Abstract
Cancer remains a leading cause of mortality and poses a substantial threat to public health. Studies have revealed that Long noncoding RNA DANCR is a cytoplasmic lncRNA whose aberrant expression plays a pivotal role in various cancer types. Within tumour biology, DANCR exerts regulatory control over crucial processes such as proliferation, invasion, metastasis, angiogenesis, inflammatory responses, cellular energy metabolism reprogramming, and apoptosis. By acting as a competitive endogenous RNA for miRNAs and by interacting with proteins and mRNAs at the molecular level, DANCR contributes significantly to cancer progression. Elevated DANCR levels have also been linked to heightened resistance to anticancer drugs. Moreover, the detection of circulating DANCR holds promise as a valuable biomarker for aiding in the clinical differentiation of different cancer types. This article offers a comprehensive review and elucidation of the primary functions and molecular mechanisms through which DANCR influences tumours.
Similar content being viewed by others
Introduction
The role of long noncoding RNAs (lncRNAs) in cancer has garnered increasing attention. LncRNAs are defined as noncoding RNAs exceeding 200 nucleotides [1]. Accumulating evidence indicates their involvement in regulating biological processes across various cancers [2], potentially exerting tumour-suppressive and tumour-promoting functions. The expression level of lncRNAs is related to tumorigenesis, tumour invasiveness, and the tumour stage, making them potential targets for cancer treatment. Differentiation antagonizing nonprotein coding RNA (DANCR or ANCR) is an oncogenic lncRNA that plays a crucial role in regulating tumours. DANCR was first found to be related to the dedifferentiation of epidermal cells in 2012 and is downregulated during the differentiation of progenitor cells. DANCR, an 855-base pair lncRNA, is notably downregulated during differentiation processes and is positioned on chromosome 4 in humans; it comprises three exons, with introns 1 and 2 housing a microRNA (MIR4449) and a small nucleolar RNA (SNORA26) [3]. Aberrant DANCR expression has been observed in various tumour types, prompting extensive investigations into its functional roles and molecular mechanisms. Numerous studies have indicated that the molecular mechanism of DANCR closely correlates with sustained proliferation signals [4], invasion and metastasis [5, 6], angiogenesis [7], the inflammatory phenotype [8], energy metabolism reprogramming [9], and resistance to cell death [10] in tumours, and has great value in clinical diagnosis. This review focuses on the biological function, regulatory mechanism, and clinical significance of DANCR in tumours. The aim is to provide new directions for the diagnosis and treatment of cancer in the clinic.
Biological function of DANCR in tumours
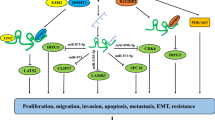
The biological function of DANCR in tumours encompasses various critical aspects. Among the identified tumour hallmarks, DANCR is intricately linked to abnormal proliferation, invasion, metastasis, angiogenesis, the inflammatory phenotype, energy metabolism reprogramming, and resistance of tumour cells to apoptosis (Fig. 1).
DANCR promotes cell proliferation
Cancer cells are characterized by their sustained proliferative signalling [11]. Studies have revealed that the upregulation of DANCR in various tumours promotes proliferation by modulating the cell cycle [12,13,14]. Arresting the cell cycle provides additional time for repair, reducing the mutation risk and preventing tumour initiation. Research has shown that inhibiting DANCR in mouse liver cancer prolongs tumour formation cycles and reduces the tumour volume, significantly impeding tumour growth [15]. Similarly, in colorectal cancer (CRC) cells, decreased DANCR levels decrease the percentage of cells in G2 phase, inducing cell cycle arrest and impeding tumour cell proliferation [16].
DANCR promotes tumour metastasis and invasion
Metastasis, the spread of cancer cells to distant organs, represents the deadliest phase of advanced cancer, accounting for 90% of cancer-related deaths. The invasion-metastasis cascade involves multiple discrete steps [17]. The epithelial-mesenchymal transition (EMT), a crucial process that converts epithelial cells into mesenchymal cells, promotes the invasion and dissemination of tumour cells [18]. Studies have shown that DANCR competitively binds to miR-874-3p, positively regulating SOX2 and enhancing the EMT in triple-negative breast cancer (TNBC) cells, promoting migration and invasion [5]. Moreover, evidence suggests that tumour metastasis relies on a small population of cancer stem cells with self-renewal abilities within tumours [19]. Knockdown of DANCR reduced lung cancer cell stemness and migration [20] but increased liver cancer cell stemness, facilitating intrahepatic and extrahepatic tumour colonization [19].
DANCR enhances tumour angiogenesis
As tumours proliferate, they demand increased oxygen and nutrients, necessitating new blood vessel formation for sustained growth [21]. Recent studies have revealed a significant association between DANCR expression and tumour angiogenesis. For example, dysfunction of ZNF750 in esophageal cancer (EC) cells can trigger DANCR upregulation, leading to enhanced angiogenic properties in human umbilical vein endothelial cells (HUVECs) and human arterial endothelial cells (HAECs) [7].
DANCR promotes tumour inflammation
The promotion of inflammation by cancer is a well-recognized hallmark [11]. Chronic inflammation activates the NF-kappa B and STAT3 signalling pathways, which are vital for tumour initiation and development. DANCR has emerged as a potent modulator of inflammation, influencing the inflammation-induced EMT and stemness in TNBC. Elevated DANCR levels notably intensify the inflammatory phenotype in breast cancer (BC) cells [8].
DANCR promotes energy metabolism reprogramming in tumour cells
Most cancer cells rely on glycolysis, even under sufficient oxygen, following the Warburg effect for proliferation advantage [22]. Recent research has linked DANCR with cancer cell glycolysis levels. Key glycolytic enzymes, such as glucose transporter type 1 (GLUT1) and pyruvate kinase M (PKM), are influenced by DANCR, regulating glycolysis and proliferation in acute myeloid leukaemia (AML) cells through the HIF-1α/GLUT1 pathway via the miR-4701-5p/PKM axis [9].
DANCR regulates tumour cell death
Tumour cells adeptly evade apoptosis to survive and proliferate malignantly. However, the impact of DANCR on cell death mechanisms is noteworthy. In BC cells, the inhibition of DANCR promotes apoptosis, hindering BC cell occurrence and proliferation [23]. Conversely, in prostate cancer (PC) cells, DANCR overexpression impedes apoptosis and promotes malignant proliferation through competitive miR-214-5p inhibition [10]. Moreover, autophagy can remove harmful proteins and excessive or damaged organelles in cells, preventing cytotoxicity and causing the malignant transformation of cells [24]. In glioma cells, DANCR is positively correlated with autophagy, enhancing malignant progression by upregulating ATG7 protein expression and promoting autophagy-mediated malignant transformation [25].
The upstream and downstream regulatory mechanisms of DANCR in tumours
Downstream regulatory mechanisms
The regulation of DANCR, a typical lncRNA, in tumours involves a multitude of intricate mechanisms. Its downstream regulatory mechanisms can be attributed to its capacity to interact with miRNAs, mRNAs, or proteins.
The competitive endogenous RNA (ceRNA) theory posits that RNA molecules containing miRNA recognition elements (MREs) can act as ceRNAs by competitively sequestering miRNAs, thereby modulating miRNA activity [26]. DANCR, which is known to bind to nearly 70 miRNAs, serves as a ceRNA for miRNAs, antagonizing their function by downregulating miRNA levels. This interaction significantly influences tumour initiation and progression. Additionally, DANCR has been shown to interact with proteins and mRNAs. For example, DANCR regulates endothelial lipase (EL/LIPG), a pivotal modulator of tumour cell metabolism, by binding to LIPG and modulating the stable expression of the LIPG protein in TNBC cells [27]. Moreover, studies have indicated that DANCR enhances the stability of the PSMD10 mRNA by interacting with the PSMD10 3’-UTR to counteract the inhibitory effects imposed by miRNAs such as miR-214 and miR-1254 [28]. Furthermore, DANCR can influence gene expression through epigenetic modifications. In cholangiocarcinoma (CCA) cells, DANCR binds to EZH2 to facilitate histone methylation of the FBP1 promoter, ultimately suppressing FBP1 expression via epigenetic mechanisms [29].
Upstream regulatory mechanisms
Moreover, the upstream regulatory mechanisms of DANCR are diverse and complex. DANCR may represent a potential drug target. Studies have confirmed that lidocaine and bupivacaine inhibit BC progression by activating the DANCR/miR-187-5p/MYB regulatory axis [30]; in addition, in terms of epigenetic modifications, Zhou et al. discovered that METTL3 enhances the stability of the DANCR mRNA through the m6A modification to promote osteosarcoma (OS) progression [31]. In summary, the regulatory mechanisms of DANCR in cancer are multifaceted and hold significant promise for cancer research and treatment (Fig. 2).
Detailed roles of DANCR in different cancers
DNACR plays crucial roles in the development and progression of numerous tumours, as well as drug resistance (Table 1).
Lung cancer
Lung cancer is the foremost contributor to global cancer mortality. Early detection of non-small cell lung cancer (NSCLC), the predominant form of lung cancer, is limited by a lack of clinical symptoms. Hence, a highly sensitive and specific diagnostic marker for lung cancer is urgently needed. Research indicates elevated DANCR expression in NSCLC tissues compared to adjacent normal tissues [6, 32,33,34]. Elevated DANCR levels stimulate the proliferation, migration, and invasion of NSCLC cells [6, 35, 36]. Increased DANCR expression is significantly correlated with a reduced overall survival time [36] and advanced clinical stage in NSCLC patients [33].
Zhen et al. illustrated that DANCR overexpression in normal cells enhances the proliferation and growth of lung epithelial cells. Additionally, DANCR overexpression counteracts the inhibitory effect of miR-216a on oncogenes (ELF4B, JAK2, and MALAT1), promoting tumorigenesis and invasion [33]. Similarly, in NSCLC, DANCR acts as a sponge for several miRNAs, including miR-496 [32], miR-214-5p [34], and miR-1225-3 [6]; Yu et al. confirmed the interaction between DANCR and miR-216a in the A549 cell line, revealing that DANCR inhibits miR-216a and leads to the activation of Wnt/β-catenin signalling [20]. Furthermore, DANCR knockdown hindered EZH2-mediated epigenetic silencing of the p21 promoter, leading to enhanced p21 expression [35]. Bai et al.‘s in vivo tumour transplantation experiments in nude mice revealed decreases in tumour weight, volume, and growth upon DANCR knockdown [36]. Intriguingly, Wang et al. observed a significant downregulation of DANCR in biopsy and circulating samples from NSCLC patients compared to normal tissues. Their findings highlighted the regulatory role of DANCR in NSCLC using the NCI-H23 and NCI-H522 cell lines, which was partially mediated by TGF-β1 downregulation [37].
DANCR has emerged as a pivotal regulator of drug resistance in NSCLC, and Nicolescu et al. reported a notable increase in DANCR expression in gefitinib-resistant NSCLC cell lines [38]. Therefore, silencing DANCR could be a promising strategy to overcome drug resistance in NSCLC.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) comprises approximately 90% of liver cancer cases, and its incidence is increasing globally [39]. Recent studies have highlighted the impact of aberrant DANCR expression on HCC progression. DANCR expression is upregulated in HCC compared to adjacent liver tissues [40, 41], it enhances stemness features in HCC cells [19], and it is correlated with an unfavorable patient prognosis [28, 42,43,44]. DANCR suppression hindered HCC cell proliferation, metastasis, and invasion both in vitro and in vivo [40], induced apoptosis, and impeded cell cycle progression [41]. Targeting DANCR expression in xenograft tumours delays tumour growth [15].
DANCR competes with miR-216a-5p [41], miR-27a-3p [42], miR-222-3p [43], miR-125b-5p [44], and miR-140-3p [45] to modulate HCC progression. Moreover, the interaction of DANCR with heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1) in HepG2 and Huh7 cells enhances HNRNPA1 expression by preventing its degradation and facilitating the EMT, invasion, and migration of liver cancer cells [45]. Additionally, DANCR competes for binding to the 3’UTRs of CTNNB1 and PSMD10, hindering the inhibitory effects of miR214, miR320a, and miR-199a on CTNNB1 and of miR-214, miR-1254, and other miRNAs on PSMD10 [19, 28].
DANCR also regulates HCC drug resistance. Liu et al. utilized BALB/c nude mice to establish a xenograft model and observed sorafenib resistance in HCC Hep3B cell-derived tumours. Studies have confirmed that DANCR is involved in a feedback loop involving the IL-6/STAT3 signalling pathway, enhancing HCC cell resistance to sorafenib [28].
Breast cancer
BC is the most prevalent cancer globally and the primary cause of cancer-related deaths among women. TNBC, in particular, is characterized by high heterogeneity, high invasiveness and a poor prognosis [46], underscoring the critical need to identify molecular targets for BC treatment. In recent years, DANCR has emerged as playing a key role in the expression and drug resistance of TNBC. Research indicates that DANCR expression is elevated in TNBC tissues compared to adjacent normal tissues [47, 48], and its high levels are significantly associated with the histological grade, lymph node metastasis, advanced TNM stage, and reduced overall survival. Suppression of DANCR not only impedes the growth and invasion of BC cells [49] but also influences the EMT, stemness traits, and inflammatory responses [8, 50] while promoting apoptosis and autophagy [51].
The molecular mechanisms by which DANCR regulates BC are varied and complex. Research indicates that DANCR exerts its inhibitory effects by competitively binding to various miRNAs, including miR-874-3p [5] and miR-4319 [23], thereby fostering the malignant progression of BC. EZH2, a protein modulated by DANCR, suppresses gene expression by catalyzing histone H3 lysine 27 trimethylation (H3K27 methylation) at the target gene promoter [52]. The interplay between DANCR and EZH2 plays a pivotal role in regulating cancer progression. In TNBC, DANCR enhances EZH2 binding to the CD44 and ABCG2 gene promoters [49]. It also facilitates EZH2 binding to the SOCS3 promoter, leading to epigenetic SOCS3 downregulation [8], while forming a complex with EZH2 that promotes increased EZH2 binding to CDK1. This process triggers ubiquitin-proteasome degradation and EZH2 degradation, ultimately halting BC progression [53]. Moreover, DANCR interacts with RXRA, promoting its phosphorylation and activating the PI3K/AKT signalling pathway, promoting TNBC progression [47]. Yang et al. [27] highlighted the role of DANCR in preserving LIPG protein stability and mitochondrial metabolism through oxidative phosphorylation (OXPHOS) in TNBC cells by binding to LIPG endothelial lipase. Their work indicated that cynaroside, a novel LIPG inhibitor, effectively suppresses DANCR expression in TNBC cells, significantly impeding tumour formation. Additionally, Wu et al. observed a notable increase in the tumour volume and weight following DANCR knockdown in a xenograft model utilizing MDA-MB-231 cells in 6-week-old female nude mice [5]. In addition, miR-29b-1/a [54], lidocaine and bupivacaine [30] were shown to downregulate DANCR expression, inhibiting cancer cell progression.
Interestingly, Li et al. documented that elevated DANCR levels in MDA-MB-231 and MCF10A cells hinder tumorigenesis and distant metastasis in breast cancer. Conversely, DANCR inhibition promotes the EMT in human breast cancer cells. Through xenograft experiments on BALB/c nude mice, researchers have shown that DANCR can restrain tumour growth and metastasis in vivo [53]. Notably, DANCR expression was downregulated in breast cancer tissues compared to that in matched normal adjacent tissues. Their study suggested that DANCR plays a role in preventing the TGF-β1-induced EMT. Reduced DANCR levels correlate with increased RUNX2 expression, fostering the invasion and metastasis of breast cancer cells [55]. Similarly, Jiang et al. reported that DANCR silencing significantly increases the tumorigenicity, proliferation, and invasion of breast cancer cells. Interestingly, DANCR expression was notably diminished in highly metastatic breast cancer cell lines [56].
In TNBC cells, DANCR interacts with Krüppel-like factor 5 (KLF5), inducing acetylation at the K369 site on KLF5. This process increases KLF5 protein levels, thereby conferring resistance to cisplatin (DDP) by inhibiting p27 transcription [57].
Colorectal cancer
CRC is the third most common cancer globally and the second leading cause of cancer-related deaths, with a growing trend in mortality rates worldwide [58, 59]. In a study by Luan et al., hypoxia-immune-related lncRNAs were screened from TCGA and GEO public databases of CRC to predict CRC survival and immunotherapy responsiveness. DANCR is highly expressed in CRC specimens and plays a pivotal role in the initiation and progression of CRC through diverse mechanisms [60]. Functioning as a tumour promoter in CRC cells, DANCR expression is significantly increased in CRC tissues compared to adjacent nontumour tissues [12]. Increased DANCR levels enhance the growth and liver metastasis of CRC tumours [61]. Moreover, DANCR is significantly correlated with the TNM stage [62], histological grade and lymph node metastasis of CRC tissues. Increased DANCR expression indicates a poor prognosis [63] and aggressive advancement [64] of CRC; conversely, DANCR knockdown can impede the proliferation of CRC cells [16] and reduce the tumorigenicity, proliferation, migration and survival of CRC cells [63]. Research has shown that DANCR can serve as an independent indicator of both overall survival and disease-free survival in patients with CRC [64].
A recent study [62] indicated that suppressing DANCR could decrease EZH2 expression, leading to the inhibition of the invasion and migration of CRC cells. Furthermore, Lian et al. revealed that DANCR could enhance the acetyltransferase activity of lysine acetyltransferase 6 A (KAT6A) by binding to it, resulting in elevated KAT6A-mediated gene expression and promoting cell cycle progression in CRC. Using female BALB/c nude mice, they established a subcutaneous CRC mouse model and showed that DANCR knockout significantly impeded CRC tumour growth [16]. Both DANCR and heat shock protein 27 (HSP27) have been found to share binding sites with miR-577 targets in HT29 and LOVO cells. DANCR promotes HSP27 expression and facilitates the proliferation and metastasis of CRC cells by inhibiting miR-577 [61]. Various microRNAs, including miR-518a-3p [63], miR-145-5p [65] and miR-185-5p [12], were reported to interact competitively with DANCR, contributing to the aggressive progression of CRC.
In terms of CRC drug resistance mechanisms, studies have highlighted that DANCR notably enhances glycolysis in DDP-resistant colon cancer cells. In contrast, DANCR knockdown in colon cancer cells can directly interact with and increase the expression of miR-125b-5p, which targets hexokinase 2 (HK2), thus suppressing glycolysis and enhancing DDP sensitivity in tumour cells. The DANCR/miR-125b-5p/HK2 glycolysis axis has emerged as a promising therapeutic target to combat DDP resistance in colon cancer [66]. Additionally, Xiong et al. identified DANCR as a target suppressed by doxorubicin, leading to the inhibition of doxorubicin-induced apoptosis in CRC cells. This regulatory function involves the interplay between the DANCR and QK proteins, influencing the expression of the oncogenic long noncoding RNA MALAT1. Consequently, this study identified DANCR as a novel effector molecule in the downstream pathway of doxorubicin, addressing doxorubicin resistance across different cancer types [67].
Gastric cancer
Gastric cancer (GC) is a prevalent malignant tumor worldwide and is the fourth most common cause of cancer-related mortality. The disease commonly manifests at advanced stages in the majority of patients, resulting in recurrent episodes that frequently lead to mortality [68]. Therefore, studies exploring new biomarkers for early GC detection and innovative treatment targets are urgently needed. Research has shown that DANCR expression in GC tissues surpasses that in adjacent tissues, with increased DANCR levels promoting tumour growth [69] and correlating with reduced patient survival rates [70]. Notably, significant associations have been observed between DANCR expression levels and the tumour size, TNM stage, lymph node metastasis, and invasion depth in patients with GC. Conversely, DANCR knockdown induces cell cycle arrest and hinders the EMT and apoptosis, thereby curtailing the proliferation, migration, and invasion of GC cells and ultimately suppressing GC growth in vivo [71].
DANCR has emerged as a pivotal factor in driving GC progression. Pan et al. reported that SALL4 activation induces DANCR expression in MGC-803 GC cells, thereby contributing to cancer promotion through the activation of the β-catenin pathway. Utilizing a male BALB/c nude mouse allogeneic transplantation model, they revealed that depleting DANCR inhibited the weight, volume, size, and proliferation of tumours derived from GC MGC-803 cells in vivo [71]. Moreover, FoxO1 has been found to stimulate macrophages to generate inflammatory factors and polarize them towards the M1 phenotype, exerting an antitumour effect. Xie et al. identified FoxO1 as a downstream target regulated by DANCR; elevated DANCR expression resulted in decreased FoxO1 expression, impeding macrophage polarization to the M1 type and ultimately fostering the invasion and metastasis of GC cells [69]. Additionally, KLF5, a zinc finger transcription factor, plays critical regulatory roles in cellular behaviour during tumorigenesis. Cheng et al. noted that suppressing KLF5 led to DANCR inhibition in male BALB/c nude mice, ultimately impeding GC progression [70].
DDP is commonly used as a primary chemotherapy agent for different stages of GC. Remarkably, DANCR exhibited increased expression in DDP-resistant GC tissues and cell lines, enhancing the proliferation and antiapoptotic activity of resistant cells such as SGC7901/DDP and BGC823/DDP cells. Conversely, suppressing DANCR can counteract the multidrug resistance observed in these resistant GC cells. Xu et al. examined the expression of genes related to multidrug resistance and reported that elevated DANCR levels substantially increased the mRNA and protein levels of MDR1 and MRP1. This discovery suggested that DANCR may play a role in the development of multidrug resistance by influencing the P-glycoprotein (P-gp) and MRP1 pathways [72].
Prostate cancer
PC ranks among the three most prevalent cancers in men [73]. Current strategies aimed at reducing PC mortality risk overdiagnosis; hence, the quest for novel biomarkers persists to enhance PC diagnosis and management [74]. Multiple studies have confirmed the oncogenic role of DANCR in PC. In PC tissues and cells, DANCR expression is elevated compared to that in normal prostate tissues and cells. DANCR enhances the invasiveness and migratory capacity of PC cells in vitro [75]. Suppression of DANCR hampers proliferation, migration, invasion, and EMT protein expression in PC cells, consequently halting cell cycle progression [76].
Androgen deprivation therapy is the cornerstone treatment for advanced PC by impeding androgen receptor (AR) signalling through either androgen deprivation or AR antagonists. Jia et al. reported that DANCR knockdown reduces the enzalutamide-induced invasion and migration of PC cells and that targeting DANCR may be promising for preventing prostate cancer metastasis and mitigating potential side effects of AR inhibitors. These findings suggest the potential of DANCR knockout to prevent the adverse effects of AR inhibitors on PC treatment. In addition, in a nude mouse xenograft model, DANCR knockdown decreased the number of metastatic lesions formed by CW22Rv1 cells; within C4-2B and CW22RV1 PC cells, DANCR and EZH2 collaborate to hinder TIMP2/3 expression by epigenetically silencing their promoters, thereby promoting tumour metastasis [75]. Lu et al. revealed that the MYC oncogene can stimulate DANCR transcription, with DANCR subsequently limiting the expression of the cell cycle inhibitor p21. They confirmed DANCR as a direct target of MYC, inhibiting p21 expression to bolster PC cell proliferation [77]. In addition, miRNAs such as miR-214-5p [10], miR-185-5p [76], and miR-135a [78] have been shown to regulate the progression of PC by competitively binding to DANCR in PC cells.
Taxol is a commonly used anticancer drug. However, the efficacy of Taxol-based chemotherapy is limited due to the development of drug resistance. Zhao et al. reported that the expression of DANCR was increased in PC tissues and cells and that DANCR knockdown led to increased sensitivity of PC cells to paclitaxel [78]. Wang and Chen reported that DANCR expression was significantly upregulated in PC tissues and cells and in a paclitaxel-resistant PC cell line. They found that DANCR positively regulates Ldha by competitively binding to miR-33B-5P, thereby promoting paclitaxel resistance in PC cells [79]; additionally, treatments incorporating docetaxel (DTX) as a standard anticancer agent have produced survival advantages for patients with castration-resistant PC. Nevertheless, individuals undergoing repeated chemotherapy often exhibit resistance. Ma et al. reported the upregulation of DANCR in DTX-resistant PC tissues and cells. Suppression of DANCR increased the sensitivity of PC cells to DTX, as supported by experiments involving male BALB/c nude mice injected subcutaneously with PC3/DTX cells transfected with sh-DANCR. The outcomes revealed that DANCR knockout significantly impeded tumour growth, including tumour dimensions and mass. Their investigation confirmed that the DANCR/miR-34a-5p axis reinforces DTX resistance in PC cells by targeting JAG1 [80].
Malignant glioma
Glioma is the most prevalent primary malignant brain tumour in adults [81]. It is characterized by rapid cell proliferation and angiogenesis. The majority of patients with WHO grade IV glioblastoma exhibit the highest malignancy level and poorest prognosis [82]. Numerous studies have indicated that DANCR may serve as an oncogenic factor crucial for glioma onset, progression, and other malignant traits. DANCR is significantly upregulated in glioma tissues and cell lines, and its elevated expression is correlated with an advanced tumour grade. Suppression of DANCR has been shown to impede glioma cell proliferation [83], migration, invasion, and angiogenesis [84]. Additionally, it can enhance apoptosis, induce cell cycle arrest [85], and is positively correlated with glioma cell proliferation and autophagy [25]. High DANCR expression is associated with increased malignancy and an unfavourable prognosis in glioma patients [86].
In glioma cells, DANCR functions as a competitive endogenous RNA for miRNAs such as miR-33a-5p, miR-33b-5p, miR-1-3p, miR-206 and miR-613 [87] to drive the malignant progression of glioma. Dysregulation of the Wnt/β-catenin signalling pathway promotes tumour stem cell renewal, proliferation, and differentiation [88]. Studies indicate that downregulating DANCR can mitigate the impact of Wnt/β-catenin activation on glioma cell proliferation and migration [83]. The PI3K/Akt/mTOR signalling pathway is implicated in tumour cell migration, invasion and angiogenesis. Wang et al. confirmed that DANCR can activate the PI3K/AKT/mTOR pathway to modulate glioma cell growth and metastasis [84]. Furthermore, DANCR epigenetically suppresses PTEN expression by binding to EZH2, thereby enhancing glioma cell invasion, migration, proliferation, and impeding apoptosis [89]. Feng et al. inoculated transfected cells into male BALB/c nude mice, and the results showed that DANCR knockdown significantly reduced tumour weight, tumour volume, and tumour growth [90].
DDP is employed as an adjuvant chemotherapy for glioma; however, the development of acquired resistance hampers its efficacy. Ma et al. silenced DANCR in female athymic BALB/c nude mice, and the apoptotic response to cisplatin was potentiated. Additionally, Ma et al. verified that DANCR enhances resistance to DDP by elevating AXL expression, which activates the PI3K/Akt/NF-kappa B pathway in glioma cells [87]. Etoposide is a well-tolerated chemotherapeutic utilized in glioblastoma treatment. Han et al. revealed that FOXO1 promotes PID1 expression, consequently enhancing DANCR stability through FOXO1 ubiquitination and contributing to glioblastoma cell resistance to etoposide [91].
Acute myeloid leukaemia
Among adult acute leukaemias, AML has the highest incidence and shortest survival time. Regrettably, the survival rate has shown little improvement in recent decades [92]. The postoperative recurrence rate of AML is notably high [93], with its aetiology displaying significant heterogeneity [94]. Consequently, the identification of supplementary AML biomarkers holds substantial importance for screening, diagnosing, predicting the prognosis, and monitoring AML, along with forecasting individual responses to treatment [95].
Research studies have validated the significant expression of DANCR in populations enriched with leukaemia stem cells. Silencing DANCR in these cells diminishes their self-renewal capacity and proliferation. Furthermore, targeting of DANCR in vivo has been shown to extend the survival period of mice after continuous transplantation in a primary mouse model of AML [96]. This finding provides novel insights for AML treatment strategies. Among FLT3 mutations, internal tandem duplication (ITD) is the most prevalent. Wu et al. reported that high DANCR expression in FLT3-ITD + AML patients and cells was correlated with unfavourable patient outcomes. IGF2BP2 plays a role in stabilizing lncRNAs and enhancing their expression. Wu et al. revealed that IGF2BP2 regulates the glycolysis levels by upregulating DANCR expression, impacting the progression of FLT3-ITD + AML [9].
The ‘3 + 7 regimen’, which combines cytarabine (Ara-C) and anthracycline drugs, has served as the cornerstone of AML treatment for decades since the discovery of its efficacy in AML [97]. Numerous studies have detailed the use of Ara-C and daunorubicin for treating newly diagnosed adult patients with AML presenting with myelodysplastic syndrome-related changes and therapy-induced AML [98]. However, investigating alternative treatment options for patients who exhibit mutations associated with chemotherapy resistance is crucial. Studies have shown that elevated DANCR levels in human AML cells correlate with Ara-C resistance. Moreover, DANCR promotes autophagy in human AML cells treated with Ara-C, thereby increasing the expression of ATG16L1, a critical autophagy component. This study conclusively showed that DANCR contributes to Ara-C resistance in human AML cells by activating the miR-874-3P/ATG16L1 axis to enhance autophagy [99].
Other types of cancer
In addition to the aforementioned exceptional cancers, DANCR is also implicated in the regulation of other types of cancer. It is upregulated in nasopharyngeal carcinoma (NPC) [100], oral cancer [101], tongue squamous cell carcinoma [102], CCA [103], EC [104], pancreatic cancer (PAAD) [105], bladder cancer (BLCA) [106], retinoblastoma [14], OS [31], ovarian cancer [107], and endometrial cancer [108] and downregulated in renal cell carcinoma (RCC) [109]. The regulation of DANCR expression is still controversial in papillary thyroid cancer (PTC) [110, 111] and cervical cancer (CC) [112, 113].
Resistance to radiation therapy remains a significant challenge in the management of advanced NPC. DANCR knockdown suppresses PTEN, a well-established tumour suppressor, thereby augmenting the radiosensitivity of NPC cells [114]. Additionally, DANCR can drive NPC tumorigenesis by interacting with RBM3, which contributes to NPC radioresistance [115]. Research on PAAD indicates a progressive increase in DANCR expression from early to advanced stages. Although MLL3 acts as a tumour suppressor protein, DANCR does not influence MLL3 regulation in early PAAD stages; however, DANCR downregulates MLL3 in advanced stages, promoting cancer progression [116]. Notably, DANCR expression is significantly increased in BLCA tissues with lymph node metastasis. Its role in modulating BLCA cell metastasis involves activating the IL-11-STAT3 signalling pathway through LRPPRC guidance to stabilize mRNAs and enhance CCND1 expression [106]. Furthermore, DANCR influences lymphangiogenesis by regulating VEGF-C expression, impacting tumour angiogenesis and progression [117]. Elevated p38MAPK levels impede metastatic tumour formation. Studies have revealed that silencing DANCR activates the p38MAPK pathway in OS cells, leading to decreased cell proliferation, migration, and invasion; conversely, inhibition of the p38MAPK pathway reverses these effects [118].
Limited research exists on DANCR in RCC. Current studies suggest that DANCR functions as a tumour suppressor in RCC, with its expression downregulated in RCC tissues. Elevated DANCR levels can suppress the proliferation, migration, and invasion of RCC cells while enhancing their apoptosis [109].
Studies have indicated a significant increase in DANCR expression in CC tissues and cells. High DANCR levels are positively correlated with the tumour size, FIGO stage, lymph node metastasis, and a poor prognosis. DANCR facilitates the proliferation, migration, and invasion of CC cells. However, Ta et al. observed downregulation of DANCR in the cervical tissue and serum of patients with HPV-negative CC compared to normal controls and patients with HPV-positive CC. They also noted that under hypoxic conditions, elevated DANCR suppressed HPV-negative CC cell proliferation by inhibiting HiF-1α [113]. In their research on PTC, Zhang et al. reported significantly lower DANCR expression in tumour tissues than in adjacent normal tissues. They further assessed DANCR expression in patients with various TNM stages of PTC and concluded that DANCR serves as an independent protective factor in TNM staging [110]. Conversely, Icduygu et al. reported markedly higher DANCR levels in PTC tissues than in adjacent normal tissues. Notably, DANCR expression did not increase significantly when the tumour diameter was ≤ 1 cm. Moreover, DANCR expression correlated with age and microcarcinoma, potentially influencing thyroid papillary carcinoma development [111].
The diagnostic value of DANCR
LncRNAs exhibit stability in serum, plasma, and various body fluids and are resistant to endogenous ribonucleases. This resilience has positioned circulating lncRNAs as promising cancer biomarkers [143]. Numerous investigations have detected DANCR in serum, plasma, and exosomes, underscoring its utility in diagnosing, predicting the prognosis, and assessing treatment responses across different conditions (Table 2).
Ma et al. [40] proposed that plasma DANCR levels could outperform AFP as a diagnostic biomarker for HCC. Notably, Table 2 shows the superior diagnostic accuracy of plasma DANCR levels over AFP levels in HCC patients. Furthermore, Wang et al. [144] highlighted the potential of circulating exosomal DANCR as a noninvasive prognostic marker for hepatitis C virus-related HCC. In CRC, Shen et al. investigated DANCR levels in both tissue samples and serum, revealing significant upregulation in both samples. They noted a decrease in serum DANCR expression among postoperative individuals compared to pretreatment and relapsed patients. Their study integrated carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) as tumour markers and concluded that the concurrent detection of DANCR, CEA, and CA199 yielded the highest sensitivity and area under the curve (AUC) compared to the detection of a single biomarker. This comprehensive approach enhanced CRC diagnostic efficacy [145]. Research on GC indicated elevated DANCR expression levels in patient serum compared to healthy controls, with a notably high AUC value, indicating of its diagnostic potential [71]. Zhang et al. explored DANCR as a tumour marker for PTC utilizing receiver operating characteristic curves to differentiate between patients with early-stage (I/II) and advanced-stage (III/IV) PTC [110]. Additionally, Shi et al. reported significantly elevated serum exosomal DANCR levels in BC patients relative to those in patients with benign breast diseases and healthy individuals. The combined detection of serum exosomal DANCR, CA153, and CEA levels markedly improved the BC diagnostic accuracy, with serum exosomal DANCR levels serving as an independent BC risk factor [50].
These studies illustrate the considerable promise of measuring the circulating DANCR level as a novel noninvasive biomarker for cancer diagnosis. Nonetheless, existing limitations in its clinical implementation underscore the need for additional research on lncRNA liquid biopsy to reinforce its viability.
Conclusions and prospects
Although DANCR is generally considered an oncogenic factor in most cancers, it is considered a tumour suppressor in RCC, and its role in some cancers, such as lung cancer and PTC, remains controversial. These discrepancies suggest that the distinct expression profiles and functional roles of DANCR across various cancer types and cell lines significantly influence disease progression and clinical outcomes. Extensive research has revealed the intricate mechanisms through which DANCR promotes tumour development, involving interactions such as a ceRNA targeting microRNAs, the modulation of mRNA or protein activities, initiation of signalling cascades, and epigenetic modulation. Despite the identified role of DANCR in mediating drug resistance in multiple cancer types, investigations into its precise contribution to this phenomenon remain in nascent stages, and the underlying mechanisms remain unclear. Notably, circulating DANCR exhibits exceptional precision in discriminating among diverse cancer types. Nevertheless, conclusive clinical investigations are imperative to confirm the implications of DANCR for early-stage diagnosis, prognostication, and pharmacotherapy (Fig. 3).
The role of DANCR in cancer. DANCR is located on chromosome 4 with 855 base pairs and consists of three exons, with introns 1 and 2 containing miR4449 and snoR26. DANCR can regulate the hallmarks of various cancers. DANCR is aberrantly expressed in a variety of tumours. Circulating DANCR has diagnostic value. DANCR regulates drug resistance in a variety of tumours. DANCR has complex molecular mechanisms. DANCR has broad application prospects
Although targeting DANCR for tumour therapy holds promise, its therapeutic application encounters various obstacles, including drug-related toxicity, off-target effects, and the development of secure and effective delivery mechanisms. Concurrently, DANCR shows potential for clinical utility in addressing tumour drug resistance. A standardized research framework encompassing the isolation of circulating DANCR and the enhancement of bioinformatic sequencing techniques characterized by increased efficiency, sensitivity, and specificity must be developed to establish DANCR as a novel biomarker.
Abbreviations
- lncRNAs:
-
Long non-coding RNAs
- DANCR/ANCR:
-
Differentiation antagonizing non-protein coding RNA
- CRC:
-
Colorectal cancer
- EMT:
-
Epithelial-mesenchymal transition
- TNBC:
-
Triple-negative breast cancer
- EC:
-
Esophageal cancer
- HUVECs:
-
Human umbilical vein endothelial cells
- HAECs:
-
Human arterial endothelial cells
- BC:
-
Breast cancer
- GLUT1:
-
Glucose transporter type 1
- PKM:
-
Pyruvate kinase M
- AML:
-
Acute myeloid leukaemia
- PC:
-
Prostate cancer
- ceRNA:
-
Competitive endogenous RNA
- MREs:
-
miRNA recognition elements
- EL/LIPG:
-
Endothelial lipase
- CCA:
-
Cholangiocarcinoma
- OS:
-
Osteosarcoma
- NSCLC:
-
Non-small cell lung cancer
- HCC:
-
Hepatocellular carcinoma
- HNRNPA1:
-
Heterogeneous nuclear ribonucleoprotein A1
- OXPHOS:
-
Oxidative phosphorylation
- KLF5:
-
Krüppel-like factor 5
- DDP:
-
Cisplatin
- KAT6A:
-
Lysine acetyltransferase 6 A
- HSP27:
-
Heat shock protein 27
- HK2:
-
Hexokinase 2
- GC:
-
Gastric cancer
- P-gp:
-
P-glycoprotein
- AR:
-
Androgen receptor
- DTX:
-
Docetaxel
- ITD:
-
Internal tandem duplication
- Ara-C:
-
Cytarabine
- NPC:
-
Nasopharyngeal carcinoma
- PAAD:
-
Pancreatic cancer
- BLCA:
-
Bladder cancer
- RCC:
-
Renal cell carcinoma
- PTC:
-
Papillary thyroid cancer
- CC:
-
Cervical cancer
- CEA:
-
Carcinoembryonic antigen
- CA199:
-
Carbohydrate antigen 199
- AUC:
-
Area under the curve
References
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9.
Wang H, Meng Q, Qian J, Li M, Gu C, Yang Y, Review. RNA-based diagnostic markers discovery and therapeutic targets development in cancer. Pharmacol Ther. 2022;234:108123.
Kretz M, Webster DE, Flockhart RJ, Lee CS, Zehnder A, Lopez-Pajares V, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–43.
Wang S, Jiang M. The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed Pharmacother. 2018;103:94–100.
Wu G, Zhou H, Li D, Zhi Y, Liu Y, Li J, et al. LncRNA DANCR upregulation induced by TUFT1 promotes malignant progression in triple negative breast cancer via mir-874-3p-SOX2 axis. Exp Cell Res. 2020;396:112331.
Huang YF, Zhang Y, Fu X. Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225-3p/ErbB2 signal. Eur Rev Med Pharmacol Sci. 2021;25:758–69.
Bi Y, Guo S, Xu X, Kong P, Cui H, Yan T, et al. Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death Dis. 2020;11:296.
Zhang KJ, Tan XL, Guo L. The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol Oncol. 2020;14:309–28.
Wu S, Chi C, Weng S, Zhou W, Liu Z. IGF2BP2 promotes lncRNA DANCR stability mediated glycolysis and affects the progression of FLT3-ITD + acute myeloid leukemia. Apoptosis. 2023;28:1035–47.
Deng H, Zhu B, Dong Z, Jiang H, Zhao X, Wu S. Mir-214-5p targeted by LncRNA DANCR mediates TGF-β signaling pathway to accelerate proliferation, migration and inhibit apoptosis of prostate cancer cells. Am J Transl Res. 2021;13:2224–40.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Lu W, Huang Z, Wang J, Liu H. Long non-coding RNA DANCR accelerates colorectal cancer progression via regulating the miR-185-5p/HMGA2 axis. J Biochem. 2022;171:389–98.
Tang Y, Cao G, Zhao G, Wang C, Qin Q. LncRNA differentiation antagonizing non-protein coding RNA promotes proliferation and invasion through regulating miR-135a/NLRP37 axis in pancreatic cancer. Invest New Drugs. 2020;38:714–21.
Wang JX, Yang Y, Li K. Long noncoding RNA DANCR aggravates retinoblastoma through miR-34c and miR-613 by targeting MMP-9. J Cell Physiol. 2018;233:6986–95.
Gan X, Ding D, Wang M, Yang Y, Sun D, Li W, et al. DANCR deletion retards the initiation and progression of hepatocellular carcinoma based on gene knockout and patient-derived xenograft in situ hepatoma mice model. Cancer Lett. 2022;550:215930.
Lian J, Zhang H, Wei F, Li Q, Lu Y, Yu B, et al. Long non-coding RNA DANCR promotes colorectal tumor growth by binding to lysine acetyltransferase 6A. Cell Signal. 2020;67:109502.
Gerstberger S, Jiang Q, Ganesh K. Metastasis Cell. 2023;186:1564–79.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Yuan Sxian, Wang J, Yang F, Tao Q, fei, Zhang J, Wang L, li, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63:499–511.
Yu JE, Ju JA, Musacchio N, Mathias TJ, Vitolo MI. Long noncoding RNA DANCR activates Wnt/β-Catenin signaling through MiR-216a inhibition in Non-small Cell Lung Cancer. Biomolecules. 2020;10:1646.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Jia H, Liang K, Liu G, Zhang Z, Shi Y, Liang H, et al. lncRNA DANCR promotes proliferation and metastasis of breast Cancer cells through sponging miR-4319 and Upregulating VAPB. Cancer Biother Radiopharm. 2022;37:650–61.
Yun CW, Lee SH. The roles of Autophagy in Cancer. Int J Mol Sci. 2018;19:3466.
Yu W, Ma L, Li X. DANCR promotes glioma cell autophagy and proliferation via the miR–33b/DLX6/ATG7 axis. Oncol Rep. 2023;49:39.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA. Language? Cell. 2011;146:353–8.
Yang R, Han S, Clayton J, Haghighatian M, Tsai CC, Yao Y, et al. A proof-of-Concept inhibitor of endothelial lipase suppresses triple-negative breast Cancer cells by hijacking the mitochondrial function. Cancers (Basel). 2022;14:3763.
Liu Y, Chen L, Yuan H, Guo S, Wu G. LncRNA DANCR promotes Sorafenib Resistance via activation of IL-6/STAT3 signaling in Hepatocellular Carcinoma cells. Onco Targets Ther. 2020;13:1145–57.
Wang N, Zhang C, Wang W, Liu J, Yu Y, Li Y, et al. Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis. 2019;10:585.
Lin CY, Tseng WT, Chang YY, Tsai MH, Chuang EY, Lu TP, et al. Lidocaine and Bupivacaine Downregulate MYB and DANCR lncRNA by Upregulating Mir-187-5p in MCF-7 cells. Front Med (Lausanne). 2021;8:732817.
Zhou X, Yang Y, Li Y, Liang G, Kang D, Zhou B, et al. METTL3 contributes to Osteosarcoma Progression by increasing DANCR mRNA Stability via m6A modification. Front Cell Dev Biol. 2021;9:784719.
Lu QC, Rui ZH, Guo ZL, Xie W, Shan S, Ren T. LncRNA-DANCR contributes to lung adenocarcinoma progression by sponging miR-496 to modulate mTOR expression. J Cell Mol Med. 2018;22:1527–37.
Zhen Q, Gao LN, Wang RF, Chu WW, Zhang YX, Zhao XJ, et al. LncRNA DANCR promotes Lung Cancer by sequestering miR-216a. Cancer Control. 2018;25:1073274818769849.
Chen YR, Wu YS, Wang WS, Zhang JS, Wu QG. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214-5p/CIZ1 axis. Eur Rev Med Pharmacol Sci. 2020;24:2539–47.
Guo L, Gu J, Hou S, Liu D, Zhou M, Hua T, et al. Long non-coding RNA DANCR promotes the progression of non-small-cell lung cancer by inhibiting p21 expression. Onco Targets Ther. 2019;12:135–46.
Bai Y, Zhang G, Chu H, Li P, Li J. The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am J Cancer Res. 2019;9:270–84.
Wang S, Lan F, Xia Y. lncRA ANCR inhibits Non-small Cell Lung Cancer Cell Migration and Invasion by inactivating TGF-b pathway. Med Sci Monit. 2018;24:6002–9.
Nicolescu C, Vaidya A, Schilb A, Lu ZR. Regulating Oncogenic LncRNA DANCR with targeted ECO/siRNA nanoparticles for Non-small Cell Lung Cancer Therapy. ACS Omega. 2022;7:22743–53.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Ma X, Wang X, Yang C, Wang Z, Han B, Wu L, et al. DANCR acts as a diagnostic biomarker and promotes Tumor Growth and Metastasis in Hepatocellular Carcinoma. Anticancer Res. 2016;36:6389–98.
Wang J, Pu J, Zhang Y, Yao T, Luo Z, Li W, et al. DANCR contributed to hepatocellular carcinoma malignancy via sponging miR-216a-5p and modulating KLF12. J Cell Physiol. 2019;234:9408–16.
Guo D, Li Y, Chen Y, Zhang D, Wang X, Lu G, et al. DANCR promotes HCC progression and regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 2019;52:e12628.
Wang X, Cheng ML, Gong Y, Ma WJ, Li B, Jiang YZ. LncRNA DANCR promotes ATG7 expression to accelerate hepatocellular carcinoma cell proliferation and autophagy by sponging miR-222-3p. Eur Rev Med Pharmacol Sci. 2020;24:8778–87.
Yang L, Jiang MN, Liu Y, Wu CQ, Liu H. Crosstalk between lncRNA DANCR and miR-125b-5p in HCC cell progression. Tumori. 2021;107:504–13.
Wen Z, Lian L, Ding H, Hu Y, Xiao Z, Xiong K, et al. LncRNA ANCR promotes hepatocellular carcinoma metastasis through upregulating HNRNPA1 expression. RNA Biol. 2020;17:381–94.
Karim AM, Eun Kwon J, Ali T, Jang J, Ullah I, Lee YG, et al. Triple-negative breast cancer: epidemiology, molecular mechanisms, and modern vaccine-based treatment strategies. Biochem Pharmacol. 2023;212:115545.
Tang J, Zhong G, Zhang H, Yu B, Wei F, Luo L, et al. LncRNA DANCR upregulates PI3K/AKT signaling through activating serine phosphorylation of RXRA. Cell Death Dis. 2018;9:1167.
Tao W, Wang C, Zhu B, Zhang G, Pang D. LncRNA DANCR contributes to tumor progression via targetting miR-216a-5p in breast cancer: lncRNA DANCR contributes to tumor progression. Biosci Rep. 2019;39:BSR20181618.
Sha S, Yuan D, Liu Y, Han B, Zhong N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open. 2017;6:1310–6.
Shi W, Jin X, Wang Y, Zhang Q, Yang L. High serum exosomal long non-coding RNA DANCR expression confers poor prognosis in patients with breast cancer. J Clin Lab Anal. 2022;36:e24186.
Zhang XH, Li BF, Ding J, Shi L, Ren HM, Liu K, et al. LncRNA DANCR-miR-758-3p-PAX6 Molecular Network regulates apoptosis and autophagy of breast Cancer cells. Cancer Manag Res. 2020;12:4073–84.
Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298:1039–43.
Li Z, Hou P, Fan D, Dong M, Ma M, Li H, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71.
Muluhngwi P, Klinge CM. Identification and roles of miR-29b-1-3p and miR29a-3p-Regulated and non-regulated lncRNAs in endocrine-sensitive and resistant breast Cancer cells. Cancers (Basel). 2021;13:3530.
Li Z et al. M D, D F, P H, H L, L L, LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer. Oncotarget [Internet]. 2017 [cited 2023 Nov 21];8. https://pubmed.ncbi.nlm.nih.gov/28978036/.
Jiang C, Shi X, Yi D, Wang R, Xu F, Guan W, et al. Long non-coding RNA anti-differentiation non-coding RNA affects proliferation, invasion, and migration of breast cancer cells by targeting miR-331. Bioengineered. 2021;12:12236–45.
Su A, Yao K, Zhang H, Wang Y, Zhang H, Tang J. DANCR induces Cisplatin Resistance of Triple-negative breast Cancer by KLF5/p27 signaling. Am J Pathol. 2023;193:248–58.
Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. 2022;7:700.
Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262–74.
Luan L, Dai Y, Shen T, Yang C, Chen Z, Liu S, et al. Development of a novel hypoxia-immune-related LncRNA risk signature for predicting the prognosis and immunotherapy response of colorectal cancer. Front Immunol. 2022;13:951455.
Wang Y, Z L. N W, J F, J Z, L L, Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Experimental & molecular medicine [Internet] 2018 [cited 2023 Nov 27];50. https://pubmed.ncbi.nlm.nih.gov/29717105/.
Yang ZY, Yang F, Zhang YL, Liu B, Wang M, Hong X, et al. LncRNA-ANCR down-regulation suppresses invasion and migration of colorectal cancer cells by regulating EZH2 expression. Cancer Biomark. 2017;18:95–104.
Sun Y, Cao B, Zhou J. Roles of DANCR/microRNA-518a-3p/MDMA ceRNA network in the growth and malignant behaviors of colon cancer cells. BMC Cancer. 2020;20:434.
LIU Z, Yx LLJL. C. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. International journal of clinical and experimental pathology [Internet] 2015 [cited 2023 Nov 27];8. https://pubmed.ncbi.nlm.nih.gov/26617879/.
Bahreini F, Saidijam M, Mousivand Z, Najafi R, Afshar S. Assessment of lncRNA DANCR, Mir-145-5p and NRAS axis as biomarkers for the diagnosis of colorectal cancer. Mol Biol Rep. 2021;48:3541–7.
Shi H, Li K, Feng J, Liu G, Feng Y, Zhang X. LncRNA-DANCR interferes with miR-125b-5p/HK2 Axis to desensitize Colon cancer cells to Cisplatin vis activating anaerobic glycolysis. Front Oncol. 2020;10:1034.
Xiong X, None MW, Z DPWH. C, H K, LncRNA DANCR represses Doxorubicin-induced apoptosis through stabilizing MALAT1 expression in colorectal cancer cells. Cell death & disease [Internet] 2021 [cited 2023 Nov 12];12. https://pubmed.ncbi.nlm.nih.gov/33414433/.
L Z, Z X, X X, B Z, H W, M W, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene [Internet] 2014 [cited 2023 Nov 29];33. https://pubmed.ncbi.nlm.nih.gov/24276240/.
Xie C, Guo Y, Lou S. LncRNA ANCR promotes Invasion and Migration of Gastric Cancer by regulating FoxO1 expression to inhibit macrophage M1 polarization. Dig Dis Sci. 2020;65:2863–72.
Cheng Z, Liu G, Huang C, Zhao X. KLF5 activates lncRNA DANCR and inhibits cancer cell autophagy accelerating gastric cancer progression. NPJ Genom Med. 2021;6:75.
Pan L, Liang W, Gu J, Zang X, Huang Z, Shi H, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9:1915–30.
Xu YD, Shang J, Li M, Zhang YY. LncRNA DANCR accelerates the development of multidrug resistance of gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23:2794–802.
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, et al. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol. 2021;18:282–301.
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate Cancer epidemiology and risk Factors-A systematic review. Eur Urol. 2023;84:191–206.
Jia J, Li F, Tang XS, Xu S, Gao Y, Shi Q, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7:37868–81.
Sun W, Zu S, Shao G, Wang W, Gong F. Long non-coding DANCR targets mir-185-5p to upregulate LIM and SH3 protein 1 promoting prostate cancer via the FAK/PI3K/AKT/GSK3β/snail pathway. J Gene Med. 2021;23:e3344.
Lu Y, Hu Z, Mangala LS, Stine ZE, Hu X, Jiang D, et al. MYC targeted long noncoding RNA DANCR promotes Cancer in Part by reducing p21 levels. Cancer Res. 2018;78:64–74.
Zhao HF, Zhang ZC, Shi BK, Jiang XZ. DANCR sponges miR-135a to regulate paclitaxel sensitivity in prostate cancer. Eur Rev Med Pharmacol Sci. 2019;23:6849–57.
Wang YY, Chen C. lncRNA-DANCR promotes Taxol Resistance of prostate Cancer cells through modulating the miR-33b-5p-LDHA Axis. Dis Markers. 2022;2022:9516774.
Ma Y, Fan B, Ren Z, Liu B, Wang Y. Long noncoding RNA DANCR contributes to docetaxel resistance in prostate cancer through targeting the miR-34a-5p/JAG1 pathway. Onco Targets Ther. 2019;12:5485–97.
Davis ME. Epidemiology and overview of Gliomas. Semin Oncol Nurs. 2018;34:420–9.
Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018;17:61.
Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/β-catenin signaling. Biomed Pharmacother. 2018;102:602–7.
Wang W, Li Y, Ma Q, Yan H, Su W. Differentiation antagonizing non-protein coding RNA modulates the proliferation, migration, and angiogenesis of glioma cells by targeting the miR-216a/LGR5 axis and the PI3K/AKT signaling pathway. Onco Targets Ther. 2019;12:2439–49.
Xu D, Yu J, Gao G, Lu G, Zhang Y, Ma P. LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci Rep. 2018;38:BSR20171664.
Yang JX, Sun Y, Gao L, Meng Q, Yang BY. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma. 2018;65:790–8.
Ma Y, Zhou G, Li M, Hu D, Zhang L, Liu P, et al. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem Int. 2018;118:233–41.
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165.
Cheng C, Dong Y, Ru X, Xia Y, Ji Y. LncRNA ANCR promotes glioma cells invasion, migration, proliferation and inhibits apoptosis via interacting with EZH2 and repressing PTEN expression. Cancer Gene Ther. 2021;28:1025–34.
Feng L, Lin T, Che H, Wang X. Long noncoding RNA DANCR knockdown inhibits proliferation, migration and invasion of glioma by regulating miR-135a-5p/BMI1. Cancer Cell Int. 2020;20:53.
Han J, Yu X, Wang S, Wang Y, Liu Q, Xu H, et al. IGF2BP2 induces U251 Glioblastoma Cell Chemoresistance by inhibiting FOXO1-Mediated PID1 expression through stabilizing lncRNA DANCR. Front Cell Dev Biol. 2021;9:659228.
Carter JL, Hege K, Yang J, Kalpage HA, Su Y, Edwards H, et al. Targeting multiple signaling pathways: the new approach to acute myeloid leukemia therapy. Signal Transduct Target Ther. 2020;5:288.
Yang D, Zhang X, Zhang X, Xu Y. The progress and current status of immunotherapy in acute myeloid leukemia. Ann Hematol. 2017;96:1965–82.
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31:63–76.
Bill M, Papaioannou D, Karunasiri M, Kohlschmidt J, Pepe F, Walker CJ, et al. Expression and functional relevance of long non-coding RNAs in acute myeloid leukemia stem cells. Leukemia. 2019;33:2169–82.
Kantarjian H. Acute myeloid leukemia–major progress over four decades and glimpses into the future. Am J Hematol. 2016;91:131–45.
Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, Daver N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020;61:288–97.
Zhang H, Liu L, Chen L, Liu H, Ren S, Tao Y. Long noncoding RNA DANCR confers cytarabine resistance in acute myeloid leukemia by activating autophagy via the miR-874-3P/ATG16L1 axis. Mol Oncol. 2021;15:1203–16.
Hao Y, Zhao H, Jin X, He P, Zhang J, Dong Q, et al. Long non–coding RNA DANCR promotes nasopharyngeal carcinoma cell proliferation and migration. Mol Med Rep. 2019;19:2883–9.
Qu XH, Shi YL, Ma Y, Bao WW, Yang L, Li JC, et al. LncRNA DANCR regulates the growth and metastasis of oral squamous cell carcinoma cells via altering miR-216a-5p expression. Hum Cell. 2020;33:1281–93.
Zheng Y, Zheng B, Meng X, Yan Y, He J, Liu Y. LncRNA DANCR promotes the proliferation, migration, and invasion of tongue squamous cell carcinoma cells through miR-135a-5p/KLF8 axis. Cancer Cell Int. 2019;19:302.
Zhu CY, Fan CR, Zhang YL, Sun QX, Yan MJ, Wei W, et al. LncRNA DANCR affected cell growth, EMT and angiogenesis by sponging mir-345-5p through modulating Twist1 in cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2020;24:2321–34.
Shi H, Shi J, Zhang Y, Guan C, Zhu J, Wang F, et al. Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J Thorac Dis. 2018;10:2573–82.
Yao Z, Chen Q, Ni Z, Zhou L, Wang Y, Yang Y, et al. Long non-coding RNA differentiation antagonizing nonprotein coding RNA (DANCR) promotes Proliferation and Invasion of Pancreatic Cancer by sponging mir-214-5p to regulate E2F2 expression. Med Sci Monit. 2019;25:4544–52.
Chen Z, Chen X, Xie R, Huang M, Dong W, Han J, et al. DANCR promotes metastasis and proliferation in bladder Cancer cells by enhancing IL-11-STAT3 Signaling and CCND1 expression. Mol Ther. 2019;27:326–41.
Pei CL, Fei KL, Yuan XY, Gong XJ. LncRNA DANCR aggravates the progression of ovarian cancer by downregulating UPF1. Eur Rev Med Pharmacol Sci. 2019;23:10657–63.
Sun J, Gao S, Lu C. Knockdown of differentiation antagonizing non-protein coding RNA exerts anti-tumor effect by up-regulating miR-214 in endometrial carcinoma. Mol Cell Biochem. 2019;460:9–15.
Jin L, Fu H, Quan J, Pan X, He T, Hu J, et al. Overexpression of long non-coding RNA differentiation antagonizing non-protein coding RNA inhibits the proliferation, migration and invasion and promotes apoptosis of renal cell carcinoma. Mol Med Rep. 2017;16:4463–8.
Zhang K, Lv J, Peng X, Liu J, Li C, Li J, et al. Down-regulation of DANCR acts as a potential biomarker for papillary thyroid cancer diagnosis. Biosci Rep. 2019;39:BSR20181616.
Icduygu FM, Akgun E, Sengul D, Ozgoz A, Alp E. Expression of SOX2OT, DANCR and TINCR long non–coding RNAs in papillary thyroid cancer and its effects on clinicopathological features. Mol Med Rep. 2022;25:120.
Cao L, Jin H, Zheng Y, Mao Y, Fu Z, Li X, et al. DANCR- mediatedmicroRNA-665regulatesproliferationand metastasisofcervicalcancerthroughtheERK/SMADpathway. Cancer Sci. 2019;110:913–25.
Ta W, Zhang Y, Zhang S, Sun P. LncRNA ANCR downregulates hypoxia–inducible factor 1α and inhibits the growth of HPV–negative cervical squamous cell carcinoma under hypoxic conditions. Mol Med Rep. 2020;21:413–9.
Ma X, Zhou J, Liu J, Wu G, Yu Y, Zhu H, et al. LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. Onco Targets Ther. 2018;11:8399–408.
Li Q, Jiang Y, Zhong G, Lu Y, Song T, Zhang Y, et al. Long noncoding RNA DANCR regulates cell proliferation by stabilizing SOX2 mRNA in nasopharyngeal carcinoma. Am J Pathol. 2020;190:2343–54.
Liu Y, Li J, Yue B, Liang L, Zhang S, Chen Y. Long non-coding RNA DANCR regulate MLL3 and thereby it determines the progression of pancreatic cancer. J BUON. 2020;25:1954–9.
Ping Q, Shi Y, Yang M, Li H, Zhong Y, Li J, et al. LncRNA DANCR regulates lymphatic metastasis of bladder cancer via the miR-335/VEGF-C axis. Transl Androl Urol. 2021;10:1743–53.
Liu B, Zhao H, Zhang L, Shi X. Silencing of long-non-coding RNA ANCR suppresses the migration and invasion of osteosarcoma cells by activating the p38MAPK signalling pathway. BMC Cancer. 2019;19:1112.
Zhang J, Jin X, Zhou C, Zhao H, He P, Hao Y, et al. Resveratrol suppresses human nasopharyngeal Carcinoma Cell Growth Via inhibiting differentiation antagonizing Non-protein Coding RNA (DANCR) expression. Med Sci Monit. 2020;26:e923622.
Ma X, Yuan Y, Lu J, Li M, Yu Y, Liu J, et al. Long noncoding RNA ANCR promotes migration, invasion, EMT progress and stemness of nasopharyngeal carcinoma cells via the miR-4731-5p/NMT1 axis. Pathol Res Pract. 2021;224:153540.
Wen X, Yp XL, Xj M, Yq Y, Pp W et al. Z, Long non-coding RNA DANCR stabilizes HIF-1α and promotes metastasis by interacting with NF90/NF45 complex in nasopharyngeal carcinoma. Theranostics [Internet]. 2018 [cited 2023 Nov 8];8. https://pubmed.ncbi.nlm.nih.gov/30555573/.
Bi C, Shan J, Li M, Zhang Q, Li C, Tong J, et al. Long noncoding RNA differentiation antagonizing nonprotein coding RNA promotes the proliferation, invasion and migration of neuroblastoma cells via targeting β-1, 4-galactosyltransferase III by sponging miR-338-3p. NeuroReport. 2021;32:965–74.
Zhang N, Jiang W. Long non–coding RNA DANCR promotes HMGA2–mediated invasion in lung adenocarcinoma cells. Oncol Rep. 2019;41:1083–90.
Wu L, Xia L, Jiang H, Hu Y, Li L, Xu L, et al. Long non–coding RNA DANCR represses the viability, migration and invasion of multiple myeloma cells by sponging miR–135b–5p to target KLF9. Mol Med Rep. 2021;24:649.
Wang B, Chen W, Zhao Z, Sun Y, Huang Y. LncRNA-DANCR promotes growth and metastasis of colorectal cancer via activating epithelial-mesenchymal transition process. Transl Cancer Res. 2019;8:2517–25.
Zhang C, Wang L, Yang J, Fu Y, Li H, Xie L, et al. MicroRNA-33a-5p suppresses esophageal squamous cell carcinoma progression via regulation of lncRNA DANCR and ZEB1. Eur J Pharmacol. 2019;861:172590.
Hao YP, Qiu JH, Zhang DB, Yu CG. Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol. 2017;39:1010428317699798.
Luo Y, Wang Q, Teng L, Zhang J, Song J, Bo W, et al. LncRNA DANCR promotes proliferation and metastasis in pancreatic cancer by regulating miRNA-33b. FEBS Open Bio. 2020;10:18–27.
Hu X, Wx P, X HZJJ. Z, D H, IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell death and differentiation [Internet] 2020 [cited 2023 Nov 12];27. https://pubmed.ncbi.nlm.nih.gov/31804607/.
Zhan Y, Chen Z, Li Y, He A, He S, Gong Y, et al. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J Exp Clin Cancer Res. 2018;37:273.
Jiang N, Wang X, Xie X, Liao Y, Liu N. J L, lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer letters [Internet] 2017 [cited 2023 Nov 12];405. https://pubmed.ncbi.nlm.nih.gov/28642170/.
Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. J Orthop Surg Res. 2017;12:103.
Wang Y, Zeng X, Wang N, Zhao W, Zhang X. S T, Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Molecular cancer [Internet] 2018 [cited 2023 Nov 12];17. https://pubmed.ncbi.nlm.nih.gov/29753317/.
Zhang W, Li JZ, Tai QY, Tang JJ, Huang YH, Gao SB. LncRNA DANCR regulates osteosarcoma migration and invasion by targeting miR-149/MSI2 axis. Eur Rev Med Pharmacol Sci. 2020;24:6551–60.
Lin X, Yang F, Qi X, Li Q, Wang D, Yi T, et al. LncRNA DANCR promotes tumor growth and angiogenesis in ovarian cancer through direct targeting of miR-145. Mol Carcinog. 2019;58:2286–96.
Huang P, Qi B, Yao H, Zhang L, Li Y, Li Q. Knockdown of DANCR suppressed the Biological behaviors of Ovarian Cancer cells treated with transforming growth Factor-β (TGF-β) by sponging MiR-214. Med Sci Monit. 2020;26:e922760.
Cui PH, Li ZY, Li DH, Han SY, Zhang YJ. SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J Med Sci. 2021;37:371–8.
Liang H, Zhang C, Guan H, Liu J, Cui Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J Cell Physiol. 2019;234:7266–78.
Tian W, Lei N, Guo R, Yuan Z, Chang L. Long non-coding RNA DANCR promotes cervical cancer growth via activation of the Wnt/ β-catenin signaling pathway. Cancer Cell Int. 2020;20:61.
Hu C, Han Y, Zhu G, Li G, Wu X. Krüppel-like factor 5-induced overexpression of long non-coding RNA DANCR promotes the progression of cervical cancer via repressing microRNA-145-3p to target ZEB1. Cell Cycle. 2021;20:1441–54.
Kamaliyan Z, Mirfakhraie R, Azizi-Tabesh G, Darbeheshti F, Omranipour R, Ahmadinejad N, et al. The role of FOXC1/FOXCUT/DANCR axis in triple negative breast cancer: a bioinformatics and experimental approach. Mol Biol Rep. 2022;49:2821–9.
Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X, Jiang H, et al. Systemic delivery of Tumor-Targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple-negative breast Cancer Therapy. Bioconjug Chem. 2019;30:907–19.
Qi P, Zhou XY, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer. 2016;15:39.
Wang SC, Li CY, Chang WT, Cheng WC, Yen CH, Tu WY, et al. Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. 2021;41:956–68.
Shen X, Xue Y, Cong H, Wang X, Fan Z, Cui X, et al. Circulating lncRNA DANCR as a potential auxillary biomarker for the diagnosis and prognostic prediction of colorectal cancer. Biosci Rep. 2020;40:BSR20191481.
Liu Z, Petinrin OO, Toseef M, Chen N, Wong KC. Construction of Immune Infiltration-related LncRNA signatures based on machine learning for the prognosis in Colon cancer. Biochem Genet 2023.
Ajorlou M, Bina-Jourshari P, Mirzaei S, Maghsoudloo M, Hashemi M, Mousavi-Niri N, et al. Evaluation of the expression level of some coding and non-coding genes in oral squamous cell carcinoma. Mol Cell Probes. 2023;70:101916.
Malakootian M, Mirzadeh Azad F, Fouani Y, Taheri Bajgan E, Saberi H, Mowla SJ. Anti-differentiation non-coding RNA, ANCR, is differentially expressed in different types of brain tumors. J Neurooncol. 2018;138:261–70.
Funding
This work was supported by the Natural Science Foundation of Hunan Province, Grant/Award Numbers:2024JJ5299; the Key Project of Education Department of Hunan Province, Grant/Award Numbers:20A364, 22A0243; and the Open Fund for National Key Laboratory Cultivation Base of Chinese Medicinal Powder & Innovative Medicinal Jointly Established by Province and Ministry, Grant/Award Numbers: 23PTKF1005.
Author information
Authors and Affiliations
Contributions
Z.X. and S.Z. discussed the content, researched the data and contributed to writing the article and to reviewing and/or editing of the manuscript. A.D. and L.S. conceptualised, supervised and revised the manuscript. R.Y. conducted the literature review and drafted the paper. X.C. helped with preparing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, R., Xu, Zj., Zhang, Sk. et al. New evidence for a role of DANCR in cancers: a comprehensive review. J Transl Med 22, 569 (2024). https://doi.org/10.1186/s12967-024-05246-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05246-z