Abstract
Background
Cervical cancer (CC) is one of the most common gynecologic tumors among women around the world. Although the etiological role of human papillomavirus (HPV) in CC is well established, other factors in CC carcinogenesis remains unclear. Here, we performed a systematic review and meta-analysis to explore the association between infections of human herpesvirus (HHVs) and CC risk.
Methods
Embase and PubMed databases were utilized to search the relevant studies. The revised JBI Critical Appraisal Tool was used to assess the quality of the included studies. Prevalence and odds ratios (ORs) with 95% confidence intervals (CI) were calculated to evaluate the association between viral infection and CC or precancerous cervical lesions (PCL).
Results
Totally 67 eligible studies involving 7 different HHVs were included in meta-analysis. We found an increased risk of CC or PCL that was associated with the overall infection of HHVs (CC, OR = 2.74, 95% CI 2.13–3.53; PCL, OR = 1.95, 95% CI 1.58–2.41). Subgroup analysis showed a trend towards positive correlations between herpes simplex virus type 2 (HSV-2) infection and CC (OR = 3.01, 95% CI 2.24 to 4.04) or PCL (OR = 2.14, 95% CI 1.55 to 2.96), and the same is true between Epstein-Barr virus (EBV) infection and CC (OR = 4.89, 95% CI 2.18 to 10.96) or PCL (OR = 3.55, 95% CI 2.52 to 5.00). However, for HSV-1 and cytomegalovirus (HCMV), there was no association between viral infection and CC or PCL. By contrast, the roles of HHV-6, HHV-7, and Kaposi sarcoma–associated herpesvirus (KSHV) in cervical lesions were unclear due to the limited number of studies.
Conclusions
This study provided evidence that HHVs infection as a whole increase the risk of CC incidence. In addition, some types of HHVs such as EBV and HSV-2 may serve as potential targets in the development of new interventions or therapeutic strategies for cervical lesions.
Similar content being viewed by others
Introduction
Cervical cancer (CC) is one of the most common gynecologic tumors among women around the world. Despite CC could have been prevented through HPV vaccination [1], screening tests, and other potent inhibitors (such as carrageenan [2]) of HPV infection, the disease burden remains high worldwide. In 2020, an estimated 604,000 cases were newly diagnosed worldwide according to the data from WHO [3]. Although the etiological role of human papillomavirus (HPV) in CC has been well recognized, more than 90% of HPV infections are cleared within 2 years [4]. Only those persistent infection with high-risk HPV can lead to cancer, indicating that HPV is necessary but not sufficient for carcinogenesis. In addition to HPV, other mucosally transmitted pathogens have been implicated in the development of CC [5, 6]. In this context, a better understanding of viral cofactors involved in malignancy and tumor progression is vital for the interventive and therapeutic development in CC.
Human herpesviruses (HHVs) are a family of DNA viruses including herpes simplex virus type 1 and 2 (HSV-1 and HSV-2), varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (HCMV), human herpesvirus 6 and 7 (HHV-6 and HHV-7), and Kaposi sarcoma–associated herpesvirus (KSHV). Like HPV, HHVs causatively linked to a spectrum of human sexually transmitted diseases. Infections of HHVs are usually asymptomatic but more likely to establish a lifelong persistent infection [7], leading to modulation of the host immune response, host genome instability, or malignant transformation in the extreme case [8]. Except for VZV, viral DNA or RNA of HHVs has been detected in exfoliated cells or tissues from CC or cervical intraepithelial neoplasia (CIN) lesions [9,10,11,12], suggesting that most of HHVs exist in cervical epithelial cells with a possible oncogenic role. Of these HHVs, HSV, EBV, and HCMV have been identified to have high correlation with abnormal cervical cytology [9,10,11].
HSV-1 and HSV-2 are historically associated with oral and genital herpes, respectively, however, HSV-1 infection in genital tract continues to increase with the changes in sexual practices in recent years [13]. In 1968, the possibility of HSV-2 as a causal agent for CC was first reported in the journal of Science [14]. Later on, some studies demonstrated that HSV-2 seropositive women have a significantly increased risk of developing CC [15,16,17,18], and HSV DNA was able to be detected in CC tissues [16, 18]. However, another prospective study further pinpointed that there is no association between HSV-2 seroconversions and the development of cervical neoplasia [19], making the role of HSV-2 in CC controversial. In contrast, EBV is a well-established oncogenic virus associated with various lymphomas and some epithelial carcinomas [20, 21]. Of note, there is a correlation between EBV infection and abnormal cervical cytology [10]; on one hand, the prevalence of EBV positivity increases with lesion severity [22]; on the other hand, CIN or CC occurs more often among EBV positive women than those without EBV infection [23]. In addition to HSV and EBV, HCMV is also implicated as a co-factor in HPV-related CC [11].
Despite the correlations described above, the roles of HHVs in HPV-related CC remains incompletely understood. In the current study, we conducted a systematic review and meta-analysis to elucidate the potential roles of HHVs as a whole in the development of CC. The association between CC and the individual herpesvirus, i.e., HSV-1, HSV-2, HCMV, and EBV, was also investigated. Additionally, the effects of the possible influencing factors on the primary outcomes including virus detection methods, specimen type, stage of the disease, and different regions divided by the human development index (HDI) [24] were included in this analysis.
Method
This study was registered in the International Prospective Register of Systmactic Reviews database (CRD42022314073) and followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) reporting guideline.
Search strategy
We searched Embase and PubMed using Medical Subject Headings (MeSH) terms and “search terms” (as listed in the Supplementary Methods). The most recent search was done on September 16, 2023. We applied no date or language restrictions. The reference list of identified papers was manually checked for additional relevant articles.
Study selection criteria
Studies meeting the following criteria were included. First, the participants were women with cervical lesions (pathologically confirmed) and women with normal cervix. Meanwhile, cervical lesions include CC and/or precancerous cervical lesions (PCL). Second, the detections of HHVs antigens or antibodies were performed in all participants. Third, studies reported prevalence of HHVs infections or addressed the adjusted odds ratio (OR) for the association between cervical lesions and HHVs.
The exclusion criteria were as follows: (1) studies that included participants with CC or PCL combined with other genital malignancies; (2) methods for viral detections without detailed descriptions, such as “manufacturer information”, “detection of targets”, or “performed as the manufacturer’s guidelines”; (3) detection of viral infection using lymphocytes immune responses to viral antigens; (4) studies that lacked a control group; (5) studies that were published as abstracts, letters, case reports, or reviews; (6) studies that were repeated research results.
Data extraction
A preconceived and standardized form was used for data collection. Extracted information included: (1) authors and year of publication; (2) countries where the research was conducted; (3) population investigated (types of cervical lesions); (4) specimen type; (5) method for viral detection; (6) relevant findings: number of individuals with cervical lesions and /or herpesviruses infections; (7) the adjusted OR values and their corresponding 95% confidence interval (CI), if applicable. Two authors (Yuan Xia and Yangxuan Lin) independently conducted study selection and data extraction, and all extracted data were cross checked by the third and fourth author (Shunli Cai and Han Zhang). Disagreements were resolved through consensus.
Quality assessment
We assessed study quality using the revised JBI Critical Appraisal Tools of 8 items (Supplementary Methods). Studies with at least seven “yes” scores were considered to be of high methodology quality, those with between four and six “yes” scores to be of moderate quality and those with less than four “yes” scores to be of low methodological quality. Three authors (Yuan Xia, Yangxuan Lin, and Shunli Cai) performed this evaluation independently and disagreement was resolved through consensus and discussion.
Statistical analysis
We analyzed the results by the pooled prevalence and odds ratio (OR). In the primary analysis, we first studied the overall association between HHVs and CC or PCL. Then, we studied individual HHV in cervical lesions of the pooled prevalence and OR value. For 9 studies that related to HSV-2 and reported adjusted effect estimates, we also conducted meta-analysis to pool the adjusted estimates.
Subsequently, univariable and multivariable random effects meta-regression analyses were performed to investigate factors associated with OR values, as well as to explain interstudy heterogeneity. According to the results of meta-analysis and mete regression, we further performed subgroup analysis for HSV-2 and EBV by different stages of disease (CIN 1, CIN 2/3, and CC), viral detection methods, and different HDI regions. In addition, we also assessed the OR value of EBV in specimen types.
The GRADE (Grading of Recommendation, Assessment, Development, and Evaluations) tool was used to assess the quality of evidence of the primary outcome [25]. The evidence was assigned a GRADE rating of very low, low, moderate or high by employing the five GRADE rating down considerations (risk of bias, heterogeneity between studies, indirectness, risk of random errors, and publication bias) and 3 factors may lead to rating up. Additionally, in the GRADE approach, observation studies start as low-quality evidence.
All statistical analyses were conducted using R statistical software version 4.2.0. Random effects model was used to calculate the pooled results and 95% CI. Heterogeneity was assessed using the I² statistic. Forest plots were generated to visualize the study-specific effect sizes along with 95% CI. We assessed publication bias using Peters test. All p values were two-sided. A p value of less than 0.05 was considered to be significant.
Results
Search results
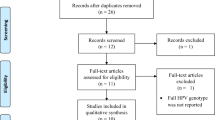
Totally 2233 publications were yielded after removal of duplicates, and 353 articles were left for full-text reading after excluding 1880 irrelevant records based on the screening of title and abstract. After full-text screening, 67 eligible publications [12, 15,16,17,18,19, 22, 23, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] were included for the subsequent analysis (Fig. 1 and Table S1). Since 2 publications included data from a very high HDI country and a high HDI country, and 1 publication across 3 different HDI countries (very high, high and low), totally 71 studies were analyzed, including 2 studies in low HDI countries, 5 were in medium HDI countries, 19 were in high HDI countries, and 45 were in very high HDI countries. Viral nucleic acid was detected using PCR-based and hybridization-based assays in 25 and 7 studies, respectively. Virus-specific antibodies in serum were measured using immunological tests in 38 studies. Two different detection methods were used in 3 studies. The characteristics of the study are summarized in Table S1.
Overall association between human herpesviruses (HHV) and CC or PCL
In all selected studies, the overall pooled prevalence of HHV among women with CC was 56% (95% CI 48–63%), whereas a significantly lower pooled proportion of 34% (95% CI 27–42%) was indicated in individuals with normal cervix (OR = 2.74, 95%CI 2.13 to 3.53) (Tables 1 and Fig. S1). Similarly, there was a significantly higher pooled prevalence (44%, 95% CI 35–54%) of HHV among women with PCL compared to normal women (28%, 95% CI 20–37%), the pooled OR was 1.95 (95% CI 1.58 to 2.41) (Tables 1 and Fig. S1).
Association between individual HHV and CC or PCL
Seven different herpesviruses including HSV-1, HSV-2, HCMV, EBV, HHV-6, HHV-7, and KSHV were included in this review. The numbers of studies and individuals used to evaluate the association between CC or PCL were summarized in Table 1. The pooled prevalence and OR of viruses among patients with CC or PCL and corresponding controls were evaluated (Table 1). We failed to perform meta-analysis for HHV-6, HHV-7, and KSHV due to the small number of studies (2 or 3).
For HSV-1 and HCMV, there was no association between viral infection and CC or PCL (Tables 1 and Fig. S2-3). By contrast, there was an association between HSV-2 infection and CC (OR = 3.01, 95% CI 2.24 to 4.04), and the same is true between HSV-2 infection and PCL (OR = 2.14, 95% CI 1.55 to 2.96) (Fig. 2). The adjusted OR from 9 studies (Fig. S4 and Table S2) also indicated that HSV-2 could be a risk factor for CC (OR = 1.53, 95% CI 0.98 to 2.38) or PCL (OR = 2.53, 95% CI 1.28 to 4.99), although the 95% CI for OR of CC included 1. Lastly, we found an association between EBV infection and CC (OR = 4.89, 95% CI 2.18 to 10.96) or PCL (OR = 3.55, 95% CI 2.52 to 5.00) (Fig. 3).
In addition, a small number of studies elucidate the presence of HHV-6, HHV-7 and KSHV in cervical samples. One study [36] from Italian women found that the prevalence of HHV-6 DNA was significantly higher in high-grade squamous intraepithelial lesions compared with normal women, whereas the prevalence of HHV-7 was low with no association with cervical lesions. Another study from Argentina [35] reported the similar results for HHV-6, but HHV-7 DNA was detected in all samples. Therefore, it seems that HHV-6 is a possible risk factor for cervical lesions. For KSHV, the positive rate for viral DNA was 8.7% in cervical biopsy samples from Chinese women with abnormal Papanicolaou smears [38]. However, no statistically significant association between KSHV and high-grade cervical lesions was found. Taken together, these findings suggest that genital tract is a possible transmission pathway for HHV-6, HHV-7, and KSHV, and their roles in cervical malignancy deserve further evaluation.
Meta-regression and subgroup analysis of association between viral Infection and cervical lesions
The results of meta-regression are shown in Table S3-S7 and Table 2. We found that the risk of HSV-2 or EBV infection for cervical lesions varied according to viral detection methods, specimen types or different HDI regions, but not the year of publication. Furthermore, based on the above finding that HSV-2 and EBV infections are risk factors for CC or PCL, we performed subgroup analyses in terms of different stages of disease, viral detection methods, HDI regions, and specimen types (Table 2).
According to the different stages of disease, results of subgroup analysis showed that HSV-2 was identified as a risk factor for CC (OR = 3.01, 95%CI 2.24–4.04) and CIN2/3 (OR = 1.64, 95%CI 1.29–2.08) except for CIN1 (OR = 1.87, 95%CI 0.95–3.70) (Tables 2 and Fig. S5A). Moreover, HSV-2 infection was associated with cervical lesions for studies using either immunological tests for detection of serum antibodies to HSV-2 (OR = 2.41, 95%CI 1.92–3.02) or PCR-based approaches for detection of genes encoding viral antigens (OR = 2.79, 95%CI 1.79–4.34) (Tables 2 and Fig. S5B). Testing for subgroup differences according to specimen types (Tables 2 and Fig. S5C) yielded the similar results as the subgroup analysis according to the viral detection methods (serum: OR = 2.50, 95%CI 1.98–3.17; brush/swab: OR = 3.34, 95%CI 2.02–5.53). We further analyzed the influence of different HDI regions on association between HSV-2 infection and cervical lesions (Tables 2 and Fig. S5D). The results showed a higher OR in low HDI countries (OR = 15.63, 95%CI 3.18–76.90), whereas lower OR values were found in medium, high, and very high HDI counties (medium: OR = 2.82, 95%CI 1.05–7.54; high: OR = 2.84, 95% CI 2.10–3.82; very high: OR = 2.23, 95% CI 1.74–2.85). In addition,
For EBV, regardless of the different stages of disease progression, subgroup analysis revealed an association of EBV infection and cervical lesions (Tables 2 and Fig. S6A). The pooled ORs were 2.31 (95%CI 1.54–3.47), 4.32 (95%CI 2.42–7.70), and 4.89 (95%CI 2.18–10.96) for CIN1, CIN2/3, and CC, respectively. In addition, we found that the studies using PCR- (OR = 3.92, 95%CI 2.84–5.41) and hybridization-based (OR = 4.85, 95%CI 1.32–17.80) assays showed significant relation between EBV infection and cervical lesions (Tables 2 and Fig. S6B). Further analysis regarding the different HDI regions showed a higher OR in medium HDI country (OR = 6.70, 95% CI 2.59–17.28), whereas lower OR values were found in high and very high HDI countries (high HDI: OR = 3.53, 95% CI 1.80–6.94; very high HDI: OR = 3.65, 95% CI 2.30–5.80) (Tables 2 and Fig. S6C). Lastly, we analyzed the effects of specimen types on association between EBV infection and CC or PCL. The results showed a higher OR in studies using formalin-fixed and paraffin-embedded (FFPE) samples (OR = 6.61, 95%CI 2.93–14.87), followed by studies using brush/swab (OR = 4.06, 95%CI 2.37–6.95) and biopsy (fresh-frozen) (OR = 3.22, 95% CI 1.24–8.33) samples (Tables 2 and Fig. S6D).
GRADE assessment
We include five outcomes in the GRADE assessment: the associations between HHVs, HSV-1, HSV-2, HCMV or EBV infection and CC or PCL. We assessed the quality of evidence from ‘very low’ to ‘moderate’ for theses outcomes (Table S8).
Publication bias
By using the Peters test, we did not find publication bias in HSV-1 (P = 0.7324), HCMV (P = 0.5436) and EBV (P = 0.7702), except for HSV-2 (P = 0.0036).
Discussion
CC is the frequently occurring cancer of the female genital tract, and HPV infection is an established cause of CC. Other than HPV, the association between other viruses such as HHV and the risk of CC remains unclear. Therefore, it is of particularly necessary to perform this meta-analysis and systematic review. Based on our analyses, we found that the pooled prevalence of HHVs among CC or PCL patients are significantly higher than normal controls, suggesting that HHVs infections are very likely to increase the risk of cervical lesions. We also conducted four meta-analyses to explore the roles of HSV-1, HSV-2, HCMV, and EBV in cervical lesions, respectively. The results showed a trend towards a positive correlation between HSV-2 or EBV infections and cervical lesions, but there is no association between HSV-1 or HCMV and cervical lesions.
As one of the most common pathogens of sexually transmitted infection, HSV-2 was shown to be a risk factor for CC (OR = 3.01, 95% CI 2.24 to 4.04) and PCL (OR = 2.14, 95% CI 1.55 to 2.96) in the present study. Actually, the association between HSV-2 and CC has been debated for a long time. One meta-analysis [19] of longitudinal studies conducted in 2002 reported that HSV-2 was not associated with the risk of CC, but this study did not follow the Meta-analysis of Observational Studies in Epidemiology Guidelines [85]. In 2014, another meta-analysis [9] revealed an association between HSV-2 infection and CC in traditional case-control studies but not in nested case-control studies. Although the nested case-control study provides a high level of evidence, the number of such studies is relatively small. Given the fact that the small number of studies and participants may have impact on the validity of the results, we included both traditional and nested case-control studies, i.e., 38 studies enrolling 3991 CC patients and 8427 control individuals. Furthermore, the pooled adjusted OR estimates from 9 studies (adjustment for multiple factors including age, HPV status, number of sexual partners, et al.) also revealed the association between HSV-2 infection and CC or/and PCL (Fig. S4). In fact, it is hard to determine whether HSV-2 infection occurs simultaneously along with carcinogenesis due to the inability of serologic assays to distinguish the current infection from the past exposure of HSV-2. In this case, we performed subgroup analysis in terms of different viral detection methods. Indeed, similar results were obtained in both immunological tests for detection of serum antibodies to HSV-2 and viral DNA detection using PCR-based assays.
Moreover, we provided strong evidence that the incidence of CC is increased approximately 5-fold upon exposure to EBV, and the incidence of PCL is also increased upon EBV infection, in line with a previous meta-analysis [10] that showed a 4- and 2-times increase in the risk of CC and PCL incidence with EBV infection, respectively. Nonetheless, compared to the previous study [10], our meta-analysis included studies containing one or more control groups. On the other hand, we included more recent studies and performed more subgroup analyses. Of note, the subgroup analysis in terms of different stages of disease showed a positive correlation between the risk of EBV infection and lesion grade, further supporting the involvement of EBV in the development of CC. In addition, EBV detected with hybridization-based assays showed a higher pooled OR value than that using PCR-based assays, suggesting that EBV is a reliable cofactor in CC progression, since the former is the gold standard for EBV detection in tissues. To date, EBV infection in cervix is associated with an increased frequency of reactivation of EBV, viral shedding, and inflammation in the genital tract [86]. Previous studies suggested a potential cooperation of EBV with CC development by two possible mechanisms including synergizing with HPV and inducing local immunosuppression by infecting tissue-infiltrating lymphocytes [87]. Further elucidation of the mechanisms underlying the EBV-mediated tumorigenesis in CC is required.
Another interesting finding of our meta-analysis is that the risk of cervical lesions with HSV-2 or EBV infections negatively correlated to HDI distribution (Fig. S5D and Fig. S6C). For instance, the OR estimate for HSV-2 or EBV associated cervical lesions (included both CC and PCL) was obviously higher among low or medium HDI countries than high and very high HDI countries. According to these results, the women infected with HSV-2 or EBV in countries defined within the low ranking of the HDI are more likely to develop cervical lesions. This trend is also in agreement with the distribution of CC incidence worldwide [3]. One possible explanation is the unique socio-demographic characteristics of the lower HDI countries that might enhance the impact of HHVs infections on CC, which need to be taken into account in the future study.
Lastly, there are several limitations of this study. First, HPV infection is the main cause of CC, but the most studies in our analysis did not provide the data of HHVs and HPV co-infection. Thus, we failed to take the HPV infection into account in the subgroup analysis. Second, although we performed analyses in terms of different means of detection among studies, more detailed factors were not included in our analysis. For example, immunological tests for HSV-2 specific serum antibodies include ELISA, neutralization, complement fixation tests, radioimmunoassay, etc. In addition, different type of antibodies (IgA or IgG) with different cut-off values were applied. Thus, we cannot exclude the impacts of the above factors on the results.
Collectively, our results revealed the effects of HHVs infections on CC or PCL. We found a robust positive correlation between EBV infection and CC risk. Although the individual HHV-6, HHV-7, or KSHV was not independently analyzed, their potential roles in CC require further investigations. Importantly, our findings suggest HHVs (e.g., EBV or HSV-2) as potential targets in the development of new interventions or therapeutic strategies, including but not limited to vaccines and microbicides, for cervical lesions.
Data Availability
The original contributions presented in the study are included in the article or Supplementary Material.
References
Giuseppina Capra LG, Domenica Matranga C, Bellavia MF, Guarneri T, Fasciana G, Scaduto A, Firenze. Alessandra Vassiliadis, Antonio Perino: potential impact of a nonavalent HPV vaccine on HPV related low-and high-grade cervical intraepithelial lesions: a referral hospital-based study in Sicily. Hum Vaccin Immunother. 2017;13:1839–43.
Gloria Calagna MM, Consiglio Paola G, Capra A, Perino V, Chiantera. Gaspare Cucinella: ‘Secondary prevention’ against female HPV Infection: literature review of the role of carrageenan. Rev Expert Rev Anti Infect Ther. 2020;18:865–74.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chap. 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):342–51.
Bosch FX, de Sanjose S. The epidemiology of human papillomavirus Infection and Cervical cancer. Dis Markers. 2007;23:213–27.
Guidry JT, Scott RS. The interaction between human papillomavirus and other viruses. Virus Res. 2017;231:139–47.
Cohen JI. Herpesvirus latency. J Clin Invest. 2020;130:3361–9.
Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–89.
Cao S, Gan Y, Dong X, Lu Z. Herpes simplex virus type 2 and the risk of Cervical cancer: a meta-analysis of observational studies. Arch Gynecol Obstet. 2014;290:1059–66.
de Lima MAP, Neto PJN, Lima LPM, Goncalves Junior J, Teixeira Junior AG, Teodoro IPP, Facundo HT, da Silva CGL, Lima MVA. Association between Epstein-Barr virus (EBV) and cervical carcinoma: a meta-analysis. Gynecol Oncol. 2018;148:317–28.
Szostek S, Zawilinska B, Kopec J, Kosz-Vnenchak M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim Pol. 2009;56:337–42.
Chavoshpour-Mamaghani S, Shoja Z, Mollaei-Kandelous Y, Sharifian K, Jalilvand S. The prevalence of human herpesvirus 8 in normal, premalignant, and malignant cervical samples of Iranian women. Virol J. 2021;18:144.
Feng Min GY, Yang Yali L. Epidemiological characteristics of herpes simplex virus. Chin J Microbiol Immunol. 2019;39:937–50.
Rawls WE, Tompkins WA, Figueroa ME, Melnick JL. Herpesvirus type 2: association with carcinoma of the cervix. Science. 1968;161:1255–6.
Adelusi B, Fabiyi A, Osunkoya BO. Herpes type-2 viruses and gynaecological malignancies. Int J Gynaecol Obstet. 1976;14:209–12.
Bahena-Roman M, Sanchez-Aleman MA, Contreras-Ochoa CO, Lagunas-Martinez A, Olamendi-Portugal M, Lopez-Estrada G, Delgado-Romero K, Guzman-Olea E, Madrid-Marina V, Torres-Poveda K. Prevalence of active Infection by herpes simplex virus type 2 in patients with high-risk human papillomavirus Infection: a cross-sectional study. J Med Virol. 2020;92:1246–52.
de Sanjose S, Munoz N, Bosch FX, Reimann K, Pedersen NS, Orfila J, Ascunce N, Gonzalez LC, Tafur L, Gili M, et al. Sexually transmitted agents and cervical neoplasia in Colombia and Spain. Int J Cancer. 1994;56:358–63.
Zhao Y, Cao X, Zheng Y, Tang J, Cai W, Wang H, Gao Y, Wang Y. Relationship between cervical Disease and Infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2. J Med Virol. 2012;84:1920–7.
Lehtinen M, Koskela P, Jellum E, Bloigu A, Anttila T, Hallmans G, Luukkaala T, Thoresen S, Youngman L, Dillner J, Hakama M. Herpes simplex virus and risk of Cervical cancer: a longitudinal, nested case-control study in the nordic countries. Am J Epidemiol. 2002;156:687–92.
Su ZY, Siak PY, Leong CO, Cheah SC. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front Microbiol. 2023;14:1116143.
Tavakoli A, Monavari SH, Solaymani Mohammadi F, Kiani SJ, Armat S, Farahmand M. Association between Epstein-Barr virus Infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20:493.
Aromseree S, Pientong C, Swangphon P, Chaiwongkot A, Patarapadungkit N, Kleebkaow P, Tungsiriwattana T, Kongyingyoes B, Vendrig T, Middeldorp JM, Ekalaksananan T. Possible contributing role of Epstein-Barr virus (EBV) as a cofactor in human papillomavirus (HPV)-associated cervical carcinogenesis. J Clin Virol. 2015;73:70–6.
Cameron JE, Rositch AF, Vielot NA, Mugo NR, Kwatampora JKL, Waweru W, Gilliland AE, Hagensee ME, Smith JS. Epstein-Barr Virus, high-risk human papillomavirus and abnormal cervical cytology in a prospective cohort of African female sex workers. Sex Transm Dis. 2018;45:666–72.
United Nations Development Programme (UNDP). Human Development Report 2021/2022. pp. 272–275. https://hdr.undp.org/system/files/documents/global-report-document/hdr2021-22pdf_1.pdf; 2022:272–275.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Soumya D, Nagaraja KMM, Rishi Gowtham R, Umapathi N, Madhavi Latha PM, Padmalatha AM, Prakash P. Usha Kalawat: Detection of Chlamydia trachomatis and Herpes Simplex Virus-2 Infections Among Clinically Suspected Women with Cervical Cancer or Precancerous Lesions. Indian Journal of Gynecologic Oncology 2023, 21:1–7.
Adam E, Sanders EK, Melnick JL, Levy AH, Rawls WE. Antibodies to herpesvirus type 2 in Breast cancer and Cervical cancer patients. Cancer. 1974;33:147–52.
Adam E, Kaufman RH, Adler-Storthz K, Melnick JL, Dreesman GR. A prospective study of association of herpes simplex virus and human papillomavirus Infection with cervical neoplasia in women exposed to diethylstilbestrol in utero. Int J Cancer. 1985;35:19–26.
Ammatuna P, Giovannelli L, Giambelluca D, Mancuso S, Rubino E, Colletti P, Mazzola G, Belfiore P, Lima R. Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J Med Virol. 2000;62:410–5.
Arivananthan M, Yadav M, Kumar S. Detection of HHV-6 genotypes by in situ hybridization with variant-specific oligonucleotide probes. J Virol Methods. 1997;66:5–14.
Arnheim Dahlstrom L, Andersson K, Luostarinen T, Thoresen S, Ogmundsdottir H, Tryggvadottir L, Wiklund F, Skare GB, Eklund C, Sjolin K, et al. Prospective seroepidemiologic study of human papillomavirus and other risk factors in Cervical cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2541–50.
Aurelian L, Davis HJ, Julian CG. Herpesvirus type 2-induced, tumor-specific antigen in cervical carcinoma. Am J Epidemiol. 1973;98:1–9.
Becker TM, Lee F, Daling JR, Nahmias AJ. Seroprevalence of and risk factors for antibodies to herpes simplex viruses, Hepatitis B, and Hepatitis C among southwestern hispanic and non-hispanic white women. Sex Transm Dis. 1996;23:138–44.
Bell RB, Aurelian L, Cohen GH. Proteins of herpes virus type 2 IV. Leukocyte inhibition responses to type common antigen(s) in cervix cancer and recurrent herpetic Infections. Cell Immunol. 1978;41:86–102.
Biganzoli P, Frutos MC, Venezuela F, Mosmann J, Kiguen A, Pavan J, Ferreyra L, Cuffini C. Detection of human herpesvirus 6 (HHV-6) and human herpesvirus 7 (HHV-7) DNA in endocervical samples from a positive and negative HPV woman of Cordoba, Argentina. J Clin Pathol. 2020;73:30–4.
Broccolo F, Cassina G, Chiari S, Garcia-Parra R, Villa A, Leone BE, Brenna A, Locatelli G, Mangioni C, Cocuzza CE. Frequency and clinical significance of human beta-herpesviruses in cervical samples from Italian women. J Med Virol. 2008;80:147–53.
Brock KE, MacLennan R, Brinton LA, Melnick JL, Adam E, Mock PA, Berry G. Smoking and infectious agents and risk of in situ Cervical cancer in Sydney, Australia. Cancer Res. 1989;49:4925–8.
Chan PK, Li WH, Chan MY, Cheng AF. Detection of human herpesvirus 8 in cervical cells of Chinese women with abnormal papanicolaou smears. Clin Infect Dis. 1999;29:1584–5.
Choi NW, Shettigara PT, Abu-Zeid HA, Nelson NA. Herpesvirus Infection and cervical anaplasia: a seroepidemiological study. Int J Cancer. 1977;19:167–71.
Dale GE, Coleman RM, Best JM, Benetato BB, Drew NC, Chinn S, Papacosta AO, Nahmias AJ. Class-specific herpes simplex virus antibodies in sera and cervical secretions from patients with cervical neoplasia: a multi-group comparison. Epidemiol Infect. 1988;100:445–65.
Daxnerova Z, Berkova Z, Kaufman RH, Adam E. Detection of human cytomegalovirus DNA in 986 women studied for human papillomavirus-associated cervical neoplasia. J Low Genit Tract Dis. 2003;7:187–93.
de Abreu AL, Malaguti N, Souza RP, Uchimura NS, Ferreira EC, Pereira MW, Carvalho MD, Pelloso SM, Bonini MG, Gimenes F, Consolaro ME. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am J Cancer Res. 2016;6:1371–83.
Farivar TN, Johari P, Shafei S, Najafipour R. Lack of association between herpes simplex virus type 2 Infection and Cervical cancer–taq man realtime PCR assay findings. Asian Pac J Cancer Prev. 2012;13:339–42.
Feng M, Duan R, Gao Y, Zhang H, Qiao Y, Li Q, Zhao F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Intraepithelial Neoplasia in Chinese women Living with HIV. Front Cell Infect Microbiol. 2021;11:703259.
Ferrera A, Baay MF, Herbrink P, Figueroa M, Velema JP, Melchers WJ. A sero-epidemiological study of the relationship between sexually transmitted agents and Cervical cancer in Honduras. Int J Cancer. 1997;73:781–5.
Graham S, Rawls W, Swanson M, McCurtis J. Sex partners and herpes simplex virus type 2 in the epidemiology of cancer of the cervix. Am J Epidemiol. 1982;115:729–35.
Gupta MM, Jain R, Parashari A, Singh V, Satyanarayana L. HSV-IgA serum antibodies in cervical intraepithelial neoplasia and invasive cancer patients, and in their spouses: a case control study. APMIS. 1992;100:598–604.
Janda Z, Kanka J, Vonka V, Svoboda B. A study of herpes simplex type 2 antibody status in groups of patients with cervical neoplasia in Czechoslovakia. Int J Cancer. 1973;12:626–30.
Kahla S, Oueslati S, Achour M, Kochbati L, Chanoufi MB, Maalej M, Oueslati R. Correlation between Ebv co-infection and HPV16 genome integrity in Tunisian Cervical cancer patients. Braz J Microbiol. 2012;43:744–53.
Kalimo K, Terho P, Honkonen E, Gronroos M, Halonen P. Chlamydia trachomatis and herpes simplex virus IgA antibodies in cervical secretions of patients with cervical atypia. Br J Obstet Gynaecol. 1981;88:1130–4.
Kawana T, Cornish JD, Smith MF, Aurelian L. Frequency of antibody to a virus-induced tumor-associated antigen (AG-4) in Japanese sera from patients with Cervical cancer and controls. Cancer Res. 1976;36:1910–4.
Kessler II, Kulcar Z, Rawls WE, Smerdel S, Strnad M, Lilienfeld AM. Cervical cancer in Yugoslavia. I. antibodies to genital herpesvirus in cases and controls. J Natl Cancer Inst. 1974;52:369–76.
Khenchouche A, Sadouki N, Boudriche A, Houali K, Graba A, Ooka T, Bouguermouh A. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol J. 2013;10:340.
Kienka T, Varga MG, Caves J, Smith JS, Sivaraman V. Epstein-Barr virus, but not human cytomegalovirus, is associated with a high-grade human papillomavirus-associated cervical lesions among women in North Carolina. J Med Virol. 2019;91:450–6.
Kumar A, Selim MS, Madden DL, Wallen WC, Vasquez HH, Nankervis GA. Humoral-and cell-mediated immune responses to herpesvirus antigens in patients with cervical carcinoma. Gynecol Oncol. 1980;10:18–25.
Landers RJ, O’Leary JJ, Crowley M, Healy I, Annis P, Burke L, O’Brien D, Hogan J, Kealy WF, Lewis FA, et al. Epstein-Barr virus in normal, pre-malignant, and malignant lesions of the uterine cervix. J Clin Pathol. 1993;46:931–5.
Lehtinen M, Hakama M, Knekt P, Heinonen PK, Lehtinen T, Paavonen J, Teppo L, Leinikki P. Lack of serum antibodies to the major HSV-2 specified DNA-binding protein before diagnosis of cervical neoplasia. J Med Virol. 1989;27:131–6.
Lehtinen M, Hakama M, Aaran RK, Aromaa A, Knekt P, Leinikki P, Maatela J, Peto R, Teppo L. Herpes simplex virus type 2 Infection and Cervical cancer: a prospective study of 12 years of follow-up in Finland. Cancer Causes Control. 1992;3:333–8.
Lehtinen M, Dillner J, Knekt P, Luostarinen T, Aromaa A, Kirnbauer R, Koskela P, Paavonen J, Peto R, Schiller JT, Hakama M. Serologically diagnosed Infection with human papillomavirus type 16 and risk for subsequent development of cervical carcinoma: nested case-control study. BMJ. 1996;312:537–9.
Marinho-Dias J, Ribeiro J, Monteiro P, Loureiro J, Baldaque I, Medeiros R, Sousa H. Characterization of cytomegalovirus and epstein-barr virus Infection in cervical lesions in Portugal. J Med Virol. 2013;85:1409–13.
McCormick TM, Canedo NH, Furtado YL, Silveira FA, de Lima RJ, Rosman AD, Almeida Filho GL, Carvalho Mda G. Association between human papillomavirus and Epstein - Barr virus DNA and gene promoter methylation of RB1 and CDH1 in the cervical lesions: a transversal study. Diagn Pathol. 2015;10:59.
McDonald AD, Williams MC, Manfreda J, West R. Neutralizing antibodies to herpesvirus types 1 and 2 in carcinoma of the cervix, carcinoma in situ and cervical dysplasia. Am J Epidemiol. 1974;100:130–5.
Mendis LN, Best JM, Banatvala JE. Class-specific antibodies (IgG and IgA) to membrane antigens of herpes simplex type 2-infected cells in patients with cervical dysplasia and neoplasia. Int J Cancer. 1981;27:669–77.
Mendis LN, Best JM, Senarath L, Chiphangwi J, Vestergaard BF, Banatvala JE. A geographical study of antibodies to membrane antigens of HSV-2-infected cells and HSV-2-specific antibodies in patients with Cervical cancer. Int J Cancer. 1981;28:535–42.
Munoz N, Kato I, Bosch FX, De Sanjose S, Sundquist VA, Izarzugaza I, Gonzalez LC, Tafur L, Gili M, Viladiu P, et al. Cervical cancer and herpes simplex virus type 2: case-control studies in Spain and Colombia, with special reference to immunoglobulin-G sub-classes. Int J Cancer. 1995;60:438–42.
Nahmias AJ, Naib ZM, Josey WE. Epidemiological studies relating genital herpetic Infection to cervical carcinoma. Cancer Res. 1974;34:1111–7.
Najem SN, Vestergaard BF, Potter CW. Herpes simplex virus type-specific antibodies detected by indirect and competition ELISA. Comparison of sera from patients with carcinoma of the uterine cervix, age matched controls and patients with recurrent genital herpes. Acta Pathol Microbiol Immunol Scand B. 1983;91:205–7.
Peng HQ, Liu SL, Mann V, Rohan T, Rawls W. Human papillomavirus types 16 and 33, herpes simplex virus type 2 and other risk factors for Cervical cancer in Sichuan Province, China. Int J Cancer. 1991;47:711–6.
Perez LO, Barbisan G, Abba MC, Laguens RM, Dulout FN, Golijow CD. Herpes simplex virus and human papillomavirus Infection in cervical Disease in Argentine women. Int J Gynecol Pathol. 2006;25:42–7.
Santos NB, Villanova FE, Andrade PM, Ribalta J, Focchi J, Otsuka AY, Dale Silva I. Epstein-Barr virus detection in invasive and pre-invasive lesions of the uterine cervix. Oncol Rep. 2009;21:403–5.
Sasagawa T, Shimakage M, Nakamura M, Sakaike J, Ishikawa H, Inoue M. Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive Cervical cancer: a comparative study with human papillomavirus (HPV) Infection. Hum Pathol. 2000;31:318–26.
Se Thoe SY, Wong KK, Pathmanathan R, Sam CK, Cheng HM, Prasad U. Elevated secretory IgA antibodies to Epstein-Barr virus (EBV) and presence of EBV DNA and EBV receptors in patients with cervical carcinoma. Gynecol Oncol. 1993;50:168–72.
Seo SS, Kim WH, Song YS, Kim SH, Kim JW, Park NH, Kang SB, Lee HP. Epstein-Barr virus plays little role in cervical carcinogenesis in Korean women. Int J Gynecol Cancer. 2005;15:312–8.
Shimakage M, Sasagawa T. Detection of Epstein–Barr virus-determined nuclear antigen-2 mRNA by in situ hybridization. J Virol Methods. 2001;93:23–32.
Shoji Y, Saegusa M, Takano Y, Hashimura M, Okayasu I. Detection of the Epstein-Barr virus genome in cervical neoplasia is closely related to the degree of infiltrating lymphoid cells: a polymerase chain reaction and in situ hybridization approach. Pathol Int. 1997;47:507–11.
Silver MI, Paul P, Sowjanya P, Ramakrishna G, Vedantham H, Kalpana B, Shah KV, Gravitt PE. Shedding of Epstein-Barr virus and cytomegalovirus from the genital tract of women in a periurban community in Andhra Pradesh, India. J Clin Microbiol. 2011;49:2435–9.
Skinner GR, Whitney JE, Hartley C. Prevalence of type-specific antibody against type 1 and type 2 herpes simplex virus in women with abnormal cervical cytology: evidence towards pre-pubertal vaccination of sero-negative female subjects. Arch Virol. 1977;54:211–21.
Szkaradkiewicz A, Wal M, Kuch A, Pieta P. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) cervical Infections in women with normal and abnormal cytology. Pol J Microbiol. 2004;53:95–9.
Thiry L, Sprecher-Goldberger S, Hannecart-Pokorni E, Gould I, Bossens M. Specific non-immunoglobulin G antibodies and cell-mediated response to herpes simplex virus antigens in women with cervical carcinoma. Cancer Res. 1977;37:1301–6.
Vass-Sorensen M, Abeler V, Berle E, Pedersen B, Davy M, Thorsby E, Norrild B. Prevalence of antibodies to herpes simplex virus and frequency of HLA antigens in patients with preinvasive and invasive Cervical cancer. Gynecol Oncol. 1984;18:349–58.
Vestergaard BF, Hornsleth A, Pedersen SN. Occurrence of herpes- and adenovirus antibodies in patients with carcinoma of the cervix uteri. Measurement of antibodies to herpesvirus hominis (types 1 and 2), cytomegalovirus, EB-virus, and adenovirus. Cancer. 1972;30:68–74.
Vonka V, Kanka J, Hirsch I, Zavadova H, Krcmar M, Suchankova A, Rezacova D, Broucek J, Press M, Domorazkova E, et al. Prospective study on the relationship between cervical neoplasia and herpes simplex type-2 virus. II. Herpes simplex type-2 antibody presence in sera taken at enrollment. Int J Cancer. 1984;33:61–6.
Wilkie NM, Eglin RP, Sanders PG, Clements JB. The association of herpes simplex virus with squamous carcinoma of the cervix, and studies of the virus thymidine kinase gene. Proc R Soc Lond B Biol Sci. 1980;210:411–21.
Zhang Wei JS, Li Junyao L, Lihua XM, Xiaohong W, Ming S, Airu W, Jianheng S, Xixia W. Zhang Wenhua, Liu Zhiming: the Infection of EBV for cervical Epithelium-a New Caustive Agent in the development of cervical carcinomas? Chin J Cancer Res. 1992;4:23–9.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Blanco R, Carrillo-Beltran D, Munoz JP, Osorio JC, Tapia JC, Burzio VA, Gallegos I, Calaf GM, Chabay P, Aguayo F. Characterization of high-risk HPV/EBV co-presence in pre-malignant cervical lesions and squamous cell carcinomas. Microorganisms. 2022. 10.
Blanco R, Carrillo-Beltran D, Osorio JC, Calaf GM, Aguayo F. Role of Epstein-Barr Virus and Human Papillomavirus Coinfection in Cervical Cancer: Epidemiology, mechanisms and perspectives. Pathogens 2020, 9.
Funding
This work was supported by the CAMS Initiative for Innovative Medicine to MF (grant number 2021-I2M-1-004); the Program of Medical Discipline Leader in Yunnan Health System (No. D-2019023) and the Technological Innovation Talents of Yunnan Province (No.2019HB044) to M.F.; the Program of Medical Discipline Leader in Yunnan Health System (No. D-2019027) to H.Z.
Author information
Authors and Affiliations
Contributions
HZ and SC, research collection, cross-checking, data analysis, manuscript drafting and review. YX and YL, research collection, cross-checking, data analysis. GZ, data analysis and manuscript review. MF and YY, concept, design, research collection, data analysis, manuscript drafting and review. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Cai, S., Xia, Y. et al. Association between human herpesvirus infection and cervical carcinoma: a systematic review and meta-analysis. Virol J 20, 288 (2023). https://doi.org/10.1186/s12985-023-02234-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02234-5