Abstract
Objective
The purpose of this study is to evaluate whether dose to PET-defined active regions of the bone marrow is a better predictor of hematologic toxicity than dose to the total bone as a surrogate of the bone marrow.
Methods
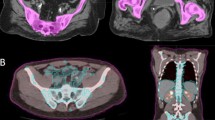
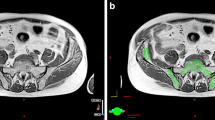
Twenty-one patients with stage IB-IVB cervical cancer who had baseline PET imaging and underwent definitive cisplatin-based chemoradiation from 2010 to 2015 were reviewed. Total bone marrow (TBM) volume was defined as the bone from L4 to the coccyx, the pelvic bones, and proximal femoral heads. Active bone marrow (ABM) was defined by PET as the volume of the bone within the TBM greater than or equal to the mean total body standard uptake value (SUV). Dosimetric parameters evaluated were mean dose, V10, V20, and V40, which were compared by a t test. Hematologic toxicity was graded using Common Terminology Criteria for Adverse Events (CTCAEv4). Receiver operating characteristic (ROC) analysis was used to assess predictors of grade 3 or higher (grade 3+) hematologic toxicity.
Results
ABM volume (mean = 1227 mL, range 793–1671 mL) was significantly smaller than the TBM volume (mean = 1553 mL, range 1117–1920 mL) [p = 0.0001]. ROC analysis identified ABM volume (AUC = 0.800, p = 0.002) and V40 to the ABM (AUC = 0.800, p = 0.008) as the best predictors for grade 3+ hematologic toxicity. All ten patients with an ABM volume < 1201 mL had grade 3+ toxicity compared to 5/11 with an ABM volume > 1201 mL (Fisher’s p = 0.012).
Conclusion
A lower volume of ABM at a baseline (<1201 mL) was highly predictive of grade 3+ hematologic toxicity.

Similar content being viewed by others
References
Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Williams CJ (2001) Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 358(9284):781–786. doi:10.1016/S0140-6736(01)05965-7
Rose BS, Liang Y, Lau SK, Jensen LG, Yashar CM, Hoh CK, Mell LK (2012) Correlation between radiation dose to (1)(8)F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 83(4):1185–1191. doi:10.1016/j.ijrobp.2011.09.048
Liang Y, Bydder M, Yashar CM, Rose BS, Cornell M, Hoh CK, Lawson JD, Einck J, Saenz C, Fanta P, Mundt AJ, Bydder GM, Mell LK (2013) Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys 85(2):406–414. doi:10.1016/j.ijrobp.2012.04.044
Zhu H, Zakeri K, Vaida F, Carmona R, Dadachanji KK, Bair R, Aydogan B, Hasan Y, Yashar CM, Mell LK (2015) Longitudinal study of acute haematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J Med Imaging Radiat Oncol 59(3):386–393 . doi:10.1111/1754-9485.12297quiz 394
Klopp AH, Moughan J, Portelance L, Miller BE, Salehpour MR, Hildebrandt E, Nuanjing J, D’Souza D, Souhami L, Small W Jr, Gaur R, Jhingran A (2013) Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 86(1):83–90. doi:10.1016/j.ijrobp.2013.01.017
Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, Roeske J (2011) Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys 79(4):1043–1047. doi:10.1016/j.ijrobp.2009.12.025
Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, Hurteau JA, Collins YC, Lengyel E, Mundt AJ (2006) Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 66(5):1356–1365. doi:10.1016/j.ijrobp.2006.03.018
Rose BS, Aydogan B, Liang Y, Yeginer M, Hasselle MD, Dandekar V, Bafana R, Yashar CM, Mundt AJ, Roeske JC, Mell LK (2011) Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 79(3):800–807. doi:10.1016/j.ijrobp.2009.11.010
Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, Jani AB, Kindler HL, Mundt AJ, Roeske JC, Chmura SJ (2008) Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 70(5):1431–1437. doi:10.1016/j.ijrobp.2007.08.074
Bazan JG, Luxton G, Mok EC, Koong AC, Chang DT (2012) Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 84(3):700–706. doi:10.1016/j.ijrobp.2011.12.072
Son SH, Choi BO, Ryu MR, Kang YN, Jang JS, Bae SH, Yoon SK, Choi IB, Kang KM, Jang HS (2010) Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys 78(4):1073–1080. doi:10.1016/j.ijrobp.2009.09.009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There were no funding sources utilized in the conduction or completion of this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review board at the University of Cincinnati.
Informed consent
Statement of Informed Consent was not obtained because use of identifiable information in the conduct of this research study was approved under a waiver of authorization.
Rights and permissions
About this article
Cite this article
Khullar, K., Sudhoff, M., Elson, J. et al. A comparison of dosimetric parameters in PET-based active bone marrow volume and total bone volume in prediction of hematologic toxicity in cervical cancer patients treated with chemoradiation. J Radiat Oncol 6, 161–165 (2017). https://doi.org/10.1007/s13566-016-0270-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-016-0270-7