Abstract
Background
Colorectal cancer (CRC) is an illness strongly influenced by sex and gender, with mortality rates in males significantly higher than females. There is still a dearth of understanding on where sex differences exist along the pathway from presentation to survival. The aim of this review is to identify where actions are needed to improve outcomes for both sexes, and to narrow the gap for CRC.
Methods
A cross-sectional review of national data was undertaken to identify sex differences in incidence, screening uptake, route to diagnosis, cancer stage at diagnosis and survival, and their influence in the sex differences in mortality.
Results
Overall incidence is higher in men, with an earlier age distribution, however, important sex differences exist in anatomical site. There were relatively small differences in screening uptake, route to diagnosis, cancer staging at diagnosis and survival. Screening uptake is higher in women under 69 years. Women are more likely to present as emergency cases, with more men diagnosed through screening and two-week-wait. No sex differences are seen in diagnosis for more advanced disease. Overall, age-standardised 5-year survival is similar between the sexes.
Conclusions
As there are minimal sex differences in the data from routes to diagnosis to survival, the higher mortality of colorectal cancer in men appears to be a result of exogenous and/or endogenous factors pre-diagnosis that lead to higher incidence rates. There are however, sex and gender differences that suggest more targeted interventions may facilitate prevention and earlier diagnosis in both men and women.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the UK’s third most common cancer in men (after prostate and lung cancer) and women (after breast and lung cancer) [1] costing the UK approximately £1.6bn [2]. CRC is a disease that has both biological sex differences and socio-cultural gender components [3,4,5,6,7,8,9,10]. Greater awareness of how sex and gender impact on CRC may therefore lead to new insights into how improvements in prevention, early diagnosis, treatment and survival can be made.
More males develop CRC, with age-standardised rates (ASRs) of 86.1 per 100,000 males compared to 56.9 per 100,000 female in the UK in 2014 (which equates to 22,844 male and 18,421 female new cases annually) [11]. CRC mortality rates are also higher in men (ASRs of 33.9 per 100,000 males c.f. 21.8 per 100,000 females) [11]. Mortality rates are significantly higher for males than for females in all age groups from 45 to 49 and over, and the gap is widest at the ages of 70–74, when the male:female age-specific mortality rate ratio is around 1.7:1 [11]. There is also a global trend for men to have both higher incidence (746,298 vs 614,304 [20.6 vs14.3 ASR]) and mortality (373,639 vs 320,294 [10 vs 6.9 ASR]) for CRC [12].
Previous research has reported that sex differences in CRC exist with regard to its type [13], location [5, 14, 15] and survival [16] and in the health behaviour of men and women with regard to lifestyle-related risk factors [17,18,19,20], awareness of risk [21], and screening behaviour [22, 23]. This review however, critically explores sex differences for data across the CRC cancer pathway (screening uptake, the route to diagnosis, cancer staging at diagnosis and survival), and how they might play a part in the sex differences in mortality. These differences are explored alongside a review of the literature on biological awareness and behaviour differences to give direction towards strategies to increase early detection and reduce cancer burden and mortality.
Method
The data used for the review comprised a cross-sectional study of CRC, compiling national data available for the UK (or for England where UK data for all countries combined were not available). Data were sourced from a range of publicly available datasets (or via personal communication where possible where data were not published), and peer reviewed literature. The following metrics and sources were used:
-
CRC incidence rates, by age (2012–2014) and by anatomical site (2010–2012) in the UK [11]
-
Screening uptake and positivity rates of the guaiac-based faecal occult blood test (gFOBT), for 60–74 years olds combined and by five-year age band, in England 2014–2015 [24]
-
Routes to diagnosis data which show the proportion of CRC patients who were diagnosed through the different pathways up to the point of diagnosis, England 2006–2013 [25]
-
Stage at diagnosis data, England 2012 [26]
-
Stage at diagnosis by route to diagnosis 2012–2013 [27]
-
1 and 5-year age-standardised net survival for patients diagnosed in England 2011–2015 [28]
-
Age-standardised net survival by stage at diagnosis: one-year survival for patients diagnosed in England 2012–2014 [29], 5-year survival for patients diagnosed in the former Anglia Cancer Network area 2006–2010 [30]
Where available, 95% confidence intervals (CIs) were used as per the CIs provided by data sources, and examined for overlap to identify statistically significant differences between sexes. Where CIs were not provided (screening uptake/positivity rates and proportions by stage at diagnosis), the two-sample test of proportions was used to identify statistically significant differences between sexes.
Results
Incidence of Colorectal cancer
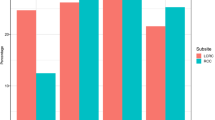
In the UK, 2012–2014 incidence rates in adults aged 45 and over were significantly higher for males than females and this gap was widest at ages 70–74 where the male:female incidence ratio of age-specific rates was 1.7:1 [11] (Fig. 1).
Bowel Cancer (C18-C20): 2012–2014. Average Number of New Cases Per Year and Age-Specific Incidence Rates per 100,000 Population, UK [11] (with permission to publish from CRUK)
A breakdown of CRC incidence by anatomical site (Table 1) shows that the proportions of CRC cases in the rectum and sigmoid colon are higher in males (31.5% and 23.1%, respectively) than females (23.1% and 20.4%, respectively). The proportion of cases in the caecum and ascending colon are higher in females (17.2% and 9.8%, respectively) than males (12.2% and 7.3%, respectively) [11], which are harder to detect and diagnose.
Screening
Overall screening uptake is higher in women than men; for the financial year 2014–15, uptake of gFOBT screening in England (Table 2) was higher amongst women aged 60–74 (60.9%) than men (55.5%) (p < 0.001). By 5-year age group: uptake is higher for women aged 60–64, 65–69 and 70–74 compared with men in the same age bands (all p < 0.001), but this gap appears to narrow with age. Positivity rates (proportion of adequately completed gFOBT tests coming back as ‘positive’/‘abnormal’) are higher in men than women (p < 0.001), with the total positivity rate for men 2.2% and for women 1.5% in the 60–74 age range [24].
Route to diagnosis
There are interesting sex differences demonstrated in the pathway to the point of diagnosis of men and women. For cancers diagnosed in England in 2006–2013, a higher proportion of CRCs in males were diagnosed via the bowel screening program (8.1% of male CRCs c.f. 5.1% of female CRCs) and the “Two Week Wait” (TWW) pathway for urgent GP referrals for suspected cancer (29.0% c.f. 26.6%) (Table 3) [24]. Both these routes are associated with a higher three-year relative survival than average across all routes for CRC.
There was also a sex difference for emergency presentations (EPs), the route associated with the worst survival, with more female CRCs being diagnosed via this route than males (27.6% female CRCs c.f. 22.1% male CRCs). This difference was greatest for patients aged 85+, with 44.8% of female cases aged 85+ diagnosed through EPs compared with 38.5% of males.
Sex differences in stage of cancer at diagnosis
There are small differences in the stage distribution of CRCs at diagnosis between males and females (Table 4) [26]. When excluding cases with an unknown stage from the denominator, data for England in 2012 show a higher proportion of CRCs in males diagnosed at stage I than females (18.2% in males c.f. 16.3% in females; p < 0.001). Conversely, more females than males were diagnosed at stage II (28.7% in females c.f. 27.1% in males; p < 0.001). When stage I and II are combined, there was no longer a difference between males and females. There were no differences between the proportions of males and females diagnosed at stages III and IV, both separately and combined (stage III p = 0.25, stage IV p = 0.08, combined p = 0.65). Staging completeness was higher for males than females (90.0% c.f. 87.7%; p < 0.001).
To explore this further, the routes to diagnosis data have been combined with staging data to see how stage distributions vary by route (Fig. 2) [27]. For patients diagnosed in England in 2012–2013, 32% of all patients diagnosed with CRC via an EP were diagnosed at stage IV, with this proportion varying between the sexes (Fig. 2). More male EPs were diagnosed at stage IV than female (34% males (33–35%) c.f. 30% (29–31%) females) such that even though more females present via an EP, males are more likely to be diagnosed at a late stage via this route. This might explain the finding of no overall difference in stage at diagnosis between the sexes despite higher EP rates in women. However, a higher proportion of female cases had an unknown stage, so difference in stage by route should be interpreted with caution.
Stage at diagnosis by route to diagnosis, adults aged 15–99, England, 2012–2013 [27] (‘Other managed’ includes ‘other outpatient’ and ‘inpatient elective) (with permission to publish from CRUK)
Out of those diagnosed via screening, more males were diagnosed at stage I [36% males (34–37%), 32% (30–34%) females], while females were more likely to be diagnosed at stage II [27% (26–29%), females, 23% (22–25%) males]. There were no other differences in stage distribution between sexes across the other routes analysed.
Survival
Overall, age-standardised one-year net CRC survival is slightly higher for males, whilst five-year net survival is similar for the sexes (Table 5). By age group, one-year net survival for CRC is similar for males and females across all ages, except for the 65–74 and 75–99 year age groups where males have a slight advantage over females (82.7% cf. 81.3% and 67.9% cf. 62.3%). Five-year net survival data are similar between males and females for all ages, except 75–99 (49.0% cf. 46.4%).
One-year age-standardised net survival at stage I and II estimates for patients diagnosed in England in 2014 are similar for males and females (Table 6). Females diagnosed at stage III and IV and unknown stage have worse survival than males.
Five-year age-standardised net survival was similar between males and females for stages I, III and IV (Table 7), but survival at stage II was better for females (87% cf. 82% males) [30].
Discussion
The UK data reflect previous studies [15, 31, 32] in showing that the overall incidence of bowel cancer is higher in males than in females. This increased vulnerability of men to developing CRC may be due to a number of biological and gender-related (behavioural) factors [31, 33,34,35]. Men are more likely to have a diet high in red and processed meat [36], be heavier consumers of alcohol [37], and more likely to smoke [20]. Men also have a greater propensity to deposit visceral fat [38] which is associated with increased risk of CRC [39,40,41].
It is important to note, however, that the female sex is more associated with hypermethylation, microsatellite instability, BRAF V600E mutation, and CpG island methylator phenotype (CIMP)-high, [7, 13], which are more likely to result in the sessile serrated polyps (SSP). These occur in the proximal colon and are more likely to be missed during colonoscopies and lead to more aggressive forms of cancer [14]. Females were also found to have higher frequency of KRAS mutations in codon 12 than males, which again are associated with more advanced adenomas [42].
Socio-economic deprivation (as based on income domain scores) appears to disproportionally affect the incidence rates for men - in England, incidence rates are 13% higher for males living in the most deprived areas compared with the least deprived, while for females the rates are similar for those living in the least and most deprived areas [43]. This may be due to men’s increased likelihood of a lifestyle associated with the risk factors mentioned above in areas of socio-economic deprivation [44, 45].
Screening
A factor relating to men’s relatively lower participation, especially with regard to their higher overall risk of CRC, in screening has been attributed to poorer knowledge about cancer and screening as compared to women, with the 2014 Cancer Awareness Measure (CAM) survey reporting that men were less likely to be aware of the bowel screening programme than women (p < 0.05) [46]. This lack of awareness has been noted elsewhere, both with regard to men’s awareness of screening generally [47, 48] and CRC specifically [10, 49].
Encouraging men to discuss bowel screening with their GP or partner on receipt of an invitation may improve uptake [50] - the role of primary care in nudging both men and women to take up cancer screening opportunities has been noted elsewhere [49, 51,52,53]. Sending enhanced information associated with the national screening programme has been shown to increase uptake of gFOBT in both sexes [54] and an Australian study showed sending men a notification letter prior to faecal immunochemical test (FIT) screening resulted in a 12% increase in uptake compared to those who were not contacted in advance [55].
The UK National Screening Committee has recommended the change from using gFOBT to the less onerous FIT as the primary test for bowel cancer screening in the UK - pilot studies show this improves uptake [22, 56] and reduces the male-female gap in uptake compared to gFOBT [57]. It is imperative that there is a timely roll out of FIT across the UK countries to benefit both men and women as soon as possible, and reduce the sex difference.
The recently introduced bowel scope screening (BSS) in England at the age of 55 overcomes some of the issues associated with faecal sampling, but retains some gender specific issues with regard to its acceptability and uptake. It has been shown that women are less likely than men to take up the opportunity [58, 59], with anticipated greater discomfort, anxiety and embarrassment from the test, and the gender of the practitioner undertaking the test seeming to be particular barriers [60]. However, uptake in women has been shown to be significantly greater compared to men when invited to self-refer (20.7% vs 8.8%; X2-test of independence - P = 0.05) which may have been facilitated by their opportunity to request a same-sex practitioner (requested by 100% of female attendees vs 67% of males P < 0.05) [61], highlighting the importance of screening programmes to acknowledge gender specific barriers.
Sex differences in the effectiveness of screening
Although women are more likely to accept an invitation to screening using gFOBT, they seem to benefit less as the number needed to screen to detect a CRC is higher in women than in men at all ages [62]. In part, this can be explained by the discrepancy in incidence between the sexes at the screening ages, but it is also now well established there are more interval cancers in women, indicating that gFOBT is less sensitive in women than in men [63]. The reason for this latter observation is not clear, but initial work on quantitative FIT for haemoglobin has demonstrated very clearly that women have significantly less haemoglobin in faeces than do men [64, 65]. Thus, if there was an aim to equalise the screening positivity rate in men and women, it would be necessary to set different thresholds for faecal haemoglobin (FHb) concentration. However, the positive predictive value (PPV) for women is less than for men, so that were a lower cut-off for triggering an invitation to colonoscopy used in women, a few more cancers might be detected at screening, but at the cost of many more negative colonoscopies. The balance between benefit and harm would therefore be altered by taking such an approach, and this has not been adequately modelled. In the future however, a risk score based on FIT-derived FHb, but incorporating sex and age, might address this issue.
It is not known why women have lower levels of FHb, and why FHb has a lower PPV for colorectal neoplasia, but it has been demonstrated that FHb concentration is strongly correlated to both all cause and non-colorectal cancer mortality [66], and may be acting as a non-specific marker for health status. This is in keeping with the observations mentioned above, that women tend to have a healthier lifestyle and are less prone to the deposition of visceral fat than men.
Route to diagnosis
Females are typically a decade older when they develop the condition, with a higher risk of co-morbidity [7], which masks symptoms and more negatively affects their survival and may explain why they have higher rates of EP. However, Abel et al. found that even after adjusting for age and deprivation differences, women still had higher rates of EPs for colon and rectal cancer compared with men [67]. This may relate to the sex difference in distribution by anatomical site, with symptom profiles likely to vary for CRC subsites.
It has been noted that men are generally less aware of cancer signs and symptoms [46, 68,69,70,71,72]. The 2014 CAM survey found men recalled and recognised fewer signs and symptoms of cancer than women (p < 0.001), including recognising persistent change in bowel/bladder habits (p < 0.05) [46]. However, although women may have higher levels of awareness it does not mean that they are more likely to have shorter delays from the onset of symptoms until consultation, with the CAM survey showing women reported more barriers to seeing a GP than men (p < 0.001). Delay in women is also evident for other cancers, including breast cancer and gynaecological cancers which have received significantly more publicity and campaign activity than CRC [73,74,75].
Although men are usually less likely to attend preventative health checks and screening than women, there is generally little evidence that they delay when experiencing actual symptoms of ill-health [76,77,78,79]. This also seems to be the case with regard to consultation relating to CRC symptoms and fits with the results presented in this paper. Apart from shorter delays in men seeking medical advice for rectal bleeding [80], no sex differences in consultation have been noted for the most positive predictive symptoms of CRC [81,82,83,84]. These include altered bowel habits with a range of other symptoms (including anaemia, weight loss, abdominal pain, diarrhoea and constipation). The more serious the symptoms, such as vomiting, abdominal pain and the presence of obstruction, the shorter the duration of delay in seeking consultation for both men and women, and the greater likelihood that they would be diagnosed through an emergency admission [67, 84, 85].
Getting partner sanction for seeking help is an important factor in early diagnosis [86]; Esteva et al. [85] and Lobchuk et al. [87] both report that women are more likely to push men to see their GP when they have CRC symptoms, whereas women are more likely to wait to see if the symptoms will clear up themselves. Ramos et al. [88] similarly observed that women were more likely not to have discussed their symptoms with their partner prior to their first consultation, which impacts on their early presentation as they procrastinate over whether or not to report the signs.
Sex differences in stage of cancer at diagnosis
These findings are contrary to those of Nguyen et al. [8], whose systematic review and meta-analysis exploring the implication of sex as a risk factor for advanced neoplasia and colorectal cancer found the pooled relative risk for advanced cancer in men was 1.83 (95% confidence interval, 1.69–1.97). The data from McPhail et al. [26] tends to suggest there is little difference in stage at diagnosis, with more men diagnosed at stage I.
A study by Lyratzopoulos et al. [89], of cancer data covering 2006–2010 from the East of England suggested that although sex was a factor in presenting with more advanced cancer generally, it was not so marked for CRC (including after adjustment for other demographic factors), which is in line with our findings. An analysis of sex disparities in cancer mortality and survival in America also came to the conclusion that though there was a higher death rate in men, this was more closely linked to higher incidence than any other sex, or gender specific factor [35].
Survival
The Eurocare 4 study found that females had a 2.2% point survival advantage over males, especially in their younger years [90], for cancer deaths due to cancer of the bowel and rectum across Europe. However, our data is more in line with the more recent Eurocare 5 data, which shows a negligible difference between the sexes for colon cancer [91].
Strengths and limitations
The major strength of this review is that the data has originated from national population based datasets so data are representative of the population.
However, a limitation is that the data were at an aggregate level and results have not been adjusted for other demographic factors that can vary between males and females, with the exception of age where indicated. Therefore, it is uncertain as to what degree some of the sex differences in the data can be accounted for by sex differences in other demographic factors. For instance, variation in the uptake of screening and CRC outcomes have previously been attributed to ethnicity and the individual’s socio-economic circumstances [92,93,94,95,96]; this was not able to be determined from our data. However, previous studies on screening uptake have demonstrated that sex differences exist even after adjustment for other factors [22, 62, 92].
A further limitation to the literature review relates to the current state of play with regard to research on sex and gender differences, with some studies included being of a smaller scale than would be preferable. However, it was felt that for this review the completeness of the overview of the state of play with regard to sex and gender differences warranted their inclusion.
Conclusion
This is one of the first reviews to start to bring together data on CRC cancer incidence, screening uptake, route to diagnosis, cancer stage and survival specifically from a sex and gender difference perspective. The data show higher incidence rates in males than females. As there are relatively small differences in the data from routes to diagnosis to 5-year survival for males and females, it suggests the higher CRC death rate in males is primarily due to the higher incidence rate. The data show minimal sex differences in survival by stage at diagnosis, which could indicate that there are not significant sex differences in access to and effectiveness of CRC treatment.
Nevertheless, exogenous and/or endogenous sex and gender differences are more apparent for the pathway up to the point of diagnosis of CRC. We highlight that bowel screening uptake is lower in men than women, whilst a higher proportion of CRC cases in women are diagnosed via an EP.
On balance, such differences between men and women mean sex differences in stage distribution are relatively small, but highlight that there could be different approaches focused onto these sex and gendered factors to take to improve early diagnosis across both males and females. Further research could focus on why incidence rates are higher in males and how much the difference is due to modifiable risk factors.
Further understanding of sex differences in CRC could come from analysing data that considers colon and rectal cancers separately, and further breakdowns such as morphology. This may help understand the higher EP rates in females, and how gFOBT/FIT screening is less effective in females than males.
Abbreviations
- ASRs:
-
Age Standardised Rates
- BRAF:
-
v-Raf murine sarcoma viral oncogene homolog B
- BSS:
-
Bowel Scope Screening
- c.f.:
-
Conferatur (Latin) – compare
- CAM:
-
Cancer Awareness Measure
- CI:
-
Confidence interval
- CIMP:
-
CpG island methylator phenotype
- CpG:
-
5’—C—phosphate—G—3
- CRC:
-
Colorectal Cancer
- EPs:
-
Emergency Presentation
- FHb:
-
Faecal haemoglobin
- FIT:
-
Faecal immunochemical test
- gFOBT:
-
guaiac faecal occult blood test
- KRAS:
-
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- PPV:
-
Positive predictive value
- TWW:
-
Two week wait
References
CRUK. Bowel cancer incidence statistics. Cancer Res. UK. 2016. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics
Leal J. Cancer costs the UK economy £15.8bn a year. 2012. Available from: http://www.ox.ac.uk/news/2012-11-07-cancer-costs-uk-economy-£158bn-year
Payne S. Not an equal opportunity disease – a sex and gender-based review of colorectal cancer in men and women: part I. J Men’s Heal Gend. 2007;4:131–9.
Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31.
Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–75.
Christy SM, Mosher CE, Rawl SM. Integrating men’s health and masculinity theories to explain colorectal cancer screening behavior. Am J Mens Health. 2014;8:54–65.
Chacko L, Macaron C, Burke CA. Colorectal cancer screening and prevention in women. Dig Dis Sci. 2015;60:698–710.
Nguyen SP, Bent S, Chen Y-H, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:676–81.e1–3.
Wang Y, Freemantle N, Nazareth I, Hunt K. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: an analysis of routinely collected UK general practice data. PLoS One. 2014;9:e101562. https://doi.org/10.1371/journal.pone.0101562.
Clarke N, Gallagher P, Kearney PM, Mcnamara D, Sharp L. Impact of gender on decisions to participate in faecal immunochemical test-based colorectal cancer screening: a qualitative study. Psycho-Oncology. 2016;25:1456–62.
CRUK. Bowel cancer incidence statistics. 2017. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-One
Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer—global burden, trends, and geographical variations. J Surg Oncol. 2017;115:619–30.
Koo JH, Leong RWL. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33–42.
Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59:A4444.
Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–75.
Kotake K, Asano M, Ozawa H, Kobayashi H, Sugihara K. Gender differences in colorectal cancer survival in Japan. Int J Clin Oncol. 2015;21:194–203.
Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, et al. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Color Dis. 2009;11:689–701.
Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80.
Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109:814–22.
Chang LC, Wu MS, Tu CH, Lee YC, Shun CT, Chiu HM. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc. 2014;79:961–9.
Moffat J, Bentley A, Ironmonger L, Boughey A, Radford G, Duffy S. The impact of national cancer awareness campaigns for bowel and lung cancer symptoms on sociodemographic inequalities in immediate key symptom awareness and GP attendances. Br J Cancer. 2015;112:S14–21.
Moss S, Mathews C, Day T, Smith S, Seaman H, Snowball J, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2016. https://doi.org/10.1136/gutjnl-2015-310691.
Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut. 2015;64:282–91.
PHE. Public Health England personal communication. 2016;
PHE. personal communication, similar data can be found: http://www.ncin.org.uk/publications/routes_to_diagnosis. 2016;
McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl):S108–15.
NCIN/CRUK. Routes to diagnosis of cancer by stage, 2012-2013. 2016. Available from: http://www.ncin.org.uk/view?rid=3071
ONS. Cancer Survival in England: adults diagnosed between 2011 and 2015 and followed up to 2016. London: Office for National Statistics; 2017. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed
ONS/PHE. Cancer survival by stage at diagnosis for England (experimental statistics): Adults diagnosed 2012, 2013 and 2014 and followed up to 2015. 2016. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbystageatdiagnosisforenglandexperimentalstatistics/adultsdiagnosed20122013and2014andfollowedupto2015
PHE. National Cancer Registration and analysis service PHE, Personal communication. 2017.
Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomark Prev. 2009;18:1174–82.
EC. The State of Men’s Health in Europe Report (Extended report). Luxembourg: European Commission; 2011.
CRUK/NCIN. Excess cancer burden in men. London: Cancer Research UK, NCIN, Leeds Met University, Men’s Health Forum; 2013.
Edgren G, Liang L, Adami H-O, Chang E. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27:187–96.
Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomark Prev. 2011;20:1629–37.
Bates B, Cox L, Nicholson S, Page P, Prentice A, Steer T, et al. National Diet and Nutrition Survey: Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013–2013/2014). London: Public Health England; 2016.
Schütze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, et al. Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. BMJ. 2011;342:d1584.
Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404.
Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, et al. Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomark Prev. 2010;19:2978–86.
Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. Nutrition and human health from a sex-gender perspective. Mol Asp Med. 2011;32:1–70.
Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomark Prev. 2007;16:2533–47.
Lorentzen JA, Grzyb K, De Angelis PM, Hoff G, Eide TJ, Andresen PA. Oncogene mutations in colorectal polyps identified in the Norwegian colorectal cancer prevention (NORCCAP) screening study. Clin Med Insights Pathol. 2016;9:19–28.
CRUK/NCIN. Cancer by deprivation in England: Incidence, 1996-2010, Mortality, 1997-2011. London; 2014. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence#heading-Eight
White A, Seims A, Newton R. The state of Men’s health in Leeds: Main report. Leeds: Leeds Beckett University, Leeds City Council; 2016.
Marmot Review Team. Marmot Indicators 2014 A preliminary summary with graphs - strategic review of health inequalities post 2010. London: Institute of Health Equity. p. 2014.
CRUK. Cancer awareness measure (CAM) key findings report; 2014 & trends analysis (2008–2014). London: Cancer Research UK; 2016.
Teo CH, Ng CJ, Booth A, White A. Barriers and facilitators to health screening in men: a systematic review. Soc Sci Med. 2016;165:168–76.
Saab MM, Reidy M, Hegarty J, O’Mahony M, Murphy M, Von Wagner C, et al. Men’s information-seeking behaviour regarding Cancer risk and screening: a meta-narrative systematic review. Psycho-Oncology. 2017;27:410–9.
Tinmouth J, Titvo P, McGregor SE, Claus D, Pasut G, Myers RE, et al. A qualitative evaluation of strategies to increase colorectal cancer screening uptake. Can Fam Physician. 2011;57:7–15.
Wilkins D. Slow on the uptake? Encouraging male participation in the NHS bowel Cancer screening Programme. London: Men’s Health Forum; 2011.
Fox SA, Heritage J, Stockdale SE, Asch SM, Duan N, Reise SP. Cancer screening adherence: does physician-patient communication matter? Patient Educ Couns. 2009;75:178–84.
Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen. 2011;18:24–9.
Hewitson P, Ward AM, Heneghan C, Halloran SP, Mant D. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer. 2011;105:475–80.
White B, Power E, Ciurej M, Lo SH, Nash K, Ormiston-Smith N. Piloting the impact of three interventions on guaiac Faecal occult blood test uptake within the NHS bowel Cancer screening Programme. Biomed Res Int. 2015;2015:928251.
Zajac IT, Duncan AC, Flight I, Wittert G, Cole SR, Young G, et al. Theory-based modifications of an advanced notification letter improves screening for bowel cancer in men: A randomised controlled trial. Soc Sci Med. 2016;165:1–9.
Steele RJ, McDonald PJ, Digby J, Brownlee L, Strachan JA, Libby G, et al. Clinical outcomes using a faecal immunochemical test for haemoglobin as a first-line test in a national programme constrained by colonoscopy capacity. United Eur Gastroenterol J. 2013;1:198–205.
Digby J, McDonald PJ, Strachan JA, Libby G, Steele RJC, Fraser CG. Use of a faecal immunochemical test narrows current gaps in uptake for sex, age and deprivation in a bowel cancer screening programe. J Med Screen. 2013;20:80–5.
Brenner H, Zwink N, Ludwig L, Hoffmeister M. Should screening colonoscopy be offered from age 50? Results from a statewide pilot project, and from a randomized intervention study. Dtsch Arztebl Int. 2017;114:94–100.
McGregor LM, Bonello B, Kerrison RS, Nickerson C, Baio G, Berkman L, et al. Uptake of bowel scope (flexible sigmoidoscopy) screening in the English National Programme: the first 14 months. J Med Screen. 2016;23:77–82.
Wardle J, Miles A, Atkin W. Gender differences in utilization of colorectal cancer screening. J Med Screen. 2005;12:20–7.
Kerrison RS, McGregor LM, Marshall S, Isitt J, Counsell N, Wardle J, et al. Use of a 12 months’ self-referral reminder to facilitate uptake of bowel scope (flexible sigmoidoscopy) screening in previous non-responders: a London-based feasibility study. Br J Cancer. 2016;114:751–8.
Quyn AJ, Fraser CG, Stanners G, Carey FA, Carden C, Shaukat A, et al. Uptake trends in the Scottish bowel screening Programme and the influences of age, sex, and deprivation. J Med Screen. 2018;25:24–31.
Steele RJC, McClements P, Watling C, Libby G, Weller D, Brewster DH, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576–81.
McDonald PJ, Strachan JA, Digby J, Steele RJC, Fraser CG. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clin Chem Lab Med. 2012;50:935–40.
Arana-Arri E, Idigoras I, Uranga B, Pérez R, Irurzun A, Gutiérrez-Ibarluzea I, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: should faecal haemoglobin cut-offs differ by age and sex? BMC Cancer. 2017;17:1–14.
Chen L-S, Yen AM-F, Fraser CG, Chiu SY-H, Fann JC-Y, Wang P-E, et al. Impact of faecal haemoglobin concentration on colorectal cancer mortality and all-cause death. BMJ Open. 2013;3:e003740.
Abel GA, Shelton J, Johnson S, Elliss-Brookes L, Lyratzopoulos G. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br J Cancer. 2015;112(Suppl):S129–36.
Sach TH, Whynes DK. Men and women: beliefs about cancer and about screening. BMC Public Health. 2009;9:431.
Moynihan C, Huddart R. Ignorance and uncertainty regarding cancer and cancer genetics in men. In: Kirby R, Carson C, White A, Kirby M, editors. Men’s health. 3rd ed. London: Informa Healthcare; 2009. p. 17–26.
McCreary DR, Gray RE, Grace SL. Gender differences in cancer mortality risk perceptions and screening behaviors among adults 40-60 years of age. Int J Men’s Heal. 2006;5:53–63.
Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, et al. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101(Suppl):S18–23.
Vart GF. How men differ from women in their attitudes towards bowel cancer screening and intention to be screened. J Men's Health. 2010;7:241–8.
Low EL, Simon AE, Waller J, Wardle J, Menon U. Experience of symptoms indicative of gynaecological cancers in UK women. Br J Cancer. 2013;109:882–7.
Linsell L, Burgess CC, Ramirez AJ. Breast cancer awareness among older women. Br J Cancer. 2008;99:1221–5.
Lo SH, Waller J, Wardle J, von Wagner C. Comparing barriers to colorectal cancer screening with barriers to breast and cervical screening: a population-based survey of screening-age women in Great Britain. J Med Screen. 2013;20:73–9.
Wyke S, Hunt K, Ford G. Gender differences in consulting a general practitioner for common symptoms of minor illness. Soc Sci Med. 1998;46:901–6.
Macintyre S, Ford G, Hunt K. Do women “over-report” morbidity? Men’s and women’s responses to structured prompting on a standard question on long standing illness. Soc Sci Med. 1999;48:89–98.
Farrimond H. Beyond the caveman: rethinking masculinity in relation to men’s help-seeking. Health. 2012;16:208–25.
Wenger LM. Beyond ballistics: expanding our conceptualization of men’s health-related help seeking. Am J Mens Heal. 2011;5:488–99.
Courtney RJ, Paul CL, Sanson-Fisher RW, Macrae FA, Attia J, McEvoy M. Factors associated with consultation behaviour for primary symptoms potentially indicating colorectal cancer: a cross-sectional study on response to symptoms. BMC Gastroenterol. 2012;12:100.
Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93:399–405.
Lawrenson R, Logie J, Marks C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care. 2006;15:267–71.
Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng K, Marshall T. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med. 2009;7:17.
Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98:60–70.
Esteva M, Leiva A, Ramos M, Pita-Fernández S, González-Luján L, Casamitjana M, et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer. 2013;13:87.
Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(Suppl):S92–101.
Lobchuk MM, Bapuji SB, McClement SE, Sisler JJ, Katz A, Martens P, et al. What is the role of family in promoting faecal occult blood test screening? Exploring physician, average-risk individual, and family perceptions. Cancer Epidemiol. 2012;36:e190–9.
Ramos M, Arranz M, Taltavull M, March S, Cabeza E, Esteva M. Factors triggering medical consultation for symptoms of colorectal cancer and perceptions surrounding diagnosis. Eur J Cancer Care. 2010;19:192–9.
Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. 2013;24:843–50.
Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–27.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:23–34.
von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40:712–8.
Ollberding NJ, Nomura AMY, Wilkens LR, Henderson BE, Kolonel LN. Racial/ethnic differences in colorectal cancer risk: the multiethnic cohort study. Int J Cancer. 2011;129:1899–906.
Møller H, Sandin F, Robinson D, Bray F, Klint S, Linklater KM, et al. Colorectal cancer survival in socioeconomic groups in England: variation is mainly in the short term after diagnosis. Eur J Cancer. 2012;48:46–53.
Raine R, Duffy SW, Wardle J, Solmi F, Morris S, Howe R, et al. Impact of general practice endorsement on the social gradient in uptake in bowel cancer screening. Br J Cancer. 2016;114:1–6.
Clarke N, McNamara D, Kearney PM, O’Morain CA, Shearer N, Sharp L. The role of area-level deprivation and gender in participation in population-based faecal immunochemical test (FIT) colorectal cancer screening. Prev Med. 2016;93:198–203.
Acknowledgements
Michael Cook NIH, CIH for helpful feedback on an earlier draft.
Sam Winters (National Cancer Registration and Analysis Service, Public Health England) for the Routes to Diagnosis data breakdown.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article, with hyperlinks to the data where applicable within the references.
Author information
Authors and Affiliations
Contributions
AW conceived the study and conducted majority of literature search and drafting initial text, LI and CC conducted data collection and analysis and critical revision and text of the manuscript; RJCS, NO-S and AS contributed critical revision, comment and text during the development of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Leeds Beckett University approval obtained. No specific permissions were needed to access the data sources.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
White, A., Ironmonger, L., Steele, R.J.C. et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 18, 906 (2018). https://doi.org/10.1186/s12885-018-4786-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4786-7