Abstract
NF-κB is generally recognized as an important regulator of ageing, through its roles in cellular senescence and inflammatory pathways. Activated in virtually all cell-cell communication networks of the immune system, NF-κB is thought to affect age-related defects of both innate and adaptive immune cells, relevant to inflamm-ageing and declining adaptive immunity, respectively. Moreover, the family of NF-κB proteins that exist as heterodimers and homodimers exert their function beyond the immune system. Given their involvement in diverse areas such as DNA damage to metabolism, NF-κB has the potential to serve as linkages between known hallmarks of ageing. However, the complexity of NF-κB dimer composition, dynamic signaling, and tissue-specific actions has received relatively little attention in ageing research. Here, we discuss some areas where further research may bear fruit in our understanding the impact of NF-κB in healthy ageing and longevity.
Similar content being viewed by others
Background
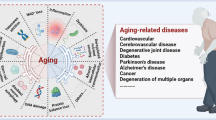
Ageing is one of the few universal features that directly impact all animals, including humans. Even though age-associated diseases or degeneration that limit lifespan may differ between individuals, there are recurring themes in the biological process of organismal ageing that had been termed the “hallmarks of ageing” [1]. While the existing concepts have fueled intense examination of several pathways such as nutrient sensing mTOR, SIRT1, p53, IGF-1, and telomere attrition, it is equally important to identify connections between seemingly unrelated mechanisms of ageing, or to discover new aspects of ageing biology. One of the master transcriptional regulators at the crossroads of immunity and ageing is nuclear factor kappa B (NF-κB), with its diverse roles implicated in nearly all the hallmarks of ageing.
In this mini review, we aim to expose relatively unexplored topics surrounding NF-κB in the spirit of ‘leave no stone unturned’ (Fig. 1). In both the innate and the adaptive immune systems, NF-κB senses danger signals and regulates the expression of cytokines and their receptors in a complex cell-cell communication cascade. Therefore, any age-related intrinsic defects of NF-κB signaling would have a direct impact on cell-cell communications within the immune system and with the surrounding microenvironments. Here we discuss evidence and ideas for the relevance of NF-κB in two concepts of immune ageing: inflamm-ageing and declining adaptive immunity (immuno-senescence). The cited studies are not necessarily drawn from the ageing research community. Rather, we attempted to compile independent reports which, when connected in the context of ageing, suggest potential age-related pathophysiological mechanisms. Thus, emphasis is on the areas that may warrant further investigations in the future, based on what has been learned so far.
Growing old alongside the complex structure and signaling of NF-κB
NF-κB is a ubiquitously expressed transcription factor which regulates expression of genes responsible for various biological processes, including immune responses, inflammation, cell proliferation, and apoptosis [2]. In mammalian cells, NF-κB exists as homodimers or heterodimers consisting of five members of the Rel family proteins which contain the DNA binding Rel homology domain (RHD), namely RelA (also known as p65), RelB, c-Rel, NF-κB1/p50, and NF-κB2/p52. The transcriptionally inactive p50 and p52 are generated from the precursors p105 and p100, respectively. These NF-κB subunits combine with each other to form different dimer molecules [3]. In unstimulated cells, the NF-κB dimers bind to the inhibitor of NF-κB proteins (IκBs), which are sequestered in the cytoplasm. Upon stimulation with various extracellular stimuli or stress, IκBs get degraded, the free NF-κB dimers translocate into the nucleus and reversibly bind to specific DNA binding motifs, and modulate the expression of hundreds of target genes [4]. As each of the dimer molecule has different affinities for consensus DNA binding motifs [5], a distinct gene expression profile might be induced depending on which dimers translocate into the nucleus and bind to the regulatory sites in the genome. Any changes in the NF-κB dimer composition would affect the DNA binding profile and consequent gene expression programs. Using the steady-state fluorescence anisotropy assay, Ramsey et al. have shown that the differential presence of IκBs alters the equilibrium concentrations of NF-κB dimers [6]. Thus, any changes in the expression of NF-κB subunits or the inhibitory IκB proteins would affect the NF-κB:DNA interactions and gene expression as a consequence.
Although alterations of the NF-κB pathway have been reported in physiological ageing, no study has been carried out to directly determine the changes in NF-κB dimer composition with ageing. Genetic perturbation studies have used knock-out or overexpression of one or more subunits of NF-κB or IκBs which alter the equilibrium dimer composition. These studies indirectly suggest that altered NF-κB dimer composition may be an important factor in ageing. Nfκb1−/− mice deficient in both the p105 precursor and p50 subunit show constitutive activation of NF-κB by RelA/RelA homodimer, which promotes premature ageing phenotype [7, 8]. Deletion of c-Rel subunit in mouse model develops Parkinson’s diseases (PD)-like neuropathology with ageing [9, 10]. On the other hand, systematic deletion of IκBα from the CNS in mice induces activation of NF-κB in neurons and astroglia and leads to increased Aβ production and promotes Alzheimer´s disease (AD) progression at an early age [11]. In Sirt6-/- mice, which have an accelerated ageing phenotype, the haploinsufficiency of RelA results in improved growth and a longer lifespan in Sirt6-/-Rela+/- mice compare to the control Sirt6-/- littermates [12]. The RelA haploinsufficiency in aged muscle-derived stem/progenitor cells (MDSPCs) led to increased myogenic potential and higher resistance to oxidative stress [13].
Direct measurements of the NF-κB dimer composition in physiological ageing require a longitudinal study of animal models using either a sensitive and high-throughput single-cell proteomics assay or live-cell analyses of dimers in individual cells. Savas Tay’s group has proposed a single-cell multiplex analysis method termed proximity-sequencing (Prox-seq) to simultaneously measure proteins and protein complexes as well as mRNAs in individual cells. This newly developed method can be leveraged for the quantitative measurement of NF-κB dimer composition in single cells and the changes with ageing [14]. Using the number and brightness assay, Martin et al. [15] have demonstrated a quantitative measurement technique for the live-cell measurement of NF-κB dimer composition, reporting that the proportion of RelA:RelA homodimers is higher than expected in the nucleus of TNF-α stimulated fibroblast cells. A few groups [16, 17] have generated fluorescent knock-in reporter mouse models expressing a fluorescent fusion of the RelA subunit from the endogenous locus. We have also generated mouse models expressing RelA and/or c-Rel subunit fused to either mEGFP and/or mScarlet. These mouse models can be used as a tool to measure the endogenous NF-κB dimer composition using fluorescent correlation or cross-correlation spectroscopy (FCS or FCCS) assays in live cells. These experimental techniques will help answer the question how NF-κB dimer composition changes with ageing.
Apart from the NF-κB dimer composition, post-transcriptional modifications such as the site-specific phosphorylation of the NF-κB subunits can affect the interactions, stability, degradation, and transcriptional activity of NF-κB dimers. Certain phosphorylation events might control the selectivity of NF-κB transcriptional activity in a gene-specific manner. The phosphorylation status of all the NF-κB subunits, the corresponding activating protein kinases, and their potential biological effects have been reviewed elsewhere [18]. However, only a handful of studies have reported age-associated changes in phosphorylation status of different NF-κB subunits. During skin ageing, increased phosphorylation of IKKα (Thr23) was observed in the nucleus which induced the phosphorylation of RelA at Ser536 and enhanced the NF-κB activity through increased DNA binding [19]. Another study reported an increase of phosphorylated RelA in hypothalamus and cortical tissues with ageing [20]. More longitudinal studies are required to understand the functional relevance of all the possible age-related changes in post-translational modifications of NF-κB subunits in ageing biology.
From stress to signaling: hypertension, mechanical tissue strain, and oxidative stress can activate NF-κB
There are various in vivo stresses that can activate the NF-κB signaling pathway including shear stress, mechanical strain, and oxidative stress. Generally, arterial endothelial cells experience mainly two types of shear stresses: pulsatile shear stress (PS) and oscillatory shear stresses (OS). In the human umbilical vein endothelial cells (HUVEC), NF-κB appeared to be activated in response to PS and OS flows [21]. During the development of atherosclerosis, shear stress in endothelial cells (ECs) and smooth muscle alters NF-κB signaling which induces pro-inflammation cytokines, chemokines, and adhesion molecules in vascular ECs, promoting monocyte recruitment and disease progression [22, 23]. In addition, acute shear stress in ECs appears to activate NF-κB in an integrin-dependent manner. The signaling downstream of integrin triggers activation of the classical NF-κB pathway [23].
Age-related diseases including osteoporosis and sarcopenia may be conditions where NF-κB is activated via mechanical strains. Exposure of articular cartilage to excessive mechanical loading is strongly associated with the pathogenesis of osteoarthritis. Gremlin-1 is a mechanical loading-inducible factor in chondrocytes and activates NF-κB signaling, resulting in a subsequent induction of catabolic enzymes [24]. Sarcopenia, defined as the loss of skeletal muscle mass and strength, is a common feature of ageing. Muscle unloading or loss of muscle innervation led to an 8-fold increase in NF-κB activation [25]. Studies also illustrate a constitutive activation of NF-κB in aged muscle, even though the mechanism in the context of sarcopenia is still unclear [26]. In addition, mechanical stimulation could activate the classical NF-κB pathway in osteoblasts and related cells [27]. Depending on the degree of the stimulus, the NF-κB pathway is either inhibited by low tensile changes or activated by high stresses [27].
NF-κB is recognized as a redox-sensitive transcription factor and involved in the cellular response to oxidative stress [28]. With ageing, oxidative stress is accumulated due to higher rates of reactive oxidative species (ROS) generation, mitochondrial dysfunction, defects in electron transport, and additional oxidative stress from other age-related conditions [28, 29]. Exposure to ROS causes damages to macromolecules, including DNA, proteins, and lipids. Interestingly, ROS are important regulator of TNF-α signaling which can promote either NF-κB activation or inhibition leading to cell survival or death [30]. NF-κB heterodimers may be modified when cells accumulate oxidative stress. For example, Cys-62 in the RHD domain of p50 subunit may be oxidized, potentially leading to increased DNA binding [31]. Phosphorylation of Ser-276 on RelA has been shown to be ROS-dependent, which is required for transcriptional activation of some NF-κB target genes [31].
Tissue-specific senescence and SASP of macrophages and their vulnerability to DNA damage in ageing
Cellular senescence is a state of stable cell cycle arrest, generally considered to fuel the ageing process [32]. Senescent cells secrete various pro-inflammatory cytokines, chemokines, growth and angiogenic factors, referred to as senescence-associated secretory phenotype (SASP) [33, 34]. SASP recruits other immune cells via chemotaxis, one such cell type being macrophages. Macrophages are professional phagocytic cells that play a key role in the physiological clearance of dying cells and senescent cells [35, 36]. But the other side of the story is that macrophages themselves may become senescent in age-related inflammation. Expression of p16INK4a and senescence-associated beta-galactosidase (SA-β-Gal) in macrophages increase with age and might amplify the cellular senescence and the SASP during ageing [37,38,39,40]. There is a two-way interaction between the senescent cells and macrophages, known as paracrine senescence [38], where either of the cells can be the first to show increased p16INK4a expression. Also, senescent cells can skew macrophage phenotypes in a context-dependent manner. p53-expressing senescent stellate cells induced M1 polarization of macrophages [41], whereas senescent thyrocytes induced M2 polarization in macrophages [42]. The microenvironment within the ageing tissue can fuel the senescence in tissue-resident macrophages such as microglia [43], Kupffer cells [44], splenic macrophages [45], alveolar macrophages [46] and peritoneal macrophages [47]. Increased cell volume, shortened life-span, and disordered distribution of tissue-resident macrophages with age may reduce their interaction with surrounding cells and hamper their function [48]. However, the in vivo effects of senescent tissue-resident macrophages need to be investigated further.
Another factor that triggers senescence is accumulated DNA damage. Various pharmacological agents (chemotherapeutics), genotoxic agents (ionizing UV radiation), and oxidative stress are known to induce senescence. Senescent cells carry persistent nuclear DNA damage foci called as DNA-SCARSs (DNA segments with chromatin alterations reinforcing senescence). DNA-SCARSs lack the DNA repair proteins RPA and RAD51 and associate with promyelocytic leukemia (PML) nuclear bodies. They also harbor activated CHK2 and p53 molecules [49]. Protein damage or proteotoxicity induced by molecules such as ROS, protein tyrosine phosphatases, and Lipofuscin can also influence the senescence process [50, 51]. Senescent cells are also reported to show altered lipid metabolism, such as ROS-induced lipid damage, lipid deposition, and lipid modifications [52, 53].
The ataxia telangiectasia mutated (ATM) kinase is thought to play a critical role in the initiation of SASP through the DNA damage response [54, 55]. ATM activation triggers several downstream pathways including NEMO, an NF-κB essential modulator. NEMO is phosphorylated at Ser85 by activated ATM, resulting in sumoylation and mono-ubiquitination of NEMO at Lys277 and 309. After post-translational modifications, the ATM/NEMO complexes are exported from the nucleus to the cytoplasm, activating the IKKα/β complex, leading to NF-κB signaling [55, 56].
Overall, cellular senescence has been recognized to promote ageing and an array of diseases. However, the heterogeneity and incomplete information about macrophage senescence in terms of cause and effects raise concerns in designing the drugs or chemotherapeutic agents that targets all senescent cells rather than a sub-population of maladaptive macrophages.
Do NF-κB signaling dynamics get muffled as the language of cell-cell communication in ageing?
Cell-cell communications through direct interactions or messenger molecules are essential for the integrity and stress responses of multicellular organisms. Dysregulation of cell communication pathways is associated with ageing and thought to promote age-related diseases [57]. Although senescent cells can be found in almost all the tissues of aged animals and humans, the senescent populations might originate initially from a small number of damaged cells, and propagate through the neighboring and remote cells or tissues via SASP [57,58,59]. NF-κB acts as a major transcription factor on the chromatin of senescent cells, and thus controls both the cell-autonomous and non-cell-autonomous aspects of the senescence program [58]. The cell-to-cell communication and propagation of NF-κB signaling are also crucial for activation of innate immunity against bacterial infections. After infection with entero-invasive bacterium Shigella flexneri in Shigelosis, the host intestinal epithelial cells (IECs) show activation of NF-κB with IL-8 induction which propagates from the infected to uninfected adjacent cells and generates rapid amplification of IL-8 production by uninfected bystander cells [60]. Dysregulation of NF-κB signaling and consequent defects in balanced cellular communication thought to occur with ageing compromise the effectiveness of innate immune system function. Emerging evidence also suggest that infected immune cells can transfer the pathogen associated molecular patterns (PAMPs) and derived signaling molecules to the non-infected cells, which then activate the bystander cells to mount a self-sustaining and amplified innate immune response [61].
NF-κB signaling is widely studied in macrophages where they get activated by PAMPs and DAMPs and secrete a large array of cytokines and chemokines. NF-κB promotes the differentiation of macrophages into M1 and M2 phenotypes, and also regulates the differentiation of naive CD4 T cells into Th1 and Th17 subtypes [62, 63]. Both subunits RelA and c-Rel play crucial roles in mediating the TCR signaling in naïve T cells during their activation and differentiation into Th1, Th2, Th17, Tregs, and Tfh cells [64]. Collectively, both canonical and noncanonical NF-κB pathways are responsible for the generation and function of effectors of adaptive immunity. A key feature of bridging the innate and adaptive immunity, which is crucial for the immune system, is that antigen-presenting cells (APCs) prime and train the naïve T cells via MHC (Major Histocompatibility Class) I and II molecules. For example, the c-Rel subunit of NF-κB is important for the delicate interactions between T cells and APCs [65]. NF-κB is one of the key signaling pathways that support the successful interplay of different immune and non-immune cells for a functional host response acting on both inter-cellular and intra-cellular levels. Overall, altered features of NF-κB signaling dynamics, such as peak timing, amplitude, or duration, which may occur in ageing might have substantial effects on the above-mentioned cell-to-cell communications in vivo. It is possible that such quantitative changes, albeit subtle, may be sufficient for the emergence of senescence phenotypes and impairment of innate and adaptive immune responses [66, 67].
Weakened tissue barriers may prime inflammatory NF-κB signaling
The physical barriers that prevent exchange of content between different tissue compartments are critically important for organismal health. Examples of such tissue boundaries include the blood-brain barrier (BBB), the gut epithelium, and the skin epidermis. The barrier function is particularly essential for tissues that are exposed to the environmental microbes and preventing them from reaching deeper tissues. However, the integrity of these tissue barriers is compromised in ageing [68, 69], which is thought to promote a low-grade chronic inflammatory state, termed inflamm-ageing.
The gut epithelium is exposed to the microbiota which undergoes age-associated compositional changes [70, 71]. Gut inflammation can be modulated by a variety of factors, including the longevity-associated factor SIRT1 that inhibits NF-κB inflammatory signaling [72]. Ageing or alcohol consumption may increase gut permeability and cause a leakage of microbial products, contributing to liver inflammation. However, the dose of alcohol that causes leaky gut is still debated [73]. Given that red wine consumption is generally considered beneficial due to the reported benefits of resveratrol for healthy ageing [74], there might be a trade-off between the effects of alcohol and resveratrol in wine. Notably, the doses of resveratrol that promote healthy ageing have not been determined [75,76,77,78].
The dose and timing of prior exposure to microbial products can skew subsequent responses with either tolerogenic or priming effects in macrophages [79]. While adaptive immune memory is generally advantageous for the host, “innate memory”, a more recently introduced concept, may be a double-edged sword [80]. Innate memory, albeit shorter-term than adaptive memory, may be constantly reinforced by elevated endotoxins leaking through the aged barriers, driving inflammaging at sites such as BBB, gut, and skin [68, 81].
Circadian disruption and NF-κB in ageing
In populations with western-style diets, chronic inflamm-ageing processes may be accelerated by further inflammatory signaling and the alteration of circadian rhythm [82, 83]. In high fat diet (HFD)-induced obesity, the adipose tissue-resident macrophages (ATMs) play a pivotal role in modulation of pro-inflammatory cytokines such as TNF-α and IL-1β [84]. ATMs may be therapeutically targeted to ameliorate the obesogenic potential of HFD in ageing populations. Better understanding of how inflammatory signaling and circadian clock are altered in ATMs during HFD-induced obesity will be important for developing strategies that combine drugs, dietary interventions, and chronotherapy to counter age-related obesity.
The circadian clock system may also be involved in longevity-inducing mechanisms of calorie restriction (CR). In a recent study using accurate monitoring of animal behaviors, Joseph Takahashi and colleagues revealed an important caveat of CR studies. Calorie-restricted mice were binge-feeding on a narrow time window, confounding the interpretation of previous CR data for the role of food intake amount only [85]. Because time-restricted feeding within the metabolically active phase confers resistance to obesity [86], future studies should decouple the effects of CR versus circadian regulation in examination of diet effects on ageing.
Circadian rhythm and inflammatory signaling have generally been examined separately, and more studies need to uncover how the two pathways intersect with each other. Their crosstalk is gaining more attention after studies showed convincing evidence of direct interactions between transcriptional regulators of the two systems [87, 88]. Perhaps a most direct link is provided by the transcriptional activation of NF-κB by the Clock, a key regulator of the circadian clock [89]. Conversely, NF-κB was found to be required for maintaining circadian rhythms in mice [90].
Conclusions
NF-κB is an essential transcription factor for regulating rapid innate responses and long-term adaptive immune responses and memory through T and B lymphocytes. Hence, it is uniquely positioned to affect the opposite aspects of the age-related immune dysregulation: inflamm-ageing (mediated largely by innate immune cells of the myeloid lineage) and declining adaptive immunity (through intrinsic defects of lymphocytes in signaling and proliferation). Building upon the exciting discoveries about the ageing of the immune system to date, the research community may find it rewarding to go beyond the “usual suspects”. For example, novel insights may come from studies of NF-κB and related mechanisms underlying the inter-relationships between different hallmarks of ageing. Resolving the relevant questions will require innovative technical approaches, improved animal models, as well as new conceptual frameworks.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- ATM:
-
Ataxia telangiectasia mutated
- ATMs:
-
Adipose tissue macrophages
- BBB:
-
Blood brain barrier
- CNS:
-
Central nervous system
- CR:
-
Calorie restriction
- EC:
-
Endothelial cell
- DNA-SCARS:
-
DNA segments with chromatin alterations reinforcing senescence
- FCCS:
-
Fluorescence cross-correlation spectroscopy
- HFD:
-
High fat diet
- IEC:
-
Intestinal epithelial cell
- IL:
-
Interleukin
- mEGFP:
-
Monomeric enhanced green fluorescent protein
- NF-κB:
-
Nuclear factor kappaB
- PAMP:
-
Pathogen-associated molecular pattern
- RHD:
-
Rel homology domain
- ROS:
-
Reactive oxygen species
- SASP:
-
Senescence-associated secretory phenotype
- TCR:
-
T cell receptor
- Th:
-
T helper cell
- TNF:
-
Tumor necrosis factor
- UV:
-
Ultraviolet
References
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153(6):1194–217.
Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes & diseases. 2021;8(3):287–97.
Hoffmann A, Baltimore D. Circuitry of nuclear factor κB signaling. Immunological reviews. 2006;210(1):171–86.
Ghosh S, HAYDEN M, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–62.
Siggers T, Chang AB, Teixeira A, Wong D, Williams KJ, Ahmed B, Ragoussis J, Udalova IA, Smale ST, Bulyk ML. Principles of dimer-specific gene regulation revealed by a comprehensive characterization of NF-κB family DNA binding. Nature immunology. 2012;13(1):95–102.
Ramsey KM, Chen W, Marion JD, Bergqvist S, Komives EA. Exclusivity and Compensation in NFκB Dimer Distributions and IκB Inhibition. Biochemistry. 2019;58(21):2555–63.
Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature communications. 2014;5(1):1–14.
Bernal GM, Wahlstrom JS, Crawley CD, Cahill KE, Pytel P, Liang H, Kang S, Weichselbaum RR, Yamini B. Loss of Nfkb1 leads to early onset aging. Aging (Albany NY). 2014;6(11):931.
Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V. Late-onset Parkinsonism in NFκB/c-Rel-deficient mice. Brain. 2012;135(9):2750–65.
Parrella E, Bellucci A, Porrini V, Benarese M, Lanzillotta A, Faustini G, Longhena F, Abate G, Uberti D, Pizzi M. NF-κB/c-Rel deficiency causes Parkinson’s disease-like prodromal symptoms and progressive pathology in mice. Translational neurodegeneration. 2019;8(1):1–20.
Lian H, Yang L, Cole A, Sun L, Chiang AC-A, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85(1):101–15.
Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74.
Proto JD, Lu A, Dorronsoro A, Scibetta A, Robbins PD, Niedernhofer LJ, Huard J. Inhibition of NF-κB improves the stress resistance and myogenic differentiation of MDSPCs isolated from naturally aged mice. Plos one. 2017;12(6):e0179270.
Vistain L, Van Phan H, Jordi C, Chen M, Reddy ST, Tay S: Quantification of proteins, protein complexes and mRNA in single cells by proximity-sequencing. bioRxiv 2020.
Martin EW, Chakraborty S, Presman DM, Tomassoni Ardori F, Oh K-S, Kaileh M, Tessarollo L, Sung M-H. Assaying Homodimers of NF-κB in Live Single Cells. Frontiers in immunology. 2019;10:2609.
Cheng QJ, Ohta S, Sheu KM, Spreafico R, Adelaja A, Taylor B, Hoffmann A. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science. 2021;372(6548):1349–53.
De Lorenzi R, Gareus R, Fengler S, Pasparakis M. GFP-p65 knock‐in mice as a tool to study NF‐κB dynamics in vivo. Genesis. 2009;47(5):323–9.
Christian F, Smith E, Carmody R. The regulation of NF-kappaB subunits by phosphorylation. Cells. 2016;5(1):12. https://doi.org/10.3390/cells5010012.
Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. The NF-κB activation in lymphotoxin β receptor signaling depends on the phosphorylation of p65 at serine 536. Journal of Biological Chemistry. 2003;278(2):919–26.
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497(7448):211–6.
Maurya MR, Gupta S. Li JY-S, Ajami NE, Chen ZB, Shyy JYJ, Chien S, Subramaniam S: Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proceed Natl Acad Sci. 2021;118(4):e2023236118.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2017;2(1):17023.
Ward AO, Caputo M, Angelini GD, George SJ, Zakkar M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis. 2017;265:266–74.
Chang SH, Mori D, Kobayashi H, Mori Y, Nakamoto H, Okada K, Taniguchi Y, Sugita S, Yano F, Chung U-, et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat Commun. 2019;10(1):1442.
Jeremy S, Tilstra CLCLJNPDR. NF-κB in aging and disease. Agi Dis. 2011;2(6):449–65.
Thoma A, Lightfoot AP: NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. In: Muscle Atrophy. Edited by Xiao J. Singapore: Springer Singapore; 2018: 267–279.
Novack DV. Role of NF-κB in the skeleton. Cell Research. 2011;21(1):169–82.
Mercurio F, Manning AM. NF-κB as a primary regulator of the stress response. Oncogene. 1999;18(45):6163–71.
Shields HJ, Traa A, Van Raamsdonk JM. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front Cell Develop Biol. 2021;9:628157.
Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016;26(4):249–61.
Lingappan K. NF-κB in oxidative stress. Current Opinion in Toxicology. 2018;7:81–6.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621.
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179(4):813–27.
Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94.
Egashira M, Hirota Y, Shimizu-Hirota R, Saito-Fujita T, Haraguchi H, Matsumoto L, Matsuo M, Hiraoka T, Tanaka T, Akaeda S, et al. F4/80 + Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology. 2017;158(7):2344–53.
Mevorach D, Trahtemberg U, Krispin A, Attalah M, Zazoun J, Tabib A, Grau A, Verbovetski-Reiner I. What do we mean when we write “senescence,“ “apoptosis,“ “necrosis,“ or “clearance of dying cells". Ann N Y Acad Sci. 2010;1209:1–9.
Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, et al. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY). 2016;8(7):1294–315.
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–90.
Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, Bulavin DV. Defined p16(High) Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020;32(1):87-99 e86.
Zealley B. Commentary on Some Recent Theses Relevant to Combating Aging: December 2021. Rejuvenation Res. 2021;24(6):464–9.
Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153(2):449–60.
Mazzoni M, Mauro G, Erreni M, Romeo P, Minna E, Vizioli MG, Belgiovine C, Rizzetti MG, Pagliardini S, Avigni R, et al. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J Exp Clin Cancer Res. 2019;38(1):208.
Trias E, Beilby PR, Kovacs M, Ibarburu S, Varela V, Barreto-Nunez R, Bradford SC, Beckman JS, Barbeito L. Emergence of Microglia Bearing Senescence Markers During Paralysis Progression in a Rat Model of Inherited ALS. Front Aging Neurosci. 2019;11:42.
Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14(2):156–65.
Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32(1):18–26.
Hinojosa CA, Akula Suresh Babu R, Rahman MM, Fernandes G, Boyd AR, Orihuela CJ. Elevated A20 contributes to age-dependent macrophage dysfunction in the lungs. Exp Gerontol. 2014;54:58–66.
Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47(7):507–18.
Hefendehl JK, Neher JJ, Suhs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014;13(1):60–9.
Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124(Pt 1):68–81.
Hohn A, Weber D, Jung T, Ott C, Hugo M, Kochlik B, Kehm R, Konig J, Grune T, Castro JP. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501.
Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, Kouloukoussa M, Kittas C, Georgakilas AG, Gorgoulis VG. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther. 2019;193:31–49.
Ademowo OS, Dias HKI, Burton DGA, Griffiths HR. Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontology. 2017;18(6):859–79.
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691.
Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cellular Signalling. 2012;24(4):835–45.
Miyamoto S. Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Research. 2011;21(1):116–30.
McCool KW, Miyamoto S. DNA damage-dependent NF-κB activation: NEMO turns nuclear signaling inside out. Immunological Reviews. 2012;246(1):311–26.
Fafián-Labora JA, O’Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends in Cell Biology. 2020;30(8):628–39.
Childs BG, Durik M, Baker DJ, Van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature medicine. 2015;21(12):1424–35.
Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO. Transplanted senescent cells induce an osteoarthritis-like condition in mice. The Journals of Gerontology: Series A. 2017;72(6):780–5.
Kasper CA, Sorg I, Schmutz C, Tschon T, Wischnewski H, Kim ML, Arrieumerlou C. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33(5):804–16.
Nguyen TA, Pang KC, Masters SL. Intercellular communication for innateimmunity. Mol Immunol. 2017;86:16–22.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Frontiers in immunology. 2014;5:614.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122(3):787–95.
Oh H, Ghosh S. NF-κB: roles and regulation in different CD 4 + T‐cell subsets. Immunological reviews. 2013;252(1):41–51.
Visekruna A, Volkov A, Steinhoff U. A key role for NF-κB transcription factor c-Rel in T-lymphocyte-differentiation and effector functions. Clinical and Developmental Immunology. 2012;2012:239368.
Thompson HL, Smithey MJ, Uhrlaub JL, Jeftic I, Jergovic M, White SE, Currier N, Lang AM, Okoye A, Park B, et al. Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates. Aging Cell. 2019;18(1):e12865.
Davies JS, Thompson HL, Pulko V, Padilla Torres J, Nikolich-Zugich J. Role of Cell-Intrinsic and Environmental Age-Related Changes in Altered Maintenance of Murine T Cells in Lymphoid Organs. J Gerontol A Biol Sci Med Sci. 2018;73(8):1018–26.
Hu L, Mauro TM, Dang E, Man G, Zhang J, Lee D, Wang G, Feingold KR, Elias PM, Man M-Q. Epidermal Dysfunction Leads to an Age-Associated Increase in Levels of Serum Inflammatory Cytokines. Journal of Investigative Dermatology. 2017;137(6):1277–85.
Hussain B, Fang C, Chang J. Blood-Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Frontiers in Neuroscience. 2021;15:688090.
Shintouo CM, Mets T, Beckwee D, Bautmans I, Ghogomu SM, Souopgui J, Leemans L, Meriki HD, Njemini R. Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Experimental Gerontology. 2020;141:111079.
Kim K-A, Jeong J-J, Yoo S-Y, Kim D-H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiology. 2016;16(1):9.
Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, Czopik A, Shanahan MT, Kang A, Chen W, et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology. 2017;153(3):772–86.
McMahan RH, Najarro KM, Mullen JE, Paul MT, Orlicky DJ, Hulsebus HJ, Kovacs EJ. A novel murine model of multi-day moderate ethanol exposure reveals increased intestinal dysfunction and liver inflammation with age. Immunity & Ageing. 2021;18(1):37.
Weiskirchen S, Weiskirchen R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv Nutr. 2016;7(4):706–18.
da Luz PL, Tanaka L, Brum PC, Dourado PMM, Favarato D, Krieger JE, Laurindo FRM. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis. 2012;224(1):136–42.
Anton SD, Ebner N, Dzierzewski JM, Zlatar ZZ. GurkaMJ, Dotson VM, Kirton J, Mankowski RT, Marsiske M, Manini TM: Effects of 90 days of resveratrol supplementation on cognitive function in elders: a pilot study. J Alternative Complement Med. 2018;24(7):725–32.
Chang C-C, Chang C-Y, Lin P-C, Huang J-P, Chen K-H, Yen T-H, Hung L-M. Administration of low-dose resveratrol attenuated hepatic inflammation and lipid accumulation in high cholesterol-fructose diet-induced rat model of nonalcoholic fatty liver disease. Chinese Journal of Physiology. 2020;63(4):149–55.
Mankowski RT, You L, Buford TW, Leeuwenburgh C, Manini TM, Schneider S, Qiu P, Anton SD. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults – A pilot study. Experimental Gerontology. 2020;131:110821.
Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–8.
Netea Mihai G, Joosten Leo AB, Latz E, Mills Kingston HG, Natoli G, Stunnenberg Hendrik G, O’Neill Luke AJ, Xavier Ramnik J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098.
Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? Journal of Experimental Medicine. 2017;214(11):3151–69.
Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. British Journal of Nutrition. 2011;106(S3):S1–78.
Budai Z, Balogh L, Sarang Z. Short-term high-fat meal intake alters the expression of circadian clock-, inflammation-, and oxidative stress-related genes in human skeletal muscle. International Journal of Food Sciences and Nutrition. 2019;70(6):749–58.
Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–17.
Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metabolism. 2017;26(1):267-277.e262.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong Eric A, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick James AJ, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metabolism. 2012;15(6):848–60.
Gachon F, Yeung J, Naef F. Cross-regulatory circuits linking inflammation, high-fat diet, and the circadian clock. Genes & Development. 2018;32(21–22):1359–60.
Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature Medicine. 2014;20(8):919–26.
Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, et al. Core circadian protein CLOCK is a positive regulator of NF-κB–mediated transcription. Proceed Natl Acad Sci. 2012;109(37):E2457–65.
Hong H-K, Maury E, Ramsey KM, Perelis M, Marcheva B, Omura C, Kobayashi Y, Guttridge DC, Barish GD, Bass J. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes & Development. 2018;32(21–22):1367–79.
Acknowledgements
We thank Kyu-Seon Oh for critical comments and suggestions on the manuscript.
Funding
Open Access funding provided by the National Institutes of Health (NIH). This work was supported entirely by the Intramural Research Program of the NIH, at National institute on Aging.
Author information
Authors and Affiliations
Contributions
P.S., M.A., S.M.T.R., and M.-H.S. wrote the manuscript. S.M.T.R. prepared Fig. 1 with input from all authors. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Songkiatisak, P., Rahman, S.M.T., Aqdas, M. et al. NF-κB, a culprit of both inflamm-ageing and declining immunity?. Immun Ageing 19, 20 (2022). https://doi.org/10.1186/s12979-022-00277-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-022-00277-w