Abstract
Prostate cancer (PCa) is a common malignant tumor with increasing incidence and high heterogeneity among males worldwide. In the era of big data and artificial intelligence, the paradigm of biomarker discovery is shifting from traditional experimental and small data-based identification toward big data-driven and systems-level screening. Complex interactions between genetic factors and environmental effects provide opportunities for systems modeling of PCa genesis and evolution. We hereby review the current research frontiers in informatics for PCa clinical translation. First, the heterogeneity and complexity in PCa development and clinical theranostics are introduced to raise the concern for PCa systems biology studies. Then biomarkers and risk factors ranging from molecular alternations to clinical phenotype and lifestyle changes are explicated for PCa personalized management. Methodologies and applications for multi-dimensional data integration and computational modeling are discussed. The future perspectives and challenges for PCa systems medicine and holistic healthcare are finally provided.
Similar content being viewed by others
Background
Prostate cancer (PCa) is a malignant solid tumor commonly occurred with high incidence and mortality worldwide. According to the report of Cancer Statistics in 2019, it is the second leading cause of cancer-related death among males in the United States [1]. In Asian countries, the number of PCa patients is also increasing these years. Extensive evidences indicated that the risk of PCa is positively correlated with the age of people [2, 3], the screening of specific PCa signature is therefore of great significance for clinical decision, especially in the aging society.
Starting from normal prostate epithelium, the development of PCa is usually very slow. It may take a long time period for the growth from intraepithelial neoplasia to clinically localized PCa, and eventually progressing into the metastatic status. Unfortunately, most of the newly diagnosed PCa patients are often at the advanced stage with distant metastasis due to the limitation of typical symptoms for early detection. Although the tests of prostate-specific antigen (PSA), imaging and prostate biopsy are well conducted in PCa clinical screening, overdiagnosis and overtreatment still widely exist [4, 5]. It is generally known that the initiation and progression of PCa is extremely heterogeneous, where interactions between genetics, lifestyle and environmental factors contribute to the evolution of PCa cells [6,7,8]. These complex interactions bring us both opportunities and challenges for systems-level deciphering of PCa carcinogenesis and developing novel methodologies to model PCa development for biomarker discovery and clinical management [9].
The recent advances in biotechnologies and computational sciences have accumulated multi-dimensional data for PCa precision medicine and healthcare. For instance, based on next-generation sequencing the genetic architecture and gene expression patterns of normal and PCa population can be tested at low cost. The mutations and anomalous expression of genes have been witnessed to be oncogenetic or anticarcinogenic in PCa progression and invasion, which are useful for PCa risk prediction and classification [10,11,12,13,14,15]. The clinical physiological data collected from digital and smart mobile devices promote the development of preventive and participatory medicine. Using B-ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI), the size and severity of tumors can be evaluated and measured in a direct way. As an emerging and interdisciplinary subject, the concept of translational informatics has been advocated for medical study. Compared with bioinformatics and biomedical informatics which mainly focus on the data at molecular and individual levels [16], translational informatics covers a broad range of topics and it integrates biomedical data including genome, transcriptome, proteome, metabolome and imaging spectrum with multi-variable social network information, such as the mechanisms from lifestyle and environmental factors positively or negatively affecting PCa [17, 18].
Systems medicine is also a data-driven paradigm for disease management. Under the framework of systems biology and medicine theory, systems medicine emphasizes the holistic properties of diseases and bridges the genotype–phenotype associations for PCa precision healthcare [19,20,21]. For example, the genetic screening helps the early detection of PCa risk and environmental changes regulate PCa growth through an epigenetic manner [22, 23]. Image parameters play insightful roles in PCa diagnosis and prevention from lifestyle medicine encourages the personalized treatment of PCa patients [24]. Since the complexity and variety of PCa big data, one of the key issues needed to be addressed is how to integrate the different data resources for computational modeling, and to simulate the dynamical changes during PCa development. The identification and prioritization of specific biomarkers and functionally driven players from the noisy and multi-structural data is the goal of translational informatics for PCa systems medicine, and this would promote the translation of basic biomedical research into clinical applications and decisions.
This review aims to summarize state of the art for data-driven translational prostate cancer research. First, the heterogeneity in PCa development and clinical strategies for PCa precision theranostics are briefly explicated. Then biomarkers and risk factors for PCa personalized management are sequentially introduced from three aspects, i.e., molecular alterations, clinical phenotype features, and environmental effects. The data resources, computational models available for PCa knowledge discovery and translational application are comprehensively summarized, and the latest techniques in artificial intelligence (AI) and the fifth generation (5G) mobile networks for PCa clinical practice are discussed. Future perspectives and challenges related to PCa systems medicine are provided in the end.
Heterogeneity in PCa development and theranostics
PCa development and evolution
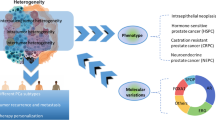
PCa is a kind of chronic consumptive diseases. It may take several years or even decades for the development from normal prostate tissue to malignant symptoms. However, under some circumstances it shows a high degree of invasiveness, which can be transferred to lymph nodes, bone, brain and other organs. As illustrated in Fig. 1, PCa originates in normal prostate epithelium. Based on the stress from both genetic and external environments, the out-of-control division and proliferation of tumor cells promote the formation of clinically localized PCa, and further develop into metastatic and castration-resistant states. Compared with primary localized PCa, metastatic PCa (mPCa) and metastatic castration-resistance PCa (mCRPC) have higher aggressiveness, and they always lead to poor prognosis and shorter overall survival time.
PCa development and theranostics. PCa prostate cancer, PSA prostate specific antigen, tPSA total PSA, fPSA free PSA, PSAD PSA density, PSAV PSA velocity, CT computed tomography, MRI magnetic resonance imaging, MRS magnetic resonance spectroscopy, ECT emission computed tomography, TNM tumor node metastasis
The development and evolution of PCa is a complex and heterogeneous process. For example, the incidence of PCa has obviously geographical and ethnic differences, where Western population shows a significantly higher incidence rate than that of Asian. The age, race and genetic background are risk factors of PCa. Ishak et al. [25] performed a comprehensive review on PCa susceptibility genetic variants in men with high risks based on genome-wide association studies. They found that the risk of PCa could be associated with single nucleotide polymorphisms (SNPs) in genes. In Chinese Han population, Xu et al. [26] identified two risk-associated loci for PCa on chromosomes 9q31.2 (rs817826) and 19q13.4 (rs103294), respectively. These findings improve the understanding of gene-level susceptibility heterogeneity between Chinese and Western population. In addition, extensive efforts demonstrated that the lifestyle, living environment and career of people have positive or negative effects on PCa occurrence and progression [27, 28].
Clinical strategies for PCa diagnosis and therapy
In general, there are no typical symptoms in the early stage of PCa. With the growth of tumor, lower urinary tract symptoms will appear when the urethra and bladder have been invaded. As described in Fig. 1, there are a series of clinical strategies can be used for PCa screening, including digital rectal examination (DRE), PSA testing, transrectal ultrasonography, CT, emission computed tomography (ECT), and MRI. In particular, prostate biopsy is considered as the gold standard for PCa diagnosis. Based on the testing results, the Gleason score and tumor node metastasis (TNM) stage are defined and measured to evaluate the risk level of PCa patients.
The selection of therapeutic methods depends on the stage of PCa development. The difference between the incidence and mortality of PCa varies dramatically. According to the routine autopsy reports, approximately 60–70% of the elderly men suffer from histological PCa, but most of them are silent and have no clinical progress [29]. For patients with low PCa risk, watchful waiting and active surveillance are preferred before positive indications increased. Currently, prostatectomy is one of the most effective ways for the treatment of localized PCa, and these patients often have good prognosis. However, the survival rate and quality of life in patients with advanced mPCa are seriously reduced. Most of the patients have missed right surgical opportunity. Although endocrine-guided therapy can delay the progression of mPCa to a certain extent, many patients develop to be castration-resistant after the treatment for a period of time, and eventually move into mCRPC states. To fight against this, Docetaxel-based chemotherapy and Abiraterone-based new endocrine plans light the longing for improving the living quality. Unfortunately, patients need to balance the advantages and drawbacks among drug sensitivity, side effects and the cost.
As reported by Hanahan et al. [30], avoiding immune destruction is one of the hallmarks of cancers. Owing to the high specificity and long-lasting anti-cancer effects, immunotherapy is becoming a hot area in cancer treatment [31]. There are currently two immunotherapy options approved by Food and Drug Administration for PCa, i.e., Pembrolizumab [32] and Sipuleucel-T [33], and they have shown great significance in PCa clinical management. For translational research, Jafari et al. [34] reviewed the latest findings in the field of immune checkpoints for PCa application, such as CTLA-4, PD-1, PD-L1, B7-H3, etc., and potential biomarkers correlated with immune infiltrates in tumor microenvironment are also identified for providing candidate PCa therapeutic targets [35].
It should be noticed that the present clinical indices and therapeutic approaches still have limitations on PCa personalized medicine. For example, the specificity of serum total PSA is not powerful enough to indicate the true states of PCa development, since the benign prostatic hyperplasia and inflammation also hold the potential to increase the expression level slightly. It is generally known that PCa is a complex disease resulting from a combination of disorder between genetic and environmental factors. Hence the integration of both molecular and clinical data would contribute to the dynamical modeling of disease changes for actionable player identification, thereby bringing a systems medicine avenue toward ‘P4’, i.e., predictive, preventive, personalized and participatory, medicine spectrum in the era of translational informatics [36].
Biomarkers and risk factors for PCa personalized management
Susceptibility and prevention from molecular alterations
The identification of biomarkers with high sensitivity and specificity for personalized PCa management is an important direction of translational informatics studies. Compared with traditional computational framework inferring candidate players from a hypothesis-driven trail to experiment-guided validation, translational informatics learns the knowledge of diseases based on big data modeling and simulating, which increases the possibility for the holistic description of dynamical characteristics during disease development and evolution [17].
According to systems biology paradigm, biomarkers can be classified at the molecular, clinical phenotype and environmental level [37]. As listed in Table 1, accumulating studies identified that the mutation and abnormal expression of biological molecules are functional for PCa risk prediction, prognosis and targeted therapy, and some of them have been successfully applied to clinical genetic testing and auxiliary diagnosis. For example, pathogenic sequence variants in BRCA1 and BRCA2 are reported to be linked with PCa risk and severity. Compared with the reference bin c.1001–c.7913, Patel et al. [38] found that such variants in the 3′ region of BRCA2 (c.7914+) had a significant association with the increased risk of PCa development, and it also held the power for aggressiveness indication. For patients with mPCa, the incidence of inherited DNA-repair gene mutations was significantly higher than that in localized PCa, and the variants in DNA-repair associated genes such as BRCA1, BRCA2, ATM, CHEK2, RAD51D and PALB2 were involved in the process of PCa metastasis [39]. In addition to genetic mutations, epigenetic alteration is also reported to be associated with PCa development. For example, the methylations in GBX2 and CDO1, respectively, were associated with the risk of PCa biochemical recurrence and biochemical recurrence-free survival of PCa patients [40, 41].
Most of the studies nowadays raise the attention to non-coding RNAs (ncRNAs) in PCa carcinogenesis, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs). Although these members do not encode proteins, they can regulate gene expression at the post-transcriptional level. In particular, lncRNAs, circRNAs and messenger RNAs (mRNAs) are known to regulate each other by competitively sharing the same miRNA response elements and finally affect the expression of down-stream genes based on competitive endogenous RNA (ceRNA) mechanisms [42]. Bidarra et al. [43] introduced that the levels of circulating miR-182-5p and miR-375-3p were candidate biomarkers to predict the advanced pathologic stage and metastasis of PCa with favorable sensitivity and specificity. Since Docetaxel-resistance limits the therapy of mCRPC patients, Corcoran et al. [44] found that miR-34a was a key player in PCa cells to regulate BCL-2 and to further affect Docetaxel response. Moreover, some of the targets of this miRNAs, e.g., SNCA, SCL7A5, had strong relationships with PCa poor prognosis. As a specific lncRNA, PCA3 was widely investigated in PCa studies. It modulated the survival of PCa cells by regulating AR signaling [45], and was recognized as a significant predictor for PCa grade reclassification [46]. In therapeutics, APP and MALAT1 played oncogenic roles in the migration of PCa cells, where the former sponged miR-218 to facilitate the expression of ZEB2/CDH2 and the latter modulated PCa progression via the MALAT1-miR-1-KRAS axis [47, 48]. In contrast to miRNAs and lncRNAs, circRNAs are a class of ncRNAs with special circle structures. Although the functions have not been clearly deciphered, they are reported functionally important to the tumorigenesis. For example, the up-regulation of circFOXO3 was positively correlated with the Gleason score and PCa progression level, whereas the down-expression of circ_001206 could predict the poor clinicopathologic features for PCa patients [49, 50].
In addition to genomic, epigenomic and proteomic studies, transcriptomic and metabolomic signatures are functional in indicating the significant changes during PCa development. For example, Emami et al. [51] trained cis-regulatory models using transcriptome-wide gene expression data from PCa and normal European ancestry men. They found that the increased expression of TMPRSS2 was associated with PCa risk and many genes showed the pattern for allele-specific transcriptional activation by PCa-associated regulators such as AR. Since the metabolite profiling is now widely used in screening biomarkers for cancer prediction, Kelly et al. [52] reviewed the metabolic biomarkers identified for PCa diagnosis, prognosis and treatment. Based on a replicated experiment, they found that the metabolite profiling could precisely discriminate PCa and benign patients, tumor aggressiveness, recurrence groups, and cases with good responses to therapeutics. Vandergrift et al. [53] performed a retrospective study on three groups of patients, i.e., PCa, benign prostatic hyperplasia, and the normal control, to identify metabolomic biomarkers for PCa aggressiveness prediction, and the result indicated the association between the increased myo-inositol level and highly aggressive PCa. Besides, hyperpolarized 13C lactate, spermine, and citrate are also convinced to serve as biomarkers for measuring the degree of PCa aggressiveness [54, 55].
Recent studies showed that plasmatic nanovesicles called exosomes may be highly helpful in the near future for early diagnosis and follow-up of PCa patients, because some RNAs, proteins or other molecules extracted from exosomes could serve as biomarkers for cancer detection. Logozzi et al. [56] conducted a prospective clinical study using two different methodological approaches, such as Immunocapture-based ELISA and nanoscale flow cytometry, to characterize plasmatic exosomes. They provided a clear evidence that plasmatic exosome expressing PSA could distinguish PCa patients not only from healthy individuals but also from patients affected by benign prostate hypertrophy. Due to the microenvironmental acidity of tumors, the increased plasmatic exosome expressing PSA was captured in PCa patients. In fact, culturing human PCa cells at different potential of hydrogen (pH) conditions, the result showed that low pH induced an amazing increase in ‘PSA + exosome’ release, which indicated the significance of environmental effects on biological activities [57].
With the coming age of big data, more functional molecules can be screened, and the interactions between various genetic components as well as metabolites from different sample sources will contribute to the systems understanding of PCa pathogenesis and clinical translation.
Clinical phenotypes toward dynamical monitoring
Clinical phenotype biomarkers consist of image signatures captured by digital devices like B-ultrasound, CT, MRI, etc, special symptoms such as lower urinary tract symptoms, abdominal pain, fever and weight loss. Compared with molecular biomarkers, changes in clinical phenotypes present an intuitive picture to describe the distribution of PCa tumors, and reflect the real-time status for dynamical monitoring of PCa patients.
As shown in Table 2, there are many phenotypes can be used for PCa clinical management. Yuan et al. [58] found that zinc-specific ion chemical exchange saturation transfer MRI was a significant image factor to indicate PCa development. They supposed that the concentration of mobile zinc in healthy prostate is relatively high, so the decrease of prostate zinc content could be applied to predict the initial development of PCa. Further in vivo experiments using mouse models convinced this hypothesis [58]. Based on MRI screening, the diffusion-weighted imaging (DWI) signal is sensitive for the response of PCa bone metastasis and androgen deprivation therapy (ADT). For example, Perez-Lopez et al. [59] performed the correlation analysis between multi-parametric MRI and mCRPC bone metastases. The clinical data demonstrated that DWI signal was significantly higher in the cohort with bone metastasis. Kim et al. [60] tested the DWI signal, i.e., apparent diffusion coefficient, on PCa patients before and after ADT treatment. The result indicated the potential of DWI as a non-invasive biomarker for monitoring the dynamical changes in patients responding to ADT therapy. As an important predictor in clinical routine examination, the change in bone scan index (BSI) has been acknowledged to be powerful for measuring the prognosis and overall survival of advanced PCa patients [61,62,63]. In particular, it was also effective in evaluating the outcome of PCa patients under Abiraterone therapy [61].
Clinical symptoms especially the lower urinary tract symptoms provide valuable guidance for PCa diagnosis, though the specificity still needs to be investigated. Several studies focused on the urinary problems associated with the recurrence and life quality of PCa patients after radiotherapy [64,65,66]. Meanwhile sexual dysfunction and depression caused by different therapeutic regimes would be important for patient prognosis tracking [67, 68]. In some cases, the clinical symptom is a consequence of molecular disorders, thus the characterization of genotype–phenotype associations for PCa systems medicine is of the significance.
Lifestyle and environmental factors for healthcare
Lifestyle is easily to be adjusted for the improvement of PCa healthcare. As described in Table 3, daily lifestyles can affect PCa development through either positive or negative ways. For example, daidzein and genistein in soy foods are protective factors for PCa [69]. The regular taking of fresh vegetables, fruits, fish, and nuts as Mediterranean diet can reduce PCa risk [70]. However, pickled vegetables, fermented soy products, salted fish, and preserved meats seem to be harmful [71]. Similar to public awareness, unhealthy habits such as tobacco consumption and alcohol intake threaten the normal function of prostate, and may increase the development of PCa based on epigenetic mechanisms [72, 73]. Interestingly, the influence of some lifestyle elements on PCa is inconsistent and controversial across different research reports. For example, Salem et al. [74] conducted a multi-center study to analyze the association between serum calcium concentration and PCa risk. They concluded that calcium was helpful for protecting against PCa. By contrast, Jackson et al. [75] found a positive linear relationship between calcium level and PCa risk, which indicated the negative effects of calcium on prostate health. To better understand the latent reasons, meta-analysis should be performed and genetic background as well as family history is urgently to be considered.
The surrounding environment may affect the development of PCa in silence. Parent et al. [76] designed a case–control study in Montreal, and uncovered that the ground-level nitrogen dioxide from traffic-related air pollution could increase the PCa risk. Based on a combination of observational and experimental studies, Ali et al. [77] suggested that environmental polychlorinated biphenyls played a negative role in the development of high-grade PCa. Chia et al. [78] studied the Singapore PCa cases and found that excessive sun exposure could increase the risk of PCa. Last but not least, related to the pH-associated exosome release discussed above [79], low pH microenvironment is a common phenotype of all cancers [80,81,82]. Some preclinical reports have shown that in a model of spontaneous PCa simply administering buffers or even alkalinizing water could prevent the formation of PCa [83, 84], which brings a new vision for clinical management and holistic healthcare of PCa patients.
In recent years, more attention has been paid to develop healthy lifestyle habits and living environments for disease prevention and healthcare. To unravel the deep linkage between lifestyle, environment and PCa pathogenesis, exclusive use of single-level biomedical data is inadequate, molecular resources together with the information from social networks and population studies are crucial for pluralistic integration [17].
Translational PCa research in big biomedical data era
Standardization and integration of data resources for knowledge discovery
In big biomedical data era, the standardization and integration of information from different resources is the first step toward systems modeling and knowledge discovery. In fact, the types of PCa data are diverse [85], including: (1) molecular data regarding to the genetic structure and metabolic process of PCa patients, such as mutations, abnormal expression and dysfunctional regulation of genes, RNAs, proteins and metabolites; (2) image data screened by B-ultrasound, CT, MRI and other digitized techniques. These data provide a clear map for personalized PCa signal identification; (3) demographic and clinical phenotype data collected from electronic medical records, laboratory tests and the history of diagnosis and therapeutics; (4) lifestyle and environmental data associated with the diet, physical sports, profession, hobby interests, and surrounding environments of PCa patients.
As illustrated in Table 4, it is encouraging that a large number of databases and knowledge bases have been designed for PCa translational studies. For example, The Dragon Database of Genes associated with Prostate Cancer (DDPC) and Prostate cancer proteomics (PCP) database, respectively, increase the convenience for PCa genomics and proteomics studies [86, 87]. Reznik et al. [88] analyzed metabolomics data from tissue samples of seven cancers and developed a large-scale online resource for investigating the change in metabolite levels across cancers. At the image and clinical level, The Cancer Imaging Archive (TCIA) provides a platform for the advanced medical imaging of PCa and other types of cancer [89]. Chen et al. [90] developed the first lifestyle database (PCaLiStDB) for PCa prevention. In the current version, a total of 2290 lifestyles were manually collected via PubMed literature mining, and the related genes, biochemical indexes as well as drug responses were reasonably annotated for the lifestyle-wide association studies of PCa. In addition, Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) is a specific database focusing on documenting the impact of PCa, and information including clinical outcomes and health-related quality of life can be recorded [91]. To promote a standardized landscape of PCa, a data resource called Prostate Cancer Ontology (PCaO) was built by referencing the authoritative clinical guidelines to extract the core concepts associated with PCa evolution, including 637 main classes and more than 2000 synonyms (http://pcaontology.net/).
Although the resources are abundant and publicly applicable, the standardization should be carefully considered before data integration and characterization since the formats of data types are highly heterogeneous. For example, the gene expression profiles are screened and calculated with different output formats, based on the experimental instruments and equipment used. Compared to the data with structural contexts, the image and environmental data on different biological layers are hard to be quantitatively measured. As noticed, the lack of standardized rules for multi-data organization limits the procedure of clinical translation. Moreover, data sharing for collaborative research is still a typical obstacle for nation-wide and multi-center clinical evaluation, and efforts need to protect the privacy and security of patient personal information. Systems medicine highlights the global and dynamical properties hidden in data. Many studies isolate the underlying interactions among different meta-resources, and solely emphasis the static patterns for further analysis. For translational applications, the integration of cross-level data in a continuous series of points would be important for the real-time monitoring and precision healthcare of PCa patients.
Computer-aided modeling and simulating for translational applications
With the accumulation of biomedical data, computer-aided modeling and simulating for the complex PCa process is now becoming available. As described in Fig. 2, multi-level PCa data drive the development of computational models from evidence-based feature characterization to mechanism-guided biomarker discovery. Three approaches, i.e., statistics, network modeling, and machine learning, have been widely applied to screen key players for personalized PCa management. Validation and carcinogenic studies are performed for translational analysis afterwards.
Biological networks are powerful for measuring the potential relationship among different molecular elements. As shown in Table 4, topological patterns hidden in protein–protein interaction (PPI) network, miRNA-mRNA regulatory network and gene co-expression network contribute to the implementation of PCa bioinformatics modeling. For example, Foj et al. [92] performed the differential expression analysis on miRNA and mRNA datasets downloaded from Gene Expression Omnibus (GEO) database. Based on PPI network information and miRNA-target prediction, a total of 12 miRNAs regulating no less than two of the 10 hub genes in PPI network were identified as candidate signature for high-grade (Gleason score ≥ 7) PCa detection. According to the pathway enrichment analysis, the 10 hub genes were closely associated with PI3K-Akt signaling, which was involved in the process of cell proliferation and survival. The identified miRNAs, therefore, were inferred to be functional in PCa invasion and aggressiveness. Compared with this method, Li et al. [93] integrated the gene expression data and PPI network for PCa-associated gene module biomarker identification. The difference is that the model directly mapped the transformed expression value of genes onto PPI, and extracted sub-networks specific to PCa progression based on greedy algorithm. Finally, top-ranked sub-networks identified from each PCa dataset were merged as module biomarker for PCa screening. Further enrichment analysis convinced the role of genes in AR nuclear signaling and EGFR pathway. Since miRNAs and lncRNAs are important post-transcriptional regulators, Lin et al. [94] focused on special regulatory structures in human miRNA-mRNA network, and defined the single-line regulation of miRNAs for biomarker characterization. Combined with the function of targeted genes, five miRNAs, i.e., miR-101-3p, miR-145-5p, miR-204-5p, miR-198 and miR-152, were identified as candidate biomarkers for PCa metastasis. Cui et al. [95] applied the weighted gene co-expression network analysis to cluster lncRNAs as module biomarkers for PCa diagnosis and prognosis. The correlation between the principle component of each identified module and PCa phenotype convinced the predictive power of the lncRNAs.
In addition to network-based methods, machine learning models such as support vector machine (SVM), random forest (RF), convolutional neural network (CNN) and deep learning are used for predicting the behavior of PCa. For example, Toth et al. [96] presented a RF model to predict the aggressiveness of PCa. The DNA methylation data in PCa cohorts with good or poor prognosis were selected as the input, and genes were ranked for evaluating the relevance between the loss of methylation in partially methylated domain regions and PCa progression. Lin et al. [97] integrated the RF with SVM and Cox proportional hazard model, and developed a Charlson comorbidity index-reinforced computational framework for predicting the recurrence-related death of localized PCa. To analyze the MRI data, Ishioka et al. [98] used a CCN algorithm for automated detection of PCa cases. Eminaga et al. [99] introduced an approach for dividing PCa patients into clinically meaningful groups based on deep learning. The result demonstrated its classification ability and clinical decision possibility. Xu et al. [100] decoded crucial clues from network topology and SVM model, and prioritized candidate miRNAs for PCa diagnosis and tumorigenesis analysis.
Considering the methods for PCa translational studies, few of them uncovered the holistic features for knowledge discovery and model training. The development of PCa is complex and dynamical, thus the use of small discrete data would limit the generalization and robustness of proposed models in a big testing space. To achieve better understandings, the traditional paradigm for biomarker identification from a small set of data should be shifted into the mode of big data-based systems modeling [17]. Meanwhile, integration of molecular genotype with clinical phenotype data is essential for the translation of informatics to practical use.
AI-based and 5G-supported clinical decision making
As shown in Fig. 3, clinical management of PCa still faces difficulties in terms of the risk prediction, diagnosis, therapy and follow-up. For example, over-diagnosis and over-treatment are concerned by the public all the time due to the limitation in current indices clinically used. Prostate biopsy and prostatectomy, respectively, are the most effective way for PCa screening and treatment, however, they are both invasive, and should not be inappropriately performed on patients without carefully considering their personalized disease history.
With the rapid progress in computer sciences, AI is now changing social lives in many ways, including the medicine. As a machine with strong ability for intelligent decision, AI is able to carry out cognitive tasks based on continuous learning and refining specific skills and knowledge from provided big data. The development of machine learning algorithms and advanced image processing systems using cloud storage and computing platforms contributes to the training of computational models to automatically conduct complex studies in molecular testing, medical imaging and translational informatics, thereby improving the accuracy and clinical workflow of PCa diagnosis and therapy [101, 102]. In particular, the application of AI to screen tiny lesions from image data for early detection of digital pathology enables the advanced characterization of PCa development based on a combinative assessment of radiology and pathology [103]. In therapy, radical radiotherapy offers the long-term outcomes for high-risk PCa patients. However, the use of radiotherapy especially the whole-pelvis radiotherapy (WPRT) remains controversial due to the noise in the mixed data and some patients may not eventually benefit from such therapeutic effects. Based on the comparison and training of big data from thousands of patients treated with WPRT, AI identifies key disease regions for WPRT optimization, and improves the effectiveness and safety of pelvic radiotherapy [104]. To achieve better performance, AI techniques need to be constantly strengthened using large-scale clinical data. Nir et al. [105] compared cross-validation approaches in AI for PCa grading from digitized histopathologic images. They found that patient-based cross-validation and multi-expert evaluation could reduce the biases of AI classifiers and improve the accuracy of estimation in contrast to patch-wise cross-validation and single-expert training.
The newly developed 5G technology aims to build a network interconnection of all things and it holds the promise for high performance computing of multi-dimensional biomedical data to clinical translation. With the support of 5G, remote medical treatment has become applicable. For example, the remote surgeries can be precisely conducted by surgeons in other locations since 5G makes high-resolution videos and AI-assisted decisions with no time-lag possible. Moreover the integrated framework of ‘AI + 5G’ promotes novel medical modes for holistic health administration, such as patient engagement, mobile health, and Internet hospital, which contribute to the realization and boom of medical diversity.
Perspectives and challenges on PCa systems medicine
The term of big data and translational informatics creates an unprecedented chance for PCa systems medicine, as illustrated in Fig. 4. The concept of ‘systems’ mainly covers two aspects of contexts: first, identifying and characterizing PCa signatures and carcinogenesis at the systems or network level; second, integrating molecular testing, image screening and lifestyle prevention for PCa precision medicine and holistic healthcare based both on computational and experimental techniques [106]. To achieve this goal, perspectives and challenges are needed to be concerned.
Perspective and challenge 1: standardizing and integrating cross-level biomedical data for database construction and systems analysis
It should be admitted that data resources are the foundation and driving force for computational modeling and experimental validating of complex status associated with PCa evolution. Standardization and integration of big biomedical data for database construction and systems analysis are important for knowledge discovery and key player identification. On one hand, the development of ontology is an essential step for PCa standardized and overall description. On the other hand, data cleaning needs to be performed before the integration and application of noisy data. In addition, data sharing from multi-center studies is advocated under the promise of protecting the personal information of PCa patients [102].
Perspective and challenge 2: proposing principles and general rules for PCa feature characterization and dynamical modeling
Until now, principles and general rules are limited for PCa feature characterization and dynamical modeling from a holistic perspective. For example, most of the studies only take the conventional parameters into account and ignore the hidden structures within the data. Systems-guided screening of biomarkers and risk factors provides functional insights in PCa pathogenesis and heterogeneity. However, it is still a challenge for translational informatics because of the considerable data sample and complexity in computer modeling. Compared with traditional modes for PCa analysis, systems biology emphasizes the identification of PCa-related genes, RNAs, proteins, pathways and non-genetic factors under an interactive and dynamical framework. The computational approaches should not only focus on the genotype changes in PCa patients, phenotype indices and clinical physiological signals are also significant for model training and testing.
Perspective and challenge 3: integrating computational and experimental methods for population-based validation and refining the models based on big data training
Traditional methods used for biomarker validation usually start from the evaluation of candidate molecules through computational simulation, followed by biological experiments using cell lines, model animals and human prostate samples for differential expression analysis. Finally, molecules with significant expression-level alternations are selected for carcinogenesis decoding. Serving for clinical use is the ultimate aim of translational studies, however, informatics models often fails in the last phase of trials because the features previously obtained do not overcome the fact of clinical complexity and diversity [107]. Hence population-based validation ranging from a small set of subjects to big groups of patients may help refine the models, and the models in turn need the big data for continuously training and learning.
Perspective and challenge 4: developing potential scenarios and practical standards for translational assessment and clinical utility of identified biomarkers
Although biomarkers ranging from molecular alternations to clinical phenotype changes are accumulated in PCa studies, the efficiency and precision still need to be considered for translational application. Currently, a growing number of biomarkers have been identified and shown the performance on PCa risk stratification in vitro, however, most of them tend to have low quality and their value should be carefully validated and documented in clinical utility. To achieve better outcomes and indicate the complex heterogenicity during PCa evolution, practical standards are necessary to be provided for biomarker comparison and moreover, the question regarding the optimal combination of biomarkers with other clinical indices is expected to be defined and answered [108].
Owing to the perspectives on potential risk screening and personalized healthcare, translational informatics would be a powerful tool against PCa in future clinical practice. The existed challenges encourage the development of novel theories and technologies for PCa precision medicine, and contribute to the transition from PCa management toward ‘P4’ continuum for systems health promoting [109, 110].
Conclusion
In this review, the recent progress and future perspective in translational informatics for PCa systems medicine are comprehensively introduced and discussed. In the era of big biomedical data, computer-aided biomarker discovery ranging from genetic factors to environmental changes gives insights in PCa heterogeneity understanding and contributes to precision medicine and holistic healthcare of PCa patients.
Availability of data and materials
The data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- AI:
-
Artificial intelligence
- 5G:
-
The fifth generation mobile networks
- mPCa:
-
Metastatic prostate cancer
- mCRPC:
-
Metastatic castration-resistance prostate cancer
- SNP:
-
Single nucleotide polymorphism
- DRE:
-
Digital rectal examination
- ECT:
-
Emission computed tomography
- TNM:
-
Tumor node metastasis
- ncRNA:
-
Non-coding RNA
- miRNA:
-
microRNA
- lncRNA:
-
Long non-coding RNA
- circRNA:
-
Circular RNA
- mRNA:
-
Messenger RNA
- ceRNA:
-
Competitive endogenous RNA
- DWI:
-
Diffusion weighted imaging
- ADT:
-
Androgen deprivation therapy
- GEO:
-
Gene expression omnibus
- DDPC:
-
The dragon database of genes associated with prostate cancer
- PCP:
-
Prostate cancer proteomics
- pH:
-
The negative of the base 10 logarithm of the molar concentration of hydrogen ions in the solution
- TCIA:
-
The cancer imaging archive
- PCaLiStDB:
-
Prostate cancer lifestyle database
- CaPSURE:
-
Cancer of the prostate strategic urologic research endeavor
- PCaO:
-
Prostate cancer ontology
- PPI:
-
Protein-protein interaction
- SVM:
-
Support vector machine
- RF:
-
Random forest
- CNN:
-
Convolutional neural network
- WPRT:
-
Whole-pelvis radiotherapy
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Bernard B, Burnett C, Sweeney CJ, Rider JR, Sridhar SS. Impact of age at diagnosis of de novo metastatic prostate cancer on survival. Cancer. 2019. https://doi.org/10.1002/cncr.32630.
Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–41.
Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, Agoritsas T, Dahm P. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519.
Verbeek JFM, Nieboer D, Steyerberg EW, Roobol MJ. Assessing a patient’s individual risk of biopsy-detectable prostate cancer: be aware of case mix heterogeneity and a priori likelihood. Eur Urol Oncol. 2019. https://doi.org/10.1016/j.euo.2019.07.012.
Karan D, Thrasher JB, Lubaroff D. Prostate cancer: genes, environment, immunity and the use of immunotherapy. Prostate Cancer Prostatic Dis. 2008;11:230–6.
Tang Y, Yan W, Chen J, Luo C, Kaipia A, Shen B. Identification of novel microRNA regulatory pathways associated with heterogeneous prostate cancer. BMC Syst Biol. 2013;7(Suppl 3):S6.
Wang Y, Chen J, Li Q, Wang H, Liu G, Jing Q, Shen B. Identifying novel prostate cancer associated pathways based on integrative microarray data analysis. Comput Biol Chem. 2011;35:151–8.
Lin Y, Chen J, Shen B. Interactions between genetics, lifestyle, and environmental factors for healthcare. Adv Exp Med Biol. 2017;1005:167–91.
Ta HQ, Whitworth H, Yin Y, Conaway M, Frierson HF Jr, Campbell MJ, Raj GV, Gioeli D. Discovery of a novel long noncoding RNA overlapping the LCK gene that regulates prostate cancer cell growth. Mol Cancer. 2019;18:113.
Wang Y, Wang J, Zhang L, Karatas OF, Shao L, Zhang Y, Castro P, Creighton CJ, Ittmann M. RGS12 Is a novel tumor-suppressor gene in African American prostate cancer that represses AKT and MNX1 expression. Cancer Res. 2017;77:4247–57.
Hashemi M, Amininia S, Ebrahimi M, Simforoosh N, Basiri A, Ziaee SAM, Narouie B, Sotoudeh M, Mollakouchekian MJ, Rezghi Maleki E, et al. Association between polymorphisms in TP53 and MDM2 genes and susceptibility to prostate cancer. Oncol Lett. 2017;13:2483–9.
Jiang J, Cui W, Vongsangnak W, Hu G, Shen B. Post genome-wide association studies functional characterization of prostate cancer risk loci. BMC Genomics. 2013;14(Suppl 8):S9.
Jiang J, Jia P, Zhao Z, Shen B. Key regulators in prostate cancer identified by co-expression module analysis. BMC Genomics. 2014;15:1015.
Zhang W, Zang J, Jing X, Sun Z, Yan W, Yang D, Shen B, Guo F. Identification of candidate miRNA biomarkers from miRNA regulatory network with application to prostate cancer. J Transl Med. 2014;12:66.
Chen J, Zhang D, Yan W, Yang D, Shen B. Translational bioinformatics for diagnostic and prognostic prediction of prostate cancer in the next-generation sequencing era. Biomed Res Int. 2013;2013:901578.
Shen B, Lin Y, Bi C, Zhou S, Bai Z, Zheng G, Zhou J. Translational informatics for Parkinson’s disease: from big biomedical data to small actionable alterations. Genomics Proteomics Bioinformatics. 2019. https://doi.org/10.1016/j.gpb.2018.10.007.
Domenech-Abella J, Lara E, Rubio-Valera M, Olaya B, Moneta MV, Rico-Uribe LA, Ayuso-Mateos JL, Mundo J, Haro JM. Loneliness and depression in the elderly: the role of social network. Soc Psychiatry Psychiatr Epidemiol. 2017;52:381–90.
Auffray C, Chen Z, Hood L. Systems medicine: the future of medical genomics and healthcare. Genome Med. 2009;1:2.
Sarah C. Systems medicine: understanding wellness and disease. Nat Rev Drug Discov. 2017;16:602.
Wu R, Lin Y, Liu X, Zhan C, He H, Shi M, Jiang Z, Shen B. Phenotype–genotype network construction and characterization: a case study of cardiovascular diseases and associated non-coding RNAs. Database. 2020. https://doi.org/10.1093/database/baz147/5706767.
Wu L, Wang J, Cai Q, Cavazos TB, Emami NC, Long J, Shu XO, Lu Y, Guo X, Bauer JA, et al. Identification of novel susceptibility loci and genes for prostate cancer risk: a transcriptome-wide association study in over 140,000 European descendants. Cancer Res. 2019;79:3192–204.
Cheong A, Zhang X, Cheung YY, Tang WY, Chen J, Ye SH, Medvedovic M, Leung YK, Prins GS, Ho SM. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016;11:674–89.
Zuniga KB, Chan JM, Ryan CJ, Kenfield SA. Diet and lifestyle considerations for patients with prostate cancer. Urol Oncol. 2019. https://doi.org/10.1016/j.urolonc.2019.06.018.
Ishak MB, Giri VN. A systematic review of replication studies of prostate cancer susceptibility genetic variants in high-risk men originally identified from genome-wide association studies. Cancer Epidemiol Biomarkers Prev. 2011;20:1599–610.
Xu J, Mo Z, Ye D, Wang M, Liu F, Jin G, Xu C, Wang X, Shao Q, Chen Z, et al. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44:1231–5.
Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22:187–99 (quiz 200-182).
Boehm K, Sun M, Larcher A, Blanc-Lapierre A, Schiffmann J, Graefen M, Sosa J, Saad F, Parent ME, Karakiewicz PI. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: results from the North-American case-control study Prostate Cancer & Environment Study. Urol Oncol. 2015;33(494):e491–7.
Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866–71.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Cha HR, Lee JH, Ponnazhagan S. Revisiting immunotherapy: a focus on prostate cancer. Cancer Res. 2020. https://doi.org/10.1158/0008-5472.CAN-19-2948.
Higa J, Wilenius K, Savino S, Larsen C, Scholz M, Vogelzang N. Real world experience with pembrolizumab in recurrent or advanced prostate cancer. Clin Genitourin Cancer. 2019. https://doi.org/10.1016/j.clgc.2019.12.009.
Higano CS, Armstrong AJ, Sartor AO, Vogelzang NJ, Kantoff PW, McLeod DG, Pieczonka CM, Penson DF, Shore ND, Vacirca J, et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer. 2019;125:4172–80.
Jafari S, Molavi O, Kahroba H, Hejazi MS, Maleki-Dizaji N, Barghi S, Kiaie SH, Jadidi-Niaragh F. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell Mol Life Sci. 2020. https://doi.org/10.1007/s00018-020-03459-1.
Wang Z, Wang Y, Peng M, Yi L. UBASH3B is a novel prognostic biomarker and correlated with immune infiltrates in prostate cancer. Front Oncol. 2019;9:1517.
Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory. N Biotechnol. 2012;29:613–24.
Lin Y, Qian F, Shen L, Chen F, Chen J, Shen B. Computer-aided biomarker discovery for precision medicine: data resources, models and applications. Brief Bioinform. 2019;20:952–75.
Patel VL, Busch EL, Friebel TM, Cronin A, Leslie G, McGuffog L, Adlard J, Agata S, Agnarsson BA, Ahmed M, et al. Association of genomic domains in BRCA1 and BRCA2 with prostate cancer risk and aggressiveness. Cancer Res. 2019;80:624–38.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53.
Jeyapala R, Savio AJ, Olkhov-Mitsel E, Kamdar S, Zhao F, Cuizon C, Liu RSC, Zlotta A, Fleshner N, van der Kwast T, Bapat B. GBX2 methylation is a novel prognostic biomarker and improves prediction of biochemical recurrence among patients with prostate cancer negative for intraductal carcinoma and cribriform architecture. Eur Urol Oncol. 2019;2:231–8.
Meller S, Zipfel L, Gevensleben H, Dietrich J, Ellinger J, Majores M, Stein J, Sailer V, Jung M, Kristiansen G, Dietrich D. CDO1 promoter methylation is associated with gene silencing and is a prognostic biomarker for biochemical recurrence-free survival in prostate cancer patients. Epigenetics. 2016;11:871–80.
Qi X, Lin Y, Chen J, Shen B. Decoding competing endogenous RNA networks for cancer biomarker discovery. Brief Bioinform. 2019. https://doi.org/10.1093/bib/bbz006/5304351.
Bidarra D, Constancio V, Barros-Silva D, Ramalho-Carvalho J, Moreira-Barbosa C, Antunes L, Mauricio J, Oliveira J, Henrique R, Jeronimo C. Circulating microRNAs as biomarkers for prostate cancer detection and metastasis development prediction. Front Oncol. 2019;9:900.
Corcoran C, Rani S, O’Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate. 2014;74:1320–34.
Lemos AEG, Matos ADR, Ferreira LB, Gimba ERP. The long non-coding RNA PCA3: an update of its functions and clinical applications as a biomarker in prostate cancer. Oncotarget. 2019;10:6589–603.
Tosoian JJ, Patel HD, Mamawala M, Landis P, Wolf S, Elliott DJ, Epstein JI, Carter HB, Ross AE, Sokoll LJ, Pavlovich CP. Longitudinal assessment of urinary PCA3 for predicting prostate cancer grade reclassification in favorable-risk men during active surveillance. Prostate Cancer Prostatic Dis. 2017;20:339–42.
Shi X, Zhang W, Nian X, Lu X, Li Y, Liu F, Wang F, He B, Zhao L, Zhu Y, et al. The previously uncharacterized lncRNA APP promotes prostate cancer progression by acting as a competing endogenous RNA. Int J Cancer. 2020;146:475–86.
Chang J, Xu W, Du X, Hou J. MALAT1 silencing suppresses prostate cancer progression by upregulating miR-1 and downregulating KRAS. Onco Targets Ther. 2018;11:3461–73.
Kong Z, Wan X, Lu Y, Zhang Y, Huang Y, Xu Y, Liu Y, Zhao P, Xiang X, Li L, Li Y. Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR-29a-3p. J Cell Mol Med. 2019;24:799–813.
Song Z, Zhuo Z, Ma Z, Hou C, Chen G, Xu G. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47:2449–64.
Emami NC, Kachuri L, Meyers TJ, Das R, Hoffman JD, Hoffmann TJ, Hu D, Shan J, Feng FY, Ziv E, et al. Association of imputed prostate cancer transcriptome with disease risk reveals novel mechanisms. Nat Commun. 2019;10:3107.
Kelly RS, Vander Heiden MG, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol Biomarkers Prev. 2016;25:887–906.
Vandergrift LA, Decelle EA, Kurth J, Wu S, Fuss TL, DeFeo EM, Halpern EF, Taupitz M, McDougal WS, Olumi AF, et al. Metabolomic prediction of human prostate cancer aggressiveness: magnetic resonance spectroscopy of histologically benign tissue. Sci Rep. 2018;8:4997.
Keshari KR, Sriram R, Van Criekinge M, Wilson DM, Wang ZJ, Vigneron DB, Peehl DM, Kurhanewicz J. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate. 2013;73:1171–81.
Giskeodegard GF, Bertilsson H, Selnaes KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS ONE. 2013;8:e62375.
Logozzi M, Angelini DF, Giuliani A, Mizzoni D, Di Raimo R, Maggi M, Gentilucci A, Marzio V, Salciccia S, Borsellino G, et al. Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from Benign prostatic hyperplasia: a prospective study. Cancers (Basel). 2019;11:1449.
Logozzi M, Angelini DF, Iessi E, Mizzoni D, Di Raimo R, Federici C, Lugini L, Borsellino G, Gentilucci A, Pierella F, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–29.
Yuan Y, Wei Z, Chu C, Zhang J, Song X, Walczak P, Bulte JWM. Development of zinc-specific iCEST MRI as an imaging biomarker for prostate cancer. Angew Chem Int Ed Engl. 2019;58:15512–7.
Perez-Lopez R, Nava Rodrigues D, Figueiredo I, Mateo J, Collins DJ, Koh DM, de Bono JS, Tunariu N. Multiparametric magnetic resonance imaging of prostate cancer bone disease: correlation with bone biopsy histological and molecular features. Invest Radiol. 2018;53:96–102.
Kim AY, Kim CK, Park SY, Park BK. Diffusion-weighted imaging to evaluate for changes from androgen deprivation therapy in prostate cancer. AJR Am J Roentgenol. 2014;203:W645–50.
Reza M, Ohlsson M, Kaboteh R, Anand A, Franck-Lissbrant I, Damber JE, Widmark A, Thellenberg-Karlsson C, Budaus L, Steuber T, et al. Bone scan index as an imaging biomarker in metastatic castration-resistant prostate cancer: a multicentre study based on patients treated with Abiraterone acetate (zytiga) in clinical practice. Eur Urol Focus. 2016;2:540–6.
Armstrong AJ, Anand A, Edenbrandt L, Bondesson E, Bjartell A, Widmark A, Sternberg CN, Pili R, Tuvesson H, Nordle O, et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018;4:944–51.
Li D, Lv H, Hao X, Dong Y, Dai H, Song Y. Prognostic value of bone scan index as an imaging biomarker in metastatic prostate cancer: a meta-analysis. Oncotarget. 2017;8:84449–58.
Weight CJ, Kim SP, Jacobson DJ, McGree ME, Boorjian SA, Thompson RH, Leibovich BC, Karnes RJ, St Sauver J. The effect of benign lower urinary tract symptoms on subsequent prostate cancer testing and diagnosis. Eur Urol. 2013;63:1021–7.
Miyake M, Tanaka N, Asakawa I, Tatsumi Y, Nakai Y, Anai S, Torimoto K, Aoki K, Yoneda T, Hasegawa M, et al. Changes in lower urinary tract symptoms and quality of life after salvage radiotherapy for biochemical recurrence of prostate cancer. Radiother Oncol. 2015;115:321–6.
Hsiao CP, Chen MK, Meyers KJ, Saligan LN. Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy. Fam Med Community Health. 2017;5:119–28.
Tomaszewski EL, Moise P, Krupnick RN, Downing J, Meyer M, Naidoo S, Holmstrom S. Symptoms and impacts in non-metastatic castration-resistant prostate cancer: qualitative study findings. Patient. 2017;10:567–78.
Chen YZ, Chiang PK, Lin WR, Chen M, Chow YC, Chiu AW, Tsai WK. The relationship between androgen deprivation therapy and depression symptoms in patients with prostate cancer. Aging Male. 2019. https://doi.org/10.1080/13685538.2018.1560404.
Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–8.
Pascual-Geler M, Urquiza-Salvat N, Cozar JM, Robles-Fernandez I, Rivas A, Martinez-Gonzalez LJ, Ocana-Peinado FM, Lorente JA, Alvarez-Cubero MJ. The influence of nutritional factors on prostate cancer incidence and aggressiveness. Aging Male. 2018;21:31–9.
Jian L, Zhang DH, Lee AH, Binns CW. Do preserved foods increase prostate cancer risk? Br J Cancer. 2004;90:1792–5.
Ho T, Howard LE, Vidal AC, Gerber L, Moreira D, McKeever M, Andriole G, Castro-Santamaria R, Freedland SJ. Smoking and risk of low- and high-grade prostate cancer: results from the REDUCE study. Clin Cancer Res. 2014;20:5331–8.
Sawada N, Inoue M, Iwasaki M, Sasazuki S, Yamaji T, Shimazu T, Tsugane S. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int J Cancer. 2014;134:971–8.
Salem S, Hosseini M, Allameh F, Babakoohi S, Mehrsai A, Pourmand G. Serum calcium concentration and prostate cancer risk: a multicenter study. Nutr Cancer. 2013;65:961–8.
Jackson MD, Tulloch-Reid MK, Lindsay CM, Smith G, Bennett FI, McFarlane-Anderson N, Aiken W, Coard KC. Both serum 25-hydroxyvitamin D and calcium levels may increase the risk of incident prostate cancer in Caribbean men of African ancestry. Cancer Med. 2015;4:925–35.
Parent ME, Goldberg MS, Crouse DL, Ross NA, Chen H, Valois MF, Liautaud A. Traffic-related air pollution and prostate cancer risk: a case-control study in Montreal, Canada. Occup Environ Med. 2013;70:511–8.
Ali I, Julin B, Glynn A, Hogberg J, Berglund M, Johansson JE, Andersson SO, Andren O, Giovannucci E, Wolk A, et al. Exposure to polychlorinated biphenyls and prostate cancer: population-based prospective cohort and experimental studies. Carcinogenesis. 2016;37:1144–51.
Chia SE, Wong KY, Cheng C, Lau W, Tan PH. Sun exposure and the risk of prostate cancer in the Singapore Prostate Cancer Study: a case-control study. Asian Pac J Cancer Prev. 2012;13:3179–85.
Logozzi M, Mizzoni D, Angelini DF, Di Raimo R, Falchi M, Battistini L, Fais S. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers (Basel). 2018;10:370.
Gillies RJ, Pilot C, Marunaka Y, Fais S. Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer. 2019;1871:273–80.
Pillai SR, Damaghi M, Marunaka Y, Spugnini EP, Fais S, Gillies RJ. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019;38:205–22.
Logozzi M, Spugnini E, Mizzoni D, Di Raimo R, Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;38:93–101.
Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, Gatenby RA. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol. 2012;188:624–31.
Astigiano S, Puglisi A, Mastracci L, Fais S, Barbieri O. Systemic alkalinisation delays prostate cancer cell progression in TRAMP mice. J Enzyme Inhib Med Chem. 2017;32:363–8.
Hulsen T. An overview of publicly available patient-centered prostate cancer datasets. Transl Androl Urol. 2019;8:S64–77.
Maqungo M, Kaur M, Kwofie SK, Radovanovic A, Schaefer U, Schmeier S, Oppon E, Christoffels A, Bajic VB. DDPC: dragon database of genes associated with prostate cancer. Nucleic Acids Res. 2011;39:D980–5.
Shishkin SS, Kovalyov LI, Kovalyova MA, Lisitskaya KV, Eremina LS, Ivanov AV, Gerasimov EV, Sadykhov EG, Ulasova NY, Sokolova OS, et al. “Prostate cancer proteomics” database. Acta Naturae. 2010;2:95–104.
Reznik E, Luna A, Aksoy BA, Liu EM, La K, Ostrovnaya I, Creighton CJ, Hakimi AA, Sander C. A landscape of metabolic variation across tumor types. Cell Syst. 2018;6(301–313):e303.
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, Moore S, Phillips S, Maffitt D, Pringle M, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging. 2013;26:1045–57.
Chen Y, Liu X, Yu Y, Yu C, Yang L, Lin Y, Xi T, Ye Z, Feng Z, Shen B. PCaLiStDB: a lifestyle database for precision prevention of prostate cancer. Database. 2020. https://doi.org/10.1093/database/baz154/5707341.
Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, Carroll PR. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773–7.
Foj L, Filella X. Identification of potential miRNAs biomarkers for high-grade prostate cancer by integrated bioinformatics analysis. Pathol Oncol Res. 2019;25:1445–56.
Li Y, Vongsangnak W, Chen L, Shen B. Integrative analysis reveals disease-associated genes and biomarkers for prostate cancer progression. BMC Med Genomics. 2014;7(Suppl 1):S3.
Lin Y, Chen F, Shen L, Tang X, Du C, Sun Z, Ding H, Chen J, Shen B. Biomarker microRNAs for prostate cancer metastasis: screened with a network vulnerability analysis model. J Transl Med. 2018;16:134.
Cui W, Qian Y, Zhou X, Lin Y, Jiang J, Chen J, Zhao Z, Shen B. Discovery and characterization of long intergenic non-coding RNAs (lincRNA) module biomarkers in prostate cancer: an integrative analysis of RNA-Seq data. BMC Genomics. 2015;16(Suppl 7):S3.
Toth R, Schiffmann H, Hube-Magg C, Buscheck F, Hoflmayer D, Weidemann S, Lebok P, Fraune C, Minner S, Schlomm T, et al. Random forest-based modelling to detect biomarkers for prostate cancer progression. Clin Epigenetics. 2019;11:148.
Lin YT, Lee MT, Huang YC, Liu CK, Li YT, Chen M. Prediction of recurrence-associated death from localized prostate cancer with a Charlson comorbidity index-reinforced machine learning model. Open Med (Wars). 2019;14:593–606.
Ishioka J, Matsuoka Y, Uehara S, Yasuda Y, Kijima T, Yoshida S, Yokoyama M, Saito K, Kihara K, Numao N, et al. Computer-aided diagnosis of prostate cancer on magnetic resonance imaging using a convolutional neural network algorithm. BJU Int. 2018;122:411–7.
Eminaga O, Al-Hamad O, Boegemann M, Breil B, Semjonow A. Combination possibility and deep learning model as clinical decision-aided approach for prostate cancer. Health Inform J. 2019. https://doi.org/10.1177/1460458219855884.
Xu J, Li CX, Lv JY, Li YS, Xiao Y, Shao TT, Huo X, Li X, Zou Y, Han QL, et al. Prioritizing candidate disease miRNAs by topological features in the miRNA target-dysregulated network: case study of prostate cancer. Mol Cancer Ther. 2011;10:1857–66.
Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol. 2019;16:391–403.
Chen J, Qian F, Yan W, Shen B. Translational biomedical informatics in the cloud: present and future. Biomed Res Int. 2013;2013:658925.
Harmon SA, Tuncer S, Sanford T, Choyke PL, Turkbey B. Artificial intelligence at the intersection of pathology and radiology in prostate cancer. Diagn Interv Radiol. 2019;25:183–8.
Tharmalingam H, Choudhury A, Van Herk M, McWilliam A, Hoskin PJ. New approaches for effective and safe pelvic radiotherapy in high-risk prostate cancer. Nat Rev Urol. 2019;16:523–38.
Nir G, Karimi D, Goldenberg SL, Fazli L, Skinnider BF, Tavassoli P, Turbin D, Villamil CF, Wang G, Thompson DJS, et al. Comparison of artificial intelligence techniques to evaluate performance of a classifier for automatic grading of prostate cancer from digitized histopathologic images. JAMA Netw Open. 2019;2:e190442.
Shen B, Shen HB, Tian T, Lu Q, Hu G. Translational bioinformatics and computational systems medicine. Comput Math Methods Med. 2013;2013:375641.
Wiens J, Saria S, Sendak M, Ghassemi M, Liu VX, Doshi-Velez F, Jung K, Heller K, Kale D, Saeed M, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med. 2019;25:1337–40.
Cooperberg MR, Carroll PR, Dall’Era MA, Davies BJ, Davis JW, Eggener SE, Feng FY, Lin DW, Morgan TM, Morgans AK, et al. The state of the science on prostate cancer biomarkers: the San Francisco consensus statement. Eur Urol. 2019;76:268–72.
Sagner M, McNeil A, Puska P, Auffray C, Price ND, Hood L, Lavie CJ, Han ZG, Chen Z, Brahmachari SK, et al. The P4 health spectrum—a predictive, preventive, personalized and participatory continuum for promoting healthspan. Prog Cardiovasc Dis. 2017;59:506–21.
Shen L, Ye B, Sun H, Lin Y, van Wietmarschen H, Shen B. Systems health: a transition from disease management toward health promotion. Adv Exp Med Biol. 2017;1028:149–64.
Acknowledgements
The authors gratefully thank the academic editor and the anonymous reviewers for their insightful comments to improve this manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 31670851), the Key Program of Jiangsu Health Commission (Grant No. H2019040), and the Natural Science Foundation of Jiangsu Province (Grant No. BK20190170).
Author information
Authors and Affiliations
Contributions
YL and XZ contributed equally to the study. YL, XZ, ZM, ZL and XW collected and reviewed the data. YL, JP, JH, and BS drafted and revised the manuscript. BS and JH conceived and supervised the study jointly. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Y., Zhao, X., Miao, Z. et al. Data-driven translational prostate cancer research: from biomarker discovery to clinical decision. J Transl Med 18, 119 (2020). https://doi.org/10.1186/s12967-020-02281-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-020-02281-4