Abstract
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has introduced the medical community to the phenomenon of long COVID, a condition characterized by persistent symptoms following the resolution of the acute phase of infection. Among the myriad of symptoms reported by long COVID sufferers, chronic fatigue, cognitive disturbances, and exercise intolerance are predominant, suggesting systemic alterations beyond the initial viral pathology. Emerging evidence has pointed to mitochondrial dysfunction as a potential underpinning mechanism contributing to the persistence and diversity of long COVID symptoms. This review aims to synthesize current findings related to mitochondrial dysfunction in long COVID, exploring its implications for cellular energy deficits, oxidative stress, immune dysregulation, metabolic disturbances, and endothelial dysfunction. Through a comprehensive analysis of the literature, we highlight the significance of mitochondrial health in the pathophysiology of long COVID, drawing parallels with similar clinical syndromes linked to post-infectious states in other diseases where mitochondrial impairment has been implicated. We discuss potential therapeutic strategies targeting mitochondrial function, including pharmacological interventions, lifestyle modifications, exercise, and dietary approaches, and emphasize the need for further research and collaborative efforts to advance our understanding and management of long COVID. This review underscores the critical role of mitochondrial dysfunction in long COVID and calls for a multidisciplinary approach to address the gaps in our knowledge and treatment options for those affected by this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic, triggered by the emergence of the novel coronavirus SARS-CoV-2, has precipitated an unprecedented global health crisis, the repercussions of which continue to resonate worldwide. While a significant proportion of those infected with COVID-19 recover, a startling observation has emerged: more than 70% of survivors experience lingering symptoms 4 months post-infection, giving rise to what is now recognized as “Long COVID Syndrome” or post-acute sequelae SARS-CoV-2 infection (PASC) [1,2,3,4,5,6,7,8,9,10,11,12]. This term encapsulates the enduring or emerging signs and symptoms that persist for weeks or months following the acute phase of COVID-19. Chronic fatigue emerges as the most frequently reported and debilitating symptom among survivors, as evidenced by numerous cross-sectional and cohort studies [13,14,15,16,17]. Individuals grappling with long COVID also report an extensive spectrum of symptoms in addition to chronic fatigue, including dyspnea, arthralgia, sleep disturbances, mood disorders such as depression and anxiety [18], headaches, dizziness, cognitive impairments colloquially termed as “brain fog,” and cardiac symptoms. The profound and diverse nature of these symptoms significantly undermines daily functioning and diminishes quality of life, imposing considerable personal, societal, and economic challenges. The persistent symptomatology of long COVID indicates that the impact of COVID-19 extends well beyond the respiratory system, implicating multiple organ systems and bodily functions, and highlights an urgent need for a comprehensive understanding of its long-term consequences on human health.
Mitochondria, the cellular organelles known as the powerhouses of the cell, are central to this discussion. They play a crucial role in cellular energy production through the process of oxidative phosphorylation, in addition to their involvement in oxidative stress, apoptosis (programmed cell death), induction of cellular senescence, and the modulation of immune responses. The function of mitochondria is essential for maintaining cellular and systemic homeostasis. Disruptions in mitochondrial function can lead to a decrease in energy production, increased production of reactive oxygen species (ROS), and initiation of inflammatory pathways, all of which can contribute to the pathophysiology of various diseases. The significance of exploring mitochondrial dysfunction in long COVID lies in the potential overlap between the symptoms of this condition and known consequences of mitochondrial impairment [19, 20]. Understanding how SARS-CoV-2 infection may lead to mitochondrial dysfunction could provide valuable insights into the mechanisms underlying long COVID symptoms. This knowledge could pave the way for novel therapeutic approaches aimed at mitigating these symptoms by targeting mitochondrial health and function. Thus, examining the role of mitochondrial dysfunction in long COVID not only offers a promising avenue for research but also holds the potential to improve the management and treatment of individuals suffering from this debilitating condition.
Evidence of mitochondrial dysfunction in long COVID
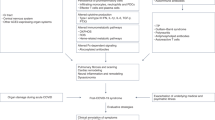
The emergence of long COVID as a distinct post-acute phase of SARS-CoV-2 infection has prompted investigations into its underlying pathophysiological mechanisms. Among the various avenues explored, evidence pointing towards mitochondrial dysfunction has garnered significant attention [19,20,21,22,23]. A growing body of research suggests that mitochondrial impairment may play a crucial role in the constellation of symptoms observed in long COVID patients [19,20,21,22,23,24,25,26,27,28,29,30,31,32] (Fig. 1). Several studies have documented mitochondrial dysfunction in individuals suffering from long COVID, providing insights into how this condition may perpetuate the diverse and persistent symptoms associated with the syndrome. For instance, research has identified abnormalities in mitochondrial respiration and bioenergetics and mitochondria-related gene expression in peripheral blood mononuclear cells (PBMCs) from long COVID patients [33,34,35,36,37]. These abnormalities are indicative of compromised mitochondrial energy production, which could underlie symptoms of fatigue and muscle weakness. Moreover, studies employing magnetic resonance spectroscopy (MRS) have observed alterations in muscle tissue and the brain indicative of mitochondrial dysfunction in long COVID sufferers [38,39,40,41], although data obtained in the heart are inconclusive [33, 42]. Such findings align with clinical reports of exercise intolerance and post-exertional malaise among these individuals [43].
Interplay of mitochondrial dysfunction and long COVID: pathophysiological mechanisms and therapeutic approaches. This figure presents a comprehensive overview of mitochondrial dysfunction in long COVID, illustrating the relationship between mitochondrial impairment and the diverse symptoms of long COVID, as well as potential therapeutic interventions. COVID-19-related mitochondrial dysfunction leads to impaired cellular energy production, increased oxidative stress, and the induction of inflammatory responses. Mitochondrial dysfunction is causally linked to key symptoms associated with long COVID—cognitive disturbances (brain), fatigue and muscle weakness (muscle), breathlessness (lung), and cardiac symptoms (heart)—linked to mitochondrial dysfunction through mechanisms such as energy production deficits, oxidative stress, immune response dysregulation, metabolic disruptions, and vascular and endothelial dysfunction. Therapeutic strategies targeting mitochondrial health, including antioxidants, exercise, dietary modifications, and pharmacological interventions, may mitigate the aforementioned symptoms emphasizing the multifaceted approach required to address long COVID. The importance of further research into mitochondrial function as a potential therapeutic target for long COVID is emphasized, highlighting areas for future investigation such as biomarker identification, longitudinal studies, and the development of novel therapeutics
Biomarkers of mitochondrial dysfunction observed in long COVID
Further evidence of mitochondrial involvement in long COVID comes from biomarker studies. Elevated levels of circulating biomarkers associated with oxidative stress and mitochondrial damage, such as F2-isoprostanes, malondialdehyde, and reduced levels of antioxidants like coenzyme Q10, have been reported [20, 21, 44,45,46,47,48]. These biomarkers point towards oxidative stress as a contributing factor to mitochondrial dysfunction in long COVID. Additionally, genomic studies have identified expression changes in genes associated with mitochondrial function and the cellular response to viral infections in COVID-19 patients [49,50,51,52]. These changes may reflect direct viral effects on mitochondrial integrity and function or secondary effects related to the immune response to the infection. Collectively, these studies offer compelling evidence that mitochondrial dysfunction is a significant contributor to the pathophysiology of long COVID. By impairing energy production and exacerbating oxidative stress, mitochondrial dysfunction could explain the wide array of symptoms experienced by patients, from fatigue and muscle weakness to cognitive disturbances and beyond. This growing evidence base underscores the importance of further research into mitochondrial health as a potential target for therapeutic interventions in long COVID.
Comparison with mitochondrial dysfunction in other post-infectious syndromes
The concept of post-infectious syndromes, where symptoms persist long after the acute phase of an infection has resolved, is not unique to COVID-19. Several other infections have been associated with chronic sequelae, where mitochondrial dysfunction has been proposed as a contributing factor. Understanding these conditions can provide valuable insights into the mechanisms that might also underlie long COVID. Here are a few notable examples.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
ME/CFS is perhaps the most well-studied condition in the context of post-infectious syndromes and mitochondrial dysfunction. The symptoms of long COVID syndrome are very similar to those of ME/CFS [35, 53,54,55,56]. It is a chronic, complex disorder characterized by profound fatigue, sleep abnormalities, pain, and other symptoms that are worsened by exertion. The condition often follows viral infections, suggesting a post-infectious origin for some patients. Several studies have implicated mitochondrial dysfunction in ME/CFS, including evidence of structural changes and dysfunctional energy producing pathways, impaired ATP production, abnormalities in mitochondrial respiratory chain function, and elevated lactate levels suggestive of metabolic stress [57,58,59,60,61,62,63,64,65,66,67]. These findings parallel some of the mitochondrial abnormalities observed in long COVID patients, suggesting a common pathway of disease development. Mitochondria are a major source of reactive oxygen species (ROS) such as H2O2 and superoxide in the cell, making them extremely vulnerable to oxidative stress. In individuals with ME/CFS, elevated levels of peroxide and superoxide correlate with symptom severity [68, 69]. There is evidence that redox imbalance contributes to the pathogenesis of both (ME/CFS) and long COVID syndrome [70]. Proposed mechanisms underlying mitochondrial dysfunction associated with ME/CSF may include chronic immune activation and autoimmune responses that target mitochondrial components.
Post-treatment Lyme disease syndrome (PTLDS)
PTLDS refers to a condition where symptoms such as fatigue, pain, and cognitive impairment persist after treatment for Lyme disease, caused by the bacterium Borrelia burgdorferi [71]. While the exact mechanisms underlying PTLDS are not fully understood, it is possible that mitochondrial dysfunction may play a role. Research has shown alterations in mitochondrial morphology and function in patients with Lyme disease [72], which may contribute to the chronic symptoms experienced by some individuals after treatment. Likely mechanisms underlying mitochondrial dysfunction associated with PTLDS may involve lingering bacterial debris or immune complexes that continue to stimulate an immune response, affecting mitochondrial function.
Epstein-Barr virus (EBV) and other herpesviruses
Infections with EBV and other members of the herpesvirus family have been linked to chronic symptoms in some individuals, including fatigue and cognitive dysfunction [73,74,75]. These viruses encode proteins that can interfere with mitochondrial function, including inducing mitochondrial fragmentation and impairing mitochondrial bioenergetics [73, 76,77,78]. The chronic activation of the immune system in response to latent viral infection could also lead to sustained mitochondrial damage and dysfunction, contributing to the persistence of symptoms.
Chronic Q fever fatigue syndrome (QFS)
Chronic Q fever, caused by the bacterium Coxiella burnetii, can lead to Q fever fatigue syndrome (QFS), where patients experience severe fatigue, muscle pain, and concentration problems for years after the acute infection [79]. While specific studies on mitochondrial function in QFS are limited [80], the symptomatology suggests a potential role for mitochondrial dysfunction, similar to ME/CFS and other chronic conditions. Likely mechanisms include chronic inflammation triggered by Coxiella burnetii. Persistent immune activation may continue to disrupt mitochondrial function.
These examples illustrate that the association between chronic post-infectious symptoms and mitochondrial dysfunction is not unique to long COVID. The investigation into mitochondrial health in these conditions suggests a potential common pathway where viral or bacterial infections lead to mitochondrial damage, resulting in energy production deficits and oxidative stress, which in turn contribute to chronic symptoms. Understanding the mechanisms of mitochondrial impairment in these syndromes may offer insights into potential therapeutic targets and interventions for long COVID and other post-infectious conditions.
Potential mechanisms of persistent mitochondrial dysfunction post-COVID-19
Long-lasting structural damage to mitochondria
During the acute phase of COVID-19, SARS-CoV-2 can directly interact with mitochondria, exploiting mitochondrial dynamics for virus proliferation [81, 82] and causing structural damage [32, 83,84,85,86]. This damage includes alterations in mitochondrial morphology, such as swelling and changes in size and number, driven by the effects of the virus on mitochondrial physiology. In theory, structural damage could impair mitochondrial function long-term, even after the virus has been cleared from the circulation [22, 85].
Persistent dysregulation of mitochondrial bioenergetics
Interaction between SARS-CoV-2 and mitochondrial components might lead to prolonged dysregulation of mitochondrial bioenergetics [29]. For example, viral proteins may interfere with the mitochondrial antiviral-signaling protein (MAVS), disrupting its normal function in the antiviral response and leading to chronic mitochondrial dysfunction [87, 88]. This persistent dysregulation could result from epigenetic modifications induced by the virus or from prolonged imbalances in proteins that regulate mitochondrial biogenesis and function.
Autoimmunity triggered by COVID-19
There is growing evidence that COVID-19 can trigger autoimmune responses [89,90,91,92,93,94,95,96,97] that may exacerbate or prolong mitochondrial dysfunction [98]. The immune system’s response to SARS-CoV-2 might include the development of autoantibodies that mistakenly target mitochondrial proteins, a phenomenon possibly driven by molecular mimicry [99]. These autoimmune responses can lead to continuous immune-mediated damage to mitochondria, maintaining a state of inflammation and mitochondrial impairment that persists well beyond the acute phase of the infection.
Mechanisms linking mitochondrial dysfunction to long COVID symptoms
In the complex landscape of long COVID, mitochondrial dysfunction emerges as a prominent feature, potentially contributing to the diverse and persistent symptoms observed in patients.
One of the hallmark symptoms of long COVID is chronic fatigue, which can be directly linked to deficits in mitochondrial ATP production [100]. When mitochondrial function is compromised, ATP production is reduced, leading to energy deficits that manifest as profound and persistent fatigue [101, 102]. This energy shortfall impacts muscle function, cognitive processes, and overall physical capacity, significantly affecting quality of life. Impaired mitochondrial function and disruption of the electron transport chain promote the genesis of reactive oxygen species (ROS) leading to oxidative stress. Increased levels of ROS can cause damage to cellular structures, including lipids, proteins, and DNA, contributing to the tissue damage observed in long COVID [20, 31, 45, 46]. Oxidative stress is also implicated in aging and various chronic diseases [103], suggesting a common pathway through which mitochondrial dysfunction can exacerbate long COVID symptoms. Fatigue in long COVID bears resemblance to the fatigue experienced by heart failure patients, a condition where mitochondrial dysfunction has also been implicated [104, 105]. In heart failure, compromised mitochondrial efficiency significantly impairs cardiac function, directly contributing to symptoms of fatigue and limited exercise capacity [106,107,108,109,110,111]. Given the heart’s reliance on mitochondrial oxidative phosphorylation for its substantial energy needs, any reduction in this process can result in marked energy deficits. This mitochondrial inefficiency in heart failure parallels the potential role of mitochondrial dysfunction in exacerbating the fatigue observed in long COVID patients. Such similarities underscore a possible common pathway of disease impact through mitochondrial compromise, enhancing our understanding of long COVID’s debilitating effects.
Mitochondria play a crucial role in modulating immune responses and inflammation. Mitochondrial dysfunction and increased levels of mitochondria-derived ROS can activate Nf-KB and the NLRP3 inflammasome, leading to the production of pro-inflammatory cytokines and chronic inflammation. This inflammatory response can perpetuate tissue damage and contribute to the systemic symptoms of long COVID, such as fatigue and malaise [46, 48, 112]. The chronic activation of the immune system, driven by ongoing mitochondrial dysfunction, may also hinder recovery and prolong the duration of symptoms. Mitochondrial dysfunction affects various metabolic pathways, including glucose metabolism, lipid oxidation, and amino acid turnover. These disruptions can lead to metabolic imbalances that contribute to the symptoms of long COVID, such as weight changes, exercise intolerance, and nutritional deficiencies. The impact on metabolism further complicates the clinical picture of long COVID, making management and treatment more challenging.
In the context of COVID-19, accumulating evidence suggests that SARS-CoV-2 directly targets endothelial cells, highlighting a critical aspect of the virus’s pathogenicity [113,114,115,116,117,118,119,120,121,122,123,124,125,126]. Emerging evidence suggests that mitochondrial dysfunction may contribute to vascular issues seen in long COVID, including endothelial dysfunction and impaired blood flow. Mitochondria are involved in the regulation of endothelial cell function and vascular tone [127,128,129,130,131,132,133,134,135,136,137]. Mitochondrial dysfunction can lead to increased oxidative stress and inflammation within the vascular system, contributing to the development of endothelial dysfunction. This can result in a range of symptoms, including blood pressure variations, dizziness, and an increased risk of thrombosis, all of which have been reported in long COVID patients.
In expanding our understanding of the cellular targets SARS-CoV-2, it is crucial to consider not only the endothelium but also intestinal enterocytes, which express the ACE2 receptor utilized by the virus for cell entry [138, 139]. The infection of intestinal enterocytes can lead to significant clinical implications, including malabsorption, which may result in deficiencies in essential nutrients critical for mitochondrial function and overall energy metabolism. Moreover, persistent enterocyte dysfunction can perpetuate chronic inflammation, both locally within the gut and systemically [138, 139]. This ongoing inflammatory response may also exacerbate mitochondrial dysfunction, contributing to the energy deficits and general malaise.
In summary, mitochondrial dysfunction is intricately linked to the diverse symptoms of long COVID through its impact on energy production, oxidative stress, immune response, metabolic processes, and vascular health. Understanding these mechanisms is crucial for developing targeted interventions to mitigate the effects of long COVID and improve patient outcomes.
Serum peroxiredoxin-3: a novel biomarker of mitochondrial dysfunction in long COVID
Peroxiredoxin-3 is a member of the peroxiredoxin family of antioxidant enzymes, which are crucial for reducing oxidative stress within cells. Specifically, PRDX3 is located in the mitochondria, where it plays a key role in detoxifying hydrogen peroxide (H2O2) and protecting cells from oxidative damage [140]. Recent research indicates that PRDX3 is responsible for removal of up to 90% of the H2O2 generated in mitochondria [141]. Given its mitochondrial localization and its role in defending against oxidative stress, PRDX3 is used as a marker for mitochondrial oxidative stress and dysfunction [142, 143].
The utility of PRDX3 as a mitochondrial biomarker lies in its ability to reflect changes in mitochondrial health and oxidative stress levels. Increased levels of PRDX3 in serum or other bodily fluids can indicate heightened oxidative stress within mitochondria, potentially signaling mitochondrial dysfunction. This makes it a valuable tool for studying oxidative stress and mitochondrial impairment in long COVID.
Study objectives and methods
In light of the emerging significance of PRDX3, we aimed to investigate a possible association between serum PRDX3 levels measured in post-COVID patients and the characteristics of their fatigue syndrome. This objective was pursued to further understand the role of mitochondrial dysfunction in the persistence of fatigue symptoms in long COVID, providing insights into potential diagnostic and therapeutic strategies. We conducted a prospective, single-center cohort study at the University of Pécs Clinical Center, Hungary, collaborating closely with General Practitioners (GPs) within the university’s operational area. Patients who presented with persistent post-COVID symptoms at their GP’s office were referred to our outpatient clinic for a baseline visit. Inclusion criteria were as follows: (i) a minimum of 30 days since symptom onset at the time of the outpatient visit; (ii) confirmation of SARS-CoV-2 infection via a positive PCR or antigen test; (iii) presence of symptoms at the time of the outpatient appointment; and (iv) age 18 years or older. We excluded individuals with the following: (i) pre-existing malignant or active autoimmune diseases; (ii) ongoing immunosuppressive therapy; (iii) acute coronary syndrome; (iv) vaccination against SARS-CoV-2; and (v) any condition potentially confounding the assessment of fatigue or cognitive state. Based on the duration from acute SARS-CoV-2 infection onset to study enrollment (4–12 weeks vs. > 12 weeks), patients were stratified in accordance with the National Institute of Care and Health Excellence (NICE) guidelines [144]. Approved by the Hungarian Medical Research Council (IV/2505–3/2021/EKU), our study adhered to the ethical guidelines of the 1975 Declaration of Helsinki. All participants provided written informed consent.
Following our previous methodologies, fatigue was assessed using the Chalder Fatigue Scale (CFQ-11) [17, 145]. Patients reported their general condition over the past 3 days, with scores ranging from 0 to 3 via the Likert scoring system, enabling calculation of a global score (max 33) and subscales for physical (0–21) and psychological fatigue (0–12) [146, 147]. Severe fatigue was defined using a bimodal scoring system, with a score ≥ 4 indicating significant fatigue [146, 147]. The Post-COVID-19 Functional Status (PCFS) Scale measured the general impact of post-COVID symptoms on daily life, considering only symptoms present within the week prior to the baseline visit [146, 147]. Peripheral blood samples were collected, centrifuged, and the supernatant stored at − 80 °C until analysis. We measured antibodies against SARS-CoV-2 and PRDX3 concentrations using an ELISA kit from ABNOVA Corp., Taipei, Taiwan. Data were evaluated using SPSS (version 11.5; IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was applied to check for normality. To analyze demographic and clinical factors, the chi-square test was used for categorical data while the Student t test was applied to quantitative values. Non-normally distributed data were presented as median and interquartile range (25–75th percentiles) and were compared with the use of Mann–Whitney test. Correlation analysis was performed by calculating Spearman’s correlation coefficient (rho). After dichotomisation of the subjects based on age, binary logistic regression analysis was performed to explore predictors of various symptoms. The best cut-off value of predictors was determined based on ROC analysis. A p-value < 0.05 was considered statistically significant.
Link between PRDX3 levels and long COVID symptoms
In our investigation into the potential role of levels as a biomarker for long COVID symptoms, we initially screened 218 patients, ultimately including 139 in the study after applying our exclusion criteria (Fig. 2). Table 1 shows the demographic and clinical characteristics as well as the systemic levels of anti-Spike (anti-S-Ig) and anti-Nucleocapsid (anti-NC-Ig) immunoglobulins of cohort according to the severity of post-COVID fatigue. Our analysis revealed a distinct pattern among those suffering from severe versus non-severe post-COVID fatigue. Notably, the total number of post-COVID symptoms (severe: 7, IQR: 4–10 vs. non-severe 2, 0–4, p < 0.001) as well as the number of symptoms related to acute SARS-CoV-2 infection reported by patients (severe: 7, IQR: 4–8 vs. non-severe: 5, 3–6, p = 0.001) were significantly higher in patients with severe fatigue when compared to those with less severe fatigue. Both anti-S-Ig (severe: 122 U/mL, IQR: 37–232 vs. non-severe: 189 U/mL, 99–474, p = 0.009) and anti-NC-Ig (severe: 43.4 U/mL, IQR: 17–101 vs. non-severe: 99 U/mL, IQR: 50–117, p = 0.002) antibody levels were significantly lower in the group of patients with severe fatigue than in those with non-severe fatigue.
Intriguingly, when we examined the baseline serum PRDX3 levels in relation to the severity of post-COVID fatigue, we found no significant association. This absence of correlation extended to other parameters commonly associated with long COVID syndrome, such as scores on the Post-COVID-19 Functional Status (PCFS) Scale and the total number of reported symptoms (Fig. 3). These findings initially suggested that PRDX3 levels might not serve as a straightforward biomarker for the overall severity of long COVID symptoms. However, a deeper dive into the data uncovered a significant relationship between PRDX3 levels and the presence of dizziness among the study participants. Patients experiencing post-COVID dizziness had markedly higher PRDX3 levels compared to those without this symptom (median: 58.4 ng/ml; IQR: 48.0–66.9 vs 47.9; 39.8–60.4; p = 0.026). Individuals with post-COVID dizziness presented with more severe fatigue based on either PCFS, or each Chalder Fatigue (physical and psychological) scales (all p < 0.001). After dichotomization of patients under 50 years (n = 71), PRDX3 was also proved to be an independent predictor of dizziness (n = 15) after including other confounders such as age, gender, other CNS-related symptoms (memory loss, headache), anti-spike, and nucleocapsid antibody titers, formerly used antiviral medications (e.g., remdesivir, favipiravir) (OR, 1.06; 95%CI, 1.004–1.118; p = 0.03). In this subgroup analysis, significantly higher PRDX3 level was detected in patients with post-COVID dizziness (n = 15) (median: 60.4 ng/ml, IQR: 49.4–68.3 vs 45.9, 35.5–57.6, p = 0.02). This specific association points to a nuanced role for PRDX3 in the post-COVID symptom complex, suggesting that while it may not correlate with the broader spectrum of long COVID symptoms, it could be particularly relevant to understanding and potentially managing specific symptoms like dizziness.
Association of serum peroxiredoxin-3 levels between patients with different degrees of fatigue observed in different phases of long COVID syndrome. SF-OSC, severe fatigue in patients with ongoing symptomatic COVID (N = 64); SF-PCS, severe fatigue in patients with post-COVID syndrome (N = 30); NSF-OSC, non-severe fatigue in patients with ongoing symptomatic COVID disease (N = 31); NSF-PCS, non-severe fatigue in patients with post-COVID syndrome (N = 14); OSC, patients who still have symptoms between 4 and 12 weeks after the start of acute symptoms; PCS, people who still have symptoms for more than 12 weeks after the start of acute symptoms. A total binary Chalder Fatigue Scale of 3 or less represents scores of those who have non-severe fatigue (NSF), with scores of 4 or more equating to severe fatigue (SF). Data are presented as individual dots and median with interquartile range. *Significant value (p < 0.05)
While initial findings highlighted PRDX3’s potential in reflecting oxidative stress within mitochondria, our results specifically connect elevated PRDX3 levels to the symptom of dizziness rather than a broader range of long COVID symptoms such as fatigue. This points to a more nuanced understanding of PRDX3’s role, necessitating a careful interpretation of its utility as a biomarker.
The association between increased PRDX3 levels and dizziness suggests a possible specific mitochondrial stress response in certain neurological contexts linked to long COVID. This finding underlines PRDX3’s sensitivity to particular pathological states—specifically, those involving enhanced oxidative stress, which is a known trigger for dizziness in various neurological disorders. The lack of a similar correlation between PRDX3 levels and other long COVID symptoms such as fatigue indicates that mitochondrial dysfunction, as measured by PRDX3, does not uniformly affect all symptoms of long COVID. This discrepancy highlights the need for a more symptom-specific approach in using PRDX3 as a mitochondrial biomarker.
Given the selective elevation of PRDX3 in patients experiencing dizziness, it is imperative to explore why this biomarker does not similarly elevate in relation to other symptoms. One hypothesis is that different long COVID symptoms may be driven by varying degrees of mitochondrial involvement or different pathophysiological processes within the mitochondria. For example, the mechanisms underlying fatigue might involve broader bioenergetic dysfunction not solely captured by oxidative stress markers such as PRDX3. This suggests the potential involvement of other mitochondrial functions or even non-mitochondrial pathways.
To further elucidate the role of mitochondrial dysfunction in long COVID, comprehensive studies should be conducted to expand biomarker panels, including a range of mitochondrial and oxidative stress markers to assess their correlation with a diverse spectrum of long COVID symptoms. Future studies should investigate the specific mitochondrial pathways that are implicated in different long COVID symptoms, which may help in understanding the selective impact on symptoms like dizziness. Further, clinical trials for mitochondria targeted therapies are needed to explore the effectiveness of antioxidants and mitochondrial support therapies in specifically treating the symptoms correlated with mitochondrial dysfunction, such as dizziness, to assess their therapeutic benefits and limitations.
Potential therapeutic implications for mitochondrial health in long COVID
The recognition of the important role of mitochondrial dysfunction in long COVID opens new avenues for therapeutic intervention aimed at restoring mitochondrial health and function [19, 20]. Effective management of mitochondrial dysfunction and restoration of cellular energetics could potentially alleviate some of the persistent symptoms experienced by long COVID patients, such as fatigue, cognitive impairment, and muscle weakness. Strategies to improve mitochondrial function involve a combination of pharmacological interventions, lifestyle modifications, and nutritional support. Antioxidants, such as coenzyme Q10, MitoQ, N-acetylcysteine, resveratrol, and alpha-lipoic acid, have been suggested to reduce oxidative stress in mitochondria, thereby improving their function [148,149,150,151].
Additionally, compounds like L-carnitine, which facilitate fatty acid transport into mitochondria for energy production, could also prove beneficial [152, 153]. NAD+ boosters, such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), have garnered attention for their potential to enhance mitochondrial function by increasing the levels of nicotinamide adenine dinucleotide (NAD+), a critical coenzyme involved in cellular energy production and repair processes [132, 154,155,156,157,158,159]. By boosting NAD+ levels, these supplements may help counteract mitochondrial dysfunction and improve energy metabolism, offering a promising therapeutic avenue for conditions like long COVID [160]. Some of these compounds, including Q10 [67, 161, 162], MitoQ (NCT05373043), alpha-lipoic acid [163], nicotinamide riboside (NCT05703074), resveratrol (NCT05601180), are currently being tested in clinical trials to assess their effectiveness in treating patients with long COVID, reflecting the growing interest in targeting mitochondrial dysfunction as a therapeutic strategy for this condition. Exercise and physical rehabilitation programs tailored to long COVID patients may help enhance mitochondrial biogenesis, promoting the growth and division of existing mitochondria [164,165,166,167,168]. Nutritional interventions focusing on a diet rich in nutrients known to support mitochondrial health, including omega-3 fatty acids, B vitamins, and minerals like magnesium, could further support recovery.
Currently, treatments aimed at improving mitochondrial function are being explored in the context of various chronic diseases, including neurodegenerative disorders and metabolic syndromes. For instance, mitochondrial-targeted antioxidants have shown promise in reducing symptoms and improving quality of life in conditions characterized by mitochondrial dysfunction. While direct evidence of their efficacy in long COVID is still emerging, these treatments offer a plausible therapeutic approach given the shared underlying mechanisms of mitochondrial impairment.
Future research should focus on the development of targeted therapies that can directly enhance mitochondrial function or protect mitochondria from damage. This includes the exploration of novel pharmacological agents that can increase mitochondrial biogenesis, enhance their energy production capabilities, or reduce mitochondrial-related inflammation and apoptosis. Clinical trials specifically designed to evaluate the efficacy of mitochondrial-targeted therapies in long COVID populations are critically needed. Additionally, the identification of biomarkers indicative of mitochondrial dysfunction could help tailor therapeutic approaches to individual patient needs, improving outcomes.
Moreover, understanding the interaction between mitochondrial dysfunction and other pathophysiological processes in long COVID, such as immune dysregulation and endothelial dysfunction, could lead to the development of combination therapies that address multiple facets of the disease. Integrative approaches that combine pharmacological treatments with lifestyle and nutritional interventions may offer the most promise for comprehensive management of long COVID, aiming not only to alleviate symptoms but also to restore overall health and well-being.
Challenges and future directions
Diagnosing and studying mitochondrial dysfunction in long COVID presents numerous challenges. The condition’s multifaceted symptomatology and the absence of standardized diagnostic criteria for mitochondrial dysfunction complicate the identification and treatment of affected individuals. Furthermore, the need for longitudinal studies is paramount to unravel the long-term implications of mitochondrial dysfunction in long COVID patients. Studies focusing on the biochemical pathways involved in mitochondrial energy production and oxidative stress in post-COVID-19 patients are warranted. These would include assessments of mitochondrial DNA, electron transport chain activity, and oxidative damage markers to better understand the extent and nature of mitochondrial dysfunction. Such studies are essential to understand the persistence of symptoms over time and their impact on patients’ quality of life. Research aimed at identifying and characterizing autoantibodies against mitochondrial antigens in long COVID patients could help establish a link between autoimmunity and persistent symptoms, guiding the development of immunomodulatory treatments. Identifying potential biomarkers for the early detection and monitoring of mitochondrial dysfunction in long COVID is another critical area of research. Biomarkers could offer invaluable insights into the disease’s progression and response to treatment, facilitating personalized therapeutic approaches.
Conclusion
The exploration of mitochondrial dysfunction in the context of long COVID has unveiled a critical aspect of the condition’s underlying pathology. This review underscores the pivotal role that mitochondrial impairment plays in contributing to the wide array of symptoms associated with long COVID, ranging from chronic fatigue and cognitive disturbances to metabolic and vascular dysfunctions. Mitochondrial health emerges as a promising target for alleviating some persistent symptoms experienced by long COVID patients. The potential for interventions—ranging from pharmacological treatments to lifestyle modifications, exercise, and dietary approaches—offers hope for those struggling with this condition. Yet, our journey towards fully understanding and effectively addressing long COVID is far from over. Significant gaps remain in our comprehension of how SARS-CoV-2 infection precipitates mitochondrial dysfunction and the manner in which this dysfunction perpetuates the wide range of long COVID symptoms. Addressing these challenges and moving forward requires a multidisciplinary collaboration to delve deeper into the role of mitochondrial dysfunction in long COVID. Such endeavors will not only broaden our understanding but also expedite the development of targeted and effective treatments, ultimately offering hope and improved outcomes for patients worldwide.
Data availability
The datasets used and or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Chilunga FP, Appelman B, van Vugt M, Kalverda K, Smeele P, van Es J, Wiersinga WJ, Rostila M, Prins M, Stronks K, et al. Differences in incidence, nature of symptoms, and duration of long COVID among hospitalised migrant and non-migrant patients in the Netherlands: a retrospective cohort study. Lancet Reg Health Eur. 2023;29:100630. https://doi.org/10.1016/j.lanepe.2023.100630.
Qi C, Osborne T, Bailey R, Cooper A, Hollinghurst JP, Akbari A, Crowder R, Peters H, Law RJ, Lewis R, et al. Impact of COVID-19 pandemic on incidence of long-term conditions in Wales: a population data linkage study using primary and secondary care health records. Br J Gen Pract. 2023;73:e332–9. https://doi.org/10.3399/BJGP.2022.0353.
Frallonardo L, Segala FV, Chhaganlal KD, Yelshazly M, Novara R, Cotugno S, Guido G, Papagni R, Colpani A, De Vito A, et al. Incidence and burden of long COVID in Africa: a systematic review and meta-analysis. Sci Rep. 2023;13:21482. https://doi.org/10.1038/s41598-023-48258-3.
Beretta S, Cristillo V, Camera G, Morotti Colleoni C, Pellitteri G, Viti B, Bianchi E, Gipponi S, Grimoldi M, Valente M, et al. Incidence and long-term functional outcome of neurologic disorders in hospitalized patients with COVID-19 infected with pre-omicron variants. Neurology. 2023;101:e892–903. https://doi.org/10.1212/WNL.0000000000207534.
Tsai J, Grace A, Espinoza R, Kurian A. Incidence of long COVID and associated psychosocial characteristics in a large U.S. city. Soc Psychiatry Psychiatr Epidemiol. 2023;59(4):611–9. https://doi.org/10.1007/s00127-023-02548-3.
Sedgley R, Winer-Jones J, Bonafede M. Long COVID incidence in a large US ambulatory electronic health record system. Am J Epidemiol. 2023;192:1350–7. https://doi.org/10.1093/aje/kwad095.
Jacobs MM, Evans E, Ellis C. Racial, ethnic, and sex disparities in the incidence and cognitive symptomology of long COVID-19. J Natl Med Assoc. 2023;115:233–43. https://doi.org/10.1016/j.jnma.2023.01.016.
Di Gennaro F, Belati A, Tulone O, Diella L, Fiore Bavaro D, Bonica R, Genna V, Smith L, Trott M, Bruyere O, et al. Incidence of long COVID-19 in people with previous SARS-Cov2 infection: a systematic review and meta-analysis of 120,970 patients. Intern Emerg Med. 2023;18:1573–81. https://doi.org/10.1007/s11739-022-03164-w.
Mansell V, Hall Dykgraaf S, Kidd M, Goodyear-Smith F. Long COVID and older people. Lancet Healthy Longev. 2022;3:e849–54. https://doi.org/10.1016/S2666-7568(22)00245-8.
Bhattacharjee N, Sarkar P, Sarkar T. Beyond the acute illness: exploring long COVID and its impact on multiple organ systems. Physiol Int. 2023;110:291–310. https://doi.org/10.1556/2060.2023.00256.
Gado K, Kovacs AK, Domjan G, Nagy ZZ, Bednarik GD. COVID-19 and the elderly. Physiol Int. 2022. https://doi.org/10.1556/2060.2022.00203.
Peterfi A, Meszaros A, Szarvas Z, Penzes M, Fekete M, Feher A, Lehoczki A, Csipo T, Fazekas-Pongor V. Comorbidities and increased mortality of COVID-19 among the elderly: a systematic review. Physiol Int. 2022. https://doi.org/10.1556/2060.2022.00206.
Egger M, Wimmer C, Stummer S, Reitelbach J, Bergmann J, Muller F, Jahn K. Reduced health-related quality of life, fatigue, anxiety and depression affect COVID-19 patients in the long-term after chronic critical illness. Sci Rep. 2024;14:3016. https://doi.org/10.1038/s41598-024-52908-5.
Laguarta-Val S, Varillas-Delgado D, Lizcano-Alvarez A, Molero-Sanchez A, Melian-Ortiz A, Cano-de-la-Cuerda R, Jimenez-Antona C. Effects of aerobic exercise therapy through nordic walking program in lactate concentrations, fatigue and quality-of-life in patients with long-COVID syndrome: a non-randomized parallel controlled trial. J Clin Med. 2024;13(4):1035. https://doi.org/10.3390/jcm13041035.
Lau B, Wentz E, Ni Z, Yenokyan K, Vergara C, Mehta SH, Duggal P. Physical health and mental fatigue disability associated with long COVID: baseline results from a US nationwide cohort. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.08.009.
Lee JS, Choi Y, Joung JY, Son CG. Clinical and laboratory characteristics of fatigue-dominant long-COVID subjects: a cross-sectional study. Am J Med. 2024. https://doi.org/10.1016/j.amjmed.2024.01.025.
Molnar T, Varnai R, Schranz D, Zavori L, Peterfi Z, Sipos D, Tokes-Fuzesi M, Illes Z, Buki A, Csecsei P. Severe fatigue and memory impairment are associated with lower serum level of anti-SARS-CoV-2 antibodies in patients with post-COVID symptoms. J Clin Med. 2021;10(19):4337. https://doi.org/10.3390/jcm10194337.
Zhang J, Shu T, Zhu R, Yang F, Zhang B, Lai X. The long-term effect of COVID-19 disease severity on risk of diabetes incidence and the near 1-year follow-up outcomes among postdischarge patients in Wuhan. J Clin Med. 2022;11(11):3094. https://doi.org/10.3390/jcm11113094.
Chen TH, Chang CJ, Hung PH. Possible pathogenesis and prevention of long COVID: SARS-CoV-2-induced mitochondrial disorder. Int J Mol Sci. 2023;24(9):8034. https://doi.org/10.3390/ijms24098034.
Noonong K, Chatatikun M, Surinkaew S, Kotepui M, Hossain R, Bunluepuech K, Noothong C, Tedasen A, Klangbud WK, Imai M, et al. Mitochondrial oxidative stress, mitochondrial ROS storms in long COVID pathogenesis. Front Immunol. 2023;14:1275001. https://doi.org/10.3389/fimmu.2023.1275001.
Grossini E, Concina D, Rinaldi C, Russotto S, Garhwal D, Zeppegno P, Gramaglia C, Kul S, Panella M (2021) Association between plasma redox state/mitochondria function and a flu-like syndrome/COVID-19 in the elderly admitted to a long-term care unit. Front Physiol. 12: 707587 https://doi.org/10.3389/fphys.2021.707587
Chang X, Ismail NI, Rahman A, Xu D, Chan RWY, Ong SG, Ong SB. Long COVID-19 and the heart: is cardiac mitochondria the missing link? Antioxid Redox Signal. 2023;38:599–618. https://doi.org/10.1089/ars.2022.0126.
Pintos I, Soriano V. Mitochondrial damage as cause of long COVID. AIDS Rev. 2023;26:145–9. https://doi.org/10.24875/AIDSRev.M23000063.
Srinivasan K, Pandey AK, Livingston A, Venkatesh S. Roles of host mitochondria in the development of COVID-19 pathology: could mitochondria be a potential therapeutic target? Mol Biomed. 2021;2:38. https://doi.org/10.1186/s43556-021-00060-1.
Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol. 2020;319:C258–67. https://doi.org/10.1152/ajpcell.00224.2020.
Ryback R, Eirin A. Mitochondria, a missing link in COVID-19 heart failure and arrest? Front Cardiovasc Med. 2021;8:830024. https://doi.org/10.3389/fcvm.2021.830024.
Chernyak BV, Popova EN, Zinovkina LA, Lyamzaev KG, Zinovkin RA. Mitochondria as targets for endothelial protection in COVID-19. Front Physiol. 2020;11:606170. https://doi.org/10.3389/fphys.2020.606170.
Chen ZZ, Johnson L, Trahtemberg U, Baker A, Huq S, Dufresne J, Bowden P, Miao M, Ho JA, Hsu CC, et al. Mitochondria and cytochrome components released into the plasma of severe COVID-19 and ICU acute respiratory distress syndrome patients. Clin Proteomics. 2023;20:17. https://doi.org/10.1186/s12014-023-09394-0.
Chang YY, Wei AC. Transcriptome and machine learning analysis of the impact of COVID-19 on mitochondria and multiorgan damage. PLoS ONE. 2024;19:e0297664. https://doi.org/10.1371/journal.pone.0297664.
Bizjak DA, Ohmayer B, Buhl JL, Schneider EM, Walther P, Calzia E, Jerg A, Matits L, Steinacker JM. Functional and morphological differences of muscle mitochondria in chronic fatigue syndrome and post-COVID syndrome. Int J Mol Sci. 2024;25(3):1675. https://doi.org/10.3390/ijms25031675.
Bhowal C, Ghosh S, Ghatak D, De R. Pathophysiological involvement of host mitochondria in SARS-CoV-2 infection that causes COVID-19: a comprehensive evidential insight. Mol Cell Biochem. 2023;478:1325–43. https://doi.org/10.1007/s11010-022-04593-z.
Akbari H, Taghizadeh-Hesary F. COVID-19 induced liver injury from a new perspective: mitochondria. Mitochondrion. 2023;70:103–10. https://doi.org/10.1016/j.mito.2023.04.001.
Streng L, de Wijs CJ, Raat NJH, Specht PAC, Sneiders D, van der Kaaij M, Endeman H, Mik EG, Harms FA. In vivo and ex vivo mitochondrial function in COVID-19 patients on the intensive care unit. Biomedicines. 2022;10(7):1746. https://doi.org/10.3390/biomedicines10071746.
Silva BSA, Pereira T, Minuzzi LG, Padilha CS, Figueiredo C, Olean-Oliveira T, Dos Santos IVM, von Ah Morano AE, Marchioto Junior O, Ribeiro JPJ, et al. Mild to moderate post-COVID-19 alters markers of lymphocyte activation, exhaustion, and immunometabolic responses that can be partially associated by physical activity level- an observational sub-analysis fit- COVID study. Front Immunol. 2023;14:1212745. https://doi.org/10.3389/fimmu.2023.1212745.
Peppercorn K, Edgar CD, Kleffmann T, Tate WP. A pilot study on the immune cell proteome of long COVID patients shows changes to physiological pathways similar to those in myalgic encephalomyelitis/chronic fatigue syndrome. Sci Rep. 2023;13:22068. https://doi.org/10.1038/s41598-023-49402-9.
Nikesjo F, Sayyab S, Karlsson L, Apostolou E, Rosen A, Hedman K, Lerm M. Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells. Clin Epigenetics. 2022;14:172. https://doi.org/10.1186/s13148-022-01398-1.
De Vitis C, Capalbo C, Torsello A, Napoli C, Salvati V, Loffredo C, Blandino G, Piaggio G, Auciello FR, Pelliccia F, et al. Opposite effect of thyroid hormones on oxidative stress and on mitochondrial respiration in COVID-19 patients. Antioxid (Basel). 2022;11(10):1998. https://doi.org/10.3390/antiox11101998.
Ernst T, Ryan MC, Liang HJ, Wang JP, Cunningham E, Saleh MG, Kottilil S, Chang L. Neuronal and glial metabolite abnormalities in participants with persistent neuropsychiatric symptoms after COVID-19: a brain proton magnetic resonance spectroscopy study. J Infect Dis. 2023;228:1559–70. https://doi.org/10.1093/infdis/jiad309.
Ranisavljev M, Todorovic N, Ostojic J, Ostojic SM. Reduced tissue creatine levels in patients with long COVID-19: a cross-sectional study. J Postgrad Med. 2023;69:162–3. https://doi.org/10.4103/jpgm.jpgm_65_23.
Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, Loo RL, Begum S, Boughton B, Yang R, et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. 2021;20:3315–29. https://doi.org/10.1021/acs.jproteome.1c00224.
Finnigan LEM, Cassar MP, Koziel MJ, Pradines J, Lamlum H, Azer K, Kirby D, Montgomery H, Neubauer S, Valkovic L, Raman B. Efficacy and tolerability of an endogenous metabolic modulator (AXA1125) in fatigue-predominant long COVID: a single-centre, double-blind, randomised controlled phase 2a pilot study. EClinicalMedicine. 2023;59:101946. https://doi.org/10.1016/j.eclinm.2023.101946.
Gorecka M, Jex N, Thirunavukarasu S, Chowdhary A, Corrado J, Davison J, Tarrant R, Poenar AM, Sharrack N, Parkin A, et al. Cardiovascular magnetic resonance imaging and spectroscopy in clinical long-COVID-19 syndrome: a prospective case-control study. J Cardiovasc Magn Reson. 2022;24:50. https://doi.org/10.1186/s12968-022-00887-9.
Jamieson A, Al Saikhan L, Alghamdi L, Hamill Howes L, Purcell H, Hillman T, Heightman M, Treibel T, Orini M, Bell R, et al. Mechanisms underlying exercise intolerance in long COVID: an accumulation of multisystem dysfunction. Physiol Rep. 2024;12:e15940. https://doi.org/10.14814/phy2.15940.
Karim A, Muhammad T, Iqbal MS, Qaisar R. Elevated plasma CAF22 are incompletely restored six months after COVID-19 infection in older men. Exp Gerontol. 2023;171:112034. https://doi.org/10.1016/j.exger.2022.112034.
Mikuteit M, Baskal S, Klawitter S, Dopfer-Jablonka A, Behrens GMN, Muller F, Schroder D, Klawonn F, Steffens S, Tsikas D. Amino acids, post-translational modifications, nitric oxide, and oxidative stress in serum and urine of long COVID and ex COVID human subjects. Amino Acids. 2023;55:1173–88. https://doi.org/10.1007/s00726-023-03305-1.
Vollbracht C, Kraft K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: implications for the benefit of high-dose intravenous vitamin C. Front Pharmacol. 2022;13:899198. https://doi.org/10.3389/fphar.2022.899198.
Trimarco V, Izzo R, Mone P, Trimarco B, Santulli G. Targeting endothelial dysfunction and oxidative stress in Long-COVID. Pharmacol Res. 2022;184:106451. https://doi.org/10.1016/j.phrs.2022.106451.
Mrakic-Sposta S, Vezzoli A, Garetto G, Paganini M, Camporesi E, Giacon TA, Dellanoce C, Agrimi J, Bosco G. Hyperbaric oxygen therapy counters oxidative stress/inflammation-driven symptoms in long COVID-19 patients: preliminary outcomes. Metabolites. 2023;13(10):1032. https://doi.org/10.3390/metabo13101032.
Medini H, Zirman A, Mishmar D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience. 2021;24(12):103471. https://doi.org/10.1016/j.isci.2021.103471.
Blandova G, Janostiakova N, Kodada D, Pastorek M, Liptak R, Hodosy J, Sebekova K, Celec P, Krasnanska G, Elias V, et al. Mitochondrial DNA variability and Covid-19 in the Slovak population. Mitochondrion. 2023;75:101827. https://doi.org/10.1016/j.mito.2023.101827.
Adamyan L, Elagin V, Vechorko V, Stepanian A, Dashko A, Doroshenko D, Aznaurova Y, Sorokin M, Suntsova M, Drobyshev A, et al. COVID-19-associated inhibition of energy accumulation pathways in human semen samples. F S Sci. 2021;2:355–64. https://doi.org/10.1016/j.xfss.2021.07.004.
Wang T, Cao Y, Zhang H, Wang Z, Man CH, Yang Y, Chen L, Xu S, Yan X, Zheng Q, Wang YP. COVID-19 metabolism: mechanisms and therapeutic targets. MedComm. 2020;2022(3):e157. https://doi.org/10.1002/mco2.157.
Versace V, Tankisi H. Long-COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): potential neurophysiological biomarkers for these enigmatic entities. Clin Neurophysiol. 2023;147:58–9. https://doi.org/10.1016/j.clinph.2023.01.001.
Tziastoudi M, Cholevas C, Stefanidis I, Theoharides TC. Genetics of COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review. Ann Clin Transl Neurol. 2022;9:1838–57. https://doi.org/10.1002/acn3.51631.
McLaughlin M, Sanal-Hayes NEM, Hayes LD, Berry EC, Sculthorpe NF. People with long COVID and myalgic encephalomyelitis/chronic fatigue syndrome exhibit similarly impaired vascular function. Am J Med. 2023. https://doi.org/10.1016/j.amjmed.2023.09.013.
Bonilla H, Quach TC, Tiwari A, Bonilla AE, Miglis M, Yang PC, Eggert LE, Sharifi H, Horomanski A, Subramanian A, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol. 2023;14:1090747. https://doi.org/10.3389/fneur.2023.1090747.
Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83:61–5. https://doi.org/10.1007/BF00294431.
Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Dis Transl Med. 2021;7:14–26. https://doi.org/10.1016/j.cdtm.2020.11.002.
Wang PY, Ma J, Kim YC, Son AY, Syed AM, Liu C, Mori MP, Huffstutler RD, Stolinski JL, Talagala SL, et al. WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2023;120:e2302738120. https://doi.org/10.1073/pnas.2302738120.
Tsilioni I, Natelson B, Theoharides TC. Exosome-associated mitochondrial DNA from patients with myalgic encephalomyelitis/chronic fatigue syndrome stimulates human microglia to release IL-1beta. Eur J Neurosci. 2022;56:5784–94. https://doi.org/10.1111/ejn.15828.
Sweetman E, Kleffmann T, Edgar C, de Lange M, Vallings R, Tate W. A SWATH-MS analysis of myalgic encephalomyelitis/chronic fatigue syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J Transl Med. 2020;18:365. https://doi.org/10.1186/s12967-020-02533-3.
Nilsson I, Palmer J, Apostolou E, Gottfries CG, Rizwan M, Dahle C, Rosen A. Metabolic dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome not due to anti-mitochondrial antibodies. Front Med (Lausanne). 2020;7:108. https://doi.org/10.3389/fmed.2020.00108.
Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29:19–36. https://doi.org/10.1007/s11011-013-9435-x.
Maksoud R, Balinas C, Holden S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of nutraceutical interventions for mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2021;19:81. https://doi.org/10.1186/s12967-021-02742-4.
Holden S, Maksoud R, Eaton-Fitch N, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. 2020;18:290. https://doi.org/10.1186/s12967-020-02452-3.
Billing-Ross P, Germain A, Ye K, Keinan A, Gu Z, Hanson MR. Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2016;14:19. https://doi.org/10.1186/s12967-016-0771-6.
Mantle D, Hargreaves IP, Domingo JC, Castro-Marrero J. Mitochondrial dysfunction and coenzyme Q10 supplementation in post-viral fatigue syndrome: an overview. Int J Mol Sci. 2024;25(1):574. https://doi.org/10.3390/ijms25010574.
Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis / chronic fatigue syndrome. Neuro Endocrinol Lett. 2009;30:715–22.
Maes M, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Sci Monit. 2011;17(4):SC11–5. https://doi.org/10.12659/msm.881699.
Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2021;118:34. https://doi.org/10.1073/pnas.2024358118.
Geebelen L, Lernout T, Devleesschauwer B, Kabamba-Mukadi B, Saegeman V, Belkhir L, De Munter P, Dubois B, Westhovens R, Humtick Hospital G, et al. Non-specific symptoms and post-treatment Lyme disease syndrome in patients with Lyme borreliosis: a prospective cohort study in Belgium (2016–2020). BMC Infect Dis. 2022;22:756. https://doi.org/10.1186/s12879-022-07686-8.
Peacock BN, Gherezghiher TB, Hilario JD, Kellermann GH. New insights into Lyme disease. Redox Biol. 2015;5:66–70. https://doi.org/10.1016/j.redox.2015.03.002.
Vernon SD, Whistler T, Cameron B, Hickie IB, Reeves WC, Lloyd A. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis. 2006;6:15. https://doi.org/10.1186/1471-2334-6-15.
Pedersen M, Asprusten TT, Godang K, Leegaard TM, Osnes LT, Skovlund E, Tjade T, Oie MG, Wyller VBB. Predictors of chronic fatigue in adolescents six months after acute Epstein-Barr virus infection: a prospective cohort study. Brain Behav Immun. 2019;75:94–100. https://doi.org/10.1016/j.bbi.2018.09.023.
Brodwall EM, Pedersen M, Asprusten TT, Wyller VBB. Pain in adolescent chronic fatigue following Epstein-Barr virus infection. Scand J Pain. 2020;20:765–73. https://doi.org/10.1515/sjpain-2020-0031.
LaJeunesse DR, Brooks K, Adamson AL. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 alter mitochondrial morphology during lytic replication. Biochem Biophys Res Commun. 2005;333:438–42. https://doi.org/10.1016/j.bbrc.2005.05.120.
Krishna G, Soman Pillai V, Valiya VM. Upregulation of GLS1 isoforms KGA and GAC facilitates mitochondrial metabolism and cell proliferation in Epstein-Barr virus infected cells. Viruses. 2020;12(8):811. https://doi.org/10.3390/v12080811.
Kawanishi M, Tada-Oikawa S, Kawanishi S. Epstein-Barr virus BHRF1 functions downstream of Bid cleavage and upstream of mitochondrial dysfunction to inhibit TRAIL-induced apoptosis in BJAB cells. Biochem Biophys Res Commun. 2002;297:682–7. https://doi.org/10.1016/s0006-291x(02)02261-1.
Raijmakers RPH, Roerink ME, Jansen AFM, Keijmel SP, Gacesa R, Li Y, Joosten LAB, van der Meer JWM, Netea MG, Bleeker-Rovers CP, Xu CJ. Multi-omics examination of Q fever fatigue syndrome identifies similarities with chronic fatigue syndrome. J Transl Med. 2020;18:448. https://doi.org/10.1186/s12967-020-02585-5.
Raijmakers RPH, Jansen AFM, Keijmel SP, Ter Horst R, Roerink ME, Novakovic B, Joosten LAB, van der Meer JWM, Netea MG, Bleeker-Rovers CP. A possible role for mitochondrial-derived peptides humanin and MOTS-c in patients with Q fever fatigue syndrome and chronic fatigue syndrome. J Transl Med. 2019;17:157. https://doi.org/10.1186/s12967-019-1906-3.
Elesela S, Lukacs NW. Role of mitochondria in viral infections. Life (Basel). 2021;11(3):232. https://doi.org/10.3390/life11030232.
Dutta S, Das N, Mukherjee P. Picking up a fight: fine tuning mitochondrial innate immune defenses against RNA viruses. Front Microbiol. 2020;11:1990. https://doi.org/10.3389/fmicb.2020.01990.
Valdes-Aguayo JJ, Garza-Veloz I, Vargas-Rodriguez JR, Martinez-Vazquez MC, Avila-Carrasco L, Bernal-Silva S, Gonzalez-Fuentes C, Comas-Garcia A, Alvarado-Hernandez DE, Centeno-Ramirez ASH, et al. Peripheral blood mitochondrial DNA levels were modulated by SARS-CoV-2 infection severity and its lessening was associated with mortality among hospitalized patients with COVID-19. Front Cell Infect Microbiol. 2021;11:754708. https://doi.org/10.3389/fcimb.2021.754708.
Scozzi D, Cano M, Ma L, Zhou D, Zhu JH, O’Halloran JA, Goss C, Rauseo AM, Liu Z, Sahu SK, et al. Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight. 2021;6:4. https://doi.org/10.1172/jci.insight.143299.
Valdes-Aguayo JJ, Garza-Veloz I, Badillo-Almaraz JI, Bernal-Silva S, Martinez-Vazquez MC, Juarez-Alcala V, Vargas-Rodriguez JR, Gaeta-Velasco ML, Gonzalez-Fuentes C, Avila-Carrasco L, Martinez-Fierro ML. Mitochondria and mitochondrial DNA: key elements in the pathogenesis and exacerbation of the inflammatory state caused by COVID-19. Med (Kaunas). 2021;57(9):928. https://doi.org/10.3390/medicina57090928.
Archer SL, Dasgupta A, Chen KH, Wu D, Baid K, Mamatis JE, Gonzalez V, Read A, Bentley RE, Martin AY, et al. SARS-CoV-2 mitochondriopathy in COVID-19 pneumonia exacerbates hypoxemia. Redox Biol. 2022;58:102508. https://doi.org/10.1016/j.redox.2022.102508.
Stewart H, Lu Y, O’Keefe S, Valpadashi A, Cruz-Zaragoza LD, Michel HA, Nguyen SK, Carnell GW, Lukhovitskaya N, Milligan R, et al. The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity. iScience. 2023;26(11):108080. https://doi.org/10.1016/j.isci.2023.108080.
Li X, Hou P, Ma W, Wang X, Wang H, Yu Z, Chang H, Wang T, Jin S, Wang X, et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell Mol Immunol. 2022;19:67–78. https://doi.org/10.1038/s41423-021-00807-4.
Sotzny F, Filgueiras IS, Kedor C, Freitag H, Wittke K, Bauer S, Sepulveda N, Mathias da Fonseca DL, Baiocchi GC, Marques AHC, et al. Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in post COVID syndrome correlate with symptom severity. Front Immunol. 2022;13:981532. https://doi.org/10.3389/fimmu.2022.981532.
Fagyas M, Nagy B Jr., Raduly AP, Manyine IS, Martha L, Erdosi G, Sipka S, Jr., Enyedi E, Szabo AA, Polik Z, et al. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Gerosci. 2022;44:2347–60. https://doi.org/10.1007/s11357-022-00649-6.
Seibert FS, Stervbo U, Wiemers L, Skrzypczyk S, Hogeweg M, Bertram S, Kurek J, Anft M, Westhoff TH, Babel N. Severity of neurological Long-COVID symptoms correlates with increased level of autoantibodies targeting vasoregulatory and autonomic nervous system receptors. Autoimmun Rev. 2023;22:103445. https://doi.org/10.1016/j.autrev.2023.103445.
Nersesjan V, Amiri M, Nilsson AC, Wamberg C, Jensen VVS, Petersen CB, Hejl AM, Lebech AM, Theut AM, Jorgensen CS, et al. SARS-CoV-2 and autoantibodies in the cerebrospinal fluid of COVID-19 patients: prospective multicentre cohort study. Brain Commun. 2023;5(5):fcad274. https://doi.org/10.1093/braincomms/fcad274.
Lee SJ, Yoon T, Ha JW, Kim J, Lee KH, Lee JA, Kim CH, Lee SW, Kim JH, Ahn JY, et al. Prevalence, clinical significance, and persistence of autoantibodies in COVID-19. Virol J. 2023;20:236. https://doi.org/10.1186/s12985-023-02191-z.
Fonseca DLM, Filgueiras IS, Marques AHC, Vojdani E, Halpert G, Ostrinski Y, Baiocchi GC, Placa DR, Freire PP, Pour SZ, et al. Severe COVID-19 patients exhibit elevated levels of autoantibodies targeting cardiolipin and platelet glycoprotein with age: a systems biology approach. NPJ Aging. 2023;9:21. https://doi.org/10.1038/s41514-023-00118-0.
Credle JJ, Gunn J, Sangkhapreecha P, Monaco DR, Zheng XA, Tsai HJ, Wilbon A, Morgenlander WR, Rastegar A, Dong Y, et al. Unbiased discovery of autoantibodies associated with severe COVID-19 via genome-scale self-assembled DNA-barcoded protein libraries. Nat Biomed Eng. 2022;6:992–1003. https://doi.org/10.1038/s41551-022-00925-y.
Casciola-Rosen L, Thiemann DR, Andrade F, Trejo-Zambrano MI, Leonard EK, Spangler JB, Skinner NE, Bailey J, Yegnasubramanian S, Wang R, et al. IgM anti-ACE2 autoantibodies in severe COVID-19 activate complement and perturb vascular endothelial function. JCI Insight. 2022;7:9. https://doi.org/10.1172/jci.insight.158362.
Cabral-Marques O, Halpert G, Schimke LF, Ostrinski Y, Vojdani A, Baiocchi GC, Freire PP, Filgueiras IS, Zyskind I, Lattin MT, et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat Commun. 2022;13:1220. https://doi.org/10.1038/s41467-022-28905-5.
Montenegro YHA, Bobermin LD, Sesterheim P, Salvato RS, Anschau F, de Oliveira MJS, Wyse ATS, Netto CA, Goncalves CS, Quincozes-Santos A, Leipnitz G. Serum of COVID-19 patients changes neuroinflammation and mitochondrial homeostasis markers in hippocampus of aged rats. J Neurovirol. 2023;29:577–87. https://doi.org/10.1007/s13365-023-01156-w.
Di Florio DN, Beetler DJ, McCabe EJ, Sin J, Ikezu T, Fairweather D. Mitochondrial extracellular vesicles, autoimmunity and myocarditis. Front Immunol. 2024;15:1374796. https://doi.org/10.3389/fimmu.2024.1374796.
Saito S, Shahbaz S, Luo X, Osman M, Redmond D, Cohen Tervaert JW, Li L, Elahi S. Metabolomic and immune alterations in long COVID patients with chronic fatigue syndrome. Front Immunol. 2024;15:1341843. https://doi.org/10.3389/fimmu.2024.1341843.
Sumbalova Z, Kucharska J, Palacka P, Rausova Z, Langsjoen PH, Langsjoen AM, Gvozdjakova A. Platelet mitochondrial function and endogenous coenzyme Q10 levels are reduced in patients after COVID-19. Bratisl Lek Listy. 2022;123:9–15. https://doi.org/10.4149/BLL_2022_002.
Prasada Kabekkodu S, Chakrabarty S, Jayaram P, Mallya S, Thangaraj K, Singh KK, Satyamoorthy K. Severe acute respiratory syndrome coronaviruses contributing to mitochondrial dysfunction: implications for post-COVID complications. Mitochondrion. 2023;69:43–56. https://doi.org/10.1016/j.mito.2023.01.005.
Fekete M, Major D, Feher A, Fazekas-Pongor V, Lehoczki A. Geroscience and pathology: a new frontier in understanding age-related diseases. Pathol Oncol Res. 2024;23:30. https://doi.org/10.3389/pore.2024.1611623.
Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067–73. https://doi.org/10.1016/s0735-1097(97)00015-6.
Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Bronstad E, Rognmo O, Mangner N, Linke A, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–72. https://doi.org/10.1002/ejhf.239.
Sack MN. Tackling inflammation at its source in heart failure: are mitochondria the key? JACC Basic Transl Sci. 2022;7:1197–9. https://doi.org/10.1016/j.jacbts.2022.07.008.
Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41. https://doi.org/10.1016/j.yjmcc.2012.09.002.
Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. https://doi.org/10.1093/cvr/cvq240.
Ren L, Gopireddy RR, Perkins G, Zhang H, Timofeyev V, Lyu Y, Diloretto DA, Trinh P, Sirish P, Overton JL, et al. Disruption of mitochondria-sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure. Proc Natl Acad Sci U S A. 2022;119:e2206708119. https://doi.org/10.1073/pnas.2206708119.
Marin-Garcia J, Akhmedov AT, Moe GW. Mitochondria in heart failure: the emerging role of mitochondrial dynamics. Heart Fail Rev. 2013;18:439–56. https://doi.org/10.1007/s10741-012-9330-2.
Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. https://doi.org/10.1016/j.jacc.2012.08.1021.
Greene C, Connolly R, Brennan D, Laffan A, O’Keeffe E, Zaporojan L, O’Callaghan J, Thomson B, Connolly E, Argue R, et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat Neurosci. 2024. https://doi.org/10.1038/s41593-024-01576-9.
Carnevale S, Beretta P, Morbini P. Direct endothelial damage and vasculitis due to SARS-CoV-2 in small bowel submucosa of COVID-19 patient with diarrhea. J Med Virol. 2021;93:61–3. https://doi.org/10.1002/jmv.26119.
Falcon-Cama V, Montero-Gonzalez T, Acosta-Medina EF, Guillen-Nieto G, Berlanga-Acosta J, Fernandez-Ortega C, Alfonso-Falcon A, Gilva-Rodriguez N, Lopez-Nocedo L, Cremata-Garcia D, et al. Evidence of SARS-CoV-2 infection in postmortem lung, kidney, and liver samples, revealing cellular targets involved in COVID-19 pathogenesis. Arch Virol. 2023;168:96. https://doi.org/10.1007/s00705-023-05711-y.
Guo Y, Kanamarlapudi V. Molecular analysis of SARS-CoV-2 spike protein-induced endothelial cell permeability and vWF secretion. Int J Mol Sci. 2023;24(6):5664. https://doi.org/10.3390/ijms24065664.
Jud P, Gressenberger P, Muster V, Avian A, Meinitzer A, Strohmaier H, Sourij H, Raggam RB, Stradner MH, Demel U, et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection-a cross-sectional study. Front Cardiovasc Med. 2021;8:750887. https://doi.org/10.3389/fcvm.2021.750887.
Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, Appelt-Menzel A, Cubukova A, Barenberg J, Leu J, et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports. 2022;17:307–20. https://doi.org/10.1016/j.stemcr.2021.12.011.
Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, Worsdorfer P, Midkiff CC, Mudd J, Belyaeva E, et al. SARS-CoV-2 infects endothelial cells in vivo and in vitro. Front Cell Infect Microbiol. 2021;11:701278. https://doi.org/10.3389/fcimb.2021.701278.
Mezoh G, Crowther NJ. Endothelial dysfunction as a primary consequence of SARS-CoV-2 infection. Adv Exp Med Biol. 2021;1321:33–43. https://doi.org/10.1007/978-3-030-59261-5_3.
Motta CS, Torices S, da Rosa BG, Marcos AC, Alvarez-Rosa L, Siqueira M, Moreno-Rodriguez T, Matos ADR, Caetano BC, Martins J, et al. Human brain microvascular endothelial cells exposure to SARS-CoV-2 leads to inflammatory activation through NF-kappaB non-canonical pathway and mitochondrial remodeling. Viruses. 2023;15(3):745. https://doi.org/10.3390/v15030745.
Nishijima Y, Hader SN, Hanson AJ, Zhang DX, Sparapani R, Gutterman DD, Beyer AM. Prolonged endothelial-dysfunction in human arterioles following infection with SARS-CoV-2. Cardiovasc Res. 2021. https://doi.org/10.1093/cvr/cvab339.
Pelisek J, Reutersberg B, Greber UF, Zimmermann A. Vascular dysfunction in COVID-19 patients: update on SARS-CoV-2 infection of endothelial cells and the role of long non-coding RNAs. Clin Sci (Lond). 2022;136:1571–90. https://doi.org/10.1042/CS20220235.
Pesti A, Danics K, Glasz T, Varkonyi T, Barbai T, Reszegi A, Kovalszky I, Valyi-Nagy I, Dobi D, Lotz G, et al. Liver alterations and detection of SARS-CoV-2 RNA and proteins in COVID-19 autopsies. Gerosci. 2023;45:1015–31. https://doi.org/10.1007/s11357-022-00700-6.
Wagner JUG, Bojkova D, Shumliakivska M, Luxan G, Nicin L, Aslan GS, Milting H, Kandler JD, Dendorfer A, Heumueller AW, et al. Increased susceptibility of human endothelial cells to infections by SARS-CoV-2 variants. Basic Res Cardiol. 2021;116:42. https://doi.org/10.1007/s00395-021-00882-8.
Wenzel J, Lampe J, Muller-Fielitz H, Schuster R, Zille M, Muller K, Krohn M, Korbelin J, Zhang L, Ozorhan U, et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–33. https://doi.org/10.1038/s41593-021-00926-1.
Yang RC, Huang K, Zhang HP, Li L, Zhang YF, Tan C, Chen HC, Jin ML, Wang XR. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J Neuroinflammation. 2022;19:149. https://doi.org/10.1186/s12974-022-02514-x.
Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174:34–43. https://doi.org/10.2353/ajpath.2009.080650.
Coletta C, Modis K, Olah G, Brunyanszki A, Herzig DS, Sherwood ER, Ungvari Z, Szabo C. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18:511. https://doi.org/10.1186/s13054-014-0511-3.
Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105(4):1333–41.
Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–24. https://doi.org/10.1161/CIRCRESAHA.111.246140.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020. https://doi.org/10.1007/s11357-020-00165-5.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. https://doi.org/10.1016/j.redox.2019.101192.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:2. https://doi.org/10.1111/acel.12731.
Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876-1881. https://doi.org/10.1152/ajpheart.00375.2009.
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67. https://doi.org/10.1161/CIRCRESAHA.118.311378.
Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121-2128.
Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37-47.
Xu E, Xie Y, Al-Aly Z. Long-term gastrointestinal outcomes of COVID-19. Nat Commun. 2023;14:983. https://doi.org/10.1038/s41467-023-36223-7.
Fernandez-de-Las-Penas C, Martin-Guerrero JD, Navarro-Pardo E, Torres-Macho J, Guijarro C, Pellicer-Valero OJ. Exploring the recovery curve for gastrointestinal symptoms from the acute COVID-19 phase to long-term post-COVID: The LONG-COVID-EXP-CM Multicenter Study. J Med Virol. 2022;94:2925–7. https://doi.org/10.1002/jmv.27727.
Karpenko IL, Valuev-Elliston VT, Ivanova ON, Smirnova OA, Ivanov AV. Peroxiredoxins-the underrated actors during virus-induced oxidative stress. Antioxid (Basel). 2021;10(6):977. https://doi.org/10.3390/antiox10060977.
Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2009;425:313–25. https://doi.org/10.1042/BJ20091541.
Qiao B, Wang J, Xie J, Niu Y, Ye S, Wan Q, Ye Q. Detection and identification of peroxiredoxin 3 as a biomarker in hepatocellular carcinoma by a proteomic approach. Int J Mol Med. 2012;29:832–40. https://doi.org/10.3892/ijmm.2012.916.
Shi L, Wu LL, Yang JR, Chen XF, Zhang Y, Chen ZQ, Liu CL, Chi SY, Zheng JY, Huang HX, et al. Serum peroxiredoxin3 is a useful biomarker for early diagnosis and assessemnt of prognosis of hepatocellular carcinoma in Chinese patients. Asian Pac J Cancer Prev. 2014;15:2979–86. https://doi.org/10.7314/apjcp.2014.15.7.2979.
COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE). In National Institute for Health and Care Excellence: Clinical Guidelines. 2020.
Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. J Psychosom Res. 1993;37:147–53. https://doi.org/10.1016/0022-3999(93)90081-p.
Klok FA, Boon G, Barco S, Endres M, Geelhoed JJM, Knauss S, Rezek SA, Spruit MA, Vehreschild J, Siegerink B. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1):2001494. https://doi.org/10.1183/13993003.01494-2020.
Doykov I, Hallqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ - the detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. https://doi.org/10.12688/f1000research.27287.2.
Bradbury J, Wilkinson S, Schloss J. Nutritional support during long COVID: a systematic scoping review. J Integr Complement Med. 2023;29:695–704. https://doi.org/10.1089/jicm.2022.0821.
Akanchise T, Angelova A. Potential of nano-antioxidants and nanomedicine for recovery from neurological disorders linked to long COVID syndrome. Antioxid (Basel). 2023;12(2):393. https://doi.org/10.3390/antiox12020393.
Pavlidou E, Poulios E, Papadopoulou SK, Fasoulas A, Dakanalis A, Giaginis C. Clinical evidence on the potential beneficial effects of diet and dietary supplements against COVID-19 infection risk and symptoms’ severity. Med Sci (Basel). 2024;12(1):11. https://doi.org/10.3390/medsci12010011.
Liao MT, Wu CC, Wu SV, Lee MC, Hu WC, Tsai KW, Yang CH, Lu CL, Chiu SK, Lu KC. Resveratrol as an adjunctive therapy for excessive oxidative stress in aging COVID-19 patients. Antioxid (Basel). 2021;10(9):1440. https://doi.org/10.3390/antiox10091440.
Vaziri-Harami R, Delkash P. Can l-carnitine reduce post-COVID-19 fatigue? Ann Med Surg (Lond). 2022;73:103145. https://doi.org/10.1016/j.amsu.2021.103145.
Naureen Z, Dautaj A, Nodari S, Fioretti F, Dhuli K, Anpilogov K, Lorusso L, Paolacci S, Michelini S, Guda T, et al. Proposal of a food supplement for the management of post-COVID syndrome. Eur Rev Med Pharmacol Sci. 2021;25:67–73. https://doi.org/10.26355/eurrev_202112_27335.
Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–28. https://doi.org/10.1016/j.cmet.2017.11.002.
Frederick DW, Davis JG, Davila A Jr, Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, Baur JA. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290:1546–58. https://doi.org/10.1074/jbc.M114.579565.
Davila A, Liu L, Chellappa K, Redpath P, Nakamaru-Ogiso E, Paolella LM, Zhang Z, Migaud ME, Rabinowitz JD, Baur JA. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. Elife. 2018;12(7):e33246. https://doi.org/10.7554/eLife.33246.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–46. https://doi.org/10.1007/s11357-020-00165-5.
Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–39. https://doi.org/10.1007/s11357-019-00095-x.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41:619–30. https://doi.org/10.1007/s11357-019-00074-2.
Block T, Kuo J. Rationale for nicotinamide adenine dinucleotide (NAD+) metabolome disruption as a pathogenic mechanism of post-acute COVID-19 syndrome. Clin Pathol. 2022;15:2632010X2211069. https://doi.org/10.1177/2632010X221106986.
Kow CS, Ramachandram DS, Hasan SS. Coenzyme Q10 therapy in patients with post COVID-19 condition. Lancet Reg Health Eur. 2023;25:100567. https://doi.org/10.1016/j.lanepe.2022.100567.
Hansen KS, Mogensen TH, Agergaard J, Schiottz-Christensen B, Ostergaard L, Vibholm LK, Leth S. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur. 2023;24:100539. https://doi.org/10.1016/j.lanepe.2022.100539.
Barletta MA, Marino G, Spagnolo B, Bianchi FP, Falappone PCF, Spagnolo L, Gatti P. Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med. 2023;23:667–78. https://doi.org/10.1007/s10238-022-00871-8.
Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–40. https://doi.org/10.1073/pnas.1019581108.
Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505-511.
Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89:467S-471S. https://doi.org/10.3945/ajcn.2008.26717D.
Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564-1572. https://doi.org/10.1152/ajpregu.00396.2005.
Campos JC, Marchesi Bozi LH, Krum B, Grassmann Bechara LR, Ferreira ND, Arini GS, Albuquerque RP, Traa A, Ogawa T, van der Bliek AM, et al. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc Natl Acad Sci U S A. 2023;120:e2204750120. https://doi.org/10.1073/pnas.2204750120.
Acknowledgements
We would like to acknowledge the GPs involved in the recruitment of these patients and to thank the staff of the Institute of Biotechnology, University of Pecs, for correct and accurate laboratory measurements. Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1). The funding sources had no role in the writing of the manuscript and in the decision to submit the article for publication.
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Study was approved by the Hungarian Medical Research Council (IV/2505–3/2021/EKU). All procedures were performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Molnar, T., Lehoczki, A., Fekete, M. et al. Mitochondrial dysfunction in long COVID: mechanisms, consequences, and potential therapeutic approaches. GeroScience (2024). https://doi.org/10.1007/s11357-024-01165-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01165-5