Abstract
Background
Patients undergoing non-major orthopedic surgery often face an increased risk of venous thromboembolism due to the necessity of immobilization postoperatively. Current guidelines commonly recommend the use of low-molecular-weight heparin (LMWH) for prophylaxis, but it is associated with low patient compliance and certain side effects. We conducted a meta-analysis of randomized controlled trials (RCTs) to assess the effectiveness and safety of rivaroxaban or LMWH for thromboprophylaxis following non-major orthopedic surgery.
Method
Relevant literature was systematically searched in PubMed, Web of Science, Cochrane Library, and Embase from their inception to October 1, 2023, to evaluate the effectiveness and safety of rivaroxaban or LMWH in RCTs for thromboprophylaxis following non-major orthopedic surgery.
Results
A total of 5 randomized controlled trials involving 5,101 patients were included. There was no statistically significant difference in the preventive effect against venous thromboembolism (VTE) when using rivaroxaban or LMWH following non-major orthopedic surgery (RR 0.80; 95%CI 0.31 to 2.07). In terms of safety, there was also no statistically significant difference in the incidence of bleeding events in patients undergoing non-major orthopedic surgery when using rivaroxaban or LMWH (RR 1.15; 95% CI 0.75 to 1.76).
Conclusion
In non-major orthopedic surgery, the risk of venous thromboembolism and bleeding complications is similar when using rivaroxaban or LMWH.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE) is a chronic condition, encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), affecting approximately 10 million people annually [1]. Thromboprophylaxis has always been an important topic in orthopedic surgery [2,3,4,5,6,7]. Therefore, the nineth ACCP guidelines recommend the use of anticoagulants for at least 14 days after major orthopedic surgery to reduce the risk of VTE [8]. Nonmajor orthopedic surgery refers to orthopedic procedures that do not include total hip replacement, total knee replacement, or hip-fracture surgery(9). These surgeries are generally less invasive and lower-risk, mainly involving minor bone or soft tissue repairs, ligament procedures, or arthroscopic operations(9). For non-major orthopedic surgery, thromboprophylaxis was also a noticeable topic. Research has indicated that without thromboprophylaxis following non-major orthopedic surgery, it will lead to about 3% of the risk of VTE [10].
However, there was controversy over the optimism thromboprophylaxis strategy. In European countries, thromboprophylaxis following non-major orthopedic surgery remains a standard care practice. Guidelines recommend the application of individualized low-molecular-weight heparin (LMWH) prophylaxis strategies for patients with one or more thrombotic risk factors and a thrombotic event risk exceeding the risk of bleeding events [11,12,13]. In contrast, guidelines from the United States and other regions, such as ACCP 2012 and the International Consensus Meeting on Venous Thromboembolism, do not recommend the use of thromboprophylaxis for non-major orthopedic surgery [8, 14]. LMWH is a classical thromboprophylactic agent with well-established efficacy but requires subcutaneous injection [15]. Moreover, LMWH use may lead to a decrease in platelet count (< 100 × 10^9/L), known as heparin-induced thrombocytopenia (HIT), which can lead to venous or arterial thrombosis [16]. The above shortcomings could result in poor patient compliance and pose significant challenges for outpatient use. Over the past decade, direct oral anticoagulants (DOACs) such as rivaroxaban have demonstrated superior thromboprophylactic effects. Compared to LMWH, rivaroxaban does not require injections or coagulation monitoring, leading to higher patient compliance and satisfaction [17,18,19,20]. Multiple studies indicate that in major orthopedic surgery, rivaroxaban carries a lower combined risk of symptomatic venous thromboembolism and death for any reason when compared to LMWH [21, 22]. Furthermore, meta-analyses suggest that in major orthopedic surgeries, rivaroxaban outperforms LMWH in preventing DVT following hip and knee arthroplasties [23]. In recent years, a large-scale randomized controlled trial (RCT) with 3604 participants compared the effectiveness of using rivaroxaban or LMWH for thromboprophylaxis following non-major orthopedic surgery [24]. However, this study has not undergone a meta-analysis and did not incorporate relevant guideline.
There has been no meta-analysis comparing the effectiveness of rivaroxaban and LMWH in non-major orthopedic surgery. To better guide clinical practice, we firstly conducted a meta-analysis of randomized controlled trials comparing the efficacy and safety of rivaroxaban and LMWH as thromboprophylactic strategies in non-major orthopedic surgery. Our meta-analysis aims to fill this gap in the literature and provide evidence to support the selection of appropriate anticoagulation strategies following non-major orthopedic surgery.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [25]. The study protocol was registered on PROSPERO (CRD42023469040).
Search strategy and selection criteria
PubMed, Web of Science, Cochrane Library, and Embase were systematically searched for relevant literature from their inception up to October 1, 2023, using the predefined search strategy outlined in the appendix. The search was conducted again when this study was finished on May 1, 2024. There were no language or geographic restrictions. After removing duplicates, four reviewers independently screened the literature based on inclusion and exclusion criteria Inclusion criteria were as follows: (1) Studies comparing rivaroxaban and LMWH treatment groups; (2) Providing data on the number of patients with thromboembolic events and bleeding after follow-up; (3) RCTs. Exclusion criteria were as follows: (1) Studies involving patients undergoing major orthopedic surgery; (2) Inability to extract data; (3) Studies based on animal or computer models. Any disagreement was resolved by discussing with a senior author .
Data extraction
Data extraction was independently performed by four reviewers based on a standardized form, including: (1) Basic characteristics of the included studies (author, publication year, study country, sample size in each group, follow-up duration, etc.); (2) Basic characteristics of the included patients (age, gender ratio, etc.); (3) Efficacy outcome measures (incidence of VTE, DVT, and PE); (4) Safety outcome measures (incidence of bleeding events, including overall bleeding events, major bleeding, and non-major clinically relevant bleeding).
Quality assessment
Two researchers independently assessed the risk of bias of relevant literature using Cochrane’s Risk of Bias tool [26]. The Cochrane Risk of Bias assessment consists of seven domains, which are: (1) Generation of the random sequence; (2) Allocation concealment (whether the study participants were aware of their group assignment); (3) Blinding of researchers and participants (double-blind, single-blind, or unblinded); (4) Blinding of outcome assessment (whether those assessing the study outcomes were blinded); (5) Follow-up bias (completeness of outcome data); (6) Reporting bias (selective reporting of study results); (7) Other bias (potential sources of bias). A standardized data extraction form was used to record the assessment of each domain in the Risk of Bias table as high risk, low risk, or unclear risk of bias. In case of disagreements, resolution was achieved through discussion with a senior author (CGW).
Statistical analysis
The occurrence rates of VTE, DVT, PE, and bleeding events in the included studies were all dichotomous data, which was extracted in the form as an absolute number and patient number. Outcomes were presented as a risk ratio (RR) with 95% confidence interval (CI). The Mantel-Haenszel (M-H) method was used Heterogeneity was quantitatively assessed using I². Considering the existence of substantial heterogeneity from surgery type, thromboprophylaxis strategy, random-effect model was used for synthesis. Sensitivity analysis was conducted using leave-one-out method. Funnel plot was used for assessment of publication bias. Egger’s test was also employed to assess publication bias. The overall quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) criteria. All analyses were performed using Review Manager (version 5.4).
Using Trial Sequential Analysis (TSA) software version 0.9.5.10 beta, we performed a TSA analysis on the outcome VTE and total bleeding to verify the reliability of the meta-analysis results. The required information size (RIS) on the relative calculated effect size for the intervention was calculated considering a type I error of 5% and a power of 80%. If the cumulative Z-curve does not exceed the RIS, further studies are needed.
Results
Literature search
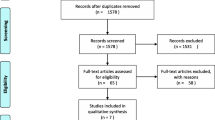
A total of 3,597 potentially relevant references were retrieved. After removing 2,976 duplicates by searching for author names, publication years, titles, etc., and excluding 600 articles based on title and abstract screening, 21 studies underwent full-text review. Subsequently, 16 studies were excluded based on inclusion and exclusion criteria. Ultimately, our final analysis included 5 RCTs that met the criteria [9, 24, 27,28,29] (See Fig 1).
Study characteristics
In the 5 RCTs, the total number of patients included was 5,101, with 2,568 patients in the rivaroxaban treatment group and 2,533 patients in the LMWH treatment group. The follow-up duration ranged from 2 weeks to six months. LMWH used in different studies was various and included Bemiparin [27], Parnaparin [28], and Enoxaparin [9, 24, 29]. The dosage and duration of drug use also varied among different studies. The basic characteristics of these included studies are summarized in Table 1.
Quality assessment
Among the 5 studies, 1 study was assessed as having low risk of bias [9], while the remaining 4 studies had an unclear risk of bias [9, 24, 27,28,29]. The main sources of bias uncertainty were related to unclear methods for generating random sequences, whether allocation concealment was implemented, and whether blinding was applied to participants, trial personnel, and outcome assessors. The distribution of risk of bias was shown in Fig. 2.
The efficacy outcomes
All five RCTs reported the number of individuals who experienced VTE after using either rivaroxaban or LMWH [9, 24, 27,28,29]. The meta-analysis results revealed no statistically significant difference in the preventive effect against VTE following non-major orthopedic surgery between the use of rivaroxaban or LMWH (RR 0.80; 95%CI 0.31 to 2.07; P = 0.05; I² = 52%) (Fig. 3A). The trial sequential analysis with 80% power showed the α-spending adjusted CI was 0.02 to 38.65. Considering the limited information, the required information size was not calculated (eFigure1). Among these, four studies reported the number of individuals who developed DVT [9, 24, 27,28,29], and the pooled effect difference also showed no statistically significant difference (RR 0.63; 95%CI 0.27 to 1.45; P = 0.05; I² = 8%) (Fig. 3B). Subsequently, an analysis was conducted on three studies that reported the number of individuals who experienced PE, with the statistical outcome showing no statistically significant difference between the two groups (RR 0.60; 95%CI 0.07 to 4.90; P = 0.05; I² = 0%)(9, 27, 28) (Fig. 3C).
The safety outcomes
Major bleeding events associated with anticoagulant therapy are mainly classified as major bleeding (lethal, critical, clinically significant bleeding, or bleeding at surgical sites requiring intervention) and non-major clinically relevant bleeding. All five studies [9, 24, 27,28,29] reported the number of total bleeding events. The meta-analysis results indicated no statistically significant difference between rivaroxaban and LMWH in terms of total bleeding events (RR 1.15; 95%CI 0.75 to 1.76; P = 0.05; I² = 0%) (Fig. 4A). The trial sequential analysis with 80% power showed the α-spending adjusted CI was 0.20 to 6.62. The required information size was 74,019 (eFigure2).Among these, three studies [9, 24, 28] demonstrated no statistically significant difference in the preventive effect of rivaroxaban and LMWH on major bleeding events (RR 1.03; 95%CI 0.49 to 2.17; P = 0.05; I² = 0%) (Fig. 4B). The included studies revealed that both rivaroxaban and low-molecular-weight heparin could lead to non-major clinically relevant bleeding, such as epistaxis, gastrointestinal bleeding, and incision hematomas. However, in three studies comparing rivaroxaban and LMWH [9, 27, 28], there was no statistically significant difference in non-major clinically relevant bleeding between rivaroxaban and LMWH (RR 1.27; 95%CI 0.72 to 2.22; P = 0.05; I² = 0%) (Fig. 4C).
Sensitivity analysis
A leave-one-out sensitivity analysis was conducted on outcomes in above outcomes, and no changes were observed in these results.
Publication bias
Funnel plots (Fig. 5) and Egger tests were conducted on the aforementioned outcomes, and no statistically significant evidence of publication bias was found.
GRADE ratings
GRADE ratings for above outcomes were assessed. The quality of evidence ranged from low to moderate (Table 2).
Discussion
This study represents the comparison of the effectiveness and safety of rivaroxaban and LMWH in preventing VTE and bleeding events following non-major orthopedic surgery. Our meta-analysis results indicate that there is no significant difference in the occurrence rates of VTE (including DVT and PE) and bleeding events (including major bleeding and non-major clinically relevant bleeding) when patients undergoing non-major orthopedic surgery use rivaroxaban or LMWH.
LMWH has demonstrated excellent efficacy in preventing thrombosis after orthopedic surgery [29,30,31,32,33]. Guidelines recommend the use of LMWH for the prevention of VTE in patients undergoing non-major orthopedic surgery who have a high VTE risk. For example, in cases where the total anesthesia time for knee arthroscopy exceeds 90 min or when the patient’s risk of developing DVT outweighs the risk of bleeding, LMWH can be used within 6–12 h post-surgery and continued for 14 days. For patients with isolated lower leg injuries requiring immobilization and a higher DVT risk compared to bleeding risk, continuous use of LMWH or fondaparinux for up to 42 days is recommended to prevent thrombosis. ACCP’s evidence-based guidelines also recommend using LMWH as a measure to prevent secondary VTE in cases of acute spinal cord injury [8, 10,11,12,13, 34]. Additionally, the risk of VTE after foot and ankle surgery varies between 0.8% and 23.5% [35,36,37,38,39,40,41]. The clinical practice guidelines of the American Academy of Orthopaedic Surgeons do not provide specific recommendations for VTE prevention after such surgeries [42]. However, the guidelines of the American Orthopaedic Foot & Ankle Society suggest that the use of LMWH significantly reduces the incidence of VTE in patients undergoing foot and ankle surgery with specific risk factors and may also reduce the incidence of postthrombotic syndrome (PTS) [43, 44]. Apart from LMWH, various anticoagulants can be used for VTE prevention in non-major orthopedic surgery patients. However, there is limited high-evidence clinical research available, and the recommendations in the guidelines are relatively weak. Therefore, the choice of which medication to use for VTE prevention remains a subject for further exploration.
Multiple clinical trials and systematic reviews have confirmed that the efficacy of rivaroxaban in preventing VTE after major orthopedic surgery is significantly superior to LMWH [21, 41, 45, 46]. Rivaroxaban is a novel oral anticoagulant that inhibits thrombin and thrombus formation by affecting the activity of coagulation factor Xa [47]. Rivaroxaban can be administered orally, and it has stable dosing and good pharmacokinetics. The drug concentration in the patient’s body can be well-controlled without the need for monitoring, making it a more convenient option for clinical use [48]. While drugs like LMWH take effect immediately after injection, they require subcutaneous administration by a nurse, which may result in complications such as subcutaneous bruising or nodules [15]. From an economic perspective, oral anticoagulants are more cost-effective than LMWH [49] and can effectively reduce the financial burden on patients who require long-term anticoagulation.
In a large RCT that included 3604 participants, Samama et al. [9] found that after non-major orthopedic surgery, patients using rivaroxaban had a lower incidence of major venous thromboembolism compared to patients using LMWH (RR 0.25; 95%CI 0.09 to 0.75; P = 0.01). Previous RCT [50] had also indicated that after major orthopedic surgery, patients using rivaroxaban experienced lower rates of DVT, PE, and mortality compared to those using LMWH. In our meta-analysis, the use of rivaroxaban or LMWH after non-major orthopedic surgery showed comparable effectiveness in preventing VTE, DVT, and PE. This similarity may be related to the limited number of studies included, and the wide confidence intervals, along with an I²value exceeding 50%, which suggests significant heterogeneity among the included studies. Therefore, caution should be exercised when interpreting our results. We also conducted an analysis of the safety of these two types of anticoagulants after non-major orthopedic surgery. The results indicated that the incidence of various bleeding events with rivaroxaban or LMWH was similar. However, previous studies [50, 51] have reported different results, suggesting that rivaroxaban is more likely to lead to major bleeding events such as gastrointestinal bleeding, retinal bleeding, or intracranial bleeding compared to LMWH. This discrepancy may be attributed to variations in the definitions and practices related to bleeding events across different studies, as well as differences in patient characteristics and the types of surgeries performed.
Our study compared the thromboprophylactic effects of rivaroxaban and low-molecular-weight heparin (LMWH) following nonmajor orthopedic surgery. Our findings indicate that rivaroxaban and LMWH have comparable efficacy in thromboprophylaxis, and their bleeding risks are similar. Therefore, our results suggest that both rivaroxaban and LMWH are equally effective for patients undergoing nonmajor orthopedic surgery and can be used interchangeably in clinical practice. Physicians may choose between the two based on the cost-effectiveness and convenience of administration of each medication. In 2022, Douillet et al. conducted a network meta-analysis on the prevention of venous thromboembolism (VTE) in patients with lower leg immobilization after trauma [52]. Their study found a favorable benefit/risk ratio for thromboprophylaxis in these patients, with rivaroxaban having the highest level of evidence. Our study also demonstrates the excellent efficacy of rivaroxaban in preventing VTE following non-major orthopedic surgery. Unlike their study, our research not only included patients with lower leg immobilization after trauma but also other non-major orthopedic surgeries such as knee arthroscopy and lumbar spine surgery. Additionally, in contrast to their study, we only included high-quality randomized controlled trials, excluding observational studies. In summary, based on the current evidence, the incidence of lower limb venous thrombosis following nonmajor orthopedic surgery is not as high as that observed after major orthopedic surgery. Therefore, for patients requiring prophylactic anticoagulation, both rivaroxaban and low-molecular-weight heparin (LMWH) can be considered appropriate treatment strategies.
This study has several limitations: (1) The quality of the included studies was not consistently high, as many studies did not mention the implementation of blinding methods. LMWH requires subcutaneous injection to take effect, whereas rivaroxaban is an orally administered anticoagulant. Implementing blinding procedures for treatment measures can be challenging, potentially leading to unclear bias risk. (2) In this study, the dosages and durations of rivaroxaban and LMWH varied, and the specific LMWH drugs used also differed, which may contribute to the heterogeneity observed. (3) Some articles had unclear or missing data when included. (4) When comparing the effectiveness of rivaroxaban and LMWH in preventing PE after non-major orthopedic surgery, only three RCTs were included, and these studies exhibited some heterogeneity, resulting in wider confidence intervals for the final risk ratio (RR) values. (5) The study by Samama et al., as the most important one, was terminated early due to recruitment issues, and most events were asymptomatic or distal deep vein thrombosis (DVT), with a low overall event rate. There is still debate in current guidelines regarding the risk of isolated distal DVT and whether treatment is necessary. Most studies use lower limb DVT as the outcome without further analysis of proximal and isolated distal thrombosis. Therefore, in our study, using lower limb DVT as the outcome does not allow us to further explore the effectiveness of the two anticoagulation strategies in preventing different types of thrombosis. Therefore, caution should be exercised when interpreting the results of this meta-analysis. (6) The results of the trial sequential analysis indicate that the current studies fall significantly short of the required information size, and the number of included studies is relatively small. Therefore, more research is needed in the future to further explore this topic.
Conclusion
In non-major orthopedic surgery, rivaroxaban showed comparable efficacy of thromboprophylaxis compared to LMWH. Also, rivaroxaban and LMWH showed comparable bleeding risks. More high-quality research is needed in this field for further exploration.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- LMWH:
-
Low-molecular-weight heparin
- VTE:
-
Venous thromboembolism
- DVT:
-
Deep vein thrombosis
- PE:
-
Pulmonary embolism
References
Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet (London England). 2021;398(10294):64–77.
An VV, Phan K, Levy YD, Bruce WJ. Aspirin as Thromboprophylaxis in hip and knee arthroplasty: a systematic review and Meta-analysis. J Arthroplast. 2016;31(11):2608–16.
Anderson DR, Dunbar M, Murnaghan J, Kahn SR, Gross P, Forsythe M, et al. Aspirin or Rivaroxaban for VTE Prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378(8):699–707.
Longo UG, Maffulli N, Denaro V. Rivaroxaban versus enoxaparin after total knee arthroplasty. Lancet (London England). 2009;374(9691):681–2. author reply 3.
Migliorini F, Maffulli N, Eschweiler J, Knobe M, Tingart M, Betsch M. Tourniquet use during knee arthroplasty: a bayesian network meta-analysis on pain, function, and thromboembolism. Surgeon: J Royal Colleges Surg Edinb Irel. 2022;20(4):241–51.
Maffulli N, Aicale R. Proximal femoral fractures in the Elderly: a few things to know, and some to forget. Med (Kaunas Lithuania). 2022;58(10).
Migliorini F, Maffulli N, Velaj E, Bell A, Kämmer D, Hildebrand F, et al. Antithrombotic prophylaxis following total hip arthroplasty: a level I bayesian network meta-analysis. J Orthop Traumatology: Official J Italian Soc Orthop Traumatol. 2024;25(1):1.
Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):eS278–325.
Samama CM, Laporte S, Rosencher N, Girard P, Llau J, Mouret P, et al. Rivaroxaban or Enoxaparin in Nonmajor Orthopedic surgery. N Engl J Med. 2020;382(20):1916–25.
Chapelle C, Rosencher N, Jacques Zufferey P, Mismetti P, Cucherat M, Laporte S. Prevention of venous thromboembolic events with low-molecular-weight heparin in the non-major orthopaedic setting: meta-analysis of randomized controlled trials. Arthroscopy: J Arthroscopic Relat Surg : Official Publication Arthrosc Association North Am Int Arthrosc Association. 2014;30(8):987–96.
Samama CM, Gafsou B, Jeandel T, Laporte S, Steib A, Marret E, et al. [French Society of Anaesthesia and Intensive Care. Guidelines on perioperative venous thromboembolism prophylaxis. Update 2011. Short text]. Annales francaises d’anesthesie. et de Reanimation. 2011;30(12):947–51.
National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. Copyright © NICE 2020. London: National Institute for Health and Care Excellence (NICE); 2019.
Afshari A, Ageno W, Ahmed A, Duranteau J, Faraoni D, Kozek-Langenecker S, et al. European guidelines on perioperative venous thromboembolism prophylaxis: executive summary. Eur J Anaesthesiol. 2018;35(2):77–83.
Swiontkowski M, Parvizi J. International Consensus Meeting on venous thromboembolism. J bone Joint Surg Am Volume. 2022;104(Suppl 1):1–3.
Lyman GH, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2015;11(3):e442–4.
O’Donnell J. Anticoagulants: therapeutics, risks, and toxicity–special emphasis on heparin-induced thrombocytopenia (HIT). J Pharm Pract. 2012;25(1):22–9.
Haac BE, O’Hara NN, Mullins CD, Stein DM, Manson TT, Johal H, et al. Patient preferences for venous thromboembolism prophylaxis after injury: a discrete choice experiment. BMJ open. 2017;7(8):e016676.
Wilke T. Patient preferences for an oral anticoagulant after major orthopedic surgery: results of a German survey. Patient. 2009;2(1):39–49.
Wilke T, Moock J, Müller S, Pfannkuche M, Kurth A. Nonadherence in outpatient thrombosis prophylaxis with low molecular weight heparins after major orthopaedic surgery. Clin Orthop Relat Res. 2010;468(9):2437–53.
Gao Y, Long A, Xie Z, Meng Y, Tan J, Lv H, et al. The compliance of thromboprophylaxis affects the risk of venous thromboembolism in patients undergoing hip fracture surgery. SpringerPlus. 2016;5(1):1362.
Beyer-Westendorf J, Lützner J, Donath L, Tittl L, Knoth H, Radke OC, et al. Efficacy and safety of thromboprophylaxis with low-molecular-weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO-TEP registry. Thromb Haemost. 2013;109(1):154–63.
Turpie AG, Lassen MR, Eriksson BI, Gent M, Berkowitz SD, Misselwitz F, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105(3):444–53.
Russell RD, Huo MH. Apixaban and rivaroxaban decrease deep venous thrombosis but not other complications after total hip and total knee arthroplasty. J Arthroplast. 2013;28(9):1477–81.
Shafiei M, Sabouri M, Aminmansour B, Rezvani M, Mahmoodkhani M, Rahmani P, et al. Comparison between rivaroxaban versus enoxaparin for venous thromboembolism prophylaxis following spine surgeries, a randomized clinical trial. J Clin Neuroscience: Official J Neurosurgical Soc Australasia. 2022;105:51–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (London England). 2021;88:105906.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2011;343:d5928.
Muñoa L, González AB, Díaz de Rada P, Valentí A, Valentí JR. Rivaroxaban is as efficient and safe as bemiparin as thromboprophylaxis in knee arthroscopy. Musculoskelet Surg. 2014;98(1):21–5.
Du W, Zhao C, Wang J, Liu J, Shen B, Zheng Y. Comparison of rivaroxaban and parnaparin for preventing venous thromboembolism after lumbar spine surgery. J Orthop Surg Res. 2015;10:78.
John MP 2nd, Streufert BD, Downes K, Chase CB, Mir HR. A prospective randomized controlled trial comparing enoxaparin & rivaroxaban for venous thromboembolism Prophylaxis in Orthopaedic Trauma. J Orthop Trauma. 2022;36(12):615–22.
Lui A, Park C, Chryssikos T, Radabaugh H, Patel A, Aabedi AA, et al. Safety and comparative efficacy of initiating low-molecular-weight heparin within 24 hours of injury or surgery for venous thromboembolism prophylaxis in patients with spinal cord injury: a prospective TRACK-SCI registry study. NeuroSurg Focus. 2023;55(4):E17.
Argandykov D, Proaño-Zamudio JA, Lagazzi E, Rafaqat W, Abiad M, Renne AM, et al. Low-molecular-weight heparin is superior to unfractionated heparin in lowering the risk of venous thromboembolism after traumatic lower extremity amputation. Surgery. 2023;174(4):1026–33.
Song YX, Li X, Nie SD, Hu ZX, Zhou D, Sun DY, et al. Extracellular vesicles released by glioma cells are decorated by Annexin A2 allowing for cellular uptake via heparan sulfate. Cancer Gene Ther. 2023;30(8):1156–66.
Qing L, Luo G, Li X, Wu P, Tang J. Individualized design of thoracodorsal artery perforator chimeric flap for customized reconstruction of complex three-dimensional defects in the extremities. J Orthop Surg Res. 2023;18(1):367.
Wang B, Xiang J, He B, Tan S, Zhou W. Enhancing bioavailability of natural extracts for nutritional applications through dry powder inhalers (DPI) spray drying: technological advancements and future directions. Front Nutr. 2023;10:1190912.
Makhdom AM, Cota A, Saran N, Chaytor R. Incidence of symptomatic deep venous thrombosis after Achilles tendon rupture. J foot Ankle Surgery: Official Publication Am Coll Foot Ankle Surg. 2013;52(5):584–7.
Calder JD, Freeman R, Domeij-Arverud E, van Dijk CN, Ackermann PW. Meta-analysis and suggested guidelines for prevention of venous thromboembolism (VTE) in foot and ankle surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1409–20.
Ahmad J, Lynch MK, Maltenfort M. Incidence and risk factors of venous thromboembolism after orthopaedic foot and ankle surgery. Foot Ankle Spec. 2017;10(5):449–54.
Huntley SR, Abyar E, Lehtonen EJ, Patel HA, Naranje S, Shah A. Incidence of and risk factors for venous thromboembolism after foot and ankle surgery. Foot Ankle Spec. 2019;12(3):218–27.
Pedersen MH, Wahlsten LR, Grønborg H, Gislason GH, Petersen MM, Bonde AN. Symptomatic venous thromboembolism after Achilles Tendon rupture: a nationwide Danish cohort study of 28,546 patients with Achilles Tendon rupture. Am J Sports Med. 2019;47(13):3229–37.
Richey JM, Ritterman Weintraub ML, Schuberth JM. Incidence and risk factors of symptomatic venous thromboembolism following foot and ankle surgery. Foot Ankle Int. 2019;40(1):98–104.
Chen CX, Zhu J, Zeng Z. Use of Ultrasound to Observe Mycosis fungoides: a Case Report and Review of Literature. Curr Med Imaging. 2022;18(7):771–5.
Jacobs JJ, Mont MA, Bozic KJ, Della Valle CJ, Goodman SB, Lewis CG, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J bone Joint Surg Am Volume. 2012;94(8):746–7.
Fleischer AE, Abicht BP, Baker JR, Boffeli TJ, Jupiter DC, Schade VL. American College of Foot and Ankle surgeons’ clinical consensus statement: risk, prevention, and diagnosis of venous thromboembolism disease in foot and ankle surgery and injuries requiring immobilization. J foot Ankle Surgery: Official Publication Am Coll Foot Ankle Surg. 2015;54(3):497–507.
Meng J, Liu W, Wu Y, Xiao Y, Tang H, Gao S. Is it necessary to wear compression stockings and how long should they be worn for preventing post thrombotic syndrome? A meta-analysis of randomized controlled trials. Thromb Res. 2023;225:79–86.
Huang HF, Li SS, Yang XT, Xie Q, Tian XB. Rivaroxaban versus enoxaparin for the prevention of venous thromboembolism after total knee arthroplasty: a meta-analysis. Medicine. 2018;97(48):e13465.
Zeng W, Yu L, Wu J, Wang F, Liu X, Ren S, et al. Clinical characteristics and long-term follow-up outcomes of myelin oligodendrocyte glycoprotein antibody-associated disease in Han Chinese participants. Medicine. 2023;102(40):e35391.
Ray WA, Chung CP, Stein CM, Smalley W, Zimmerman E, Dupont WD, et al. Association of Rivaroxaban vs Apixaban with Major ischemic or hemorrhagic events in patients with Atrial Fibrillation. JAMA. 2021;326(23):2395–404.
Houghton DE, Lekah A, Macedo TA, Hodge D, Saadiq RA, Little Y, et al. Resolution of acute lower extremity deep vein thrombosis with rivaroxaban compared to warfarin. J Thromb Thrombolysis. 2020;49(2):199–205.
Gulati S, Eckman MH. Anticoagulant therapy for Cancer-Associated thrombosis: a cost-effectiveness analysis. Ann Intern Med. 2023;176(1):1–9.
Beyer-Westendorf J, Förster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood. 2014;124(6):955–62.
Ning GZ, Kan SL, Chen LX, Shangguan L, Feng SQ, Zhou Y. Rivaroxaban for Thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. 2016;6:23726.
Douillet D, Chapelle C, Ollier E, Mismetti P, Roy PM, Laporte S. Prevention of venous thromboembolic events in patients with lower leg immobilization after trauma: systematic review and network meta-analysis with meta-epsidemiological approach. PLoS Med. 2022;19(7):e1004059.
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
The work was supported by the Hunan Provincial Education Commission Foundation (20A056,22C0669,23A0664); The Hunan Provincial Health Commission Foundation (No.202112041226, D202302088596); the Innovation and Entrepreneurship Education Base of Public Health and Preventive Medicine (Hunan Education Bureau Notice 2019 No.333 − 93); and the Funding by young backbone teachers of Hunan province training program foundation of Changsha Medical University (Hunan Education Bureau Notice 2021 No.29 − 26).
Author information
Authors and Affiliations
Contributions
LMZ, PPB, MXQ and BSH: proposed the design, searched the literature, collected, analysed and interpret the data, and wrote the report; LMZ, BHZ, SQL, and JST searched and collected the literature; LMZ, BHZ, SQL, JST and BSH analysed and interpreted the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, L., Zhu, B., Bing, P. et al. Effectiveness and safety of rivaroxaban or low-molecular-weight heparin in non-major orthopedic surgery: a meta-analysis of randomized controlled trials. J Orthop Surg Res 19, 609 (2024). https://doi.org/10.1186/s13018-024-05087-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-05087-y