Abstract
Background
Long non-coding RNAs (lncRNAs) are implicated in many pathophysiological processes, including cancers. In particular, lncRNA DANCR is regarded as a cancer-associated lncRNA exerting various regulatory mechanisms. However, the expressions, functions, and mechanisms of action of DANCR in cervical cancer are still unclear.
Methods
The expressions of DANCR in cervical cancer tissues and cell lines were evaluated using qRT-PCR. Correlations between DANCR expression and clinicopathological features and prognosis were analyzed. The roles of DANCR in cervical cancer growth were evaluated by in vitro CCK-8 and EdU assay, and in vivo xenograft assay. The regulatory effects of DANCR on Wnt/β-catenin signaling pathway were evaluated using nuclear proteins extraction, western blot, and qRT-PCR.
Results
DANCR is increased in cervical cancer tissues and cell lines. Increased expression of DANCR is associated with large tumor size, advanced FIGO stage, and poor overall survival of cervical cancer patients. Functional experiments showed that enhanced expression of DANCR promotes cervical cancer cell proliferation in vitro and xenograft growth in vivo. Conversely, DANCR knockdown inhibits cervical cancer cell proliferation in vitro and xenograft growth in vivo. Mechanistic investigation demonstrated that DANCR upregulates the expressions of FRAT1 and FRAT2 and activates the Wnt/β-catenin signaling pathway. Blocking the Wnt/β-catenin signaling pathway abolishes the pro-proliferative roles of DANCR overexpression and anti-proliferative roles of DANCR knockdown.
Conclusions
Our findings suggest DANCR as an oncogenic lncRNA in cervical cancer through activating the Wnt/β-catenin signaling pathway, and imply that DANCR may be a promising prognostic biomarker and therapeutic target for cervical cancer.
Similar content being viewed by others
Background
Cervical cancer is the second most common cancer in female, next to breast cancer, accounting for approximately 527,000 new cancer cases and 265,000 cancer deaths each year worldwide [1]. With great development of diagnostic and therapeutic strategies, including cervical cancer early screening, surgical resection, chemotherapy, and radiotherapy, the 5-year survival rate of cervical cancer has reached to 60–70% [2]. However, considerable cervical cancer patients’ long-term survivals are dismal [3]. Therefore, it is urgent to investigate the molecular mechanisms underpinning the initiation and progression of cervical cancer and develop better therapeutic modalities for cervical cancer therapy.
Accumulating evidences revealed that many non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are frequently deregulated and have critical roles in multiple cancers [4,5,6,7,8,9,10]. These non-coding RNAs could be novel candidates for prognostic biomarkers and therapeutic targets in various cancers including cervical cancer [9,10,11,12,13]. LncRNAs are a class of novel non-coding transcripts with more than 200 nucleotides in length [14, 15]. Next-generation sequencing technologies have identified more than 58,000 lncRNAs among human transcriptome, while the number of protein-coding genes is only 21,000 [16]. A variety of lncRNAs are revealed to be deregulated and associated with patients’ prognosis in several cancers including cervical cancer [17,18,19,20]. Furthermore, many lncRNAs are shown to regulate various biological aspects of cancer cells, such as cell proliferation, apoptosis, cell cycle, migration, invasion, drug-resistance, and so on [21,22,23]. These lncRNAs may be implicated in many signaling pathways critical for cancers, and regulate various oncogenes and tumor suppressors in different cancers [24,25,26,27].
LncRNA differentiation antagonizing non-protein coding RNA (DANCR), previously termed as ANCR, was first revealed to suppress progenitor differentiation [28]. Subsequent studies demonstrated that DANCR is a promising cancer-associated lncRNA [29]. DANCR is shown to be upregulated in gastric cancer, lung cancer, glioma, colorectal cancer, retinoblastoma, osteosarcoma, oesophageal cancer, breast cancer, prostate cancer, and hepatocellular carcinoma [30,31,32,33,34,35,36,37]. In these different cancers, DANCR mainly functions as an oncogene via promoting cell proliferation, invasion, migration, and/or inhibiting cell apoptosis [30,31,32,33,34,35,36,37]. However, the mechanisms underpinning the functions of DANCR in different cancers are various. DANCR was reported to modulate PI3K-Akt pathway in osteosarcoma, β-catenin pathway in hepatocellular carcinoma, miR-634-RAB1A signaling pathway in glioma, androgen-AR signaling pathway in prostate cancer [31, 32, 38, 39]. In cervical cancer, however, the expressions, roles, and mechanisms of action of DANCR are still unclear.
In the present study, we determined DANCR expression in cervical cancer tissues and cell lines, analyzed the correlation between DANCR expression and cervical cancer patients’ clinicopathological features, including prognosis. Furthermore, gain-of-function and loss-of-function assays were performed to investigate the biological roles of DANCR in cervical cancer growth. Finally, using public available dataset, combined with experimental verification, we investigated the mechanisms underlying the biological effects of DANCR in cervical cancer. We demonstrated that DANCR, which is an oncogenic lncRNA in cervical cancer through activating the Wnt/β-catenin signaling pathway, might be a promising prognostic biomarker and therapeutic target for cervical cancer.
Methods
Patient tissue samples
A total of 82 pairs of cervical cancer and adjacent noncancerous cervix tissues were obtained from cervical cancer patients with informed written consent who underwent potentially curative surgery in the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) from 2014 to 2016. All tissue samples were diagnosed by histopathological examination. Freshly resected tissue specimens were immediately frozen in liquid nitrogen and stored at − 80 ℃ until use. The Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University reviewed and approved this program in accordance with Helsinki Declaration.
Cell culture and treatment
The human normal cervical epithelial cell line HCerEpiC was purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). The cervical cancer cell lines HeLa, SiHa, C-33A, and ME-180 were purchased from American Type Culture Collection (Rockville, MD, USA). HCerEpiC cells were grown in Cervical Epithelial Cell Growth Supplement (ScienCell, Carlsbad, CA, USA). HeLa, SiHa, and C-33A cells were grown in Eagle’s Minimum Essential Medium (Invitrogen Carlsbad, CA, USA). ME-180 cells were grown in McCoy’s 5A Medium (Sigma-Aldrich, St. Louis, MO, USA). All media were added with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). Where indicated, the cervical cancer cells were treated with 5 μM ICG-001 (Selleck Chemicals, Houston, TX, USA). All cell lines were grown in a humidified incubator with 5% CO2 at 37 ℃.
RNA extraction, reverse transcription, and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from indicated tissues and cells with TRIzol Regent (Invitrogen) following the protocol. After being treated with DNase I (Takara, Dalian, China) to remove genomic DNA, the isolated RNA was subjected to reverse transcription using a PrimeScript RT Reagent Kit (Takara) following the protocol. Next, the cDNA was subjected to quantitative Real-Time PCR (qRT-PCR) using SYBR Premix Ex Taq (Takara) and gene specific primers. Primer sequences are as follows: for DANCR, 5′-GCGCCACTATGTAGCGGGTT-3′ (sense) and 5′-TCAATGGCTTGTGCCTGTAGTT-3′ (antisense); for FRAT1, 5′-TGGAAGCGAGAGTAAAAAGC-3′ (sense) and 5′-GGTCACGCCAAATAAGGAG-3′ (antisense); for FRAT2, 5′-TACCTCACTTAGCCCTTGG-3′ (sense) and 5′-ATGCGTGTCGTTAGTTTTCA-3′ (antisense); for C-myc, 5′-GCTGCTTAGACGCTGGATTT-3′ (sense) and 5′-CTCCTCCTCGTCGCAGTAGA-3′ (antisense); for Cyclin D1, 5′-TTCCTGTCCTACTACCGC-3′ (sense) and 5′-CTCCTCCTCTTCCTCCTC-3′ (antisense); and for β-actin, 5′-GGGAAATCGTGCGTGACATTAAG-3′ (sense) and 5′-TGTGTTGGCGTACAGGTCTTTG-3′ (antisense). The quantification of RNA expression was calculated following the comparative Ct method. β-actin was used as endogenous control.
Plasmids construction and transfection
DANCR expressing plasmid pcDNA3.1-DANCR was constructed as previously described [40]. Briefly, DANCR full-length sequence was PCR amplified using the PrimeSTAR HS DNA polymerase (Takara) and the primers 5′-CCCAAGCTTGCCCTTGCCCAGAGTCTTC-3′ (sense) and 5′-CGGGATCCGTCAGGCCAAGTAAGTTTATTAAC-3′ (antisense). Next, the PCR products were subcloned into the Hind III and BamH I sites of pcDNA3.1. DANCR knockdown plasmid was constructed as previously described [38]. Briefly, one pair of cDNA oligonucleotides specifically targeting DANCR was inserted into the shRNA expression plasmid pGPH1/Neo (GenePharma, Shanghai, China). The sequences of DANCR specific shRNA were: 5′-CACCAGCCAACTATCCCTTCAGTTACATTCAAGAGATGTAACTGAAGGGATAGTTGGCTTTTTTTG-3′ (sense) and 5′-GATCCAAAAAAAGCCAACTATCCCTTCAGTTACATCTCTTGAATGTAACTGAAGGGATAGTTGGCT-3′ (antisense). The sequences of control scrambled shRNA were: 5′-CACCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG-3′ (sense) and 5′-GATCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAC-3′ (antisense). Plasmids transfection was carried out using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocols.
Stable cell lines construction
For construction of DANCR stably overexpressed and control HeLa cells, DANCR expressing plasmid pcDNA3.1-DANCR or control empty plasmid pcDNA3.1 was transfected into HeLa cells. After 48 h, the transfected HeLa cells were treated with neomycin for 4 weeks. To construct DANCR stably depleted and control C-33A cells, DANCR specific shRNA or control scrambled shRNA was transfected into C-33A cells. After 48 h, the transfected C-33A cells were treated with neomycin for 4 weeks. The efficiencies of overexpression and knockdown were determined by qRT-PCR.
Cell proliferation assays
Cell proliferation was assessed by Cell Counting Kit-8 (CCK-8) and Ethynyl deoxyuridine (EdU) assays. For CCK-8 assay, DANCR stably overexpressed or depleted cervical cancer cells were resuspended and plated into 96-well plates at a density of 3000 cells per-well. After culture for indicated time, CCK-8 solution (Beyotime, Shanghai, China) was added into the wells. After 2 h of continued culture, absorbance values of optical density (OD) at 450 nm for each well were detected using automatic enzyme-linked immune detector. EdU assay was performed with the Cell-Light™ EdU Apollo®643 In Vitro Imaging Kit (RiboBio, Guangzhou, China) according to the instructions. Results were analyzed by Zeiss fluorescence photomicroscope (Carl Zeiss, Oberkochen, Germany) via randomly counting ten fields.
Mouse xenograft model
1 × 106 DANCR stably overexpressed or depleted cervical cancer cells were resuspended in 100 μl phosphate buffered saline containing 50% matrigel (Invitrogen) and then subcutaneously injected into the flanks of female BALB/c-nu mice of 6 weeks old. The mice were maintained in a sterile environment on a daily 12-h light/12-h dark cycle. Subcutaneous tumor volumes were measured every 3 days using caliper and calculated following the formula: tumor volumes = 0.5 × length × width2. At the twenty-first day after injection, the mice were sacrificed and subcutaneous tumors were resected and weighed. The Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University reviewed and approved this program. Ki67 immunohistochemical staining was carried out as we previously described with Ki67 primary antibody (Cell Signaling Technology, Boston, MA, USA) [41].
Western blot
Nuclear proteins were extracted from DANCR stably overexpressed or depleted cervical cancer cells using CelLytic™ NuCLEAR™ Extraction Kit (Sigma-Aldrich). Total proteins were extracted from indicted cervical cancer cells using RIPA buffer (Beyotime). Next, nuclear proteins or total proteins were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After being transferred onto polyvinylidene fluoride (PVDF) microporous membrane (Millipore, Boston, MA, USA), the blots were incubated with primary antibodies against β-catenin (Abcam, Hong Kong, China), histone H3 (Abcam), FRAT1 (Abcam), FRAT2 (Sigma-Aldrich), C-myc (Cell Signaling Technology), Cyclin D1 (Cell Signaling Technology), or GAPDH (Cell Signaling Technology). Histone H3 was employed as a loading control for nuclear protein, and GAPDH was employed as a loading control for total protein. The blots were visualized with chemiluminescence.
Statistical analysis
All statistical analyses were carried out using the GraphPad Prism 5.0 version (La Jolla, CA, USA). For comparisons, Wilcoxon signed-rank test, Pearson Chi square test, log-rank test, Student’s t-test, Mann–Whitney test, and Pearson correlation analysis were carried put as indicated. Results were considered as statistically significant when p < 0.05.
Results
Expression and clinical significances of DANCR in cervical cancer
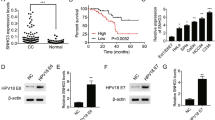
To determine the expression pattern of DANCR in cervical cancer, we firstly detected DANCR expression in 82 pairs of cervical cancer and adjacent noncancerous cervix tissues via qRT-PCR. Our results displayed that DANCR expression was markedly increased in cervical cancer tissues compared with adjacent noncancerous cervix tissues (Fig. 1a). To determine the association between DANCR expression and cervical cancer clinicopathological features, these 82 cervical cancer patients were divided into low and high DANCR expression groups based on DANCR median expression level. Pearson Chi square tests displayed that higher DANCR expression was positively associated with larger tumor size (p = 0.015) and advanced FIGO stage (p = 0.025) (Table 1). In addition, Kaplan–Meier survival analysis displayed that cervical cancer patients with higher DANCR expression had worse overall survival (p = 0.022) (Fig. 1b). Moreover, we measured DANCR expression in human normal cervical epithelial cell line HCerEpiC and cervical cancer cell lines HeLa, SiHa, C-33A, and ME-180 via qRT-PCR. As displayed in Fig. 1c, DANCR expression was obviously increased in cervical cancer cell lines compared with normal cervical epithelial cell line. Collectively, these data showed that DANCR is upregulated in cervical cancer tissues and cell lines, and increased expression of DANCR is positively correlated with larger tumor size, advanced FIGO stage, and worse prognosis.
Expression pattern and clinical significances of DANCR in cervical cancer. a The expression of DANCR in 82 pairs of cervical cancer and adjacent noncancerous cervix tissues was measured by qRT-PCR. Results are displayed as median with interquartile range. p < 0.0001 by Wilcoxon signed-rank test. b Kaplan–Meier survival analysis of the association between DANCR expression and overall survival of cervical cancer patients (n = 82) p = 0.022 by log-rank test. DANCR median expression level was used as cut-off. c The expression of DANCR in human normal cervical epithelial cell line HCerEpiC and cervical cancer cell lines HeLa, SiHa, C-33A, and ME-180 was measured by qRT-PCR. Results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01, ***p < 0.001 compared with HCerEpiC group by one-way ANOVA
Upregulation of DANCR promotes cervical cancer growth in vitro and in vivo
To determine the functional relevance of DANCR in cervical cancer, we constructed DANCR stably overexpressed and control HeLa cells through transfecting DANCR expressing plasmid (pcDNA3.1-DANCR) or control empty plasmid (pcDNA3.1). The overexpression efficiency was detected via qRT-PCR (Fig. 2a). CCK-8 assays displayed that upregulation of DANCR promoted proliferation of HeLa cells (Fig. 2b). The pro-proliferative roles of upregulation of DANCR in HeLa cells were further confirmed by EdU assays (Fig. 2c). Furthermore, we transiently overexpressed DANCR in another cervical cancer cell line SiHa (Fig. 2d). CCK-8 assays displayed that upregulation of DANCR also promoted proliferation of SiHa cells (Fig. 2e). The pro-proliferative roles of upregulation of DANCR in SiHa cells were further confirmed by EdU assays (Fig. 2f). To further investigate the functions of DANCR in vivo, subcutaneous tumor model was employed via the subcutaneous injection of DANCR stably overexpressed and control HeLa cells. As displayed in Fig. 2g, h, upregulation of DANCR promoted subcutaneous tumor growth. Furthermore, proliferation marker Ki67 staining displayed that the subcutaneous tumor formed by DANCR overexpressed HeLa cells had much more Ki67 positive cells than that formed by control HeLa cells (Fig. 2i), which further supports the pro-proliferative roles of upregulation of DANCR in vivo. Collectively, these data showed that upregulation of DANCR promotes cervical cancer growth in vitro and in vivo.
The effects of DANCR overexpression on cervical cancer growth. a The expression of DANCR in DANCR stably overexpressed (OV) and control HeLa cells was measured by qRT-PCR. b Cell proliferation of OV and control HeLa cells was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. c Cell proliferation of OV and control HeLa cells was analyzed by EdU assays. Red colour represents EdU-positive and proliferative cells. Scale bars = 100 μm. d After transient transfection of DANCR expressing plasmid or control empty plasmid into SiHa cells, the expression of DANCR in the transfected cells was measured by qRT-PCR. e After transient transfection of DANCR expressing plasmid or control empty plasmid into SiHa cells, cell proliferation of the transfected cells was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. f After transient transfection of DANCR expressing plasmid or control empty plasmid into SiHa cells, cell proliferation of the transfected cells was analyzed by EdU assays. Red colour represents EdU-positive and proliferative cells. Scale bars = 100 μm. For a–f, results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01 compared with Control group by Student’s t-test. g OV and control HeLa cells were subcutaneously injected into nude mice. Tumor volumes were measured every 3 days. h Subcutaneous tumor weights were detected at the twenty-first day after injection. i Proliferation marker Ki67 immunohistochemical staining in subcutaneous tumors formed by OV and control HeLa cells. Scale bars = 50 μm. For g–i, results are displayed as mean ± SD of n = 5 mice in each group. **p < 0.01 compared with Control group by Mann–Whitney test
Knockdown of DANCR suppresses cervical cancer growth in vitro and in vivo
To further validate the functional relevance of DANCR in cervical cancer, we constructed DANCR stably depleted and control C-33A cells via transfecting DANCR specific shRNA (KD-DANCR) or control scrambled shRNA (KD-control). The knockdown efficiency was detected via qRT-PCR (Fig. 3a). CCK-8 assays displayed that knockdown of DANCR suppressed proliferation of C-33A cells (Fig. 3b). The anti-proliferative roles of knockdown of DANCR in C-33A cells were further confirmed by EdU assays (Fig. 3c). Furthermore, we transiently repressed DANCR in another cervical cancer cell line ME-180 (Fig. 3d). CCK-8 assays displayed that knockdown of DANCR also suppressed proliferation of ME-180 cells (Fig. 3e). The anti-proliferative roles of knockdown of DANCR in ME-180 cells were further confirmed by EdU assays (Fig. 3f). To investigate the roles of knockdown of DANCR in vivo, subcutaneous tumor model was employed via the subcutaneous injection of DANCR stably depleted and control C-33A cells. As displayed in Fig. 3g, h, knockdown of DANCR suppressed subcutaneous tumor growth. Furthermore, proliferation marker Ki67 staining displayed that the subcutaneous tumor formed by DANCR depleted C-33A cells had much less Ki67 positive cells than that formed by control C-33A cells (Fig. 3i), which further supports the anti-proliferative roles of knockdown of DANCR in vivo. Collectively, these data showed that knockdown of DANCR suppresses cervical cancer growth in vitro and in vivo.
The effects of DANCR knockdown on cervical cancer growth. a The expression of DANCR in DANCR stably depleted (deleted) and control C-33A cells was measured by qRT-PCR. b Cell proliferation of deleted and control C-33A cells was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. c Cell proliferation of deleted and control C-33A cells was analyzed by EdU assays. Red colour represents EdU-positive and proliferative cells. Scale bars = 100 μm. d After transient transfection of DANCR specific shRNA (KD-DANCR) or control scrambled shRNA (KD-control) into ME-180 cells, the expression of DANCR was measured by qRT-PCR. e After transient transfection, cell proliferation of KD-DANCR and KD-control ME-180 cells was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. f After transient transfection of DANCR specific shRNA (KD-DANCR) or control scrambled shRNA (KD-control) into ME-180 cells, cell proliferation of the transfected cells was analyzed by EdU assays. Red colour represents EdU-positive and proliferative cells. Scale bars = 100 μm. For a–f, results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01 compared with KD-control group by Student’s t-test. g Deleted and control C-33A cells were subcutaneously injected into nude mice. Tumor volumes were measured every 3 days. h Subcutaneous tumor weights were detected at the twenty-first day after injection. i Proliferation marker Ki67 immunohistochemical staining in subcutaneous tumors formed by deleted and control C-33A cells. Scale bars = 50 μm. For g–i, results are displayed as mean ± SD of n = 5 mice in each group. **p < 0.01 compared with KD-control group by Mann–Whitney test
DANCR upregulates FRAT1 and FRAT2 expression
To investigate the mechanisms underpinning the pro-proliferative roles of DANCR in cervical cancer, we searched the genes whose expression was correlated with that of DANCR in cervical cancer via analysing The Cancer Genome Atlas (TCGA) data using TANRIC (http://ibl.mdanderson.org/tanric/_design/basic/index.html). Among the genes positively associated with DANCR (Additional file 1: Table S1), we noted FRAT1 and FRAT2, which are positive regulator of the Wnt/β-catenin signaling pathway and have oncogenic roles in several cancers [42,43,44,45]. Therefore, we further analyzed the association between DANCR expression and FRAT1 and FRAT2 expression in 82 cervical cancer tissues used in Fig. 1. Pearson correlation analysis displayed that the expression of FRAT1 and FRAT2 were both positively associated with the expression of DANCR in cervical cancer tissues (Fig. 4a, b). We further measured the mRNA and protein expression levels of FRAT1 and FRAT2 in DANCR stably overexpressed and control HeLa cells, and DANCR stably depleted and control C-33A cells using qRT-PCR and western blot. Our results displayed that enhanced expression of DANCR upregulated the mRNA and protein expression levels of FRAT1 and FRAT2, and while knockdown of DANCR downregulated the mRNA and protein expression levels of FRAT1 and FRAT2 in cervical cancer cells (Fig. 4c, d).
The effects of DANCR on FRAT1 and FRAT2. a The correlation between FRAT1 expression and DANCR expression in cervical cancer tissues was analyzed by Pearson correlation analysis. n = 82, r = 0.5482, p < 0.0001. b The correlation between FRAT2 expression and DANCR expression in cervical cancer tissues was analyzed by Pearson correlation analysis. n = 82, r = 0.623, p < 0.0001. c The mRNA and protein expression levels of FRAT1 and FRAT2 in DANCR stably overexpressed and control HeLa cells were measured by qRT-PCR and western blot. Results are displayed as mean ± SD of n = 3 independent experiments. *p < 0.05, **p < 0.01 compared with Control group by Student’s t-test. d The mRNA and protein expression levels of FRAT1 and FRAT2 in DANCR stably depleted and control C-33A cells were measured by qRT-PCR and western blot. Results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01 compared with KD-control group by Student’s t-test
DANCR activates the Wnt/β-catenin signaling pathway
Next, we investigated the effects of DANCR on the Wnt/β-catenin signaling pathway in cervical cancer. Nuclear proteins from DANCR stably overexpressed and control HeLa cells, and DANCR stably depleted and control C-33A cells were extracted. Nuclear β-catenin levels were determined using western blot. The results displayed that enhanced expression of DANCR upregulated nuclear β-catenin levels, and while knockdown of DANCR downregulated nuclear β-catenin levels in cervical cancer cells (Fig. 5a, b). Furthermore, the mRNA and protein expression levels of Wnt target genes C-myc and Cyclin D1 in DANCR stably overexpressed and control HeLa cells, and DANCR stably depleted and control C-33A cells were measured using qRT-PCR and western blot. Our results displayed that enhanced expression of DANCR upregulated the mRNA and protein expression levels of C-myc and Cyclin D1, and while knockdown of DANCR downregulated the mRNA and protein expression levels of C-myc and Cyclin D1 in cervical cancer cells (Fig. 5c, d). Collectively, these data demonstrated that DANCR activates the Wnt/β-catenin signaling pathway.
The effects of DANCR on the Wnt/β-catenin signaling pathway. a Nuclear β-catenin expression levels in DANCR stably overexpressed and control HeLa cells was measured by western blot. Results are displayed as representative image of n = 3 independent experiments. b Nuclear β-catenin expression levels in DANCR stably depleted and control C-33A cells was measured by western blot. Results are displayed as representative image of n = 3 independent experiments. c The mRNA and protein expression levels of C-myc and Cyclin D1 in DANCR stably overexpressed and control HeLa cells were measured by qRT-PCR and western blot. Results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01 compared with Control group by Student’s t-test. d The mRNA and protein expression levels of C-myc and Cyclin D1 in DANCR stably depleted and control C-33A cells were measured by qRT-PCR and western blot. Results are displayed as mean ± SD of n = 3 independent experiments. **p < 0.01, ***p < 0.001 compared with KD-control group by Student’s t-test
Wnt pathway inhibitor ICG-001 abolishes the roles of DANCR in cervical cancer growth
To investigate whether the activation of the Wnt/β-catenin signaling pathway mediates the pro-proliferative roles of DANCR in cervical cancer, we treated DANCR stably overexpressed and control HeLa cells, and DANCR stably depleted and control C-33A cells with the canonical Wnt/β-catenin pathway inhibitor ICG-001. CCK-8 and EdU assays displayed that treatment with ICG-001 abolished the pro-proliferative roles of DANCR overexpression (Fig. 6a, b). Consistently, treatment with ICG-001 also abolished the anti-proliferative roles of DANCR knockdown (Fig. 6c, d). These data suggested that Wnt/β-catenin signaling pathway is responsible for the roles of DANCR in cervical cancer cell proliferation.
Wnt pathway inhibitor ICG-001 abolishes the roles of DANCR in cervical cancer growth. a DANCR stably overexpressed and control HeLa cells were treated with 5 μM ICG-001. Concurrently, cell proliferation was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. b DANCR stably overexpressed and control HeLa cells were treated with 5 μM ICG-001. Concurrently, cell proliferation was analyzed by EdU assays. Red color represents EdU-positive and proliferative cells. Scale bars = 100 μm. For a, b, results are displayed as mean ± SD of n = 3 independent experiments. ns, not significant, compared with Control + ICG-001 group by Student’s t-test. c DANCR stably depleted and control C-33A cells were treated with 5 μM ICG-001. Concurrently, cell proliferation was analyzed by CCK-8 assays. OD 450 values at indicated time points were displayed to indicate cell proliferation. d DANCR stably depleted and control C-33A cells were treated with 5 μM ICG-001. Concurrently, cell proliferation was analyzed by EdU assays. Red color represents EdU-positive and proliferative cells. Scale bars = 100 μm. For c, d, results are displayed as mean ± SD of n = 3 independent experiments. ns, not significant, compared with KD-control + ICG-001 group by Student’s t-test
Discussion
Genomic and molecular features of cervical cancer are complex [46, 47]. Accumulating studies have identified various genomic mutations of PIK3CA, EP300, FBXW7, PTEN, HLA-A, ARID1A and so on in different cervical cancer tissues [11, 46]. The aberrant expression of many mRNAs, including FGFR3, CTGF, TP63, IL36G, ADH7, SPINK5, and so on, have also been identified in cervical cancer [47]. Furthermore, the dysregulation of non-coding RNAs gradually attracts researchers’ attention, such as miR-205, miR-200a, miR-30a, lncRNA BCAR4, lncRNA HOTAIR, lncRNA MALAT1, lncRNA MEG3, and so on [46, 47]. Due to the huge amount of lncRNAs, the clinical significances of most of lncRNAs in cervical cancer are unclear.
In the present study, we focused on a lncRNA DANCR, which is located on chromosome 4q12. Although DANCR has been investigated in several cancers, and been regarded as a cancer-associated lncRNA [29], the functions and clinical significances of DNACR in cervical cancer are unclear. In this study, we identified DANCR is upregulated in cervical cancer tissues and cell lines compared with adjacent noncancerous cervix tissues and normal cervical epithelial cell line, respectively. High expression of DANCR is positively associated with large tumor size, advanced FIGO stage, and poor overall survival of cervical cancer patients. Functional experiments demonstrated that ectopic expression of DANCR promotes cervical cancer cell proliferation in vitro and cervical cancer xenograft growth in vivo. Conversely, DANCR knockdown inhibits cervical cancer cell proliferation in vitro and cervical cancer xenograft growth in vivo. Therefore, our data demonstrated that DANCR also functions as an oncogene in cervical cancer, further supporting DANCR as a cancer-associated lncRNA. Our findings also implied that DANCR might be a promising prognostic biomarker and therapeutic target for cervical cancer.
In this study, we identified a novel mechanism mediating the oncogenic roles of DANCR in cervical cancer, which is the activation of the Wnt/β-catenin signalling pathway via upregulation of FRAT1 and FRAT2. Both public available TCGA data and cervical cancer tissues we collected display that the expression of FRAT1 and FRAT2 are positively associated with the expression of DANCR in cervical cancer tissues, supporting the positive regulation of FRAT1 and FRAT2 by DANCR. FRAT1 and FRAT2 belong to the GSK-3-binding protein family, inhibit GSK-3-mediated β-catenin phosphorylation and degradation, promote nuclear translocation of β-catenin, and activate Wnt/β-catenin signaling pathway [44]. Indocyanine Green-001 (ICG-001) is an antagonist of β-catenin that specifically downregulates the expression of responsive genes of β-catenin [48]. Thus, we used ICG-001 to inhibit Wnt/β-catenin signaling pathway in the functional assays, which led to the abolishment of the pro-proliferation of cervical cancer cells caused by DANCR overexpression and the anti-proliferatory roles of DNACR knockdown in cervical cancer cells. The results of functional experiments suggest that the effects of DANCR on cervical cancer cells are dependent on the activation of Wnt/β-catenin signaling pathway. DANCR has previously been reported to activate the Wnt/β-catenin signaling pathway in hepatocellular carcinoma, gastric cancer, and glioma [32, 35, 48]. However, the detailed mechanisms underpinning the activation of the Wnt/β-catenin signaling pathway by DANCR in gastric cancer and glioma are unreported 9 [35, 49]. In hepatocellular carcinoma, Yuan et al. reported that DANCR directly bound to β-catenin mRNA and inhibited β-catenin mRNA degradation [32]. Several recent studies have reported the role of DANCR in cervical cancer associated with certain miRNAs [50, 51], however whether DANCR also affects the Wnt/β-catenin signalling pathway in cervical cancer has not been revealed. In this present study, we provide a novel insight that the activation of the Wnt/β-catenin signalling pathway by DANCR is associated with cervical cancer progression. In addition, we also identify that DANCR regulates the expression levels of FRAT1 and FRAT2, which are regulators of Wnt/β-catenin signalling pathway.
Conclusions
In conclusion, our findings demonstrate that lncRNA DANCR is upregulated in cervical cancer, positively associated with tumor size and FIGO stage, and a prognostic indicator of poor survival of cervical cancer patients. DANCR promotes cervical cancer growth via activating FRAT1/FRAT2-Wnt/β-catenin signaling pathway. Our results imply that DANCR may be a promising prognostic biomarker to evaluate cervical cancer progression and useful therapeutic target for cervical cancer treatment.
Availability of data and materials
All data generated or analysed during the present study are included in this published article.
Abbreviations
- lncRNA:
-
Long non-coding ribonucleic acid
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- CCK-8:
-
Cell Counting Kit-8
- EdU:
-
Ethynyl deoxyuridine
- FIGO:
-
International federation of gynecology and obstetrics
- OD:
-
Optical density
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene fluoride
- ICG-001:
-
Indocyanine Green-001
- FRAT:
-
Frequently rearranged in advanced T cell lymphomas
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Yee GP, de Souza P, Khachigian LM. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets. 2013;13:205–20.
Wang Z, Yang B, Zhang M, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33(706–20):e9.
Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81.
Lin A, Hu Q, Li C, et al. The LINK-A lncRNA interacts with PtdIns (3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19:238–51.
Grelet S, Link LA, Howley B, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19:1105–15.
Bu Y, Yoshida A, Chitnis N, et al. A PERK-miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat Cell Biol. 2018;20:104–15.
Yuan JH, Yang F, Chen BF, et al. The histone deacetylase 4/SP1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology. 2011;54:2025–35.
Golbakhsh MR, Boddouhi B, Hatami N, et al. Down-regulation of microRNA-182 and microRNA-183 predicts progression of osteosarcoma. Arch Med Sci. 2017;13:1352–6.
Mohr S, Doebele C, Comoglio F, et al. Hoxa9 and Meis1 cooperatively induce addiction to Syk Signaling by suppressing miR-146a in acute myeloid leukemia. Cancer Cell. 2017;31(549–62):e11.
Feng W, Li L, Xu X, Jiao Y, Du W. Up-regulation of the long non-coding RNA RMRP contributes to glioma progression and promotes glioma cell proliferation and invasion. Arch Med Sci. 2017;13:1315–21.
Zhao JJ, Chen PJ, Duan RQ, Li KJ, Wang YZ, Li Y. miR-630 functions as a tumor oncogene in renal cell carcinoma. Arch Med Sci. 2016;12:473–8.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41.
Zhu XT, Yuan JH, Zhu TT, Li YY, Cheng XY. Long noncoding RNA glypican 3 (GPC3) antisense transcript 1 promotes hepatocellular carcinoma progression via epigenetically activating GPC3. FEBS J. 2016;283:3739–54.
Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208.
Li JK, Chen C, Liu JY, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16:111.
Yin D, Lu X, Su J, et al. Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non-small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Mol Cancer. 2018;17:92.
Xu T, Lin CM, Cheng SQ, et al. Pathological bases and clinical impact of long noncoding RNAs in prostate cancer: a new budding star. Mol Cancer. 2018;17:103.
Li W, Dou Z, We S, et al. Long noncoding RNA BDNF-AS is associated with clinical outcomes and has functional role in human prostate cancer. Biomed Pharmacother. 2018;102:1105–10.
Yuan JH, Liu XN, Wang TT, et al. The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nat Cell Biol. 2017;19:820–32.
Hu WL, Jin L, Xu A, et al. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. Nat Cell Biol. 2018;20:492–502.
Mondal T, Juvvuna PK, Kirkeby A, et al. Sense-antisense lncRNA pair encoded by locus 6p22.3 Determines neuroblastoma susceptibility via the USP36-CHD7-SOX9 regulatory axis. Cancer Cell. 2018;33:417–34.
Zhang L, Yang F, Yuan JH, et al. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–86.
Li C, Wang S, Xing Z, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–19.
Huang JL, Cao SW, Ou QS, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer. 2018;17:93.
Wang YD, Sun XJ, Yin JJ, et al. Long non-coding RNA FEZF1-AS1 promotes cell invasion and epithelial-mesenchymal transition through JAK2/STAT3 signaling pathway in human hepatocellular carcinoma. Biomed Pharmacother. 2018;106:134–41.
Kretz M, Webster DE, Flockhart RJ, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–43.
Thin KZ, Liu X, Feng X, Raveendran S, Tu JC. LncRNA-DANCR: a valuable cancer related long non-coding RNA for human cancers. Pathol Res Pract. 2018;214:801–5.
Liu Y, Zhang M, Liang L, Li J, Chen YX. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2015;8:11480–4.
Jia J, Li F, Tang XS, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7:37868–81.
Yuan SX, Wang J, Yang F, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63:499–511.
Mao Z, Li H, Du B, et al. LncRNA DANCR promotes migration and invasion through suppression of lncRNA-LET in gastric cancer cells. Biosci Rep. 2017;37:BSR20171070.
Sha S, Yuan D, Liu Y, Han B, Zhong N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open. 2017;6:1310–6.
Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/beta-catenin signaling. Biomed Pharmacother. 2018;102:602–7.
Shi H, Shi J, Zhang Y, et al. Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J Thorac Dis. 2018;10:2573–82.
Wang S, Jiang M. The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed Pharmacother. 2018;103:94–100.
Jiang N, Wang X, Xie X, et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55.
Xu D, Yu J, Gao G, Lu G, Zhang Y, Ma P. LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci Rep. 2018;38:BSR20171664.
Ma Y, Zhou G, Li M, et al. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-kappaB signaling pathway. Neurochem Int. 2018;118:233–41.
Chang L, Guo R, Yuan Z. IL-36alpha suppresses proliferation of ovarian cancer cells. Tumour Biol. 2017;39:1010428317706918.
Yu Q, Shang LU, Yu H, Yang Z, Xu D. Silencing of FRAT1 by siRNA inhibits the proliferation of SGC7901 human gastric adenocarcinoma cells. Biomed Reports. 2016;4:223–6.
Guo G, Kuai D, Cai S, et al. Knockdown of FRAT1 expression by RNA interference inhibits human glioblastoma cell growth, migration and invasion. PLoS ONE. 2013;8:e61206.
Wang Y, Liu S, Zhu H, et al. FRAT1 overexpression leads to aberrant activation of beta-catenin/TCF pathway in esophageal squamous cell carcinoma. Int J Cancer. 2008;123:561–8.
Saitoh T, Katoh M. FRAT1 and FRAT2, clustered in human chromosome 10q24.1 region, are up-regulated in gastric cancer. Int J Oncol. 2001;19:311–5.
Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–84.
Berger AC, Korkut A, Kanchi RS, et al. A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell. 2018;33(690–705):e9.
Emami, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci. 2004;101(34):12682–7.
Pan L, Liang W, Gu J, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9:1915–30.
Liang H, Zhang C, Guan H, Liu J, Cui Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J Cell Physiol. 2019;234(5):7266–78. https://doi.org/10.1002/jcp.27484.
Cao L, Jin H, Zheng Y, Mao Y, Fu Z, Li X, Dong L. DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 2019;110(3):913–25. https://doi.org/10.1111/cas.13921.
Acknowledgements
We thank Prof. Yong Li for his advice.
Funding
This work was funded by National Natural Science Foundation of China (No. 81703029), China Postdoctoral Science Foundation (No. 2018M642795), and the Scientific and Technological Project of Henan Province (No. 182102310115 and 192102310069).
Author information
Authors and Affiliations
Contributions
CL, YZ designed the research; GR contributed to the new experimental approach; TW, LN performed the experiments and analyzed the data; CL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University reviewed and approved this program in accordance with Helsinki Declaration. The Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University reviewed and approved this program.
Consent for publication
All Authors have seen and approved the manuscript and consent publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
The genes associated with DANCR in cervical cancer from TCGA data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, W., Lei, N., Guo, R. et al. Long non-coding RNA DANCR promotes cervical cancer growth via activation of the Wnt/β-catenin signaling pathway. Cancer Cell Int 20, 61 (2020). https://doi.org/10.1186/s12935-020-1139-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-020-1139-9