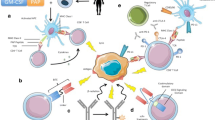
Abstract
Immunotherapy is considered as an effective method for cancer treatment owing to the induction of specific and long-lasting anti-cancer effects. Immunotherapeutic strategies have shown significant success in human malignancies, particularly in prostate cancer (PCa), a major global health issue regarding its high metastatic rates. In fact, the first cancer vaccine approved by FDA was Provenge, which has been successfully used for treatment of PCa. Despite the remarkable success of cancer immunotherapy in PCa, many of the developed immunotherapy methods show poor therapeutic outcomes. Immunosuppression in tumor microenvironment (TME) induced by non-functional T cells (CD4+ and CD8+), tolerogenic dendritic cells (DCs), and regulatory T cells, has been reported to be the main obstacle to the effectiveness of anti-tumor immune responses induced by an immunotherapy method. The present review particularly focuses on the latest findings of the immune checkpoints (ICPs), including CTLA-4, PD-1, PD-L1, LAG-3, OX40, B7-H3, 4-1BB, VISTA, TIM-3, and ICOS; these checkpoints are able to have immune modulatory effects on the TME of PCa. This paper further discusses different approaches in ICPs targeting therapy and summarizes the latest advances in the clinical application of ICP-targeted therapy as monotherapy or in combination with other cancer therapy modalities in PCa.

Similar content being viewed by others
References
Siegel RL, Miller KD (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Isaacsson Velho P, Antonarakis ES (2018) PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol 11(5):475–486
Harris E (2018) Immunotherapeutics for the treatment of prostate cancer: a patent landscape based on key therapeutic mechanisms of actions. Pharm Pat Anal 7(1):47–57
Vlachostergios PJ, Paddock M, Molina AM (2018) Molecular targeted therapies of prostate cancer. In: Precision molecular pathology of prostate cancer, Springer, New York, pp 523–546
Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J (2019) Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol 37(13):1051–1061
Curran MA, Glisson BS (2019) New hope for therapeutic cancer vaccines in the era of immune checkpoint modulation. Annu Rev Med 70:409–424
Thakur A, Vaishampayan U, Lum L (2013) Immunotherapy and immune evasion in prostate cancer. Cancers 5(2):569–590
Mo L, Chen Q, Zhang X, Shi X, Wei L, Zheng D, Li H, Gao J, Li J, Hu Z (2017) Depletion of regulatory T cells by anti-ICOS antibody enhances anti-tumor immunity of tumor cell vaccine in prostate cancer. Vaccine 35(43):5932–5938
Tang M, Diao J, Cattral MS (2017) Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell Mol Life Sci 74(5):761–776
Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A (2008) Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 26(39):5046–5057
Molavi O, Ma Z, Hamdy S, Lai R, Lavasanifar A, Samuel J (2008) Synergistic anti-tumor effects of CpG oligodeoxynucleotide and STAT3 inhibitory agent JSI-124 in a mouse melanoma tumor model. Immunol Cell Biol 86(6):506–514
Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J (2009) Immunomodulatory and anticancer effects of intra-tumoral co-delivery of synthetic lipid A adjuvant and STAT3 inhibitor, JSI-124. Immunopharmacol Immunotoxicol 31(2):214–221
Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T, Schendel DJ, Noessner E (2009) Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7–H1+ lymphocyte clusters. Eur J Cancer 45(9):1664–1672
Miller AM, Lundberg K, Özenci V, Banham AH, Hellström M, Egevad L, Pisa P (2006) CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 177(10):7398–7405
Gururajan M, Posadas EM, Chung LW (2012) Future perspectives of prostate cancer therapy. Transl Androl Urol 1(1):19
Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC (2007) LAG-3 regulates CD8+ T cell accumulation and effector function in murine self-and tumor-tolerance systems. J Clin Invest 117(11):3383–3392
Zhang Q, Vignali DA (2016) Co-stimulatory and co-inhibitory pathways in autoimmunity. Immun 44(5):1034–1051
Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria J-C, Zitvogel L, Marabelle A (2016) Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 52:50–66
Riva A, Chokshi S (2018) Immune checkpoint receptors: homeostatic regulators of immunity. Hepatol Int 12(3):223–236
Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39
Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B (2018) Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem 47(2):721–734
Fong L, Kwek SS, O'Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI (2009) Potentiating endogenous anti-tumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res 69(2):609–615
van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I (2012) Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 13(5):509–517
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP (2009) Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the anti-tumor activity of anti–CTLA-4 antibodies. J Exp Med 206(8):1717–1725
Cameron F, Whiteside G, Perry C (2011) Ipilimumab. Drugs 71(8):1093–1104
Beer T, Logothetis C, Sharma P, Gerritsen W, Ezzeddine R, Fairchild J, Gagnier P, Chin K, Cuillerot J (2011) Randomized, double-blind, phase III trial to compare the efficacy of ipilimumab (Ipi) versus placebo in asymptomatic or minimally symptomatic patients (pts) with metastatic chemotherapy-naïve castration-resistant prostate cancer (CRPC). J Clin Oncol 29(15_suppl):TPS182
Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP (2007) A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 13(6):1810–1815
Tollefson M, Karnes RJ, Thompson RH, Granberg C, Hillman D, Breau R, Allison J, Kwon E, Blute M (2010) 668 a randomized Phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. J Urol 183(4):e261
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422
Scholz M, Yep S, Chancey M, Kelly C, Chau K, Turner J, Lam R, Drake CG (2017) Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunol Target Ther 6:11
Modena A, Ciccarese C, Iacovelli R, Brunelli M, Montironi R, Fiorentino M, Tortora G, Massari F (2016) Immune checkpoint inhibitors and prostate cancer: a new frontier? Oncol Rev 10(1):293
Slovin S, Higano C, Hamid O, Tejwani S, Harzstark A, Alumkal J, Scher H, Chin K, Gagnier P, McHenry M (2013) Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 24(7):1813–1821
Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I (2013) The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 85(2):293–295
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, Van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H (2014) Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 15(7):700–712
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E (2017) VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 23(5):551
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E (2017) Investigation of mechanisms of resistance to ipilimumab therapy with a pre-surgical trial in patients with high-risk, localized prostate cancer. American Society of Clinical Oncology, New York
Jochems C, Tucker JA, Tsang K-Y, Madan RA, Dahut WL, Liewehr DJ, Steinberg SM, Gulley JL, Schlom J (2014) A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol Immunther 63(4):407–418
Cabel L, Loir E, Gravis G, Lavaud P, Massard C, Albiges L, Baciarello G, Loriot Y, Fizazi K (2017) Long-term complete remission with Ipilimumab in metastatic castrate-resistant prostate cancer: case report of two patients. J Immunother Cancer 5(1):31
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17(12):e542–e551
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G (2013) Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 31(5):616
Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ (2016) Tremelimumab: research and clinical development. Oncol Targets Ther 9:1767
Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L (2013) Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 14(11):1104–1111
McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, Liu G (2012) Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunther 61(7):1137–1147
Chamoto K, Al-Habsi M, Honjo T (2017) Role of PD-1 in immunity and diseases. In: Emerging concepts targeting immune checkpoints in cancer and autoimmunity, Springer, New York, pp 75–97
Muenst S, Soysal SD, Tzankov A, Hoeller S (2015) The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Target 19(2):201–211
Chen J, Jiang C, Jin L, Zhang X (2015) Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 27(3):409–416
Park J-J, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S (2010) B7–H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116(8):1291–1298
Philips GK, Atkins M (2014) Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 27(1):39–46
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB (2012) Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, Cetnar JP, Ey FS, Bergan RC, Slottke R (2016) Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 7(33):52810
Dolan DE, Gupta S (2014) PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 21(3):231–237
Basnet A, Khullar G, Mehta R, Chittoria N (2017) A case of locally advanced castration-resistant prostate cancer with remarkable response to nivolumab. Clin Genitourin Cancer 15(5):e881–e884
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371(11):1028–1038
Apolo AB, Mortazavi A, Stein MN, Pal SK, Davarpanah NN, Parnes HL, Ning YM, Francis DC, Cordes LM, Monk P (2017) A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. American Society of Clinical Oncology, New York
Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, Sullivan R, Dowling D, Harb R, Nirschl T, Dittamore RV (2017) Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). American Society of Clinical Oncology, New York
Hansen A, Massard C, Ott P, Haas N, Lopez J, Ejadi S, Wallmark J, Keam B, Delord J, Aggarwal R (2016) Pembrolizumab for patients with advanced prostate adenocarcinoma: preliminary results from the KEYNOTE-028 study. European Society for Medical Oncology, London
Graff J, Alumkal J, Drake C, Thomas G, Redmond W, Farhad M, Slottke R, Beer T (2016) First evidence of significant clinical activity of PD-1 inhibitors in metastatic, castration resistant prostate cancer (mCRPC). Ann Oncol 27(suppl_6):vi243–vi265
Haas NB, Stein MN, Tutrone R, Perini R, Denker A, Mauro D, Fong L (2015) Phase I-II study of ADXS31-142 alone and in combination with pembrolizumab in patients with previously treated metastatic castration-resistant prostate cancer (mCRPC): the KEYNOTE-046 trial. J Immunother Cancer 3(Suppl 2):P153
Hayes S, Petit R, Stein M, Tutrone R, Mega A, Agarwal M, Fong L, Haas N (2017) ADXS-PSA immunotherapy increases the magnitude and quality of prostate cancer-antigen-specific T cell responses in patients with metastatic castration-resistant prostate cancer. In: Proceedings of the society for immunotherapy of cancer’s (SITC) 32nd annual meeting
Rekoske BT, Olson BM, McNeel DG (2016) Anti-tumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunol 5(6):e1165377
Derer A, Frey B, Fietkau R, Gaipl US (2016) Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunther 65(7):779–786
Nardone V, Botta C, Caraglia M, Martino EC, Ambrosio MR, Carfagno T, Tini P, Semeraro L, Misso G, Grimaldi A (2016) Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther 17(11):1213–1220
Kim JW, Shaffer DR, Massard C, Powles T, Harshman LC, Braiteh FS, Conkling PR, Sarkar I, Kadel EE, Mariathasan S (2018) A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology, New York
Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A (2015) PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6(1):234
Vilalta M, Rafat M, Graves EE (2016) Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci 73(16):2999–3007
Mateo J, Hall E, Sandhu S, Omlin A, Miranda S, Carreira S, Goodall J, Gillman A, Mossop H, Ralph C (2014) Lba20antitumour activity of the Parp inhibitor olaparib in unselected sporadic castration-resistant prostate cancer (CRPC) in the Toparp trial. Ann Oncol 25(suppl_4):v1–v41
Ostrander EA, Udler MS (2008) The role of the BRCA2 gene in susceptibility to prostate cancer revisited. Cancer Epidemiol Biomarkers Prev 17(8):1843–1848
Karzai F, Madan RA, Owens H, Hankin A, Couvillon A, Cordes LM, Fakhrejahani F, Houston ND, Trepel JB, Chen C (2017) Combination of PDL-1 and PARP inhibition in an unselected population with metastatic castrate-resistant prostate cancer (mCRPC). American Society of Clinical Oncology, New York
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen M-K, Hsu J-M, Hsu JL, Yu W-H, Du Y, Lee H-H (2017) PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 23(14):3711–3720
Gianchecchi E, Fierabracci AF (2018) Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front Immunol 9:2374
Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, Chen H (2018) The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer 9(5–6):176
Andrews LP, Marciscano AE, Drake CG, Vignali DA (2017) LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 276(1):80–96
Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M (2016) Immune checkpoint modulators: an emerging antiglioma armamentarium. J Immunol Res 2016:4683607
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486
Savitsky D, Ward R, Riordan C, Mundt C, Jennings S, Connolly J, Findeis M, Sanicola M, Underwood D, Nastri H (2018) INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Res 78(13):3819–3819
Brignone C, Escudier B, Grygar C, Marcu M, Triebel F (2009) A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res:1078–0432. CCR-1009–0068
Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Maillard SA, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D (2016) Vaccination with LAG-3Ig (IMP321) and peptides induces specific CD4 and CD8 T-cell responses in metastatic melanoma patients—report of a phase I/IIa clinical trial. Clin Cancer Res 22(6):1330–1340
Ruffo E, Wu RC, Bruno TC, Workman CJ, Vignali DA (2019) Lymphocyte-activation gene 3 (LAG3): the next immune checkpoint receptor. In: Seminars in immunology. Elsevier, Amsterdam, p 101305
Elia AR, Caputo S, Bellone M (2018) Immune checkpoint-mediated interactions between cancer and immune cells in prostate adenocarcinoma and melanoma. Front Immunol 9:1786
Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72(4):917–927
Lee J, Ahn E, Kissick HT, Ahmed R (2015) Reinvigorating exhausted T cells by blockade of the PD-1 pathway. In: Forum on immunopathological diseases and therapeutics, vol 1–2. Begel House Inc., New York
Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA (2013) OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 73(24):7189–7198
Rapoport BL, Anderson R (2019) Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci 20(4):959
Kovacsovics-Bankowski M, Chisholm L, Vercellini J, Crittenden M, Lary S, Curti B, Weinberg A (2013) Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. J Immunother Cancer 1(1):P255
Li G, Quan Y, Che F, Wang L (2018) B7–H3 in tumors: friend or foe for tumor immunity? Cancer Chemother Pharmacol 81(2):245–253
Ross A, Benzon B, Zhao S, Takhar M, Haffner M, Erho N, Hurley P, Tosoian JJ, Alshalalfa M, Glavaris S (2016) The relationship of B7H3 expression to androgen and prostate cancer outcomes in a large natural history cohort of men undergoing prostatectomy. American Society of Clinical Oncology, New York
Lee H-W, Park S-J, Choi BK, Kim HH, Nam K-O, Kwon BS (2002) 4–1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol 169(9):4882–4888
Chester C, Ambulkar S, Kohrt HE (2016) 4–1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunther 65(10):1243–1248
Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP (1997) 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 186(1):47–55
Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Chen H (2012) Effects of 4–1BB signaling on the biological function of murine dendritic cells. Oncol Lett 3(2):477–481
Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z (2012) Combination immunotherapy with 4–1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin Dev Immunol 2012:439235
Bartkowiak T, Curran MA (2015) 4–1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol 5:117
Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, Di Gravio D, Huang B, Gambhire D, Chen Y (2017) Phase Ib study of utomilumab (PF-05082566), a 4–1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res 23(18):5349–5357
Norström MM, Rådestad E, Sundberg B, Mattsson J, Henningsohn L, Levitsky V, Uhlin M (2016) Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget 7(17):23581
Benzon B, Zhao S, Haffner M, Takhar M, Erho N, Yousefi K, Hurley P, Bishop J, Tosoian J, Ghabili K (2017) Correlation of B7–H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis 20(1):28
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L-F, Gondek D, Wang Y, Fava RA (2011) VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 208(3):577–592
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B (2012) TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 7(2):e30676
Piao Y, Jin X (2017) Analysis of Tim-3 as a therapeutic target in prostate cancer. Tumor Biol 39(7):1010428317716628
Amatore F, Gorvel L, Olive D (2018) Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Target 22(4):343–351
Tang DN, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P (2013) Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 1(4):229–234
Li N, Xu W, Yuan Y, Ayithan N, Imai Y, Wu X, Miller H, Olson M, Feng Y, Huang YH (2017) Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep 7(1):1485
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L (2014) VISTA regulates the development of protective anti-tumor immunity. Cancer Res 74(7):1933–1944
Assal A, Kaner J, Pendurti G, Zang X (2015) Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy 7(11):1169–1186
Madan RA, Gulley JL (2017) Prostate cancer: better VISTAs ahead? Potential and pitfalls of immunotherapy. Nat Rev Urol 14(8):455
Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318(5853):1141–1143
Japp AS, Kursunel MA, Meier S, Mälzer JN, Li X, Rahman NA, Jekabsons W, Krause H, Magheli A, Klopf C (2015) Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol Immunther 64(11):1487–1494
Omar HA, Tolba MF (2019) Tackling molecular targets beyond PD-1/PD-L1: novel approaches to boost patients’ response to cancer immunotherapy. Crit Rev Oncol Hematol 135:21–29
Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Müller S (2016) The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res 22(8):1969–1977
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563
Fay AP, Antonarakis ES (2019) Blocking the PD-1/PD-L1 axis in advanced prostate cancer: Are we moving in the right direction? Ann Transl Med 7(Suppl 1):S7
Manogue C, Cotogno P, Ledet E, Lewis B, Wyatt AW, Sartor O (2018) Biomarkers for programmed death‐1 inhibition in prostate cancer. Oncologist 24(4):444–448
Haffner MC, Guner G, Taheri D, Netto GJ, Palsgrove DN, Zheng Q, Guedes LB, Kim K, Tsai H, Esopi DM (2018) Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am J Pathol 188(6):1478–1485
Fujii T, Naing A, Rolfo C, Hajjar J (2018) Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol 130:108–120
Song Y, Li Z, Xue W, Zhang M (2019) Predictive biomarkers for PD-1 and PD-L1 immune checkpoint blockade therapy. Immunotheraphy 11(6):515–529
Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA (2014) Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 106(11):268
Mougel A, Terme M, Tanchot C (2019) Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol 10:467
Li H, Wang Z, Zhang Y, Sun G, Ding B, Yan L, Liu H, Guan W, Hu Z, Wang S (2019) The immune checkpoint regulator PDL1 is an independent prognostic biomarker for biochemical recurrence in prostate cancer patients following adjuvant hormonal therapy. J Cancer 10(14):3102
FDA US Hematology/Oncology (Cancer) Approvals and safety notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Accessed 13 Sep 2019
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS (2015) Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348(6230):124–128
Antonarakis ES (2019) A new molecular taxonomy to predict immune checkpoint inhibitor sensitivity in prostate cancer. Oncol Theoncol. 2018–0819
Bilir C, Sarisozen C (2017) Indoleamine 2, 3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci 3(2):52–56
Banzola I, Mengus C, Wyler S, Hudolin T, Manzella G, Chiarugi A, Boldorini R, Sais G, Schmidli TS, Chiffi G (2018) Expression of indoleamine 2, 3-dioxygenase induced by iFn-γ and TnF-α as potential biomarker of prostate cancer progression. Front Immunol 9:1051
Badawy AA, Guillemin G (2019) The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2, 3-dioxygenase: time for appraisal. Int J Tryptophan Res 12:1178646919868978
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2, 3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210(7):1389–1402. https://doi.org/10.1084/jem.20130066
Moon YW, Hajjar J, Hwu P, Naing A (2015) Targeting the indoleamine 2, 3-dioxygenase pathway in cancer. J Immunother Cancer 3(1):51
Acknowledgements
This work is part of a dissertation (No. 128) by S. Jafari, submitted for Ph.D. degree and supported by Biotechnology Research Center, Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafari, S., Molavi, O., Kahroba, H. et al. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell. Mol. Life Sci. 77, 3693–3710 (2020). https://doi.org/10.1007/s00018-020-03459-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03459-1