Abstract
Background
Several clinical investigations have compared different pharmacologic agents for the prophylaxis of venous thromboembolism (VTE). However, no consensus has been reached. The present investigation compared enoxaparin, fondaparinux, aspirin and non-vitamin K antagonist oral anticoagulants (NOACs) commonly used as prophylaxis following total hip arthroplasty (THA). A Bayesian network meta-analysis was performed, setting as outcomes of interest the rate of deep venous thrombosis (DVT), pulmonary embolism (PE) and major and minor haemorrhages.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting systematic reviews incorporating network meta-analyses of healthcare interventions. All randomised controlled trials (RCTs) comparing two or more drugs used for the prophylaxis of VTE following THA were accessed. PubMed, Web of Science and Google Scholar databases were accessed in March 2023 with no time constraint.
Results
Data from 31,705 patients were extracted. Of these, 62% (19,824) were women, with age, sex ratio, and body mass index (BMI) being comparable at baseline. Apixaban 5 mg, fondaparinux, and rivaroxaban 60 mg were the most effective in reducing the rate of DVT. Dabigatran 220 mg, apixaban 5 mg, and aspirin 100 mg were the most effective in reducing the rate of PE. Apixaban 5 mg, ximelagatran 2 mg and aspirin 100 mg were associated with the lowest rate of major haemorrhages, while rivaroxaban 2.5 mg, apixaban 5 mg and enoxaparin 40 mg were associated with the lowest rate of minor haemorrhages.
Conclusion
Administration of apixaban 5 mg demonstrated the best balance between VTE prevention and haemorrhage control following THA.
Level of evidence Level I, network meta-analysis of RCTs.
Similar content being viewed by others
Introduction
Hip osteoarthritis is a common cause of pain and disability [1,2,3,4]. In patients with advanced osteoarthritis, total hip arthroplasty (THA) is commonly recommended [3, 5,6,7]. THA demonstrated very good outcomes, improving patient quality of life and participation in recreational activities [8,9,10,11,12,13,14]. Although prophylaxis is recommended in all patients, symptomatic venous thromboembolism (VTE) occurs in approximately 2% of patients following lower limb arthroplasty [15,16,17,18]. In most patients, VTE may present clinically as deep vein thrombosis (DVT) or pulmonary embolism (PE) [19, 20], both of which are associated with an increased risk of disability and mortality [21, 22]. Previous VTE, varicosities, congestive heart failure, older age, female sex, higher BMI, bilateral surgery, history of prior VTE, surgical time greater than 3.5 h, factor V Leiden, antithrombin and prothrombin gene mutation, oestrogen replacement therapy, traumas and autoimmune disease such as anti-phospholipid syndrome are risk factors for VTE [23,24,25,26,27,28,29,30,31].
A 4–6 weeks, prophylaxis is recommended to reduce the risk of VTE following primary THA [32,33,34,35]. Furthermore, VTE prophylaxis increases the risk of postoperative haemorrhage [36]. Several clinical investigations have compared different types of prophylaxis and protocols for the prevention of VTE [16, 37,38,39,40,41,42,43,44,45,46,47,48,49]; however, no consensus has been reached. In a previous Bayesian network meta-analysis including 35 RCTs (53,787 patients), all anticoagulant drugs showed some effectiveness for VTE prophylaxis in total knee and hip arthroplasty, with fondaparinux and rivaroxaban being the most effective [50]. Cohen et al. [51] conducted a meta-analysis on 43 RCTs to assess the efficacy and safety of apixaban versus other anticoagulants in total knee and hip arthroplasty; apixaban, rivaroxaban, and dabigatran demonstrated similar or improved efficacy and similar safety [51].
A Bayesian network meta-analysis was conducted to compare the rate of DVT, PE and major and minor haemorrhages to identify the optimal compound as prophylaxis following THA. The compounds of interest were enoxaparin, fondaparinux, aspirin and non-vitamin K antagonist oral anticoagulants (NOACs).
Methods
Eligibility criteria
All randomised controlled trials (RCT) comparing two or more pharmacological modalities of prophylaxis of VTE following THA were accessed. Given the author language capabilities, studies in English, German, Italian, French, and Spanish were considered. Only RCTs with level I evidence, according to the Oxford Centre of Evidence-Based Medicine [52], were considered. Opinions, reviews, editorials, posters, abstracts, comments, and letters were excluded, as were animal, in vitro, and computational investigations. Studies evaluating arthroplasty in other locations were not considered. Studies which reported data on primary THA and/or revision setting in elective and/or emergency (e.g. following femoral neck fracture) surgery were considered. For studies which evaluated total joint arthroplasty in more areas, only the data from THA were collected. Studies evaluating patients who had undergone experimental surgeries or physiotherapeutic protocols were not considered. Missing quantitative data under the outcomes of interests warranted the exclusion of the study.
Search strategy
This study was conducted according to the 2015 PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions [53]. The Problem, Intervention, Comparison, Outcomes, Design (PICOD) algorithm was followed:
-
P (Problem): VTE in THA;
-
I (Intervention): Pharmacological prophylaxis;
-
C (Comparison): Apixaban, aspirin, dabigatran, edoxaban, enoxaparin, fondaparinux, rivaroxaban, ximelagatran;
-
O (Outcomes): DVT, PE, major and minor haemorrhages.
-
D (Design): Randomised controlled trial.
PubMed, Web of Science, and Google Scholar databases were accessed in May 2023 with no time constraint. The following keywords were used in each database for the search using the Boolean operator AND/OR: hip AND arthroplasty OR replacement AND prophylaxis OR prevention AND thrombosis OR thromboembolism OR pulmonary embolism OR deep vein thrombosis OR embolism OR bleeding OR haemorrhages AND rivaroxaban OR aspirin OR enoxaparin OR anticoagulant OR dabigatran OR edoxaban OR apixaban OR direct thrombin inhibitor OR fondaparinux OR NOACs OR non-vitamin K antagonist oral anticoagulants. The search was restricted to RCTs.
Data collection and extraction
Two authors (J.E. and E.V.) performed the data selection and collection. The resulting titles from the database searches were screened by hand. If the title matched the topic, the abstract was accessed. If the abstract matched the topic, the full manuscript was accessed. The bibliography of the full-text articles was also screened by hand. All resulting full texts of the articles of interest were downloaded. Both authors compared the articles resulting from the search and controversies were settled by a third author (F.M.). Data extraction was performed by a single author (E.V). in Microsoft Office Excel version 16.71 (Microsoft Corporation, Redmond, USA). Data concerning the following generalities of the included studies were extracted: name of the first author, year and journal of publication, and length of the follow-up (months). Moreover, the following patient demographics were retrieved: mean age, sex ratio, and mean BMI (kg/m2). Data concerning the following drugs were extracted: apixaban, aspirin, dabigatran, edoxaban, enoxaparin, fondaparinux, rivaroxaban and ximelagatran. With respect to the outcomes of interest, the following parameters were collected at the last follow-up: DVT, PE and major and minor haemorrhages.
Assessment of the risk of bias and quality of the recommendations
The risk of bias was evaluated in accordance with the guidelines highlighted in the Cochrane Handbook for Systematic Reviews of Interventions [54]. The risk of bias of the software Review Manager 5.3 (The Nordic Cochrane Collaboration, Copenhagen) was used. The risk of bias evaluation was conducted by two authors (J.E. and E.V.) separately. The following risks of biases were evaluated: selection, detection, performance, reporting, attrition and other biases.
Synthesis methods
The statistical analyses were performed by the main author (F.M.) following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [55]. Baseline demographics were assessed through the IBM SPSS software. Mean and standard deviation were used for continuous variables, and frequency (events/observations) for binary endpoints. Analysis of variance (ANOVA) was used to assess baseline comparability, with values of P > 0.1 considered as satisfactory. The network meta-analyses were conducted using the STATA Software/MP, version 14.1 (StataCorporation, College Station, Texas, USA). The STATA routine for Bayesian hierarchical random-effects model analysis was used. The log odds ratio (LOR) effect measure was adopted for analysis of dichotomic data. The overall inconsistency was evaluated through the equation for global linearity via the Wald test. In P-values of > 0.5, the null hypothesis could not be rejected, and the consistency assumption could be accepted at the overall level of each treatment. Both confidence (CI) and percentile (PrI) intervals were set at 95%. Edge and interval plots were obtained and evaluated. The funnel plot of each comparison was performed to assess data dispersion.
Results
Search result
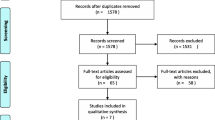
The literature search resulted in 2791 studies. Of them, 1075 were excluded as they were duplicates. An additional 1693 studies were excluded as they did not match the eligibility criteria: not matching the topic (N = 1054), type of study (N = 423), not focussed on THA (N = 175), not reporting data for each outcome separately (N = 31), experimental surgeries or physiotherapeutic protocols (N = 9) and language limitation (N = 1). Additionally, nine studies were excluded as they did not report quantitative data under the outcomes of interest (N = 11). Finally, 14 RCTs were included in the present Bayesian network meta-analysis (Fig. 1).
Risk of bias assessment
The Cochrane risk of bias tool was performed to investigate the risk of bias of RCTs. Given the high quality of the included studies, the risk of selection bias was low. Most studies performed assessors blinding; therefore, the risk of detection bias was also low. The risk of attrition and reporting biases were moderate to low, as was the risk of other biases. Concluding, the risk of bias graph evidenced a good quality of the methodological assessment of RCTs (Fig. 2).
Study characteristics and results of individual studies
Data from 31,705 patients were extracted. Of them, 62% (19,824) were women. The mean length of follow-up was 2.6 ± 0.8 years. The mean age of the patients was 68.7 ± 7.0 years, and the mean BMI was 27.5 ± 1.9 kg/m2. ANOVA found no statistically significant differences in age (P = 0.6), sex ratio (P = 0.4), and BMI (P = 0.8), attesting good baseline comparability of patient demographics. The generalities and demographics of the included studies are presented in Table 1.
Synthesis of results
Apixaban 5 mg, fondaparinux and rivaroxaban 60 mg were the most effective drugs in reducing the rate of DVT (Fig. 3).
Dabigatran 220 mg, apixaban 5 mg and aspirin 100 mg were more effective in reducing the rate of PE (Fig. 4).
Apixaban 5 mg, ximelagatran 2 mg and aspirin 100 mg were associated with the lowest rate of major haemorrhages (Fig. 5).
Rivaroxaban 2.5 mg, apixaban 5 mg and enoxaparin 40 mg were associated with the lowest rate of minor haemorrhages (Fig. 6).
Discussion
According to the main findings of the present Bayesian network meta-analysis, the administration of apixaban 5 mg following THA demonstrated the best balance between VTE prevention and haemorrhage control.
Low-molecular-weight heparins (LMWHs) have been considered the standard of care for VTE prophylaxis after total hip arthroplasty given their low rate of heparin-induced thrombocytopaenia when compared with unfractionated heparin [57]. However, a relevant number of VTEs was evidenced after arthroplasty despite the use of LMWHs such as enoxaparin [58, 59]. NOACs emerged on the market approximately two decades ago, and do not necessitate routine coagulation monitoring. Promising results from the original studies [60, 61] regarding the benefit/risk ratio of NOACs prompted a series of high-quality RCTs regarding different types of prophylaxis and protocols for the prevention of VTE after THA [11, 16, 17, 19, 34, 37,38,39,40,41, 43,44,45,46,47, 49, 56]. Additionally, the Swedish Arthroplasty Registry reported that patients undergoing unilateral THA who received NOACs demonstrated a statistically significant lower rate of VTE compared with those who received low-molecular-weight heparin (LMWH) [62]. However, to date no consensus on the optimal prophylaxis for VTE following THA has been reached. Enoxaparin binds to and enhances the activity of antithrombin III (AT-III), and was the most commonly used prophylaxis before the introduction of the NOACs. AT-III is a physiological inhibitor of the coagulation process which inhibits the coagulation factors Xa and IIa (thrombin), but also factors XIa and IXa. Enoxaparin has also been reported to lead to an AT-III-dependent inhibition of factor VIIa, the induction of endogenous tissue factor pathway inhibitor (TFPI) and the reduction of von Willebrand factor (vWF) by the vascular endothelium [63, 64]. The pentasaccharide fondaparinux is a heparinoid which also indirectly inhibits factor Xa through the activation of AT-III. Fondaparinux can thus rapidly and selectively inhibit coagulation and has a relatively long half-life which allows the drug to obtain an antithrombotic effect for 24 h [32, 65]. Several NOACs are used as prophylaxis: apixaban (half-time around 12 h), edoxaban (half-time 10-14 h), and rivaroxaban (half-time 9 h). These three substances are reversible and highly selective for the inhibition of the factor Xa. Factor Xa is involved in the formation of factor IIa (thrombin) which catalyses fibrinogen into fibrin, inhibiting clotting [66]. Among these substances, rivaroxaban was the first orally administered direct inhibitor of factor Xa. In contrast to other indirect factor Xa inhibitors, such as fondaparinux or heparin, direct factor Xa inhibitors act on both free and clot-bound factor Xa and prothrombinase activity, prolonging clotting times [67,68,69]. Factor Xa inhibitors have no direct effect on platelet aggregation, but they only indirectly inhibit thrombin-induced platelet aggregation [66, 70]. Dabigatran is a NOACs which inhibits factor IIa (thrombin) [71]. Dabigatran reversibly binds the active site of thrombin, preventing thrombin-mediated activation of clotting factors. Dabigatran also inhibits tissue factor-induced platelet aggregation in plasma, demonstrating a greater inhibitory effect than the factor Xa inhibitors rivaroxaban and apixaban [69, 71]. Ximelagatran also binds factor IIa, and is activated into melagatran in the liver. Melagatran directly inhibits the serine protease alpha-thrombin, thus preventing thrombus formation. However, given their liver toxicity, ximelagatran and melagatran were withdrawn from the market in 2006.
All previously discussed substances affect plasmatic coagulation. Aspirin, in contrast, has an inhibitory effect on platelet aggregation through irreversibly blocking prostaglandin-endoperoxide synthase (also known as platelet cyclooxygenase, COX) at the functionally amino acid serine 530, which in turn irreversibly blocks thromboxane A2 formation in platelets [72].
In the present Bayesian network meta-analysis, the administration of apixaban 5 mg demonstrated the best balance between VTE prevention and haemorrhage control following THA. Lassen et al. [47] compared more than 5400 patients using apixaban 2.5 mg twice daily (N = 2708) and enoxaparin 40 mg daily (N = 2699) as prophylaxis after THA. Apixaban showed an absolute risk reduction of 2.5% in the composite outcome of either asymptomatic or symptomatic DVT, nonfatal PE, or death from any cause during the treatment period when compared with enoxaparin [47]. At the same time, no increased risk of major bleeding was observed [47]. Similarly, edoxaban was superior to enoxaparin in preventing VTE after THA without increasing the risk of major bleeding. A dose-dependent effect was thereby reported on the rate of major postoperative bleeding [73, 74]. In a phase II study, Fuji et al. [44] compared 264 patients receiving either edoxaban 15 mg or 30 mg daily or enoxaparin 20 mg twice daily after THA, observing comparable outcomes [44]. Edoxaban-induced prolongation of prothrombin time, international normalised ratio (INR) and activated partial thromboplastin time were proportional to plasma edoxaban concentration [44]. In the following phase III study including more than 600 patients, daily administration of endoxaban 30 mg resulted more effective than enoxaparin 2000 IU subcutaneously twice daily, without an increased rate of haemorrhagic events [45]. Two RCTs compared 2.5 mg of daily fondaparinux with 40 mg of daily enoxaparin for the prevention of VTE after THA [37, 56]. In one study, 1711 patients were evaluated after THA following a fracture of the hip; another study included 2270 patients after elective THA. Both RCTs demonstrated a relative reduction risk for VTE of more than 50% for fondaparinux, without an increased risk of clinically relevant postoperative bleeding [37, 56]. Rivaroxaban was evaluated in a dose-dependent fashion (2.5 mg, 5 mg, 10 mg, 20 mg, or 30 mg daily) in an RCT of 720 patients versus enoxaparin 40 mg daily [39]. The incidence of VTEs following rivaroxaban was comparable to that of enoxaparin [39]. A slight increase in postoperative bleeding major was observed with increasing doses of rivaroxaban [39]. In another phase IIa study, the administration of 2.5–30 mg twice daily or 30 mg once daily of rivaroxaban and enoxaparin 40 mg twice daily were compared [41]. The incidence of DVTs decreased in a dose-dependent manner with rivaroxaban down to the dosage of 20 mg, whereafter the rate increased again [41]. VTEs were lower in the 10 mg rivaroxaban group than with enoxaparin [41]. Major bleeding also increased in a dose-dependent manner, with rivaroxaban up to 10% [41]. In a large phase III RCT of 4433 patients, rivaroxaban 10 mg was compared with enoxaparin 40 mg daily [34]. The absolute risk reduction for major VTE was 1.7% and 2.6% for the composite outcome of DVT, nonfatal PE or death from any cause within 36 days after surgery in the patients allocated to receive rivaroxaban. Haemorrhages, although three times more frequent in the rivaroxaban group, were not statistically significantly different between the two groups [34]. Similar findings were observed by Kakkar et al. [75]. All three studies analysed direct factor Xa inhibitors, and demonstrated a relevant risk reduction for VTEs when the proper dosage was applied with no clinically relevant increase in haemorrhages [75]. A daily administration of dabigatran 220 mg was investigated in 2055 patients and compared with daily enoxaparin 40 mg. Dabigatran was superior in VTE but non-inferior to parenteral enoxaparin for the prevention of VTE and all-cause mortality after total hip arthroplasty [43]. No significant difference was observed in haemorrhages and other adverse events [43].
One study compared 5 days of prophylaxis using 81 mg daily of aspirin versus 10 mg daily of rivaroxaban in 1804 patients. Thereafter, patients were randomly assigned to continue rivaroxaban or to switch to aspirin for an additional 30 days after THA. After 90 days, no between groups difference was observed in VTE, PE or haemorrhagic events [16]. Usually, aspirin is considered to be suitable for the secondary prevention of arterial vascular events. Its role in the primary or secondary prophylaxis of VTE is controversial, especially in Continental and Southern Europe. In the present Bayesian meta-analysis, no difference between aspirin and other established drugs such as rivaroxaban was found. These findings are also consistent with findings from studies analysing the effect of aspirin after TKA [76,77,78].
Among the total extracted patients, rivaroxaban, enoxaparin and apixaban are much more represented compared with patients undergoing prophylaxis with alternative therapies. Indeed, 42% (12,791 of 30,371) of patients received enoxaparin, 23% (6891 of 30,371) rivaroxaban, 14% (4236 of 30,371) apixaban, 7% (1989 of 30,371) fondaparinux, 6% (1707 of 30,371) aspirin, 5% (1377 of 30,371) ximelagatran, 3% (1010 of 30,371) dabigatran and 1% (370 of 30,371) edoxaban. These differences in sample size can impact the reliability of the results of the present study. Moreover, there was high heterogeneity in the administration protocols, especially in the duration of the prophylaxis and relevant dosages. Given these heterogeneities, results should be considered with caution. Almost all studies investigated VTE prevention and haemorrhage control following primary THA. One study included patients who underwent THA in both primary and revision setting [16]. However, more than 90% of these patients underwent primary THA. Whether primary or revision setting influence VTE and haemorrhage in THA is unclear.
Further studies are warranted to validate the results of the present study in a clinical setting. The mean length of the follow-up was 2–3 months in most studies. By that time, patients should have reached full weight bearing and almost regular activity levels. The dosage of the prophylaxis may be influenced by therapeutic intent, body weight and individual pharmacological clearance (e.g. estimated glomerular filtration rate). Future studies should investigate combinations of anticoagulants with an individual risk-stratified dosage. Of note, the results from RCTs usually apply a strict protocol; however, factors such as patient compliance might thus not be reflected in the results. The individual risk profile of simple patients was also not taken into account. Moreover, when deciding the anticoagulant prophylaxis to be implemented, other factors might still be considered; for example, the possibility to antagonise the drug in those rare cases of excessive bleeding. While such antagonisation is possible to at least 50% with protamine sulphate for enoxaparin [79], for NOACs it is hardly available and/or very expensive.
Conclusion
Daily administration of apixaban 5 mg resulted in the best prevention of thromboembolic events and appropriate control of the risk of haemorrhages following THA.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
References
Metcalfe D, Perry DC, Claireaux HA, Simel DL, Zogg CK, Costa ML (2019) Does this patient have hip osteoarthritis?: The rational clinical examination systematic review. JAMA 322(23):2323–2333. https://doi.org/10.1001/jama.2019.19413
Murphy NJ, Eyles JP, Hunter DJ (2016) Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther 33(11):1921–1946. https://doi.org/10.1007/s12325-016-0409-3
Descamps J, Teissier V, Graff W, Mouton A, Bouche PA, Marmor S (2023) Managing early complications in total hip arthroplasty: the safety of immediate revision. J Orthop Traumatol 24(1):38. https://doi.org/10.1186/s10195-023-00719-1
Migliorini F, Biagini M, Rath B, Meisen N, Tingart M, Eschweiler J (2019) Total hip arthroplasty: minimally invasive surgery or not? Meta-analysis of clinical trials. Int Orthop 43(7):1573–1582. https://doi.org/10.1007/s00264-018-4124-3
Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S (2018) Hip replacement. Lancet 392(10158):1662–1671. https://doi.org/10.1016/s0140-6736(18)31777-x
Pivec R, Johnson AJ, Mears SC, Mont MA (2012) Hip arthroplasty. Lancet 380(9855):1768–1777. https://doi.org/10.1016/s0140-6736(12)60607-2
Migliorini F, Cipollaro L, Cuozzo F, Oliva F, Marino AV, Maffulli N (2021) Outpatient total hip arthroplasty: a meta-analysis. App Sci 11(5):6853. https://doi.org/10.3390/app11156853
Leiss F, Götz JS, Maderbacher G, Meyer M, Reinhard J, Zeman F, Grifka J, Greimel F (2021) Excellent functional outcome and quality of life after primary cementless total hip arthroplasty (THA) using an enhanced recovery setup. J Clin Med 10:4. https://doi.org/10.3390/jcm10040621
Świtoń A, Wodka-Natkaniec E, Niedźwiedzki Ł, Gaździk T, Niedźwiedzki T (2017) Activity and quality of life after total hip arthroplasty. Ortop Traumatol Rehabil 19(5):441–450. https://doi.org/10.5604/01.3001.0010.5823
Söderman P, Malchau H, Herberts P (2001) Outcome of total hip replacement: a comparison of different measurement methods. Clin Orthop Relat Res 390:163–172. https://doi.org/10.1097/00003086-200109000-00019
Trudelle-Jackson E, Emerson R, Smith S (2002) Outcomes of total hip arthroplasty: a study of patients one year postsurgery. J Orthop Sports Phys Ther 32(6):260–267. https://doi.org/10.2519/jospt.2002.32.6.260
Szymski D, Walter N, Krull P, Melsheimer O, Schindler M, Grimberg A, Alt V, Steinbrueck A, Rupp M (2023) Comparison of mortality rate and septic and aseptic revisions in total hip arthroplasties for osteoarthritis and femoral neck fracture: an analysis of the German Arthroplasty Registry. J Orthop Traumatol 24(1):29. https://doi.org/10.1186/s10195-023-00711-9
Dubin JA, Bains SS, Chen Z, Hameed D, Moore MC, Mont MA, Nace J, Delanois RE (2023) Single center evaluation of outcomes of modular dual mobility liners during revision total hip arthroplasty: a five-year follow-up. J Orthop 43:75–78. https://doi.org/10.1016/j.jor.2023.07.016
Migliorini F, Cuozzo F, Oliva F, Eschweiler J, Hildebrand F, Maffulli N (2022) Imageless navigation for primary total hip arthroplasty: a meta-analysis study. J Orthop Traumatol 23(1):21. https://doi.org/10.1186/s10195-022-00636-9
An VV, Phan K, Levy YD, Bruce WJ (2016) Aspirin as thromboprophylaxis in hip and knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 31(11):2608–2616. https://doi.org/10.1016/j.arth.2016.04.004
Anderson DR, Dunbar M, Murnaghan J, Kahn SR, Gross P, Forsythe M, Pelet S, Fisher W, Belzile E, Dolan S, Crowther M, Bohm E, MacDonald SJ, Gofton W, Kim P, Zukor D, Pleasance S, Andreou P, Doucette S, Theriault C, Abianui A, Carrier M, Kovacs MJ, Rodger MA, Coyle D, Wells PS, Vendittoli PA (2018) Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med 378(8):699–707. https://doi.org/10.1056/NEJMoa1712746
White RH, Henderson MC (2002) Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med 8(5):365–371. https://doi.org/10.1097/00063198-200209000-00004
Hoggett L, Alexander D, Helm A, Collaborative N (2023) Post-operative complications following total hip arthroplasty for trauma: a multicentre cohort study comparing dual mobility with conventional acetabular bearings. J Orthop 40:34–37. https://doi.org/10.1016/j.jor.2023.04.013
Cao YB, Zhang JD, Shen H, Jiang YY (2010) Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 66(11):1099–1108. https://doi.org/10.1007/s00228-010-0889-z
Huang HF, Li SS, Yang XT, Xie Q, Tian XB (2018) Rivaroxaban versus enoxaparin for the prevention of venous thromboembolism after total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 97(48):e13465. https://doi.org/10.1097/md.0000000000013465
Mula V, Parikh S, Suresh S, Bottle A, Loeffler M, Alam M (2020) Venous thromboembolism rates after hip and knee arthroplasty and hip fractures. BMC Musculoskelet Disord 21(1):95. https://doi.org/10.1186/s12891-020-3100-4
Newhook TE, LaPar DJ, Walters DM, Gupta S, Jolissaint JS, Adams RB, Brayman KL, Zaydfudim VM, Bauer TW (2015) Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg 81(12):1216–1223
Lee SY, du Ro H, Chung CY, Lee KM, Kwon SS, Sung KH, Park MS (2015) Incidence of deep vein thrombosis after major lower limb orthopedic surgery: analysis of a nationwide claim registry. Yonsei Med J 56(1):139–145. https://doi.org/10.3349/ymj.2015.56.1.139
Heit JA, Silverstein MD, Mohr DN, Petterson TM, Lohse CM, O’Fallon WM, Melton LJ 3rd (2001) The epidemiology of venous thromboembolism in the community. Thromb Haemost 86(1):452–463
Sloan M, Sheth N, Lee GC (2019) Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res 477(3):523–532. https://doi.org/10.1097/corr.0000000000000615
Jaffer AK, Barsoum WK, Krebs V, Hurbanek JG, Morra N, Brotman DJ (2005) Duration of anesthesia and venous thromboembolism after hip and knee arthroplasty. Mayo Clin Proc 80(6):732–738. https://doi.org/10.1016/s0025-6196(11)61526-7
Zhang J, Chen Z, Zheng J, Breusch SJ, Tian J (2015) Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg 135(6):759–772. https://doi.org/10.1007/s00402-015-2208-8
Gathof BS, Picker SM, Rojo J (2004) Epidemiology, etiology and diagnosis of venous thrombosis. Eur J Med Res 9(3):95–103
Kearon C (2001) Epidemiology of venous thromboembolism. Semin Vasc Med 1(1):7–26. https://doi.org/10.1055/s-2001-14668
Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ (2019) Deep vein thrombosis: update on diagnosis and management. Med J Aust 210(11):516–524. https://doi.org/10.5694/mja2.50201
Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, Kline JA, Chasteen S, Snyder M, Patel P, Bhatt M, Patel P, Braun C, Begum H, Wiercioch W, Schünemann HJ, Mustafa RA (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv 2(22):3226–3256. https://doi.org/10.1182/bloodadvances.2018024828
Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, Wheeler HB (2001) Prevention of venous thromboembolism. Chest 119(1 Suppl):132s–175s. https://doi.org/10.1378/chest.119.1_suppl.132s
Mohr DN, Silverstein MD, Murtaugh PA, Harrison JM (1993) Prophylactic agents for venous thrombosis in elective hip surgery. Meta-analysis of studies using venographic assessment. Arch Intern Med 153(19):2221–2228
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358(26):2765–2775. https://doi.org/10.1056/NEJMoa0800374
Migliorini F, Driessen A, Eschweiler J, Tingart M, Maffulli N (2021) No benefits of minimally invasive total hip arthroplasty via Watson-Jones approach: a retrospective cohort study. Surgeon. https://doi.org/10.1016/j.surge.2021.07.004
Jenny JY, Bulaid Y, Boisrenoult P, Bonin N, Henky P, Tracol P, Chouteau J, Courtin C, Henry MP, Schwartz C, Mertl P, De Ladoucette A (2020) Bleeding and thromboembolism risk of standard antithrombotic prophylaxis after hip or knee replacement within an enhanced recovery program. Orthop Traumatol Surg Res 106(8):1533–1538. https://doi.org/10.1016/j.otsr.2020.02.026
Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery S (2001) Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 345(18):1298–1304. https://doi.org/10.1056/NEJMoa011100
Eriksson BI, Agnelli G, Cohen AT, Dahl OE, Lassen MR, Mouret P, Rosencher N, Kalebo P, Panfilov S, Eskilson C, Andersson M, Freij A, Group ES (2003) The direct thrombin inhibitor melagatran followed by oral ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. J Thromb Haemost 1(12):2490–2496. https://doi.org/10.1111/j.1538-7836.2003.00494.x
Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Kalebo P, Investigators OD-HS (2006) Oral, direct factor Xa inhibition with BAY 59–7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 4(1):121–128. https://doi.org/10.1111/j.1538-7836.2005.01657.x
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Muehlhofer E, Dierig C, Misselwitz F, Kalebo P, Investigators OD-HS (2006) A once-daily, oral, direct factor Xa inhibitor, rivaroxaban (BAY 59–7939), for thromboprophylaxis after total hip replacement. Circulation 114(22):2374–2381. https://doi.org/10.1161/CIRCULATIONAHA.106.642074
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Muehlhofer E, Kalebo P (2007) Dose-escalation study of rivaroxaban (BAY 59–7939)–an oral, direct factor Xa inhibitor–for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res 120(5):685–693. https://doi.org/10.1016/j.thromres.2006.12.025
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, Group RS (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358(26):2765–2775. https://doi.org/10.1056/NEJMoa0800374
Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ, Group R-NIS (2011) Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 105 (4):721–729. doi: https://doi.org/10.1160/TH10-10-0679
Fuji T, Wang CJ, Fujita S, Kawai Y, Kimura T, Tachibana S (2014) Safety and efficacy of edoxaban, an oral factor xa inhibitor, for thromboprophylaxis after total hip arthroplasty in Japan and Taiwan. J Arthroplasty 29(12):2439–2446. https://doi.org/10.1016/j.arth.2014.05.029
Fuji T, Fujita S, Kawai Y, Nakamura M, Kimura T, Fukuzawa M, Abe K, Tachibana S (2015) Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V. Thromb J 13:27. https://doi.org/10.1186/s12959-015-0057-x
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S, Investigators R (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372(9632):31–39. https://doi.org/10.1016/S0140-6736(08)60880-6
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, Investigators A (2010) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363(26):2487–2498. https://doi.org/10.1056/NEJMoa1006885
Lassen MR, Bauer KA, Eriksson BI, Turpie AG, European Pentasaccharide Elective Surgery Study Steering C (2002) Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet 359(9319):1715–1720. https://doi.org/10.1016/S0140-6736(02)08652-X
Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Wright RT, Lassen MR (2012) Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br 94(2):257–264. https://doi.org/10.1302/0301-620X.94B2.27850
He T, Han F, Wang J, Hu Y, Zhu J (2021) Efficacy and safety of anticoagulants for postoperative thrombophylaxis in total hip and knee arthroplasty: a PRISMA-compliant Bayesian network meta-analysis. PLoS ONE 16(6):e0250096. https://doi.org/10.1371/journal.pone.0250096
Cohen A, Drost P, Marchant N, Mitchell S, Orme M, Rublee D, Simon TA, Sutton A (2012) The efficacy and safety of pharmacological prophylaxis of venous thromboembolism following elective knee or hip replacement: systematic review and network meta-analysis. Clin Appl Thromb Hemost 18(6):611–627. https://doi.org/10.1177/1076029612437579
Howick J CI, Glasziou P, Greenhalgh T, Carl Heneghan, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M (2011) The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine https://www.cebmnet/indexaspx?o=5653
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784. https://doi.org/10.7326/M14-2385
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:000142. doi: https://doi.org/10.1002/14651858.ED000142
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA . Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane 2021. Available from www.training.cochrane.org/handbook. Accessed on February 2022.
Lassen MR, Bauer KA, Eriksson BI, Turpie AG (2002) Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet 359(9319):1715–1720. https://doi.org/10.1016/s0140-6736(02)08652-x
Junqueira DR, Zorzela LM, Perini E (2017) Unfractionated heparin versus low molecular weight heparins for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 4(4):CD007557. https://doi.org/10.1002/14651858.CD007557.pub3
Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW Jr (2012) Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e278S-e325S. https://doi.org/10.1378/chest.11-2404
Francis CW (2013) Prevention of VTE in patients having major orthopedic surgery. J Thromb Thrombolysis 35(3):359–367. https://doi.org/10.1007/s11239-013-0889-9
Maniscalco P, Caforio M, Imberti D, Porcellini G, Benedetti R (2015) Apixaban versus enoxaparin in elective major orthopedic surgery: a clinical review. Clin Appl Thromb Hemost 21(2):115–119. https://doi.org/10.1177/1076029614546328
Rachidi S, Aldin ES, Greenberg C, Sachs B, Streiff M, Zeidan AM (2013) The use of novel oral anticoagulants for thromboprophylaxis after elective major orthopedic surgery. Expert Rev Hematol 6(6):677–695. https://doi.org/10.1586/17474086.2013.853430
Kasina P, Wall A, Lapidus LJ, Rolfson O, Kärrholm J, Nemes S, Eriksson BI, Mohaddes M (2019) Postoperative thromboprophylaxis with new oral anticoagulants is superior to LMWH in hip arthroplasty surgery: findings from the Swedish registry. Clin Orthop Relat Res 477(6):1335–1343. https://doi.org/10.1097/corr.0000000000000714
Jupalli A, Iqbal AM (2022) Enoxaparin. In: StatPearls. StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL).
Fareed J, Hoppensteadt D, Walenga J, Iqbal O, Ma Q, Jeske W, Sheikh T (2003) Pharmacodynamic and pharmacokinetic properties of enoxaparin : implications for clinical practice. Clin Pharmacokinet 42(12):1043–1057. https://doi.org/10.2165/00003088-200342120-00003
Nadar SK, Goyal D, Shantsila E, Banerjee P, Lip GY (2009) Fondaparinux: an overview. Expert Rev Cardiovasc Ther 7(6):577–585. https://doi.org/10.1586/erc.09.19
Byon W, Garonzik S, Boyd RA, Frost CE (2019) Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet 58(10):1265–1279. https://doi.org/10.1007/s40262-019-00775-z
Singh R, Emmady PD (2022) Rivaroxaban. In: StatPearls. StatPearls Publishing
Samama MM (2011) The mechanism of action of rivaroxaban–an oral, direct factor Xa inhibitor–compared with other anticoagulants. Thromb Res 127(6):497–504. https://doi.org/10.1016/j.thromres.2010.09.008
Mujer MTP, Rai MP, Atti V, Dimaandal IL, Chan AS, Shrotriya S, Gundabolu K, Dhakal P (2020) An update on the reversal of non-vitamin k antagonist oral anticoagulants. Adv Hematol 2020:7636104. https://doi.org/10.1155/2020/7636104
Wong PC, Pinto DJ, Zhang D (2011) Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 31(4):478–492. https://doi.org/10.1007/s11239-011-0551-3
Comin J, Kallmes DF (2012) Dabigatran (Pradaxa). AJNR Am J Neuroradiol 33(3):426–428. https://doi.org/10.3174/ajnr.A3000
Altman R, Luciardi HL, Muntaner J, Herrera RN (2004) The antithrombotic profile of aspirin. Aspirin resistance, or simply failure? Thromb J 2(1):1. https://doi.org/10.1186/1477-9560-2-1
Poulakos M, Walker JN, Baig U, David T (2017) Edoxaban: a direct oral anticoagulant. Am J Health Syst Pharm 74(3):117–129. https://doi.org/10.2146/ajhp150821
Raskob G, Büller H, Prins M, Segers A, Shi M, Schwocho L, van Kranen R, Mercuri M (2013) Edoxaban for the long-term treatment of venous thromboembolism: rationale and design of the Hokusai-venous thromboembolism study–methodological implications for clinical trials. J Thromb Haemost 11(7):1287–1294. https://doi.org/10.1111/jth.12230
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372(9632):31–39. https://doi.org/10.1016/s0140-6736(08)60880-6
Colleoni JL, Ribeiro FN, Mos PAC, Reis JP, Oliveira HR, Miura BK (2018) Venous thromboembolism prophylaxis after total knee arthroplasty (TKA): aspirin vs. rivaroxaban. Rev Bras Ortop 53(1):22–27. https://doi.org/10.1016/j.rboe.2017.11.007
Jiang Y, Du H, Liu J, Zhou Y (2014) Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl) 127(12):2201–2205
Zou Y, Tian S, Wang Y, Sun K (2014) Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis 25(7):660–664. https://doi.org/10.1097/MBC.0000000000000121
Crowther MA, Berry LR, Monagle PT, Chan AK (2002) Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol 116(1):178–186. https://doi.org/10.1046/j.1365-2141.2002.03233.x
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
FM: writing, statistical analyses; NM: supervision, revision; FH: supervision; EV: literature search, data extraction, risk of bias assessment; JE: literature search, risk of bias assessment; AB: supervision; DK: supervision UKH: writing, revision. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with ethical standards.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have any competing interests in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Migliorini, F., Maffulli, N., Velaj, E. et al. Antithrombotic prophylaxis following total hip arthroplasty: a level I Bayesian network meta-analysis. J Orthop Traumatol 25, 1 (2024). https://doi.org/10.1186/s10195-023-00742-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-023-00742-2