titles and abstracts, we discarded 314 duplicate articles. Another 83 irrelevant articles were excluded based on the titles and abstracts. The full texts of the 472 remaining articles were reviewed, and 403 irrelevant articles were excluded. As a result, we identified 69 studies that met our inclusion criteria and reported SARS-CoV-2 infection in CRC patients [8,9,10,11,12,13,14,15,16,17, 23, 25,26,27,28,29,30,31,32,33,34,35,36, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. The detailed characteristics of the included studies are shown in Table 1. There were 16 case report [16, 32, 46, 48,49,50, 52, 54, 55, 59, 66, 67, 71, 75, 82, 84], 9 case series, 41 cohort [8,9,10,11,12,13,14,15,16,17, 23, 25, 26, 29, 31, 34, 43,44,45, 51, 53, 56,57,58, 60,61,62,63,64,65, 68,69,70, 73, 74, 77, 79, 80, 83, 85], 2 cross-sectional [72, 76] and 1 case–control [81] studies. These studies were conducted in China (n = 15), Italy (n = 8), United States (n = 6), United Kingdom (n = 6), Spain (n = 5), India (n = 4), France (n = 3), Turkey (n = 2), Brazil (n = 2), Japan (n = 2), Colombia (n = 1), Philippines (n = 1), Poland (n = 1), Iran (n = 1), The Netherlands (n = 1), Belgium (n = 1), United Arab Emirates (n = 1), Mexico (n = 1), Sweden (n = 1), Austria (n = 1), Hungary (n = 1), Australia (n = 1), and Chile (n = 1). Few studies were made within multi-countries (n = 3) [9, 12, 51]. The majority of the studies were single centre [11, 16, 23, 25, 27, 28, 30,31,32,33,34,35, 44,45,46,47,48,49,50, 52, 54, 55, 58, 59, 61,62,63, 65,66,67,68,69, 71, 72, 74,75,76, 78, 80, 82,83,84] and only 25 studies were multi-centre [8,9,10, 12,13,14,15,16,17, 26, 29, 36, 43, 51, 53, 56, 57, 60, 64, 70, 73, 77, 79, 81, 85]. The median NOS score for these studies was 6 (range, 5–7). Among the 69 included studies, 64 studies were moderate-quality studies (i.e., NOS scores were between 5 and 7) and 5 studies demonstrated a relatively high quality (i.e., NOS scores > 7); Table 1.
Meta-analysis of SARS-CoV-2 infection in patients with CRC
The overall pooled proportions of CRC patients who had laboratory-confirmed community-acquired and hospital-acquired SARS-CoV-2 infections were 8.1% (95% CI 6.1 to 10.1, n = 1308, 24 studies, I2 98%, p = 0.66), and 1.5% (95% CI 1.1 to 1.9, n = 472, 27 studies, I2 94%, p < 0.01), respectively; (Fig. 3, Fig. 4).
In community-acquired infected SARS-CoV-2 patients, subgroup analysis showed some difference in the rates between all patients (Asia, Europe and America groups) [8, 11, 13,14,15,16,17, 23, 28, 29, 33, 34, 43,44,45, 58, 62,63,64,65, 70, 76, 81, 86]; and the Asia group [(9.1% (95% CI 6.1 to 12.2, n = 252, 10 studies, I2 = 97%)] [11, 15,16,17, 23, 28, 45, 58, 65, 86]; Europe group [(7.7% (95% CI 3.9 to 11.4, n = 801, 8 studies, I2 = 99%)] [8, 14, 29, 33, 34, 43, 44, 64]; and America group [7.0% (95% CI 3.2 to 10.7, n = 229, 6 studies, I2 = 96%)] [13, 62, 63, 70, 76, 81], respectively; Fig. 3. In the hospital-acquired SARS-CoV-2 infected patients, subgroup analysis showed a significant difference in the rates between all patients (Europe, multi-countries, Asia and America) [9, 10, 25,26,27,28, 30, 31, 35, 36, 47, 51, 53, 56, 57, 60, 61, 65, 68, 69, 72, 74, 77, 79, 80, 83, 85]; and Europe only patients [1.1% (95% CI 0.5 to 1.6, n = 178, 12 studies, I2 = 91%)] [10, 25,26,27, 35, 47, 53, 56, 60, 61, 77, 79]; multi-countries only patients [4.7% (95% CI 3.6 to 5.9, n = 212, 2 studies, I2 = 90%)] [9, 51]; Asia only patients [0.7% (95% CI 0.0 to 1.4, n = 32, 9 studies, I2 = 69%)] [28, 31, 36, 57, 65, 68, 74, 83, 85]; and America only patients [2.3% (95% CI 0.9 to 3.6, n = 32, 4 studies, I2 = 10%)] [30, 69, 72, 80], respectively; Fig. 4.
Demographic and clinical characteristics of CRC patients with SARS‑CoV‑2 infection
The included studies had a total of 3362 CRC patients with confirmed SARS-CoV-2 infection as detailed in Table 1. Amongst these 3362 patients, all patients were adults. The median patient age ranged from 51.6 years to 80 years across studies. There was an increased male predominance in CRC patients diagnosed with SARS-CoV-2 in most of the studies [n = 2243, 66.7%] [9, 11, 14, 16, 17, 25, 27, 28, 30, 32,33,34,35, 47, 52,53,54,55,56,57,58,59, 61, 62, 71, 72, 74, 75, 77, 81,82,83,84,85] and majority of the patients belonged to White (Caucasian) (n = 262, 7.8%), Hispanic (n = 156, 4.6%) and Asian (n = 153, 4.5%) ethnicity [11, 13,14,15,16,17, 25,26,27,28,29,30,31,32,33,34, 36, 43, 44, 46,47,48,49,50, 52,53,54,55, 57,58,59,60,61, 67, 70,71,72,73, 75,76,77,78, 80, 81, 83,84,85]. Most patients were diagnosed for CRC through symptoms, endoscopy, radiological imaging, biopsies and tumor markers [8,9,10, 12,13,14, 16, 17, 23, 25,26,27,28,29,30,31,32,33,34,35,36, 43, 44, 46,47,48,49,50,51,52,53,54,55,56, 58,59,60,61, 65,66,67,68,69,70,71,72,73,74,75,76,77, 80,81,82,83,84,85]. The main source of SARS-CoV-2 infection in CRC patients was community-acquired (n = 2882, 85.7%; p = 0.014) [8, 11,12,13,14,15,16,17, 23, 28, 29, 33, 34, 43,44,45, 48, 50, 52, 55, 58, 59, 62,63,64,65,66,67, 70, 71, 75, 76, 81,82,83]. Most of those SARS-CoV-2 patients had stage III CRC (n = 725, 21.6%; p = 0.036) [9, 13, 17, 35, 45, 72, 83]; and were treated mainly with surgical resections (n = 304, 9%) and chemotherapies (n = 187, 5.6%), p = 0.008 [9,10,11, 16, 17, 23, 25, 26, 28, 29, 31,32,33,34,35,36, 43,44,45, 47, 49, 51, 53, 54, 56,57,58, 60, 61, 68, 72, 74, 77, 80, 82, 85]. The most common tumor symptoms patients experienced were change in bowel habits (n = 26, 0.8%), diarrhoea (n = 25, 0.7%), abdominal pain (n = 23, 0.7%), and nausea and vomiting (n = 21, 0.6%); p = 0.048 [11, 16, 17, 27, 46, 50, 55, 58, 61, 70, 72, 81]. Many of the CRC patients infected with COVID-19 had pre-existing hypertension (n = 68, 2%) and/or diabetes mellitus (n = 49, 1.4%), p = 0.027 [11, 17, 28, 31, 33, 34, 44, 46, 55, 57, 58, 61, 62, 71, 72, 74, 75, 77, 79, 80, 83, 85].
Patient treatment outcome and predictors of mortality
Patients were stratified based on treatment outcome (mortality or survival). A summary of the demographic, source of SARS-CoV-2 infection, CRC staging, treatment received, symptoms of tumor, comorbidities and medical complications with regards to final treatment outcome in 2787 patients who had either survived (n = 2056) or died (n = 731) is shown in Table 2.
Those patients who died were more likely to have been older in age (≥ 60 years old: 90.8% vs 0.7%; p = 0.000); and more likely to be men [male gender: 6.8% vs 2.3%; p = 0.000]. Majority of patients who died had an Asian (n = 37, 5.1%) and Hispanic ethnicity (n = 31, 4.2%; p = 0.011). CRC patients who transmitted SARS-CoV-2 from the community had a higher mortality compared to those patients who acquired the SARS-CoV-2 infection from a hospital source (88.5% vs 11.5%; p = 0.014). As expected with the CRC stating, patients with advanced stage had a high mortality [death in stage IV CRC patients occurred in n = 61 (8.3%), p = 0.036]. CRC patients infected with SARS-CoV-2 who received chemotherapy had about two-fold increased risk of mortality compared to CRC patients with SARS-CoV-2 who had surgical resections (39 (5.3%) vs 21 (2.9%); p = 0.008). The most common tumor symptoms in CRC patients with SARS-CoV-2 infection in whom mortality was reported were the change in bowel habits (n = 7, 0.9%) and diarrhoea (n = 5, 0.7%); p = 0.048. Patients with a pre-existing diabetes mellitus (n = 19, 2.6%) and hypertension (n = 14, 1.9%) had the highest mortality rate compared to other comorbidities; p = 0.027. Mortality rate was significantly very high in CRC patients infected with SARS-CoV-2 who were admitted to the intensive care unit (0.3% vs 13.1%; p = 0.000), placed on mechanical ventilation (0.1% vs 6.4%; p = 0.000) and/or suffered acute respiratory distress syndrome (0.05% vs 4%; p = 0.000).
Potential determining variables associated in survival and death groups were analysed through binary logistic regression analysis and shown in Fig. 5, Fig. 6, Fig. 7, Fig. 8 and Fig. 9. As expected, old age (≥ 60 years) (OR 1.96, 95% CI 0.94–0.96; p < 0.001), male gender (OR 1.44, 95% CI 0.41–0.47; p < 0.001), CRC patients infected with SARS-CoV-2 who came from Asia (OR 1.16, 95% CI 0.26–0.7; p = 0.01) and Europe (OR 1.14, 95% CI 0.36–0.44; p = 0.01), or transmitted the SARS-CoV-2 viral infection from a hospital source (OR 0.59, 95% CI 0.13–0.25; p < 0.001) are associated with increased odd ratio for death; Fig. 5. Among the CRC staging groups, patients who were infected with SARS-CoV-2 and presented with CRC stage III (OR 1.54, 95% CI 0.02–1.05; p = 0.041) and stage IV (OR 1.69, 95% CI 0.17–1.2; p = 0.009) had a high OR of death; Fig. 6. The odd ratios of death were also high in CRC patients infected with SARS-CoV-2 who had chemotherapy (OR 1.35, 95% CI 0.5–0.66; p = 0.023) and surgical resections (OR 1.4, 95% CI 0.8–0.73; p = 0.016); Fig. 6. Other predictors for increased risk of succumbing included admission to intensive care unit (OR 1.88, 95% CI 0.85–1.12; p < 0.001), intubation and placing on mechanical ventilation (OR 0.99, 95% CI 0.87–1.11; p < 0.001), and suffering from acute respiratory distress syndrome (OR 0.63, 95% CI 0.23–1.1; p < 0.001); Fig. 9.
These variables were considered needing further evaluation and, thus, were included in multivariate regression analysis. Nevertheless, multivariate analysis confirmed old age (≥ 60 years), male gender, CRC patients with SARS-CoV-2 infection located in Asia and Europe, who transmitted SARS-CoV-2 from hospital, CRC stage III, who had chemotherapy and surgical resections, admitted to intensive care unit, intubated and placed on mechanical ventilation and suffered acute respiratory distress syndrome were significantly associated with increased death. Although univariate analysis showed CRC stage IV patients with SARS-CoV-2 infection was significantly associated with increased mortality (p = 0.009), however, this finding was not reciprocated by multivariate analysis; Fig. 5.
Discussion
In this large systematic review and meta-analysis, we included 3362 patients with laboratory-confirmed SARS-CoV-2 infection from 69 observational studies in order to estimate the prevalence of COVID-19 disease in CRC patients. A better understanding of the prevalence of SARS-CoV-2 disease in CRC patients allows the development of more specific and more efficient ways of prevention and therapy. As expected, overall prevalence of community-acquired SARS-CoV-2 infection in CRC patients was fivefold higher compared to hospital-acquired SARS-CoV-2 infection in this group of cancer patients (8.1% vs 1.5%). This could be chiefly explained by the maintenance of good knowledge and compliance of infection prevention and control by healthcare providers [87], antimicrobial stewardship [88], and robust surveillance for hospital-acquired infections and antimicrobial resistance [89] within healthcare organizations that provide healthcare for CRC patients. Prevalence of SARS-CoV-2 infection acquired from the community in CRC patients was almost similar in Asia (9.1%, 95% CI 6.1–12.2), Europe (7.7%, 95% CI 3.9–11.4), and America (7.0%, 95% CI 3.2–10.7). However, SARS-CoV-2 infection rate acquired from the hospital in CRC patients was the highest in studies conducted in multiple countries (4.7%, 95% CI 3.6–5.9). In general, there is an approximately ninefold variation in CRC prevalence rates by world regions, with the highest rates in European regions, Australia/New Zealand, and Northern America; and rates of CRC prevalence tend to be low in most regions of Africa and in South Central Asia [90]. However, negative impact of SARS-CoV-2 infection on CRC patients should be considered as the COVID-19 pandemic has led to a sustained reduction in the number of people referred, diagnosed, and treated for CRC [22, 91,92,93]. The findings in this meta-analysis showed different results from previous systematic meta-analyses that evaluated SARS-CoV-2 infection among CRC patients [24, 94]. We reported a much lower prevalence of SARS-CoV-2 infection in CRC patients [3.43%] compared to the previous two systematic meta-analyses [45.1% and 20.5%, respectively] [24, 94]. The current meta-analysis is more comprehensive and included a total of 69 studies [8,9,10,11,12,13,14,15,16,17, 23, 25,26,27,28,29,30,31,32,33,34,35,36, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] including a total of 3362 patients; whose details on final treatment outcome were available; in comparison to smaller sample size in previous meta-analyses (sample size: n = 92 and n = 20, respectively) [24, 94]. The inclusion of 65 recently published studies [8,9,10, 12,13,14,15,16,17, 23, 25,26,27,28,29,30,31,32,33,34,35,36, 38, 43, 44, 46,47,48,49,50,51,52,53,54,55,56, 58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] contributed to the refinement on evidence of the demographic and clinical characteristics; in addition to final treatment outcome in CRC patients with SARS-CoV-2 illness.
We report no paediatric case with SARS-CoV-2 infection and CRC as the incidence of CRC is rare compared with that in adults (prevalence of CRC in patients under age 20 was reported to be 0.2%) [95]. Unlike in adults, familial cancer history is not strongly associated with CRC in children [96]. The lack of childhood cases with COVID-19 and CRC in our review can also be justified by the fact that most children with SARS-CoV-2 disease have mild symptoms or have no symptoms at all [97] and the high severity of COVID-19 tends to be much lower in children compared to adults [98]. However, CRC is more likely to be lethal in children and young adults than middle-aged adults and was explained by the higher incidence of precancerous diseases (such as polyposis, colitis) and mucinous adenocarcinoma and/or late CRC diagnosis in children [95, 96]. Hence CRC is usually diagnosed later and potentially associated with worst prognosis in young groups [95, 96], detecting CRC at an early, more treatable stage is important for cure and survival.
In our review, males gender predominated development of SARS-CoV-2 illness in CRC patients, a finding suggested in most of the reports [9, 11, 14, 16, 17, 25, 27, 28, 30, 32,33,34,35, 47, 52,53,54,55,56,57,58,59, 61, 62, 71, 72, 74, 75, 77, 81,82,83,84,85] and in contradiction with data from other reports suggesting an equal proportion of COVID-19 cases in CRC patients for both genders [14, 23, 29, 31, 60, 80] or patients with CRC and SARS-CoV-2 illness were mostly females [11, 36, 46, 49, 50, 66, 67, 76]. This review reflects previous studies in showing that the overall incidence of CRC is higher in males than in females [99,100,101]. This increased vulnerability of men to developing CRC may be due to a number of biological and gender-related (behavioural) factors [99, 102,103,104]. Men are more likely to have a diet high in red and processed meat [105], be heavier consumers of alcohol [106], and more likely to smoke [107]. Men also have a greater propensity to deposit visceral fat [108, 109] which is associated with increased risk of CRC [99,100,101, 110]. Moreover, SARS-CoV-2 has been known to infect cells via angiotensin-converting enzyme 2 receptors for entry which have been found to be highly expressed in human males and the angiotensin-converting enzyme 2 receptor gene is X-linked [111, 112]. However, male excess in CRC in our review might be attributed mainly to the differences in the inclusion criteria and the population age groups included in the studies; or can be explained by higher rates of comorbidities among men [113, 114], higher trend among females to follow hand hygiene and preventive care [115, 116], stronger immune response to infections in females who outlive men [117] or lower rates of healthcare service utilization by males [118].
We found development of COVID-19 in CRC patients was highest in people of White (Caucasian) [14, 35, 43, 44, 47, 53, 76, 77], Hispanic [13, 70, 72, 80] and Asian ethnicity [11, 15,16,17, 28, 57, 58, 83] (7.8%, 4.6% and 4.5%, respectively). Moreover, we found mortality rate in CRC patients infected with COVID-19 was significantly high in patients with Asian and Hispanic ethnicity [5.1% and 4.2%, p = 0.011]. CRC is a substantial public health burden and it is increasingly affecting populations in Asian and Hispanic countries [119, 120]. The risk of contracting COVID-19 in people with Asian and Hispanic ethnicity is known to be high and clinical prognosis in those people has been previously described to be poor [121, 122]. CRC screening has been playing an important role in reducing its disease burden [123]. The surveillance system in countries with high burden needed to provide facilities for CRC screening and public awareness education program should be considered in national and international planes to increases the self-participation of people [124]. Financial limitation and lack of authorities are still the main obstacles in the way of CRC screening in most Asian and Hispanic countries with low-income status [125, 126]. Because most of the studies included in our review that reported the ethnicity of CRC cases infected with COVID-19 were either from China, Italy, United States of America, or United Kingdom; representation of other ethnicities at risk to develop COVID-19 in CRC patients can be misleading. For instance, we report a very low prevalence of SARS-CoV-2 infection in CRC patients in Black population (n = 4, 0.12%), yet, in the United States, the incidence and mortality rates for CRC are higher among Black patients, particularly men, than among those in other racial or ethnic groups, and, among Black patients, CRC occurs at a higher rate below age 50 years [127].
During the COVID-19 pandemic, increasing age in combination with male gender might denote seriously sick patients who can potentially have more morbidity and propensity to die [128, 129]. The majority of CRC patients hospitalized with SARS-CoV-2 are older and seemed to have underlying medical conditions [11, 27, 29, 31, 33, 35, 47, 57, 59, 60, 62, 72, 77, 85], with increased age being associated with clinical severity, including case fatality. Furthermore, comorbidities [11, 16, 17, 27, 28, 31, 33, 46, 57, 58, 62, 72, 77, 83, 85] and advanced CRC stages (stage III and IV) [26, 27, 29, 31, 33, 35, 36, 46,47,48,49, 60, 72, 77] affect the prognosis of COVID-19. Although chemotherapies and surgical resections are the primary treatment modalities for early stage CRC (stage I through III) [130, 131], we report active treatment of both chemotherapies and surgical resections were associated with an increased risk for severe disease and death from COVID-19 in CRC patients, a finding which is in line with previous meta-analyses [132, 133]. Although one meta-analysis found chemotherapy was associated with an increased risk of death from COVID-19 in patients with cancer but failed to show any significant association between other treatments like surgery due to the very small number of included studies [132], our meta-analysis shown the possible increase in risks of severe COVID-19 and death in SARS-CoV-2-infected CRC patients receiving surgical resections which is in consistent with recent cohort and meta-analysis studies [133,134,135]. Chemotherapies commonly used to treat cancer, including CRC, affect not only the tumor but also the immune system [136]. Advanced COVID-19 syndrome is characterized by the uncontrolled and elevated release of pro-inflammatory cytokines and suppressed immunity, leading to the cytokine storm [137]. An impaired immune system might cause a decreased inflammatory response against SARS-CoV-2 and, thus, protecting from cytokine storm [138]. The uncontrolled and dysregulated secretion of inflammatory and pro-inflammatory cytokines in SARS-CoV-2 patients with CRC is positively associated with the severity of the viral infection and mortality rate and this cascade of events may lead to multiple organ failure, ARDS, or pneumonia and need for ICU admission and mechanical ventilation [137, 139]. Furthermore, postoperative pulmonary complications was reported to occur in half of patients with perioperative SARS-CoV-2 infection and are associated with high mortality [135], therefore, consideration should be given for postponing non-critical procedures and promoting nonoperative treatment in CRC patients to delay or avoid the need for surgery [140]. When hospitals recommence routine surgical treatments, this will be in hospital environments that remain exposed to SARS-CoV-2, so strategies should be developed to reduce in-hospital SARS-CoV-2 transmission and mitigate the risk of postoperative complications in CRC patients [135].
Limitations
First, while most of the evidence discussed were based on many cohorts, case reports, case-series and few cross-sectional and case–control studies, many of these are small and not necessarily generalizable to the current COVID-19 clinical environment in patients with CRC history. Second, to asses factors associated with mortality, larger cohort of patients is needed. Last, almost all studies included in this review were retrospective in design which could have introduced potential reporting bias due to reliance on clinical case records.
Conclusion
Patients with CRC are at increased risk of severe complications from SARS-CoV-2 which may include ARDS, or pneumonia and need for ICU admission and mechanical ventilation. Key determinants that lead to increased mortality in CRC patients infected with COVID-19 include older age (≥ 60 years old); male gender; Asian and Hispanic ethnicity; if SARS-CoV-2 was acquired from hospital source; advanced CRC (stage III and IV); if patient received chemotherapies or surgical treatment; and if patient was admitted to ICU, ventilated or experienced ARDS.
Availability of data and materials
All data generated or analysed during this study are included in this published article except for the datasets generated and analysed to explore the effect of various demographic parameters and clinical variables on patient’s final treatment outcome. These datasets are not publicly available due privacy concern but will be available, please contact the corresponding author for data requests.
Abbreviations
- ARDS::
-
Acute respiratory distress syndrome
- COVID-19::
-
Coronavirus disease 2019
- CRC: :
-
Colorectal cancer
- ICU::
-
Intensive care unit
- NOS::
-
Newcastle–Ottawa scale
- PRISMA::
-
Preferred reporting items for systematic reviews and meta-analyses
- SARS-CoV-2::
-
Severe acute respiratory syndrome coronavirus 2
References
World Health Organization. WHO coronavirus disease (COVID-19) dashboard 2022 [21 July 2022]. Available from: https://covid19.who.int/.
Centers for disease control and prevention. Risk for COVID-19 infection, hospitalization, and death by age group 2022 [21 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
Centers for disease control and prevention. Underlying medical conditions associated with high risk for severe COVID-19: Information for healthcare providers 2022 [21 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html.
Centers for Disease Control and Prevention. Science brief: Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19 2022 [21 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Fclinical-care%2Funderlying-evidence-table.html.
Sorensen RJ, Barber RM, Pigott DM, Carter A, Spencer CN, Ostroff SM, Reiner RC Jr, Abbafati C, Adolph C, Allorant A. Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: A systematic analysis. Lancet. 2022;399(10334):1469–88.
World Health orgnization. International agency for research on cancer. global cancer observatory. colorectal cancer incidence and mortality statistics worldwide and by region 2022 [02 January 2022]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf.
Balzora S, Issaka RB, Anyane-Yeboa A, Gray DM II, May FP. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest Endosc. 2020;92(4):946–50.
Bernard A, Cottenet J, Bonniaud P, Piroth L, Arveux P, Tubert-Bitter P, Quantin C. Comparison of cancer patients to non-cancer patients among covid-19 inpatients at a national level. Cancers. 2021;13(6):1436.
Collaborative C, Glasbey JC, Nepogodiev D, Simoes JF, Omar OM, Venn ML, Evans JP, Futaba K, Knowles CH, Minaya-Bravo A. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis. 2021;23(3):732–49.
Larfors G, Pahnke S, State M, Fredriksson K, Pettersson D. Covid-19 intensive care admissions and mortality among swedish patients with cancer. Acta Oncol. 2021;60(1):32–4.
Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J Infect. 2020;81(2):318.
McCarthy K, Myint PK, Moug S, Pearce L, Braude P, Vilches-Moraga A, Hewitt J, Carter B. Letter to colorectal disease: resumption of elective colorectal surgery during COVID-19 and risk of death. colorectal disease: the official journal of the association of coloproctology of great britain and Ireland. 2020.
Ospina AV, Bruges R, Mantilla W, Triana I, Ramos P, Aruachan S, Quiroga A, Munevar I, Ortiz J, Llinás N. Impact of COVID‐19 Infection on patients with cancer: experience in a latin American Country: The ACHOCC‐19 Study. The Oncologist. 2021.
Özdemir N, Dizdar Ö, Yazıcı O, Aksoy S, Dede DS, Budakoğlu B, Metan G, Alp A, Budakoğlu II, Öksüzoğlu ÖBÇ. Clinical features and outcomes of COVID-19 in patients with solid tumors: Turkish national registry data. Int J Cancer. 2021;148(10):2407–15.
Wang J, Song Q, Chen Y, Wang Z, Chu Q, Gong H, Cai S, Dong X, Xu B, Hu W. Systematic investigations of COVID-19 in 283 cancer patients. medRxiv. 2020.
Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–13.
Liu C, Wang K, Li L, Lv Q, Liu Y, Hu T, Trent JC, Sun B, Hu Q. Severity of COVID-19 in Cancer patients versus patients without cancer: a propensity score matching analysis. J Cancer. 2021;12(12):3558.
Várnai C, Palles C, Arnold R, Curley HM, Purshouse K, Cheng VW, Booth S, Campton NA, Collins GP, Hughes DJ. Mortality among adults with cancer undergoing chemotherapy or immunotherapy and infected with COVID-19. JAMA Netw Open. 2022;5(2):220130.
Leite H, Lindsay C, Kumar M. COVID-19 outbreak: implications on healthcare operations. TQM J. 2020.
Whittaker TM, Abdelrazek ME, Fitzpatrick AJ, Froud JL, Kelly JR, Williamson JS, Williams GL. Delay to elective colorectal cancer surgery and implications for survival: a systematic review and meta‐analysis. Colorectal Disease. 2021.
Sundaram S, Olson S, Sharma P, Rajendra S. A Review of the Impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):1508.
Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2021:https://doi.org/10.1007/s12029-021-00679-x.
Al-Shamsi HO, Coomes EA, Alrawi S. Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 2020;6(10):1627–8.
Antikchi MH, Neamatzadeh H, Ghelmani Y, Jafari-Nedooshan J, Dastgheib SA, Kargar S, Noorishadkam M, Bahrami R, Jarahzadeh MH. The risk and prevalence of COVID-19 infection in colorectal cancer patients: a systematic review and meta-analysis. J Gastrointest Cancer. 2021;52(1):73–9.
Berger JM, Gansterer M, Trutschnig W, Bathke AC, Strassl R, Lamm W, Raderer M, Preusser M, Berghoff AS. SARS-CoV-2 screening in cancer outpatients during the second wave of the COVID-19 pandemic. Wien Klin Wochenschr. 2021;133(17):909–14.
Filipe M, de Bock E, Geitenbeek R, Boerma D, Pronk A, Heikens J, Richir M. Impact of the COVID-19 Pandemic on Surgical Colorectal Cancer Care in the Netherlands: a Multicenter Retrospective Cohort Study. J Gastroint Surg 2021:1–3.
Khan R, Zaidi N, Chituku T, Rao M. Non-COVID fatalities in the COVID era: a paradigm shift in the face of a pandemic-lessons learnt (or not). Annals Med Surg. 2021;70: 102617.
Liu Y-L, Ren J, Yuan J-P, Zhang Z-J, Guo W-Y, Guan Y, Moeckel G, Ahuja N, Fu T. Postoperative onset and detection of sars-cov-2 in surgically resected specimens from gastrointestinal cancer patients with pre/asymptomatic COVID-19. Ann Surg. 2020;272(6): e321.
Mansi L, Spehner L, Daguindau E, Bouiller K, Almotlak H, Stein U, Bouard A, Kim S, Klajer E, Jary M. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer. 2021;150:1–9.
Martínez-Mardones M, Reyes G, Salas R, Fernández R, Melkonian E, Mordojovich E, Silva C, Suazo C. Strategies to advance recovery (STAR) protocol implemented colorectal cancer patients during the COVID-19 pandemic. Rev Med Chil. 2021;149(2):203–9.
Nakamura S, Kanemasa Y, Atsuta Y, Fujiwara S, Tanaka M, Fukushima K, Kobayashi T, Shimoyama T, Omuro Y, Sekiya N. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo. Japan Int J Clinic Oncol. 2021;26(3):485–93.
Ottaiano A, Scala S, D’Alterio C, Trotta A, Bello A, Rea G, Picone C, Santorsola M, Petrillo A, Nasti G. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. Therapeutic Adv Med Oncol. 2021;13:17588359211011456.
Pertile D, Gipponi M, Aprile A, Batistotti P, Ferrari CM, Massobrio A, Soriero D, Epis L, Scabini S. Colorectal cancer surgery during the COVID-19 pandemic: A single center experience. in vivo. 2021;35(2):1299–305.
Quaquarini E, Saltalamacchia G, Presti D, Caldana G, Tibollo V, Malovini A, Palumbo R, Teragni CM, Balletti E, Mollica L. Impact of COVID-19 outbreak on cancer patient care and treatment: data from an outpatient oncology clinic in Lombardy (Italy). Cancers. 2020;12(10):2941.
Tolley T, McGregor H, Clark J, Worwood M, Stephenson B. Reported outcome measures for colorectal cancer patients during the COVID-19 pandemic. Colorectal Disease: the Official Journal of the Association of Coloproctology of Great Britain and Ireland. 2020.
Wu Q, Chu Q, Zhang H, Yang B, He X, Zhong Y, Yuan X, Chua ML, Xie C. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. 2020;40(8):374–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. p. 1–12.
Greenwood D, Slack RC, Barer MR, Irving WL. Medical Microbiology E-Book: A Guide to Microbial Infections: Pathogenesis, Immunity, laboratory diagnosis and control. with student consult online Access: Elsevier Health Sciences; 2012.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Aschele C, Negru ME, Pastorino A, Cavanna L, Zagonel V, Barone-Adesi F, Blasi L. Incidence of SARS-CoV-2 infection among patients undergoing active antitumor treatment in Italy. JAMA Oncol. 2021;7(2):304–6.
Ayhan M, Laçin Ş, Özyükseler DT, Sürmeli H, Doğan A, Turan M, Odabas H, Turan N, Yıldırım ME. Does systemic anti-tumor therapy increase COVID-19 risk in patients with cancer? J Oncol Pharm Pract. 2021:27: 10781552211015762.
Aznab M. Evaluation of COVID 19 infection in 279 cancer patients treated during a 90-day period in 2020 pandemic. Int J Clin Oncol. 2020;25(9):1581–6.
Binet Q, Goffinet C, Etogo-Asse F-E, Shaza L. Nonbacterial thrombotic endocarditis in a patient with gastric cancer and SARS-CoV-2 infection. Clinic J Gastroenterol. 2021:14(4): 1–5.
Calvo V, Fernandez-Cruz A, Nuñez B, Blanco M, Morito A, Martínez M, Traseira C, Garitaonaindía Y, Aguado R, Ramos A. Cancer and SARS-CoV-2 Infection: a third-level hospital experience. Clin Epidemiol. 2021;13:317.
Cosma L, Sollaku S, Frantellizzi V, De Vincentis G. Early 18F-FDG PET/CT in COVID-19. J Med Imaging Radiat Oncol. 2020;64(5):671–3.
Costanzi A, Monteleone M, Confalonieri M, Colletti G, Frattaruolo C, Fingerhut A. Late bowel iischemia and colovaginal fistula after low anterior resection in a COVID-19 patient. Chirurgia (Bucharest Romania: 1990). 2020;115(5):677–80.
Gao J, Yang M, Liu L, Guo S, Li Y, Cheng C. The aggressive surgical treatment and outcome of a colon cancer patient with COVID-19 in Wuhan. China BMC Gastroenterol. 2020;20(1):1–5.
Glasbey JC, Nepogodiev D, Simoes JF, Omar O, Li E, Venn ML, Abou C, Capizzi V, Chaudhry D, Desai A. Elective cancer surgery in COVID-19–free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. 2021.
Haque F, Lillie P, Haque F, Maraveyas A. Deficient DNA mismatch repair and persistence of SARS-CoV-2 RNA shedding: a case report of hereditary nonpolyposis colorectal cancer with COVID-19 infection. BMC Infect Dis. 2021;21(1):1–4.
Joharatnam-Hogan N, Hochhauser D, Shiu K-K, Rush H, Crolley V, Wilson W, Sharma A, Muhammad A, Anwar M, Vasdev N. <? covid19?> Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer: a multi-centre North London experience. Therapeutic Adv Med Oncol. 2020;12:1758835920956803.
Johnson LN, Vesselle H. COVID-19 in an asymptomatic patient undergoing FDG PET/CT. Radiol Case Reports. 2020;15(10):1809–12.
Karam C, Badiani S, Berney CR. COVID-19 collateral damage: delayed presentation of a perforated rectal cancer presenting as Fournier’s gangrene. ANZ J Surg. 2020;90(3):1483.
Kuryba A, Boyle JM, Blake HA, Aggarwal A, Van Der Meulen J, Braun M, Walker K, Fearnhead NS. Surgical treatment and outcomes of colorectal cancer patients during the COVID-19 pandemic: a national population-based study in England. Annals Surg Open. 2021;2(2): e071.
Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7.
Liu C, Wang K, Zhang M, Hu X, Hu T, Liu Y, Hu Q, Wu S, Yue J. High expression of ACE2 and TMPRSS2 and clinical characteristics of COVID-19 in colorectal cancer patients. NPJ precision oncology. 2021;5(1):1–7.
Manlubatan SIT, Lopez MPJ, Maglangit SACA, Ozoa GM. Abdominotransanal resection of a strangulated rectal carcinosarcoma. BMJ Case Reports CP. 2021;14(8): e244501.
Martín-Bravo C, Quirós R, Blancas I, Villatoro-Roldán R, Robles M, Alcaide J, Navarro V, Pérez D, Zarcos I, Rivas-Ruiz F, editors. Incidence of COVID-19 in outpatients with cancer receiving active treatment in the context of a pandemic: An Andalusian cohort study. Seminars in Oncology; 2021: Elsevier.
Martínez ML, Borda FJG, Chavez CN, Lopez AV, Villar OG, Gomez R, Martinez RR, Vigo FdLC, Herrero EF. Impact of the Covid-19 pandemic on colorectal cancer surgery in madrid. J Coloproctol. 2021.
Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–41.
Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088.
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone G, Cavalli A, Pagano F, Ragazzi E. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N= 4532). Ann Oncol. 2020;31(8):1040–5.
Nagarkar R, Roy S, Dhondge R, Adhav A, Manke A, Banswal L, Upwanshi M, Kulkarni N, Tandale R, Bang Y. Elective surgical experience during COVID pandemic at a tertiary cancer care centre in India: a retrospective analysis. Indian J Surg Oncol. 2021;12:1–8.
Pawar T, Pokharkar A, Gori J, Pandey D, Rohila J, Dsouza A, Saklani A. The technique and justification for minimally invasive surgery in COVID-19 pandemic: laparoscopic anterior resection for near obstructed rectal carcinoma. J Laparoendosc Adv Surg Tech. 2020;30(5):485–7.
Pordány B, Herczeg G, Máté M. Colon cancer during the coronavirus pandemic-recovery from COVID-19 pneumonia of an elderly woman with multiple Co-morbidities. Orv Hetil. 2020;161(25):1059–62.
Raj Kumar B, Pandey D, Rohila J, deSouza A, Saklani A. An observational study of the demographic and treatment changes in a tertiary colorectal cancer center during the COVID-19 pandemic. J Surg Oncol. 2020;122(7):1271–5.
Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Ortiz CJF, Glickman MS. Determinants of severity in cancer patients with COVID-19 illness. Nat Med. 2020;26(8):1218.
Ruiz-Garcia E, Peña-Nieves A, Alegria-Baños J, Cornejo-Juarez P, Meneses-García A, Rivera SR, Sánchez JJ, Gerson-Cwilich R, Gerson DS, Franco HM. Prognostic factors in cancer patients infected with SARS-CoV-2: a Latin American country results. Therapeutic Adv Chronic Disease. 2021;12:20406223211047756.
Serrano MM, Pérez-Sánchez JR, Sánchez SP, De La Casa-Fages B, Jimeno VM, Tamayo IP, Grandas F. Serotonin syndrome in two COVID-19 patients treated with lopinavir/ritonavir. J Neurol Sci. 2020;415: 116944.
Sobrado LF, Nahas CSR, Marques CFS. Cotti GCdC, Imperiale AR, Averbach P, Meira JDd, Horvat N, Ribeiro-Júnior U, Cecconello I Is it safe to perform elective colorectal surgical procedures during the COVID-19 pandemic? A single institution experience with 103 patients. Clinics. 2021;76:2507.
Sorrentino L, Guaglio M, Cosimelli M. Elective colorectal cancer surgery at the oncologic hub of Lombardy inside a pandemic COVID-19 area. J Surg Oncol. 2020;122(2):117–9.
Sukumar V, Pandey D, Kumar BR, Patel S, Pawar T, Rohila J, DeSouza A, Saklani A. Colorectal services in Covid-19 times: minimally invasive surgery and enhanced recovery, the need of the hour. Indian J Surg Oncol. 2020;11(2):297–301.
Tateno Y, Harada K, Okamoto F, Katsuragawa H. Elective laparoscopic colectomy in a patient 3 weeks after coronavirus disease 2019 infection: a case report. J Med Case Reports. 2021;15(1):1–4.
Taya M, Paroder V, Redelman-Sidi G, Gangai N, Golia Pernicka JS, Gollub MJ, Javed-Tayyab S, Petkovska I, Bates DD. Abdominal imaging findings on computed tomography in patients acutely infected with SARS-CoV-2: what are the findings? Emerg Radiol. 2021;28:1–10.
Tejedor P, Simo V, Arredondo J, Lopez-Rojo I, Baixauli J, Jimenez LM, Gomez-Ruiz M, Pastor C. The impact Of SARS-CoV-2 infection on the surgical management of colorectal cancer: lessons learned from a multicenter study in Spain. Rev Esp Enferm Dig. 2021;113(2):85–91.
Tolley T, McGregor H, Clark J, Worwood M, Stephenson BM. Colorectal cancer patient reported outcome measures during COVID‐19. Colorectal Disease.
Tuech J-J, Manceau G, Ouaissi M, Denet C, Chau A, Kartheuser A, Desfourneaux V, Duchalais E, Bertrand M, Badic B. Are colorectal cancer patients at risk for COVID-19 infection during the postoperative period? The Covid-GRECCAR study. Int J Colorectal Dis. 2021;36(3):611–5.
Vicente ACR, Marinho MS, Silva PGdS, Molina RO, Manzione TdS, Godoy LGLd, Formiga FB, Bin FC. Cenário das cirurgias colorretais oncológicas eletivas em meio à pandemia de COVID-19. J Coloproctol (Rio de Janeiro). 2021;41:111–6.
Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–7.
Woźniak K, Sachs W, Boguradzki P, Basak GW, Stec R. Chemotherapy during active SARS-CoV2 infection: a case report and review of the literature. Front Oncol. 2021;11:1146.
Yu J, Ouyang W, Chua ML, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan. China JAMA Oncol. 2020;6(7):1108–10.
Ye Z, Hong Y, Wu X, Hong D, Zhang Y, Dong X, Rao Y, Lu X. Management of a colon cancer patient complicated with COVID-19. J Zhejiang Univ (Med Sci). 2020;49(2):245–8.
Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan H, Peng L, Chen Y. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan. China Annals Oncol. 2020;31(7):894–901.
Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92(10):2067–73.
Alhumaid S, Al Mutair A, Al Alawi Z, Alsuliman M, Ahmed GY, Rabaan AA, Al-Tawfiq JA, Al-Omari A. Knowledge of infection prevention and control among healthcare workers and factors influencing compliance: a systematic review. Antimicrob Resist Infect Control. 2021;10(1):1–32.
Al-Omari A, Al Mutair A, Alhumaid S, Salih S, Alanazi A, Albarsan H, Abourayan M, Al SM. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: results of a five-years pre-post analysis. Antimicrob Resist Infect Control. 2020;9(1):1–9.
Alhumaid S, Al Mutair A, Al Alawi Z, Alzahrani AJ, Tobaiqy M, Alresasi AM, Bu-Shehab I, Al-Hadary I, Alhmeed N, Alismail M. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: a 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2021;20(1):1–18.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinic. 2021;71(3):209–49.
Gheorghe A, Maringe C, Spice J, Purushotham A, Chalkidou K, Rachet B, Sullivan R, Aggarwal A. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: a national population-based modelling study in England UK. European J Cancer. 2021;152:253.
Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, Rachet B, Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–34.
Morris EJ, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, Richards M, Spencer K, Emberson J, Hollings S. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol & Hepatol. 2021;6(3):199–208.
Wang B, Huang Y. Which type of cancer patients are more susceptible to the SARS-COX-2: evidence from a meta-analysis and bioinformatics analysis. Crit Rev Oncol Hematol. 2020;153: 103032.
Hayes-Jordan AA, Sandler G, Malakorn S, Xiao L-C, Kopetz S, Rodriquez-Bigas M. Colon cancer in patients under 25 years old: a different disease? J Am Coll Surg. 2020;230(4):648–56.
hoon Ahn C, Kim SC. Two case reports: colorectal adenocarcinoma in children. Medicine. 2017;96(46):8074.
Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–9.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95.
Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Prevent Biomarker. 2009;18(6):1688–94.
Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128(7):1668–75.
White A, Ironmonger L, Steele RJ, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):1–11.
Edgren G, Liang L, Adami H-O, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27(3):187–96.
Cook MB. Excess cancer in men—a call for an increased research focus. Nat Rev Clin Oncol. 2013;10(4):186–8.
White A, Thomson C, Howard T, Shelton J. Excess cancer burden in men. London: Cancer Research; 2013.
Aykan NF. Red meat and colorectal cancer. Oncol Rev. 2015;9(1):5.
Rossi M, Jahanzaib Anwar M, Usman A, Keshavarzian A, Bishehsari F. Colorectal cancer and alcohol consumption—populations to molecules. Cancers. 2018;10(2):38.
Chang L-C, Wu M-S, Tu C-H, Lee Y-C, Shun C-T, Chiu H-M. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc. 2014;79(6):961–9.
Maskarinec G, Harmon BE, Little MA, Ollberding NJ, Kolonel LN, Henderson BE, Le Marchand L, Wilkens LR. Excess body weight and colorectal cancer survival: the multiethnic cohort. Cancer Causes Control. 2015;26(12):1709–18.
Parkin E, O’Reilly DA, Sherlock DJ, Manoharan P, Renehan AG. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev. 2014;15(5):434–51.
Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, Giles GG. Body size, weight change, and risk of colon cancer. Cancer Epidemiol Prevent Biomarker. 2010;19(11):2978–86.
Sakamoto A, Kawakami R, Kawai K, Gianatti A, Pellegrini D, Kutys R, Guo L, Mori M, Cornelissen A, Sato Y. ACE2 (angiotensin-converting enzyme 2) and TMPRSS2 (transmembrane serine protease 2) expression and localization of SARS-CoV-2 infection in the human heart. Arterioscler Thromb Vasc Biol. 2021;41(1):542–4.
Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308–9.
Fang H, Liu Q, Xi M, Xiong D, He J, Luo P, Li Z. Impact of comorbidities on clinical prognosis in 1280 patients with different types of COVID-19. J Investig Med. 2021;69(1):75–85.
Al-Omari A, Alhuqbani WN, Zaidi ARZ, Al-Subaie MF, AlHindi AM, Abogosh AK, Alrasheed AK, Alsharafi AA, Alhuqbani MN, Salih S. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2020;13(11):1639–44.
McCloskey L, Bernstein J, Winter M, Iverson R, Lee-Parritz A. Follow-up of gestational diabetes mellitus in an urban safety net hospital: missed opportunities to launch preventive care for women. J Womens Health. 2014;23(4):327–34.
Laskar AM, Deepashree R, Bhat P, Pottakkat B, Narayan S, Sastry AS, Sneha R. A multimodal intervention to improve hand hygiene compliance in a tertiary care center. Am J Infect Control. 2018;46(7):775–80.
Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. 2017;17(1):21–9.
Robertson LM, Douglas F, Ludbrook A, Reid G, van Teijlingen E. What works with men? A systematic review of health promoting interventions targeting men. BMC Health Serv Res. 2008;8(1):1–9.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91.
Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intestinal research. 2019;17(3):317.
Raharja A, Tamara A, Kok LT. Association between ethnicity and severe COVID-19 disease: a systematic review and meta-analysis. J Racial Ethn Health Disparities. 2021;8(6):1563–72.
Rodriguez-Diaz CE, Guilamo-Ramos V, Mena L, Hall E, Honermann B, Crowley JS, Baral S, Prado GJ, Marzan-Rodriguez M, Beyrer C. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol. 2020;52(46–53): e2.
Altobelli E, Lattanzi A, Paduano R, Varassi G, Di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. 2014;62:132–41.
Pourhoseingholi MA. Epidemiology and burden of colorectal cancer in Asia-Pacific region: what shall we do now. Transl Gastrointest Cancer. 2014;3(4):169–73.
Unger-Saldaña K, Saldaña-Tellez M, Potter MB, Van Loon K, Allen-Leigh B, Lajous M. Barriers and facilitators for colorectal cancer screening in a low-income urban community in Mexico City. Implement Sci Commun. 2020;1(1):1–15.
De Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol. 2016;34(1):6.
American cancer society. Colorectal cancer facts & figures 2017–2019 [10 Jan 2022]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf.
Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. The Lancet. 2020;396(10250):532–3.
Perez-Saez J, Lauer SA, Kaiser L, Regard S, Delaporte E, Guessous I, Stringhini S, Azman AS, Alioucha D, Arm-Vernez I. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva. Switzerland Lancet Infectious Diseases. 2021;21(4):e69–70.
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey J-N, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. European J Cancer. 2020.
Zou C, Huang Y, Ma Y, Yang F, Fu D. Re: A systematic review and meta-analysis: The effect of active cancer treatment on severity of COVID-19: Clinical outcomes of SARS-CoV-2-infected cancer patients undergoing surgery. European J Cancer. 2021.
Collaborative C, Collaborative G, Nepogodiev D, Simoes JF, Li E, Picciochi M, Glasbey JC, Baiocchi G, Blanco-Colino R, Chaudhry D. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76(6):748–58.
Collaborative C. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. 2020; 396(10243): 27–38.
Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, Hubert A, Baniyash M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Can Res. 2014;74(21):6022–35.
Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA, Tirupathi R, Mutair AA, Alhumaid S, Al-Omari A, Dhawan M. Role of inflammatory cytokines in COVID-19 patients: A review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436.
Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O’Mahony L, Gao Y, Nadeau K, Akdis CA. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–81.
Akboga SA, Gokce A, Hatipoglu M, Beyoglu MA, Inan K, Sezen AI, Dal HC, Akkas Y, Turan S, Kocer B. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease. Irish Journal of Medical Science (1971). 2021:1–6.
American college of surgeons. COVID-19: guidance for triage of non-emergent surgical procedures 2020 [11 Jan 2022]. Available from: https://www.facs.org/covid-19/clinical-guidance/triage.
Huang Z, Yan J, Jin T, Huang X, Zeng G, Adashek ML, Wang X, Li J, Zhou D, Wu Z. The challenges of urgent radical sigmoid colorectal cancer resection in a COVID-19 patient: a case report. Int J Surg Case Rep. 2020;71:147–50.
Acknowledgements
We would like to thank authors and their colleagues who contributed to the availability of evidence needed to compile this article. We would also like to thank the reviewers for very helpful and valuable comments and suggestions for improving the paper. We would like to thank Murtadha Alsuliman who created the cartoon.
Funding
None.
Author information
Authors and Affiliations
Contributions
SA, A A M, JS. B, N Al D, and AAl-O contributed equally to the systematic review. S A, A Al M, J S. B, and A A. R were the core team leading the systematic review. S A, A Al M, J S. B, N Al D, I A, and A A identified and selected the studies. H I. Al H, N A. Al A, H A. Al A, H A. A, S A A, and R M A did the quality assessment of the studies. S A, S A. B, A B, N A. A, W A, M Y A, A U. A, H A. Al, M M. A, A N. B, M A, M A. A, T K, J A. Al-T, and K D collected the data. S A, K M. Al m, A H. A, A M T, H A. A, and F M. AL analyzed the data. S A, A Al M, J S. B, N Al D, S A. B, Ali A. R, and A Al-O drafted the manuscript. All authors approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Ethics approval and consent to participate
This review is exempt from ethics approval because we collected and synthesized data from previous clinical studies in which informed consent has already been obtained by the investigators.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alhumaid, S., Al Mutair, A., Busubaih, J.S. et al. Colorectal cancer in patients with SARS-CoV-2: a systematic review and meta-analysis. Infect Agents Cancer 17, 49 (2022). https://doi.org/10.1186/s13027-022-00459-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-022-00459-7