Abstract
Objective
To evaluate effects of bone marrow sparing (BMS) radiotherapy on decreasing the incidence of acute hematologic toxicity (HT) for locoregionally advanced cervical cancer (LACC) patients treated by pelvic irradiation.
Materials and methods
LACC patients were recruited prospectively from May 2021 to May 2022 at a single center and were evenly randomized into the BMS group and the control group. All patients received pelvic irradiation with concurrent cisplatin (40 mg/m2 weekly), followed by brachytherapy and BM V40 < 25% in the BMS group was additionally prescribed. Acute HT was assessed weekly. Binary logistic regression model and receiver operating characteristic (ROC) curve were used for predictive value analysis. The trial was registered with Chinese clinical trial registry (ChiCTR2200066485).
Results
A total of 242 patients were included in the analysis. Baseline demographic, disease and treatment characteristics were balanced between the two groups. In the intention-to-treat population, BMS was associated with a lower incidence of grade ≥ 2 and grade ≥ 3 acute HT, leukopenia and neutropenia s(72.70% v 90.90%, P < 0.001*; 16.50% vs. 65.30%, P < 0.001*; 66.10% vs. 85.10%, P = 0.001*; 13.20% vs. 54.50%, P < 0.001*; 37.20% vs. 66.10%, P < 0.001*; 10.70% vs. 43.80%, P < 0.001*). BMS also resulted in decreased dose delivered to the organs at risk (OARs) including rectum, bladder and left and right femoral head. Univariate and multivariate analyses showed that BM V40 was an independent risk factor for grade ≥ 3 acute HT (odds ratio [OR] = 2.734, 95% confidence interval [CI] = 1.959–3.815, P < 0.001*). Cutoff value was 25.036% and area under the curve (AUC) was 0.786. The nomogram was constructed, which was rigorously evaluated and internally cross-validated, showing good predictive performance.
Conclusions
Receiving BMS pelvic irradiation could reduce the incidence of acute HT in LACC patients, and BM V40 < 25% may be a significant factor in reducing the risks of acute HT.
Similar content being viewed by others
Introduction
The morbidity and mortality rates of cervical cancer remain high among women worldwide, ranking fourth [1]. For locoregionally advanced cervical cancer (LACC) patients, treatment paradigms consist of pelvic irradiation combined with concurrent cisplatin-based chemotherapy, followed by brachytherapy [2, 3]. Nevertheless, concurrent chemoradiotherapy (CCRT) often lead to a high incidence of acute hematological toxicity (HT), which can result in interruptions and prolonged duration of radiotherapy beyond 8 weeks, negatively impacting outcomes of patients [4,5,6]. Moreover, HT can lead to infection, the need for blood transfusions, bleeding and even immunosuppression, resulting in increased hospitalization rates and treatment costs. The quality of life has been significantly compromised, a factor that holds substantial importance in contemporary cancer treatments [7]. Therefore, it is imperative to identify solutions to reduce the incidence of HT.
Bone marrow (BM) is considered an early response tissue characterized by active division and proliferation, demonstrating a high radiosensitivity similar to that of many tumors, with an alpha/beta ratio of approximately 10. In healthy adults, the active BM predominantly resides within the flat bones—including the skull, sternum, ribs, and iliac bones—as well as the vertebrae, clavicles, scapulae, and the epiphyses of long bones [8]. Patients with LACC undergoing pelvic irradiation will have significant portions of their BM within the radiation field; specifically, regions of the lumbar and sacral spine, hip bone, and both femoral heads are exposed, encompassing an estimated 51% of the active BM [9]. Dose restrictions are applied to the femoral heads, which account for around 4% of the active BM, to prevent complications such as necrosis and fractures. Notably, the pelvic BM is not typically included among the organs at risk (OARs) that are routinely protected during radiotherapy.
Imaging studies have revealed that BM damage is pronounced following pelvic irradiation [10]. Exposure to ionizing radiation (IR) leads to double-strand DNA breaks (DSBs), resulting in cell death. Direct damage arising from immediate interaction with irradiation and indirect damages caused by reactive oxygen species (ROS) are two primary mechanisms of IR-induced DNA damage [11]. Researches have demonstrated that the production of ROS within irradiated BM can be augmented by various sources, including nicotinamide adenine dinucleotide phosphate oxidases (NOXs), cyclooxygenases, mitochondrial electron transport chain-1 [12,13,14,15]. Nitric oxide (NO) is another mediator for division, survival and mobilization of hematopoietic stem and progenitor cells and the production of NO is known to increase in irradiated BM, further contributing to BM damages [16].
Injury to localized hematopoietic cells resulting from pelvic irradiation can be efficiently repaired through the recruitment of circulating pluripotent hematopoietic stem cells [17]. But concurrent chemotherapy can synergistically exacerbate such injuries, leading to acute HT. BM is a parallel organ and it is effective to limit local radiation dose, consequently pelvic BM sparing (BMS) is a promising strategy to mitigate acute HT. By limiting the radiation dose to the pelvic BM, both pathologic and radiographic damage can be lessened, and the incidence of acute HT can be significantly decreased [18, 19].
BMS has been implemented not only in cervical cancer, but also other pelvic malignancies, such as rectum cancer and anal cancer, which turned out to be effective in decreasing the incidence of acute HT [20,21,22,23]. Nevertheless, the importance of BM as an OAR has received little attention in the radiotherapy protocols for prostate and endometrial cancer [24].
The target volume of radiotherapy, despite optimization efforts, inevitably involves normal tissue to some extent. Advancements in radiation technologies serve as an effective strategy to alleviate toxicity. Intensity modulated radiotherapy (IMRT) allows beams to be segmented into thousands of tiny pencil-thin radiation beams, each with varying intensities entering the body from multiple angles, thus enabling the irradiation of complex, irregular clinical target volumes with sparing of the adjacent normal tissues. Compared to the now infrequently used conventional two-dimensional four-field pelvic radiotherapy and three-dimensional conformal radiotherapy (3DCRT), IMRT has been shown to reduce the incidence of HT [25, 26].
In two-dimensional four-field pelvic radiotherapy, 3DCRT and IMRT, BM within the treatment plan have been delineated based on the bone’s outer contour, including both active and inactive BM [25,26,27]. Image-guided IMRT (IG-IMRT) integrates with single photon emission computed tomography (SPECT)/computed tomography (CT) or 18 F-fluorodeoxyglucose positron emission tomography (18FDG-PET)/CT, which allows for PET-based BMS [28,29,30]. This strategy spares myeloid precursors concentrated in active BM, guided by the standard uptake value (SUV) [31]. Nevertheless, the utilization of PET in newly diagnosed cervical cancer patients with biopsy-proven carcinoma of the cervix is still limited. Given the widespread application of IMRT, here we conducted a prospective randomized controlled trial (RCT) to evaluate the effects of BMS-IMRT on acute HT in LACC patients.
Materials and methods
Patients
From May 2021 to May 2022, eligible LACC patients were recruited for the RCT study, who received pelvic IMRT and cisplatin-based concurrent chemotherapy, followed by brachytherapy.
The Institutional Review Board of the Second Affiliated Hospital of Xi’an Jiao Tong University approved the clinical trial (approval number: 2021-021), and the trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines [32]. Prior to the intervention, all patients were thoroughly informed about the study design and potential risks, and provided signed informed consent. The trial was registered on Chinese Clinical Trial Registry with the identifier ChiCTR2200066485.
Eligibility criteria included: (1) Patients aged between 18 and 75 years with Karnofsky performance status (KPS) ≥ 70. (2) Cervical biopsy-proven squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix. (3) International Federation of Gynecology and Obstetrics (FIGO, 2018) stage IB3, IIA2-IVA. (4) Absence of serious underlying medical conditions, including hematopoietic abnormalities and abnormal heart, lung, liver, kidney function, or immunodeficiency.
Exclusion criteria included: (1) Patients with a history of hematologic diseases or malignancies other than cervical cancer. (2) Patients who have previously received surgical treatment for cervical cancer, pelvic irradiation, interventional therapy, chemotherapy, targeted therapy, or immunotherapy. (3) Patients who were pregnant or breastfeeding. (4)Patients presenting with uncontrolled vaginal bleeding, a risk of vaginal fistula formation, or ureteral obstruction. (5) Patients concurrently enrolled in other clinical trials.
External beam radiotherapy (EBRT)
CT-based simulation
A Pelvic fixation plate and a thermoplastic mold were utilized to ensure repeatability of the EBRT procedure, and patients were immobilized in the horizontal supine position with their arms raised above their heads. A contrast-enhanced CT scan with a slice thickness of 5 mm was performed from the T12-L1 interspace to 5 cm below ischial tuberosity.
Definition of target volume and OARs
The CT image datasets were imported into the treatment planning system (TPS). The target volume and OARs were delineated by a consistent deputy chief physician following the International commission on radiation units and measurements (ICRU) related reports [33]. The gross tumor volume node (GTVnd) was defined as a lymph node with short-axis diameter ≥ 1 cm on imaging. The clinical target volume (CTV) included the gross tumor, the cervix, the entire uterus, the parauterine, the vagina, and pelvic lymph node drainage areas. The pelvic lymph node drainage areas included a 7 mm region extending beyond the edge of the blood vessel, encompassing the common iliac, extra ilia, intra ilia as well as the obturator and the presacral regions. The upper boundary of CTV was the bifurcation of the common iliac artery and extending to the renal vascular level when the para-aortic lymph nodes were incorporated into GTVnd. The lower boundary of CTV was set 3 cm beneath the most inferior vaginal involvement. The planning target volume (PTV) of the CTV was expanded by 7 mm laterally and 15 mm axially from the primary CTV; the PTV of the GTVnd (PGTVnd) was enlarged by 7 mm laterally and 15 mm axially on primary GTVnd. OARs included rectum, bladder, femoral head, spinal cord, small intestine and colon. When the upper limit of CTV extended to the renal vascular level, kidney and liver were also considered as OARs.
BM contouring
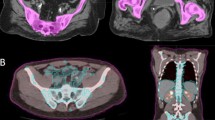
In the BMS group, for each patient, the outer contour of the pelvic bone, lumbar spine, and femoral heads were delineated as a surrogate for BM, based on the CT simulation positioning images in the TPS. The delineation extended from 4 levels above PTV to the end of the ischial nodule.
Previously, dual EBRT plans were designed for patients, using pelvic dose gradients, specifically V10, V20, V25, V30 and V40. It was observed that limiting the dose to V40 < 25% resulted in plan conformity and uniformity comparable to that of non-BMS plans, while still meeting the requirements for PTV and OARs (Fig. 1). Consequently, the BM V40 < 25% was prescribed for BMS group. In addition, it was considered that dose to the pelvic BM from intracavitary brachytherapy (ICBT) was negligible.
EBRT planning and evaluation
IMRT treatment plans for all patients were developed using TPS. Treatment was administered using Varian 21EX or Trilogy linear accelerators with 6 MV X-rays. Fixed-field static reverse IMRT was implemented with a field number of 5 or 7 coplanar fields. The prescribed dose ranged from 45.0 to 50.4 Gy delivered in 25–28 daily fractions of 1.8-2 Gy per day, five days per week, and PTV was optimized to ≥ 95% of the V(100%). For patients with positive lymph nodes, simultaneous integrated boost (SIB) was used, increasing the dose for PGTVnd to 55-61.6 Gy delivered in 25–28 daily fractions of 2.2 Gy per day. For OARs, it was prescribed that small intestine and colon V40 ≤ 30%, V50 ≤ 10%, rectal and bladder V50 ≤ 50%, left and right femoral heads V50 ≤ 5% respectively, left and right kidneys Dmean ≤ 13 Gy respectively, liver D30% ≤20 Gy, spinal cord V40 ≤ 0%.
The EBRT plan for each patient was designed by a consistent medical physicist, and was evaluated using data from dose volume histograms (DVHs). We also assessed the plan’s conformity and homogeneity. We calculated conformity index (CI) and homogeneity index (HI) as follows: CI = (VPTV, ref/VPTV)× (VPTV, ref /Vref), where VPTV, ref represents the volume of reference isodose surface encompassing the target, VPTV represents the target volume, and Vref represents the volume of reference isodose surface. An optimal conformity is indicated by a CI value closer to 1. HI = [D2% - D99% ]/D50%, where D2%, D50% and D99% correspond to the doses received by 2%, 50% and 99% of the target volume of the. Optimal homogeneity is reflected by a lower HI value.
ICBT
ICBT was administered to patients using either a two-dimensional therapy technique or image-guided brachytherapy (IGBT) technology. ICBT initiated after the delivery 40–50 Gy of EBRT. A high-dose-rate (HDR) 192iridium after-loading treatment machine was utilized with a dose fractionation schedule of either 6 Gy × 5 fractions or 7 Gy × 4 fractions, 1 to 2 times per week. EBRT and ICBT were typically completed within a duration of 6–8 weeks. In the two-dimensional therapy, point A was defined as a location 2 cm above the ectocervix and 2 cm lateral to the midline. Rectal and bladder were prescribed as Dmax ≤ 60-70%. In the IGBT, High risk CTV (HR-CTV) included the cervix, the vagina and any remaining tumor. It was prescribed that HR-CTV V(100%) ≥ 90%, rectum and bladder D2cm3 ≤ 75%. The equivalent dose in 2 Gy fractions (EQD2) for point A and HR-CTV was 80–85 Gy, increasing to 87 Gy or higher when the tumor diameter of the is 4 cm or greater.
Chemotherapy regime
Concurrently with radiotherapy, patients were administered weekly cisplatin-based chemotherapy (40 mg/m2) for a duration of 6 weeks. Patients with FIGO stage III and IVA received 2 to 4 cycles of adjuvant chemotherapy following the completion of radiotherapy. Adjuvant chemotherapy consisted of TP regimen (cisplatin 60 mg/m2 d1-2 + paclitaxel 135 mg/m2 d1), once every 21 days. Chemotherapy and radiotherapy were typically held if the white blood cell count (WBC) was < 2.0 × 109/L, the absolute neutrophil cell count (ANC) was < 1.0 × 109/L, or the platelet (PLT) count was < 50 × 109/L. Chemotherapy was additionally held if patients developed febrile neutropenia, renal failure, grade ≥ 2 neurotoxicity, or grade ≥ 3 nausea lasting > 24 h. Acute toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCTE, version 4.03) [34].
Study endpoint
The primary endpoint of this phase II randomized trial was HT during EBRT. Blood tests including WBC, ANC, hemoglobin (HGB), PLT, and lymphocyte (LYM), were conducted at least weekly throughout the treatment course, with the nadir observed during EBRT used to assess HT. Acute HT was graded according to CTCAE (version 4.03, Table 1s) [34].
Secondary endpoint included dosimetric parameters from EBRT plan’s DVHs, including PTV V(100%), CI, HI, small intestine V40 (%) and V50 (%), rectum V50(%), bladder V50(%), left and right femoral head V30 (%) and V50 (%), left and righ kidney Dmean (Gy), liver D(30%), spinal cord V40(%), and BM V10(%) to V50(%).
Study design
This study was conducted as an open-label, single-center, prospective, randomized clinical trial. Participant enrollment was achieved within a timeframe of 12 months, followed by a 3-month follow-up period. Prior to the initiation of treatment, eligible participants were randomized into two groups (BMS and control), utilizing a computer-generated random number list. Based on previously published studies, we estimated an incidence of grade ≥ 3 acute HT in the BMS group of 40%. The incidence rate of grade ≥ 3 acute HT in the control group was estimated at 62% according to a pretest in our center. To achieve a power of 90% and a two-sided type I error of 0.05, the required sample size for each group was 104 according, based on a two-sided test. Considering an anticipated maximum loss to follow-up rate of approximately 20% for this trial, the final sample size was adjusted to 127 participants for each group.
Statistical analysis
SPSS (IBM, Chicago, IL, version 28.0), R (version 4.1.0, http://www.rproject.org/) and GraphPad Prism (version 9.0) software were used for statistical analysis and to generate graphs for the study. Continuous variables were compared with parametric methods when a normal distribution was confirmed. For non-normally distributed variables and categorical data, nonparametric tests were utilized for comparisons. Univariate and multivariate analyses were conducted using a binary logistic regression model. The receiver operating characteristic (ROC) curve was used for predictive value analysis. The “rms” package in R was used to generate the nomograms. Unless otherwise stated, all analyses were performed with a 2-sided significance level of P = 0.05.
Results
Patient characteristics
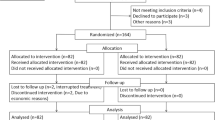
A total of 254 eligible LACC patients were enrolled in this study (Fig. 2). Of these, 6 patients withdrew their consent and another 6 did not meet the inclusion criteria (3 patients for hemoglobin < 10 g/dL; 2 patients for cervical lymphatic metastasis diagnosed by aspiration biopsy). Consequently, based on a computer-generated random number list, 242 patients were assigned to the BMS group (n = 121) and the control group (n = 121), all of whom were subsequently included in the intention-to-treat analysis. 2 patients in the BMS group were lost to follow-up.
Baseline demographic parameters and chemotherapy cycles received were balanced between two treatment groups (Table 1). All patients received a minimum of four cycles of concurrent chemotherapy. In the BMS group, 42 (34.70%) patients received a 45 Gy/25f dose of EBRT, 53 (43.80%) patients received 50 Gy/25f, and 26 (21.50%) received 50.4 Gy/28 f. In the control group, it was 45 (37.20%), 51 (42.10%) and 25 (20.70%) patients respectively. No significant difference was observed in the EBRT dose distribution between two groups. Similarly, no significant difference was observed in other radiotherapy variables, including the prescribed doses of SIB and ICBT. Furthermore, baseline routine blood test results, including WBC, ANC, HGB, PLT and LYM and HT (excluding lymphopenia) demonstrated no significant difference between the two groups.
Effects of BMS on acute HT
For patients enrolled in the trial, blood count tests were performed weekly, and nadirs during EBRT were used as data. In total, 8 (3.30%) patients did not experience any acute HT (excluding lymphopenia). 36 (14.90%), 99 (40.90%), 69 (28.50%) and 30 (12.40%) patients experienced grade 1, grade 2, grade 3 and grade 4 HT separately (Fig. 1s). Treatment was interrupted for HT in 94 patients, after which chemotherapy and radiotherapy resumed once the toxicity subsided.
Patients in the BMS group were primarily in grades 0–2 HT, while those in the control group were mostly in the grade 2–4. The incidence of grade ≥ 2 and grade ≥ 3 acute HT, leukopenia and neutropenia was significantly lower in the BMS group as compared with the control group (72.70% vs. 90.90%, P < 0.001*; 16.50% vs. 65.30%, P < 0.001*; 66.10% vs. 85.10%, P = 0.001*; 13.20% vs. 54.50%, P < 0.001*; 37.20% vs. 66.10%, P < 0.001*; 10.70% vs. 43.80%, P < 0.001*) (Table 2). In cases of anemia and thrombocytopenia, the incidence of grade ≥ 2 toxicity was significantly reduced in the BMS group (17.40% vs. 30.60%, P = 0.016*; 20.70% vs. 33.10%, P = 0.030*). For grade ≥ 2 and grade ≥ 3 lymphopenia, no significant difference was observed. When we included incidence of lymphopenia in acute HT, difference showed no significance for grade ≥ 2 and grade ≥ 3 toxicity between the two groups.
Patients were stratified into three groups based on the administered EBRT dose,and the incidence of HT across the groups was compared. The distribution of patients was 42 vs. 45, 53 vs. 51, and 26 vs. 25 for the BMS and control groups, respectively, corresponding to EBRT doses of 45 Gy/25f, 25 Gy/25f, and 50.4 Gy/28 f. Incidences of grade ≥ 2 or grade ≥ 3 acute HT (excluding lymphopenia), leukopenia and neutropenia were significantly reduced in the BMS group compared to the control group (Tables 2, 3 and 4 s, Fig. 3A-F). Incidences of Grade ≥ 2 and grade ≥ 3 anemia, thrombocytopenia, lymphopenia and acute HT (including lymphopenia) were comparable between the groups.
Evaluation of PTV and OARs
Dosimetric parameters for PTV and OARs were compared between the two groups (Table 5s). PTV V(100%) was significantly lower in the BMS group than in the control group (96.27% ± 1.07% vs. 96.75% ± 1.29%, P = 0.002*). However, the dose distribution within the PTV for each patient met the clinical requirement of at least 95% of the PTV volume receiving 100% or more of the prescribed dose. CI and HI were both comparable between the two groups. For OARs, the results were encouraging as parameters of rectum, bladder and left and right femoral heads showed a significant decrease in the BMS group compared to the control group. Dosimetric parameters for the small intestine, both kidneys, and liver demonstrated no increase in the BMS group. Spinal cord V40(%) in the two groups both satisfied requirements.
Dosimetric parameters of BM ranging from to V10(%) to V50(%) were displayed and their median values were presented (Fig. 2s-A). Median value of BM V40(%) was 24.95%. Additionally, we compared BM V10(%) to V50(%) between the BMS group and the control group. With the exception of V10(%) and V15(%), all other parameters for BMS group were higher than those for the control group(Fig. 2s-B).
Analysis of factors for acute HT
To identify clinical and dosimestric factors for grade ≥ 3 acute HT, we conducted stepwise univariate and multivariate binary logistic regression analyses (Fig. 4A-B). Clinical factors encompassed age, KPS, histologic type and chemotherapy cycles received. Dosimestric factors comprised EBRT dose, SIB prescribed, V10-50 as well as minimum dose (Dmin), maximize dose (Dmax), Dmean and Dmedian of BM. Univariate analysis showed that KPS, FIGO stage, SIB prescribed and all dosimetric parameters of BM were associated with grade ≥ 3 HT. Multivariate analysis showed that BM V20 and V40 were predictors of risk (odds ratio [OR] = 1.628, 95% confidence interval [CI] = 1.084–2.445, P = 0.019*; OR = 2.734, 95%CI = 1.959–3.815, P < 0.001*). Each incremental unit of BM V20 or V30 was associated with a 62.8% or 173.4% increased risk of grade ≥ 3 acute HT (excluding lymphopenia). We generated the ROC curves for grade ≥ 3 HT and BM V20, V30 (Fig. 4C-D). For V20, the cutoff value was 83.073% with an area under the curve (AUC) of 0.631. The sensitivity and specificity were 0.755 and 0.556, respectively. For V40, the cutoff value was 25.036% with an AUC of 0.786. The sensitivity and specificity were 0.713 and 0.798, respectively.
Independent risk factors analyses for grade ≥ 3 acute HT. Univariate (A) and multivariate (B) binary logistic regression analyses. Receiver operating characteristic (ROC) curves for BM V20 (C) and V40 (D). Abbreviation OR, odds ratio; CI, confidence interval; KPS, Karnofsky performance status; FIGO, International Federation of Gynecology and Obstetrics; EBRT, External beam radiotherapy; SIB, simultaneous integrated boost; BM, bone marrow; Vx,volume receiving x Gy; Dmin, minimum dose; Dmax, maximize dose, Dmean, mean dose; Dmedian, median dose; AUC, under the curve. * P < 0.05 was considered significant
Construction and evaluation of the nomogram. (A) Nomogram. (B) Calibration curve. (C) Decision Curve Analysis (DCA) curve. (D) 2000 times five-fold cross-validated C-indexes and Brier scores. Abbreviation BM, bone marrow; Vx,volume receiving x Gy; C-index, concordance index. * P < 0.05 was considered significant
Construction and evaluation of the nomogram
We developed a prognostic nomogram based on the independent factors identified through logistic regression analyses (Fig. 5A). The nomogram quantifed each predictor, roviding an individualized calculation of a patient’s total risk score, corresponding to the probability of grade ≥ 3 acute HT (excluding lymphopenia). The concordance index (C-index) was 0.759, and the Brier score was 0.195 A calibration curve was plotted to evaluate the consistency between the predicted and actual risk, which closely followed the 45° line, indicating good consistency (Fig. 5B). Decision Curve Analysis (DCA) demonstrated the nomogram’s good clinical utility(Fig. 5C). When the threshold probability for grade ≥ 3 HT ranged from 0.033 to 0.960, the net benefit of applying the nomogram (ranging from 0.505 to 40.983%) was significantly greater than that of the “no intervention” and “intervention for all” strategies. Furthermore, we performed 2000 times five-fold cross-validation for the C-index and Brier score, yielding a median C-index of 0.692 (interquartile range [IQR] = 0.637–0.742) and Brier score of 0.192 (IQR = 0.187–0.197) (Fig. 5D).
Discussion
It is generally accepted that EBRT and ICBT should be completed within 8 weeks, as each additional day of RT duration results in a 0.5–0.1% reduction in pelvic control [5, 35]. However, acute HT can interrupt treatment and prolong the duration, a frequent adverse effect in LACC patients undergoing CCRT. Compared with pelvic irradiation alone, CCRT remarkably improves overall survival and concurrently reduces the rates of recurrence and metastasis [4]. At the same time, the incidence of acute HT also rises, as irradiation damages not only hematopoietic cells in the pelvic BM but also those throughout the entire body due to systematic chemotherapy [36]. Encopassing a substantial patient cohort, our study verified that BMS can reduce the incidence of acute HT, which is paticularly vital for LACC patients receiving CCRT to complete the therapeutic plan timely.
Multiple studies have reported that BMS can reduce the incidence of grade ≥ 2 or grade ≥ 3 HT. However, the benefits of BMS to date have been complicated by the use of varied EBRT techniques, inconsistent definition of BM, heterogeneous trial results, and diverse control group configuration. Studies by Hui et al. and Chang et al. proved that IMRT results in milder HT than 3DCRT [26, 37]. In IMRT era, Radiation Therapy Oncology Group (RTOG) 0418 research concluded that BM V40 > 37% or Dmedian > 34.2 was correlated with a higher rate of grade ≥ 2 HT [18]. Huang et al. recommended that efforts to maintain lumbosacral spine (LSS) V10 < 87%, LSS Dmean < 39 Gy and pelvic bone (PB) V40 < 28% simultaneously may reduce the risk of grade ≥ 2 HT [38]. It was reported also that patients with BM V10 ≥ 95% or V20 > 76% were more likely to experience grade ≥ 3 leukopenia [19]. In patients with rectum cancer, Newman et al. found that pelvic mean dose ≥ 36.6 Gy in neoadjuvant IMRT was strongly correlated with the occurrence of grade3 HT during postoperative chemotherapy [21]. In patients with anal cancer, Lee et al. reported that patients receiving CCRT with BM V40 > 23% experienced a higher rate of grade ≥ 3 leukopenia [22]. However, for endometrial patients receiving postoperative IMRT alone, BM volume was not correlated significantly with the incidence of acute HT [18]. As radiation technology improved, more studies were performed to explore the effect of PET-based BMS on reducing the incidence of HT. Mell et al. defined active BM as the subregion with a SUV on PET/CT greater than the mean value over the total BM volume [39]. When pelvic marrow and active marrow mean dose were limited to < 27 Gy and < 28.5 Gy, respectively, along with V10 < 90% and V20 < 75%, the research proved that PET-BMS-IMRT significantly reduced the incidence of grade ≥ 3 neutropenia compared with standard IMRT. Khullar et al. also concluded that a lower volume of active BM defined by PET at a baseline (< 1201 mL) was highly predictive of grade ≥ 3 HT [40].
Our findings add to a large body of evidence that the incidence of acute HT can be decreased in LACC patients receiving BMS pelvic irradiation. Patients in our study were treated with IMRT and we delineated the entire pelvic bone as BM. Compared with previous studies, we included the incidence of leukopenia, neutropenia, anemia, thrombocytopenia, as well as acute HT involving the four variables above as observed results. Additionally, lymphopenia and HT including lymphopenia were analyzed. Our results indicated that the incidence of grade ≥ 2 and grade ≥ 3 acute HT excluding lymphopenia, leukopenia and neutropenia was significantly lower in the BMS group, aligning with the previously reported results. Furthermore, when patients were stratified into 3 groups according to the EBRT dose, a reduction in grade ≥ 2 or grade ≥ 3 HT was also observed in the BMS group. But the incidence of HT including lymphopenia and lymphopenia was comparable between the two groups. PTV V(100%) of all patients met the goal of ≥ 95% and no significance was observed in conformity and homogeneity between the two groups. For OARs, dosimetric parameters of patients in the BMS group were comparable or superior. It was concluded that BMS-IMRT was highly feasible. Notably, rectum V50, bladder V50, left and right femoral head V30 decreased in the BMS group compared with the control group, potentially reducing the incidence of radiation-induced proctitis, cystitis, femoral head and neck injuries. Previous studies had scarcely reported a decrease in the dose to OARs. Subsequent logistic regression analyses proved that BM V20 and V40 were independent risk factors. ROC analysis further corroborated our findings that BM V40 < 25.036% was significantly correlated with a lower incidence of grade ≥ 3 acute HT, with an AUC of 0.786. Ultimately, a nomogram was constructed and rigorously evaluated through internal cross-validation, exhibiting robust predictive performance.
Our results corroborate the reported results with significantly enhanced practical and clinical applicability regarding the EBRT techniques and BM definition. Financial predicament and technological challenges, especially in low- and middle-income countries, hinder the widespread adoption of IG-IMRT. BMS-IMRT is a promising strategy to improve acute HT, as it is both effective and cost-effective.
Our study had several limitations. First, the single-centered study may introduce regional bias. Second, although the dose to rectum, bladder, and femoral head were reduced, we did not include the incidence of complications of pelvic irradiation. Third, survival of patients and pelvic insufficiency fracture (PIF) caused by pelvic irradiation were not assessed due to the short duration of follow-up. We are currently following up patients to observe whether BMS-IMRT confers survival benefits or decrease the incidence of PIF. Fourth, our study was limited to patients with cervical cancer and did not encompass individuals with other pelvic malignancies receiving pelvic irradiation.
Conclusion
In patients with LACC receiving CCRT, BMS exhibited significant benefits in the incidence of acute HT, without compromising the efficacy of radiotherapy or damaging adjacent normal tissues. BMS takes on significance to balance the benefits and risks. Multicenter study, long-term follow-up and the inclusion of other pelvic malignancies are required in future investigations.
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Abbreviations
- 3DCRT:
-
three-dimensional conformal radiotherapy
- ANC:
-
absolute neutrophil count
- AUC:
-
area under the curve
- BM:
-
bone marrow
- BMS:
-
bone marrow paring
- CCRT:
-
concurrent chemoradiotherapy
- CI:
-
confidence interval
- CI:
-
confidence interval
- C-index:
-
the concordance index
- CT:
-
computed tomography
- CTCTE:
-
Common Terminology Criteria for Adverse Events
- CTV:
-
clinical target volume
- D2%, D50% and D99%:
-
the dose of 2%, 50% and 99% of the volume of the target
- DCA:
-
Decision Curve Analysis
- Dmax:
-
maximize dose
- Dmean:
-
mean dose
- Dmedian:
-
median dose
- Dmin:
-
minimum dose
- DSBs:
-
double strand DNA breaks
- DVHs:
-
the dose volume histograms
- EBRT:
-
external beam radiotherapy
- EQD2:
-
equivalent dose in 2 Gy fractions
- 18FDG-PET:
-
18 F-fluorodeoxyglucose positron emission tomography
- FIGO:
-
International Federation of Gynecology and Obstetrics
- GTVnd:
-
gross tumor volume node
- HDR:
-
high-dose-rate
- HGB:
-
hemoglobin
- HI:
-
homogeneity index
- HR-CTV:
-
high risk clinical target volume
- HT:
-
hematologic toxicity
- ICBT:
-
intracavitary brachytherapy
- ICRU:
-
International commission on radiation units and measurements
- ICRU:
-
International commission on radiation units and measurements
- IGBT:
-
image-guided brachytherapy
- IMRT:
-
intensity modulated radiotherapy
- IG-IMRT:
-
image guided IMRT
- IOR:
-
interquartile range
- IR:
-
ionizing radiation
- KPS:
-
Karnofsky performance status
- LACC:
-
locoregionally advanced cervical cancer
- LSS:
-
lumbosacral spine
- LYM:
-
lymphocyte
- NO:
-
nitric oxide
- NOXs:
-
nicotinamide adenine dinucleotide phosphate oxidases
- ns:
-
no significance
- OARs:
-
organs at risk
- OR:
-
odds ratio
- PB:
-
pelvic bone
- PGTVnd:
-
plan gross tumor volume node
- PIF:
-
pelvic insufficiency fracture
- PLT:
-
platelet
- PTV:
-
plan target volume
- RCT:
-
randomized controlled trial
- ROC:
-
receiver operating characteristic
- ROS:
-
reactive oxygen species
- RTOG:
-
Radiation Therapy Oncology Group
- s.d:
-
standard deviation
- SIB:
-
simultaneous integrated boost
- SPECT:
-
single photon emission computed tomography
- SUV:
-
standardized uptake value
- TPS:
-
treatment planning system
- VPTV:
-
the target volume
- VPTV:
-
ref, the volume of reference isoline wrapped around the target
- Vref:
-
the volume of reference isoline wrapped
- Vx:
-
volume receiving x Gy
- WBC:
-
white blood cell
References
Sung H, Ferlay J, Siegel RL et al. Global Cancer Statistics. 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal For Clinicians 2021; 71: 209–249.
Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43.
Rose PG. Combination therapy: new treatment paradigm for locally advanced cervical cancer? Nat Rev Clin Oncol. 2011;8:388–90.
Reducing uncertainties about the. Effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26:5802–12.
Petereit DG, Sarkaria JN, Chappell R, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995;32:1301–7.
Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet (London England). 2001;358:781–6.
Cohen P, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (London England). 2019;393:169–82.
Cristy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol. 1981;26:389–400.
ELLIS R. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–8.
Iyer R, Jhingran A. Radiation injury: imaging findings in the chest, abdomen and pelvis after therapeutic radiation. Cancer Imaging: Official Publication Int Cancer Imaging Soc. 2006;6:S131–9.
Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol (G B)). 2013;25:578–85.
Wang Y, Liu L, Pazhanisamy SK, et al. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–56.
Nuvoli B, Galati R. Cyclooxygenase-2, epidermal growth factor receptor, and aromatase signaling in inflammation and mesothelioma. Mol Cancer Ther. 2013;12:844–52.
Piccoli C, Ria R, Scrima R, et al. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–76.
Najafi M, Motevaseli E, Shirazi A, et al. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol. 2018;94:335–56.
Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–6.
Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39.
Klopp A, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90.
Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:800–7.
Jianyang W, Yuan T, Yuan T, et al. A prospective phase II study of magnetic resonance imaging guided hematopoietical bone marrow-sparing intensity-modulated radiotherapy with concurrent chemotherapy for rectal cancer. Radiol Med. 2016;121:308–14.
Newman NB, Sidhu MK, Baby R, et al. Long-term bone marrow suppression during postoperative chemotherapy in rectal Cancer patients after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2016;94:1052–60.
Lee AY, Golden DW, Bazan JG, et al. Hematologic nadirs during Chemoradiation for Anal Cancer: temporal characterization and dosimetric predictors. Int J Radiat Oncol Biol Phys. 2017;97:306–12.
Arcadipane F, Silvetti P, Olivero F et al. Bone marrow-sparing IMRT in Anal Cancer patients undergoing Concurrent Chemo-Radiation: results of the First Phase of a prospective phase II trial. Cancers 2020; 12.
Iorio GC, Spieler BO, Ricardi U, Dal Pra A. The impact of pelvic nodal radiotherapy on hematologic toxicity: a systematic review with Focus on Leukopenia, Lymphopenia and future perspectives in prostate Cancer Treatment. Crit Rev Oncol/Hematol. 2021;168:103497.
Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388–96.
Chang Y, Yang Z-Y, Li G-L, et al. Correlations between Radiation dose in bone marrow and hematological toxicity in patients with cervical Cancer: a comparison of 3DCRT, IMRT, and RapidARC. Int J Gynecol Cancer: Official J Int Gynecol Cancer Soc. 2016;26:770–6.
Corbeau A, Kuipers SC, de Boer SM, et al. Correlations between bone marrow radiation dose and hematologic toxicity in locally advanced cervical cancer patients receiving chemoradiation with cisplatin: a systematic review. Radiotherapy Oncology: J Eur Soc Therapeutic Radiol Oncol. 2021;164:128–37.
Bolan PJ, Arentsen L, Sueblinvong T, et al. Water-fat MRI for assessing changes in bone marrow composition due to radiation and chemotherapy in gynecologic cancer patients. J Magn Reson Imaging: JMRI. 2013;38:1578–84.
Wang S-B, Liu J-P, Lei K-J, et al. The volume of tc sulfur colloid SPET-defined active bone marrow can predict grade 3 or higher acute hematologic toxicity in locally advanced cervical cancer patients who receive chemoradiotherapy. Cancer Med. 2019;8:7219–26.
Elicin O, Callaway S, Prior JO, et al. [(18)F]FDG-PET standard uptake value as a metabolic predictor of bone marrow response to radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:1099–107.
Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1185–91.
World Medical Association Declaration. Of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlentherapie Und Onkologie: Organ Der Deutschen Rontgengesellschaft. 2012;188:97–9.
Department of Health and Human Services. Common Terminology Criteria for Adverse Events, Version 4.03; June 14, 2010.Available at: https://evs.nci.nih.gov/ftp1/CTCAE/About.html. In. 2010.
Lanciano RM, Pajak TF, Martz K, Hanks GE. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys. 1993;25:391–7.
Cao X, Wu X, Frassica D, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA. 2011;108:1609–14.
Hui B, Zhang Y, Shi F, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in cervical cancer patients undergoing concurrent chemoradiotherapy: comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy. Int J Gynecol Cancer: Official J Int Gynecol Cancer Soc. 2014;24:1648–52.
Huang J, Gu F, Ji T, et al. Pelvic bone marrow sparing intensity modulated radiotherapy reduces the incidence of the hematologic toxicity of patients with cervical cancer receiving concurrent chemoradiotherapy: a single-center prospective randomized controlled trial. Radiation Oncol (London England). 2020;15:180.
Williamson CW, Sirák I, Xu R, et al. Positron Emission Tomography-guided bone marrow-sparing Radiation Therapy for Locoregionally Advanced Cervix Cancer: final results from the INTERTECC phase II/III trial. Int J Radiat Oncol Biol Phys. 2022;112:169–78.
Khullar K, Sudhoff M, Elson J, et al. A comparison of dosimetric parameters in PET-based active bone marrow volume and total bone volume in prediction of hematologic toxicity in cervical cancer patients treated with chemoradiation. J Radiation Oncol. 2017;6:161–5.
Funding
This study is supported by grants from Key Research and Development Project of Shaanxi Province (No. 2021SF-013), and Key Research and Development Project of Shaanxi Province (No. 2022SF-128).
Author information
Authors and Affiliations
Contributions
W.L.: Conceptualization, Project administration, Visualization, Roles/Writing - original draft. L.M.: Data curation, Software, Resources. F.L.: Formal analysis, Validation. K.L., Y.Z.: Funding acquisition. H.R., X.B. and Y.G.: Investigation.Y.G., M.W., D.L., and Y.D.: Methodology. X.M., Z.W.: Supervision. Y.W.: Project administration, Writing - review & editing. R.Y.: Writing - review & editing.
Corresponding authors
Ethics declarations
Clinical trial information
The study was approved by the Second Affiliated Hospital of Xi’an Jiao Tong University Institutional Review Board (2021-021) and was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided informed consent. And the study was registered on Chinese Clinical Trial Registry with the identifier ChiCTR2200066485.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Ma, L., Li, F. et al. Effects of bone marrow sparing radiotherapy on acute hematologic toxicity for patients with locoregionally advanced cervical cancer: a prospective phase II randomized controlled study. Radiat Oncol 19, 46 (2024). https://doi.org/10.1186/s13014-024-02432-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-024-02432-7