Abstract
Horticulture productivity has been increasingly restricted by heat stress from growing global warming, making it far below the optimum production capacity. As a popular ornamental cultivar of tree peony, Paeonia suffruticosa ‘Yu Hong’ has also been suffering from heat stress not suitable for its optimal growth. To better understand the response mechanisms against heat stress of tree peony, investigations of phenotypic changes, physiological responses, and quantitative proteomics were conducted. Phenotypic and physiological changes indicated that 24 h of exposure to heat stress (40 °C) was the critical duration of heat stress in tree peony. The proteomic analyses revealed a total of 100 heat-responsive proteins (HRPs). According to bioinformatic analysis of HRPs, the heat tolerance of tree peony might be related to signal transduction, synthesis/degradation, heat kinetic proteins, antioxidants, photosynthesis, energy conversion, and metabolism. Our research will provide some new insights into the molecular mechanism under the response against the heat stress of tree peony, which will benefit the future breeding of heat-resistant ornamental plants.
Similar content being viewed by others
Introduction
Global temperatures have been rising in recent decades mainly due to the rapid accumulation of greenhouse gas in the atmosphere [1], making the higher temperature key to plant growth and development as an essentially abiotic limited factor [2, 3]. Tree peony (Paeonia suffruticosa, Paeoniaceae) is a deciduous shrub widely distributed around the world [4, 5]. As an excellent ornamental plant, tree peony has been adored by people for its elegant appearances and gorgeous colors, and even awarded the ‘king of flowers’ in China [6,7,8,9]. In cool climates, tree peony can grow vigorously under trees and along riverbanks. However, temperatures over 26 °C in the summer will interrupt its normal growth, which severely limits their use as ornamental plants. Therefore, improving new tree peony cultivars with heat-resistance is of great importance for ornamental breeding.
How serious the plant is affected under certain stress can be accurately reflected by some physiological and biochemical indices. Previous research has shown that plants respond to external stress by enhancing antioxidant enzyme activities, like peroxidase and superoxide dismutase, to remove reactive oxygen species (ROS) from the body and weaken lipid oxidation in the cell membrane [10, 11], as well as the synthesizing osmoregulatory substances like proline and soluble protein to maintain the water balance inside and outside the cell [12, 13]. Furthermore, the level of malondialdehyde and relative conductivity can be used to characterize the severity of cell damage [14, 15]. However, most research regarding tree peony heat tolerance focus on physiological and biochemical changes, while few are on molecular biology. A lack of understanding of the molecular mechanism of tree peony heat tolerance makes it necessary to perform molecular investigations on its heat stress response.
Proteins are critical components of cellular responses, and changes of proteomic facing stress can reveal how plants respond to stress [16, 17]. Proteomics has become a cornerstone of systematic biology and are effective in addressing numerous biological problems in recent decades along with the development of protein sequencing technology [1, 18]. Isobaric tags for relative and absolute quantification (iTRAQ) together with tandem mass spectrometry (MS/MS) has been developed as a superior choice for precise quantification of diverse proteins, especially the lowly expressed proteins [19,20,21]. For example, iTRAQ combined with MS/MS was used to investigate differentially expressed proteins associated with shoot dormancy release in tree peony under low-temperature treatment [6]; similarly iTRAQ combined with MS/MS was used to investigate the molecular mechanism of hybridization incompatibility in tree peony and its relatives [22]. However, to our knowledge, there have been no reports on the use of proteomic approaches to evaluate heat tolerance in tree peony.
Leaves are the principal trophic organs that directly sense temperature changes in the environment and have phenotypic plastic responses to such changes [23]. Temperature fluctuations also affect leaf photosynthesis and transpiration, which are the foundations of plant growth and development [24]. As a result, research focusing on the impacts of high temperatures on leaves will benefit the understanding how plants respond to heat stress. Herein, the iTRAQ-MS/MS and corresponding biological analyses to characterize the proteomic changes of the heat-tolerant tree peony ‘Yu Hong’ leaves under heat stress, and to reveal the responsive mechanism of tree peony under heat stress at the protein level, and to provide a theoretical basis for the future cultivation of new heat-tolerant tree peony germplasm.
Material and methods
Plant materials
Tree peony seeds were collected from the National Color Family Farm in Jinhua, Zhejiang Province, and the annual live seedlings of Jiangnan tree peony cultivar ‘Yu Hong’ with high heat tolerance were used in this study.
The seeds were dried naturally indoors after sampling, soaked in water for 24 h, plump seeds were chosen to be sterilized with 0.5 percent potassium permanganate solution, and sowed in the Zhejiang A & F University base in September 2018. Selected seedlings with steady growth patterns were transferred into pots with a diameter of 50 cm in March 2019. Each pot contained one plant. Routine management was conducted during the slow seedling stage.
Seedling stress treatment
Live seedlings with good uniformity and growth were chosen and separated into two groups: control (ambient treatment at 25 °C) and experimental (high temperature treatment at 40 °C). Four treatment gradients (0, 12, 24, and 36 h) were set in the control group and the experimental group respectively, and three biological replicates were set in each gradient, a total of 24 pots of plant materials. After 0, 12, 24, and 36 h of treatment, the leaf tissues were collected, marked, and rapidly placed in liquid nitrogen for subsequent measurement of the physiological indicators and protein extraction. During the experiment, the air humidity in the incubator was set to 80%, the light intensity was set to 4000 LX, and the light time was set to 12 h of light and 12 h of darkness.
Measurement of physiological parameters in seedlings
The acidic ninhydrin colorimetric technique was used to determine the content of proline (Pro) [25, 26]. The guaiacol method was used to determine the content of peroxidase (POD) [27]. The thiobarbituric acid (TBA) method was used to determine the content of malondialdehyde (MDA) [28]. The nitrogen blue tetrazolium (NBT) photochemical reduction method was used to measure the content of superoxide dismutase (SOD) [29]. The Coomassie bright blue technique was used to determine the content of soluble protein SP [30]. The relative electric conductivity (REC) of leaves after various treatments can be measured using a conductivity meter (DDS-307A, Shanghai Yoke Instrument Co., Ltd.). REC is equal to (S1/S2) 100 after measuring the initial electrolyte leakage (S1), heating the sample to 100℃, and measuring the final electrolyte leakage (S2) at room temperature.
Protein extraction, digestion, iTRAQ labeling, and data analysis
Protein extraction was performed according to the trichloro-acetic acid/acetone precipitation method [31] for the three materials under each treatment, without mixing. Samples were ground into powder in liquid nitrogen and dissolved in lysis buffer (pH = 8) containing 8 mol/L urea, 0.2% SDS, and 50 mmol/L Tris–HCl, followed by 5 min of ultrasonication on ice. The lysate was centrifuged at 13 000 r/min for 20 min at 4 °C and the supernatant was transferred to a clean tube. Extracted proteins were reduced with 2 mmol/L DTT for 1 h at 55 °C and subsequently alkylated with sufficient iodoacetic acid for 1 h at 25 °C. Then samples were mixed with 5 volumes of precooled acetone by vortexing and incubated at –20 °C for 3 h. Samples were then centrifuged and the precipitation was collected. After washing twice with cold acetone, the pellet was dissolved by dissolution buffer (pH = 8.5) containing 8 mol/L urea and 0.1 mol/L TEAB. Total protein concentration was determined by the Bradford protein assay [32].
Enzymatic digestion of trypsin to sample protein at a 20:1 ratio. After 15 h of digestion at 37 °C, peptides were desalted with a C18 cartridge to remove high-concentration urea. The six labeling reagents iTRAQ115, iTRAQ116, iTRAQ117, iTRAQ118, iTRAQ119, and iTRAQ121 of the iTRAQ reagent-8plex complex kit (Sigma, USA) were used to label the experimental (TE1, TE2, TE3) and control (CK1, CK2, CK3) desalting peptides, respectively, according to the manufacturer's instructions. The labeling reagent and peptides were dissolved with 70 µL of isopropanol and 20 µL of 0.5 mol/L TEAB, respectively. One unit of labeling reagent was used for 100 μg of peptides. After incubation for 1 h at room temperature, the reaction was stopped with 100 µL of 50 mmol/L Tris–HCl (pH = 8). Individually iTRAQ-labeled peptides were mixed in equal volumes, and then desalted and lyophilized. iTRAQ-labeled peptide mix was fractionated using a XB ridge Peptide BEH C18 column (25 cm × 4.6 mm, 5 µm) on a L3000 HPLC Cperating System at a rate of 1 mL/min; the column oven was set as 50 °C. Buffer A (2% acetonitrile, pH = 10) and B (98% acetonitrile, pH = 10) were used to develop a gradient for elution. The solvent gradient was set as follows: 0–3 min, 100% A, 0% B; 3–21 min, 95% A, 5% B; 21–27 min, 80% A, 20% B; 27–33 min, 70% A, 30% B; 33–58 min, 0% A, 100% B. Based on the peak pattern, 12 fractions were chosen. All fractions were lyophilized and reconstituted in 0.1% formic acid. Proteomics analyses were processed using an EASY-nLCTM 1200 UHPLC system (ThermoFisher) coupled with a Q Exactive HF-X mass spectrometer (ThermoFisher). A total of 2 µg of total peptides reconstituted in 0.1% formic acid was injected onto an Acclaim PepMap100 C18 NanoTrap column (100 µm × 2 cm, 5 µm). Then peptides were separated on a Reprosil-Pur 120 C18-AQ analytical column (150 µm × 15 cm, 1.9 µm), at a flow rate of 600 nL/min with a 5–100% linear gradient of eluent B (0.1% formic acid in 80% acetonitrile) in eluent A (0.1% formic acid in H2O). The detailed solvent gradient listed as follows: 10% B, 90% A, 2 min; 30% B, 70% A, 49 min; 50% B, 50% A, 2 min; 90% B, 10% A, 2 min; 100% B, 5 min.
Q Exactive HF-X mass spectrometer was operated in positive polarity mode with a capillary temperature of 320 °C and spray voltage of 2.3 kV. Full MS scans ranging from 350 to 1500 m/z were acquired at a resolution of 60 000 (at 200 m/z) with a maximum ion injection time of 20 ms and an automatic gain control target value of 3 × 106. The 40 most abundant precursor ions from the full MS scan were selected for fragmentation using higher energy collisional dissociation fragment analysis at a resolution of 15,000 (at 200 m/z) with a normalized collision energy of 32%, an automatic gain control target value of 1 × 105, an intensity threshold of 8.3 × 103, the dynamic exclusion parameter of 60 s and a maximum ion injection time of 45 ms.
Using the search engines, the obtained spectra from each fraction were individually compared to the Uniprot Oryza sativa Database from uniprotOryzasativa. 2.1 of Proteome Discoverer (PD 2.1, ThermoFisher, USA). On the peptide and protein levels, a protein was recognized as a protein having at least one distinct peptide (FDR ≤ 0.01), respectively. Different protein groups were created from proteins with similar peptide compositions that could not be distinguished based on an MS/MS study. During the search, iTRAQ quantification was done using iTRAQ 8-plex. The protein quantitation results were statistically analyzed by t-test, and the significant ratios, defined as P < 0.05 and fold change (FC) ≥ 1.5 or ≤ 0.67, were considered heat-responsive proteins (HRPs).
Bioinformatics analysis
The sequences of HRPs were compared with three public databases: KEGG (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg), COG (Clusters of Orthologous Groups of proteins, http://www.ncbi.Nlm.nih.gov/COG), GO (Gene Ontology, http://geneontology.org/). The Protein Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used for sequence similarity comparison.
Statistical analysis
One-way analysis of variance (ANOVA) with Duncan’s multiple range tests was conducted on the data, and p < 0.05 was considered significant. t-test analysis on protein quantitative results, p < 0.05 was considered significant. All the above analyses were accomplished with the IBM SPSS Statistics 21.0 software package (IBM Corporation, Armonk, NY, USA). All figures were drawn with the Origin 21.0 software (OlriginLab Co., Northampton, MA, USA).
Results
Phenotype and physiological responses to heat stress
To investigate the critical time of heat stress treatment for ‘Yu Hong’, phenotypic and physiological indices were recorded at 0, 12, 24, and 36 h of after heat treatment, respectively.
The morphology of ‘Yu Hong’ leaves changed significantly as the high temperature treatment duration was extended. Compared to treatment 0 h (Fig. 1A), slight brown spots appeared on the edge of the leaves after 12 h of heat stress (Fig. 1B), The brown spots on the edge of leaves increased significantly after 24 h of heat stress compared to 12 h, and the stems and leaves were slightly yellowed (Fig. 1C). The seedlings developed much worse and the leaves showed obvious heat damage symptoms after 36 h of heat stress, with browning of the leaf edges, curling and crinkling of the leaves, narrowing of the leaf area, and plants on the verge of withering (Fig. 1D).
The SOD activity, POD activity, proline content, and SP of ‘Yu Hong’ leaves showed a trend of increasing and then decreasing with the extension of treatment time, MDA content was gradually increasing, and the REC showed a trend of increasing and then stabilizing under heat stress. Among them, SOD activity increased significantly after 24 h of heat stress, then recovered to the treatment 0 level after 36 h. After 12 h of high temperature treatment, POD activity and SP content began to rise dramatically, peaked after 24 h, and then recovered to pre-treated levels after 36 h. After 12 h of high temperature treatment, proline content grew considerably, peaked after 24 h, and then recovered to pre-treatment levels after 36 h. After 24 h of high temperature treatment, the MDA level began to rise rapidly, peaking at 36 h. After 24 h of treatment, the REC peaked and remained steady as the treatment time was extended (Fig. 2).
Changes of physiological parameters of ‘Yu Hong’ leaves with treatment time. The physiological parameters include superoxide dismutase content (A), peroxidase content (B), proline content (C), malondialdehyde content (D), soluble protein content (E), and the relative electric conductivity (F). Different letters indicate a significant difference (p < 0.05) using Duncan’s multiple range test
Responses of HRPs to heat stress
According to the above changes of leaf phenotype and leaf physiological parameters of ‘Yu Hong’, 24 h of heat stress was the critical duration of ‘Yu Hong’ in response to heat stress. So, we chose the material after 24 h of high temperature treatment for further iTRAQ studies.
iTRAQ screening revealed a total of 1916 proteins. Compared to normothermic control samples, a total of 100 HRPs were identified under heat stress (Fig. 3).
Functional analysis of HRPs in response to heat stress
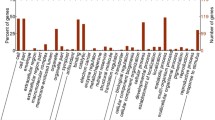
Of the 100 HRPs, 79 HRPs were categorized into 16 categories using COGs database. The largest group was Posttranslational modification, protein turnover, chaperones, followed by Amino acid transport and metabolism, Lipid transport and metabolism, Coenzyme transport and metabolism, Secondary metabolites biosynthesis, transport and catabolism, General function prediction only, Translation, ribosomal structure and biogenesis, Energy production and conversion and Carbohydrate transport and metabolism (Fig. 4).
To further characterize all heat-responsive proteins, these HRPs identified were subjected to GO analysis. Of the 100 HRPs identified under heat stress, 74 HRPs were annotated into three groups as cellular component (159), molecular function (98), and biological process (132). GO analysis showed that cell (34), cell part (33), and organelle (27) were the major cellular component terms; catalytic activity (45) and binding (44) were the dominant molecular function terms; and metabolic process (41), cellular process (40), and single-organism process (25) were the most dominant biological process terms (Fig. 5).
To further investigate the major metabolic pathways responding to heat stress, KEGG enrichment analysis was carried out with HRPs. KEGG pathway enrichment showed that 42 HRPs were annotated into 33 pathways. The protein processing pathway in endoplasmic reticulum is the most, with 22 HRPs (Fig. 6).
Through the similarity comparison of the amino acid sequences of heat response proteins through the Protein Blast tool of the NCBI online website, the 100 HRPs were classified into eight categories, i.e., a) protein synthesis/degradation (14), b) heat shock protein (23), c) defense and antioxidants (8), d) energy production and conversion (9), e) signal transduction (8), f) photosynthesis (7), g) metabolism (13), and h) other unknown functional HRPs (18) (Fig. 7). Details of HRPS can be found in Addendum 1.
All 23 HRPs classified as heat shock proteins were up-regulated. Among the 14 HRPs involved in protein synthesis, processing and degradation, 9 were up-regulated and 5 were down-regulated. There were 5 up-regulated and 3 down-regulated HRPs in those involved in antioxidants. Among the 9 HRPs involved in energy conversion, 5 were up-regulated and 4 were down-regulated. Among 7 HRPs involved signal transduction, 4 were up-regulated and 3 were down-regulated. In metabolism there were 3 up-regulated and 10 down-regulated HRPs. As to the 18 HRPs with unknown functions, 2 were up-regulated, and 16 were down-regulated (Fig. 7).
Heat stress response model
Based on the results of proteomic analyses, we comprehensively identified the key HRPs of tree peony and proposed a heat response model of proteins in tree peony (Fig. 8). HRPs were divided into three levels in this model. The heat response protein functions were divided into four groups on the first level (blue) including signal transduction, energy conversion and metabolism, protein synthesis/degradation, and defense. The second level (Orange) was a further division of energy conversion and metabolism, and defense, among which the heat stress proteins and antioxidants were under defense while photosynthesis, energy conversion, and metabolism under energy and metabolism. The heat response proteins that corresponded to each functional pathway were the third level (green) in the model.
The model of tree peony response to heat stress. Cal, Calmodulin; CDPK, Calcium-dependent protein kinase; ABC, ABC transporter family member; LHCB, Chlorophyll a / b binding protein; NDH, NADH dehydrogenase; FtsH, FtsH protease; cytP450, Cytochrome P450; CS, Citrate synthase; FBA, Fructose- 1,6- bisphosphate aldolase; MDH, Malate dehydrogenase; Cox, Cytochrome C oxidase; GS, Glutamine Synthetase; PAL, Phenylalanine ammonia lyase; ACC, Acetyl-CoA catablerboxylase; sHSP, Small heat shook protein; HSP70, Hsp70 family protein; HSP90, Hsp90 family protein; POD, Peroxidase; APX, Ascorbate peroxidase; GPX, Glutathione peroxidase; CAT, Catalase; AaAER, 2-alkenal reductase; 40S r-protein, 40S Ribosomal protein; 60S r-protein, 60 s ribosomal protein L26-1; ClpB, Chaperone-protein ClpB; PPI, Peptidylprolyl isomerase; E2-17Ka, Ubiquitin-binding enzyme E2-17Ka; UBL, Ubiquitin-like family protein
Discussion
Heat stress will cause an imbalance between ROS generation and antioxidant defenses, resulting in an enrichment of ROS in plants, which will damage plant cells and even inhibit plant growth and secondary metabolite induction [33, 34]. MDA is a lipid peroxidation product that is considerably elevated if the lipids in plant cells are damaged [35]. Pro, SP, POD, SOD, and REC are also crucial indicators of cell oxidative damage, with higher values indicating that the plant is under more severe stress [21]. Here, compared to 12 h of heat stress, tree peony leaves were on the verge of wilting at 24 h of heat stress (Fig. 1), while leaves were already browning and wilting at 36 h of heat stress (Figs. 2A, B). More importantly, all physiological indicators except malondialdehyde reached their highest levels after 24 h of heat stress, and the malondialdehyde content started to increase significantly after 24 h of heat stress. This shows that 24 h is the optimum duration of treatment for tree peony ‘Yu Hong’. The results of the proteomic analysis of the heat response under 24 h of heat stress will be discussed further below.
HRPs involved in signal transduction
Stress perception, signal transduction, and the expression of certain stress-related genes and metabolites all play a role in plant adaptation to environmental stress [36]. Signal transduction is a complex regulatory system in plants that plays an important role in sustaining their normal growth and development in response to abiotic stresses [37, 38]. The importance of Ca2+ as a second messenger in the signal transduction pathway is well understood [39]. Abiotic stress causes a transitory rise in cytoplasmic Ca2+, either through exosomal inward flow or the release of internal reserves [40]. Calmodulin is the most well-known Ca2+ sensor in signal transduction, and it is involved in heat shock signaling [37]. Calcium-dependent protein kinase is a serine/threonine protein kinase with c-terminal calmodulin-like structural domain at its c-terminus, making it both a Ca2+ sensor and an effector of Ca2+ signal transduction [39, 40]. The fact that these two proteins (c109749.graph_c1, c119803.graph_c0) are up-regulated in our study shows that they are involved in signal transduction mechanisms in response to heat stress.
Ca2+ signaling is involved in the transport of membrane proteins and vesicles in plant cells, in addition to directing ion transport [41]. Vesicular transport is well established as a key mode of communication between organelles in cells. Most of the vesicular transport is mediated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), and highlight fusion protein is a subfamily of SNAREs proteins [42,43,44]. ABC transporter family member is also a widely distributed transporter protein in plants, and ABC functions have been shown to be involved in signal transduction for physiological processes such as hormone transport, pathogen resistance, abiotic stressors, and detoxifying [45, 46]. The fact that these two proteins (c116136.graph_c0, c113074.graph_c1) up-regulated in our study shows that they were also involved in signal transduction mechanisms in response to heat stress.
HRPs involved in protein synthesis/degradation
Protein synthesis is inhibited by heat stress [17]. Plant responses to environmental stress rely heavily on maintaining a balance between intracellular protein production and breakdown [47]. A variety of proteins involved in protein synthesis were shown to be down-regulated in this study, including 1 aspartate protease family protein (c117236.graph_c1), 1 trigger factor protein (c108032.graph_c0), 1 peptidine family protein (c117276.graph_c1), 1 subtilisin-like serine protease (c92427.graph_c0), and 1 protein disulfide isomerase (c112740.graph_c0). Interestingly, many proteins were also up-regulated. Ribosomes is a huge macromolecular ribosomal protein that catalyze protein synthesis in cells and play a vital role in cell growth, development, and differentiation [21]. The effectiveness and stability of the ribosome are directly influenced by the expression of ribosomal proteins [48]. In this study, we identified 1 40S ribosomal protein (c116290.graph_c3) and 1 60 s ribosomal protein L26-1 (c110207.graph_c0) up-regulation. We also discovered the up-regulated expression of 2 chaperone-protein ClpB (c103417.graph_c0, c118249.graph_c1) and 3 peptidylprolyl isomerase (c115415.graph_c1, c111758.graph_c0, c106105.graph_c0). The most immediate hazard of heat stress is the misfolding of proteins, which causes loss of function and consequently harm [47]. So, the elimination of misfolded proteins is very important for protease stability and prevention of protein lesions [49]. The elimination of misfolded proteins is essential for protease stabilization and prevention. PPI, a protein folding enzyme that catalyzes peptidyl proline cis–trans-isomerization, may also be involved in protein complex construction and disassembly, protein transport, and protein activity control [2, 47]. ClpB is a chaperone protein found in bacteria, fungi and plants that cooperate with other chaperone proteins to rescue misfolded proteins that have aggregated—an activity that helps cells survive heat shock and other stresses [50]. Furthermore, we discovered that the expression of 1 Ubiquitin-binding enzyme E2-17Ka (c104797.graph_c0) and 1 Ubiquitin-like family protein (c93677.graph_c0) involved in the ubiquitination process had increased. The ubiquitination process is a crucial step in the selective degradation of intracellular proteins [49, 51, 52]. Increased expression of these proteins helps maintain the balance between protein production and degradation while also enhancing thermotolerance.
HRPs involved in stress defense
Heat shock protein
Plants need molecular chaperones to prevent the creation of misfolded proteins and maintain intracellular equilibrium in stressful situations [53]. HSPs are a type of molecular chaperone protein that helps plants protect themselves from stress and adapt to environmental changes [21, 54]. sHSP, HSP70 and HSP90 were all subsets of HSPs [55, 56]. Many studies have found that sHSP, HSP70, and HSP90 production was linked to plant heat tolerance, and that overexpression improves plant heat tolerance [57,58,59,60]. Our research found that all 23 HSPs discovered in the study, including 14 sHSP (c107551.graph_c0, c117526.graph_c2, c120033.graph_c1, c112621.graph_c0, c117374.graph_c1, c112949.graph_c1, c104422.graph_c0, c100675.graph_c0, c116391.graph_c0, c92835.graph_c0, c110265.graph_c0, c94118.graph_c0, c114184.graph_c1, c114184.graph_c0), 6 HSP70 (c119976.graph_c0, c65824.graph_c0, c110893.graph_c1, c17729.graph_c0, c112482.graph_c0, c114151.graph_c1), and 3 HSP90 (c101826.graph_c1, c119657.graph_c2, c120096.graph_c0), were upregulated. They clearly perform a crucial role as chaperone proteins in the tree peony's heat stress response.
Antioxidants
The equilibrium between the generation and scavenging of ROS is frequently disrupted by heat stress, which stimulates the formation of ROS, which can damage membrane systems and other cellular components [17]. Plants have evolved efficient enzymatic antioxidant systems to deal with the peroxidative damage of ROS during their long-term adaptive evolution [61]. POD (c100066.graph_c1), APX (c115075.graph_c0), GPX (c114484.graph_c0), and CAT (c116374.graph_c0), all of which are enzymatic antioxidants of the enzymatic antioxidant system, were among the up-regulated antioxidant enzymes in this study [23, 37, 38]. In response to heat stress, up-regulated production of these proteins is necessary for scavenging ROS.
Excess ROS can cause lipid oxidation, resulting in highly reactive lipid peroxidation-derived molecules like 4-hydroxy-2-nonenal, 4-hydroxy-2-hexenal, malondialdehyde, and acrolein, which are known as reactive carbonyl species (RCS) [62]. RCS in high quantities can cause irreversible cell damage and even death [63]. As a result, cellular harm might result from poor RCS clearance of reactive carbonyl molecules. We found 1 up-regulated 2-alkenal reductase (c118463.graph_c0), which detoxifies lipid-derived RCS by catalyzing the conversion of unsaturated bonds (C = C) to saturated bonds and contributes to plant cellular detoxification [64, 65].
HRPs involved in energy and metabolism
Photosynthesis
Photosynthesis is the most basic physiological process for plant growth and development, as well as the most important source of energy for plant metabolism [38, 66, 67]. Photosynthesis is frequently regarded as the most thermosensitive physiological process due to its intricate molecular mechanism and the requirement for several enzymes to participate in its regulation [17, 21, 68]. When the external temperature exceeds the optimal adaption range of plants, photosynthesis diminishes, and this decline is linked to the suppression of the RuBisCO-activating enzyme [38]. Our findings support this conclusion since we discovered 2 down-regulated Rubisco. Interestingly, 1 chlorophyll a / b binding protein (c96149.graph_c0) [69], 1 NADH dehydrogenase (c116330.graph_c0) [70], 1 FtsH protease (c108824.graph_c0) [71], and 1 cytochrome P450 (c118450.graph_c0) [72] involved with the positive regulation of photosynthesis were also found and up-regulated in this study. We hypothesize that this is a long-evolved approach adopted by plants to cope with environmental stress, with the primary goal of maintaining photosynthetic efficiency and avoiding energy imbalances produced by stress.
Energy conversion
Respiration, the main metabolic pathway metabolism of carbohydrates, is an enzymatic oxidation reaction and is an important pathway for energy conversion in plants [37]. In this study, 1 citrate synthase (c104351.graph_c0), 1 fructose- 1,6- bisphosphate aldolase (c41383.graph_c0), 1 phosphoglycerate kinase (c117676.graph_c3) and 1 Malate dehydrogenase (c112840.graph_c0) were up-regulated, and both of them are important regulatory enzymes in glycolysis pathway and tricarboxylic acid cycle pathway in carbohydrate metabolism [73]. Promoting energy conversion is also an important measure to cope with heat stress. Furthermore, 1 up-regulated cytochrome C oxidase (c119073.graph_c0) was discovered in this study, which is found in the electron transport system of mitochondria and generates two molecules of water by transferring electrons to O2−. Because mitochondria, but not chloroplasts, create ROS, defects or shortages in this enzyme can be lethal to plants [74, 75]. This finding shows that shielding mitochondria from oxidative damage produced by heat stress can aid in heat stress management.
Metabolism
Heat stress, even for a short period of time, may alter plant metabolism, which in turn may affect plant responses to stress [76, 77]. Because nitrogen is a fundamental component of amino acid composition and serves a variety of other activities, it is an important factor in influencing plant development and stress resistance [78]. In this study, 1 glutamine Synthetase (c111030.graph_c0) and 1 phenylalanine ammonia lyase (c117414.graph_c0), both involved in nitrogen metabolism, were shown to be up-regulated [79]. This could be because under heat stress, inactive nitrogen metabolism can stifle amino acid synthesis, and a lack of specific amino acids can cause plant development to be thermally inhibited [78]. As a result, increasing nitrogen metabolism can aid in plant heat stress tolerance.
Fatty acids are typical ester compounds in plants that are classified as saturated or unsaturated depending on whether they include unsaturated bonds (C = C). Many studies have found that fatty acid desaturation is a key determinant of heat tolerance, possibly because unsaturated fatty acids may eliminate ROS and harmful chemicals while also improving cell membrane stability [80,81,82]. We discovered 1 up-regulated acetyl-CoA carboxylase (c117871.graph_c2) in our work, which is the initiator and key to regulate fatty acid biosynthesis through a series of condensation, dehydration and reduction reactions that can eventually desaturate or lengthen to produce a variety of different fatty acids for a variety of biological processes [83]. Thus, ACC helps to regulate fatty acid metabolism in response to heat stress.
Conclusion
In this work, to uncover the molecular mechanisms underlying heat resistance in the tree peony cultivar ‘Yu Hong’ treated with normal and high temperature, physiological and proteomic changes were explored. A total of 100 differentially expressed proteins were identified as HRPs. These HRPs were found to be involved in signal transduction, protein synthesis/degradation, heat shock proteins, antioxidants, photosynthesis, energy conversion, and metabolism, according to bioinformatics study. Based on proteome changes, a model of tree peony response to heat stress was developed. In addition, other differentially expressed proteins with unknown functions were detected in this work, and their functions need to be confirmed in the future. Overall, our research provides valuable insights into the molecular mechanism of heat tolerance of tree peony, and may provide a useful reference for cultivating new varieties of heat resistant tree peony.
Availability of data and materials
The proteomics data used to support the findings of this study are available from the corresponding author upon request.
References
Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH. A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics. 2007;7:3369–83.
Li WM, Wei ZW, Qiao ZH, Wu ZN, Cheng LX, Wang YY. Proteomics aalysis of alfalfa response to heat stress. PLoS ONE. 2013;8:e82725.
Zhang YY, Xu L, Zhu XW, Gong YQ, Xiang F, Sun XC, Liu LW. Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.). Plant Mol Biol Rep. 2013;31:195–203.
He JY, Dong YQ, Liu XY, Wan YL, Gu TW, Zhou XF, et al. Comparison of chemical compositions, antioxidant, and anti-Photoaging activities of Paeonia suffruticosa flowers at different flowering stages. Antioxidants. 2019;8:345.
Guo LL, Guo DL, Yin WL, Hou XG. Tolerance strategies revealed in tree peony (Paeonia suffruticosa; Paeoniaceae) ecotypes differentially adapted to desiccation. Appl Plant Sci. 2018;6:e1191.
Zhang YX, Tian XL, Liu CY, Gai SP, Zheng GS. Differential expression proteins associated with bud dormancy release during chilling treatment of tree peony (Paeonia suffruticosa). Plant Biol. 2015;17:114–22.
Lv SZ, Cheng S, Wang ZY, Li SM, Jin X, Lan L, et al. Draft genome of the famous ornamental plant Paeonia suffruticosa. Ecol Evol. 2019;10:4518–30.
Gu ZY, Zhu J, Hao Q, Yuan YW, Duan YW, Men SQ, et al. A novel R2R3-MYB transcription factor contributes to petal blotch formation by regulating organ-specific expression of PsCHS in tree peony (Paeonia suffruticosa). Plant Cell Physiol. 2018;60:599–611.
Wang SL, Ren XX, Xue JQ, Xue YQ, Cheng XD, Hou XG, et al. Molecular characterization and expression analysis of the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE gene family in Paeonia suffruticosa. Plant Cell Rep. 2020;39:1425–41.
Ren RF, Li ZD, Zhang LL, Zhou H, Liu Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell, Tissue Organ Cult. 2021;144:233–46.
Zhang LX, Chang QS, Hou XG, Chen SD, Li S. Biochemical and photosystem characteristics of wild-type and Chl b-deficient mutant in tree peony (Paeonia suffruticosa). Photosynthetica. 2021;59:256–65.
Rakhmankulova ZF, Shuyskaya EV, Voronin PY. Different ways of maintaining water balance in leaves in two populations of C4 Atriplex tatarica differing in productivity and drought tolerance. Russ J Plant Physiol. 2021;68:1143–51.
Wang JY, Yuan B, Xu Y, Huang BR. Differential responses of amino acids and soluble proteins to heat stress associated with genetic variations in heat tolerance for hard fescue. J Am Soc Hortic Sci. 2018;143:45–55.
Yusuf CS, Chand R, Mishra VK, Joshi AK. The association between leaf malondialdehyde and lignin content and resistance to spot blotch in wheat. J Phytopathol. 2016;164:896–903.
Zhou BB, Sun J, Liu SZ, Jin WM, Zhang Q, Wei QP. Dwarfing apple rootstock responses to elevated temperatures: A study on plant physiological features and transcription level of related genes. J Integr Agric. 2016;15:1025–33.
Dipanjana G, Lin QS, Xu J, Hellmann HA. Editorial: How plants deal with stress: Exploration through proteome investigation. Front Plant Sci. 2017;8:1176.
Shi JZ, Chen YT, Xu Y, Ji DH, Chen CS, Xie CT. Differential proteomic analysis by iTRAQ reveals the mechanism of Pyropia haitanensis responding to high temperature stress. Sci Rep. 2017;7:44734.
Whitelegge JP, Komatsu S, Jorrin-Novo JV. Diverse facets of plant proteomics. Phytochemistry. 2011;72:961–2.
Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–69.
Ge P, Hao PC, Cao M, Guo GF, Yan YM. iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways of wheat seedling growth under hydrogen peroxide stress. Proteomics. 2013;13:3046–58.
Guo LP, Gu ZX, Jin XL, Yang RQ. iTRAQ - based proteomic and physiological analyses of broccoli sprouts in response to the stresses of heat, hypoxia and heat plus hypoxia. Plant Soil. 2017;414:355–77.
He D, Lou XY, He SL, Lei YK, Lv BV, Wang Z, et al. Isobaric tags for relative and absolute quantitation-based quantitative proteomics analysis provides novel insights into the mechanism of cross-incompatibility between tree peony and herbaceous peony. Funct Plant Biol. 2019;46:417–27.
Wang L, Ma KB, Lu ZG, Ren SX, Jiang HR, Cui JW, et al. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020;20:86.
Way DA, Yamori W. Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth Res. 2014;119:89–100.
Zdunek-Zastocka E, Grabowska A, Michniewska B, Orzechowski S. Proline concentration and its metabolism are regulated in a leaf age dependent manner but not by abscisic acid in pea plants exposed to cadmium stress. Cells. 2021;10:946.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7.
Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80.
Heath RL, Packer L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98.
Giannopolites CN, Ries SK. Superoxide dismutase occurrence in higher plants. Plant Physiol. 1977;59:309–14.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Foline phenol reagent. J Biol Chem. 1951;193:265–75.
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, et al. Proteomic analysis of arabidopsis seed germination and priming. Plant Physiol. 2001;126:835–48.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6:1720–31.
Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513–23.
Christou A, Manganaris GA, Fotopoulos V. Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ Exp Bot. 2014;107:46–54.
Huang GT, Ma SL, Bai LP, Li Z, Hui M, Jia P, et al. Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep. 2012;39:969–87.
Wang J, Liang C, Yang S, Song J, Zou X. iTRAQ-based quantitative proteomic analysis of heat stress-induced mechanisms in pepper seedlings. PeerJ. 2021;9:e11509.
Abreu IA, Farinha AP, Negrao S, Goncalves N, Fonseca C, Rodrigues M, et al. Coping with abiotic stress: Proteome changes for crop improvement. J Proteomics. 2013;93:145–68.
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell. 2002;14:S389–400.
Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Curr Sci. 2005;88:1771–80.
Sokolovski S, Hills A, Gay RA, Blatt MR. Functional interaction of the SNARE protein NtSyp121 in Ca2+ channel gating, Ca2+ transients and ABA signalling of stomatal guard cells. Mol Plant. 2008;1:347–58.
Zhang B, Wang H, Zhang YX. SNARE proteins and their role in plant ion channel regulation. Plant Growth Regul. 2020;92:443–53.
Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–6.
Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–66.
Geisler M, Hegeds T. A twist in the ABC: regulation of ABC transporter trafficking and transport by FK506-binding proteins. FEBS Lett. 2020;594:3986–4000.
Aryal B, Laurent C, Geisler M. Learning from each other: ABC transporter regulation by protein phosphorylation in plant and mammalian systems. Biochem Soc Trans. 2015;43:966–74.
Li JJ, Nadeem M, Chen LY, Wang MH, Wan MY, Qiu LJ, et al. Differential proteomic analysis of soybean anthers by iTRAQ under high-temperature stress. J Proteomics. 2020;229:103968.
Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–51.
Fang NN, Zhu M, Rose A, Wu KP, Mayor T. Deubiquitinase activity is required for the proteasomal degradation of misfolded cytosolic proteins upon heat-stress. Nat Commun. 2016;7:12907.
Carroni M, Kummer E, Oguchi Y, Wendler P, Clare DK, Sinning I, et al. Head-to-tail interactions of the coiled-coil domains regulate ClpB activity and cooperation with Hsp70 in protein disaggregation. eLife. 2014;3:e02481.
Li GQ, Zang XN, Zhang XC, Lu N, Ding Y, Gong L, et al. Cloning of ubiquitin-activating enzyme and ubiquitin-conjugating enzyme genes from Gracilaria lemaneiformis and their activity under heat shock. Gene. 2014;538:155–63.
Chanarat S. UBL5/Hub1: An Atypical Ubiquitin-Like Protein with a Typical Role as a Stress-Responsive Regulator. Int J Mol Sci. 2021;22:9384.
Voth W, Jakob U. Stress-activated chaperones: A first line of defense. Trends Biochem Sci. 2017;42:899–913.
Merret R, Carpenier MC, Favory JJ, Picart C, Descombin J, Bousquet-Antonelli C, et al. Heat shock protein HSP101 affects the release of ribosomal protein mRNAs for recovery after heat shock. Plant Physiol. 2017;174:1216–25.
Wang WX, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–52.
Vierling E. The roles of heatshock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620.
Wang J, Gao X, Dong J, Tian XY, Wang ZH. Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front Plant Sci. 2020;11:243.
Mcloughlin F, Basha E, Fowler ME, Kim M, Bordowitz J, Katiyar-Agarwal S, et al. Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol. 2016;172:1221–36.
Sung DY, Vierling E, Guy CL. Comprehensive expression profile analysis of the arabidopsis Hsp70 gene family. Plant Physiol. 2001;126:789–800.
Krishna P, Gloor G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones. 2001;6:238–46.
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30.
Yalcinkaya T, Uzilday B, Ozgur R, Turkan I, Mano J. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ Exp Bot. 2019;165:139–49.
Mano J, Khorobrykh S, Matsui K, Iijima Y, Sakurai N, Suzuki H, et al. Acrolein is formed from trienoic fatty acids in chloroplast: a targeted metabolomics approach. Plant Biotechnol. 2014;31:535–43.
Wei YK, Li JX, Hu WL, Yang CQ, Wang LJ. Isolation and characterization of AaAER, a novel double bond reductase from Artemisia annua. J Plant Biochem Biotechnol. 2015;24:331–7.
Wang Y, Zhao YX, Wang SS, Liu JF, Wang XQ, Han YL, et al. Up-regulated 2-alkenal reductase expression improves low-nitrogen tolerance in maize by alleviating oxidative stress. Plant, Cell Environ. 2021;44:559–73.
Ashraf M, Harris PJC. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51:163–90.
Lu YZ, Li RQ, Wang RC, Wang XM, Zheng WJ, Sun QX, et al. Comparative proteomic analysis of flag leaves reveals new insight into wheat heat adaptation. Front Plant Sci. 2017;8:1086.
Centritto M, Brilli F, Fodale R, Loreto F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011;31:275–86.
Xu YH, Rui L, Lu Y, Liu ZQ, Jiang SC, Shen YY, et al. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J Exp Bot. 2012;63:1095–106.
Yamori W, Shikanai T, Makino A. Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase-like complex performs a physiological role for photosynthesis at low light. Sci Rep. 2015;5:15593.
Shao S, Cardona T, Nixon PJ. Early emergence of the FtsH proteases involved in photosystem II repair. Photosynthetica. 2018;56:163–77.
Lassen LM, Nielsen AZ, Olsen CE, Bialek W, Jensen K, Møller BL, et al. Anchoring a plant cytochrome P450 via PsaM to the thylakoids in Synechococcus sp PCC 7002: Evidence for light-driven biosynthesis. Plos One. 2014;9:e102184.
Timabud T, Yin XJ, Pongdontri P, Komatsu S. Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J Proteomics. 2016;133:1–19.
Dinler BS, Gunduzer E, Tekinay T. Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biologica Cracoviensia S Botanica. 2016;58:29–41.
Dahan J, Tcherkez G, Macherel D, Benamar A, Belcram K, Quadrado M, et al. Disruption of the CYTOCHROME C OXIDASE DEFICIENT1 gene leads to cytochrome c oxidase depletion and reorchestrated respiratory metabolism in Arabidopsis thaliana. Plant Physiol. 2014;166:1788–802.
Liu X, Khajuria C, Li J, Trick HN, Huang L, Gill BS, et al. Wheat Mds-1 encodes a heat-shock protein and governs susceptibility towards the Hessian fly gall midge. Nat Commun. 2013;4:2070.
Currie Y, Moch J, Underwood J, Kharabsheh H, Quesenberry A, Miyagi R, et al. Transient heat stress compromises the resistance of wheat (Poales: Poaceae) seedlings to hessian fly (Diptera: Cecidomyiidae) infestation. J Econ Entomol. 2014;107:389–95.
Rossi S, Chapman C, Yuan B, Huang BR. Improved heat tolerance in creeping bentgrass by γ-aminobutyric acid, proline, and inorganic nitrogen associated with differential regulation of amino acid metabolism. Plant Growth Regul. 2021;93:231–42.
Liu LF, Wang J, Han ZH, Sun XC, Li HX, Zhang JH, et al. Molecular analyses of tomato GS, GOGAT and GDH gene families and their response to abiotic stresses. Acta Physiol Plant. 2016;38:229.
Hu LL, Bi AY, Hu ZR, Amombo E, Li HY, Fu JM. Antioxidant metabolism, photosystem II, and fatty acid composition of two tall fescue genotypes with different heat tolerance under high temperature stress. Front Plant Sci. 2018;9:1242.
Li Q, Shen WY, Zheng Q, Fowler DB, Zou JT. Adjustments of lipid pathways in plant adaptation to temperature stress. Plant Signal Behav. 2016;11:e1058461.
Wang HS, Chao Y, Tang XF, Wang LY, Dong XC, Meng QW. Antisense-mediated depletion of tomato endoplasmic reticulum omega-3 fatty acid desaturase enhances thermal tolerance. J Integr Plant Biol. 2010;52:568–77.
Chen F, Dong GJ, Wang F, Shi YQ, Zhu JY, Zhang YL, et al. A beta-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021;232:655–72.
Funding
The research was supported by grants from Zhejiang Provincial Natural Science Foundation of China [Grant No. LY21C160001], Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding [Grant No. 2021C02071-4].
Author information
Authors and Affiliations
Contributions
Cui-Hua Gu and Jie Wang contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jin Ma, Qun Wang, Ling-Ling Wei, Si-Dan, Hong, Yu Zhao, Guo-Zhe Zhang. The first draft of the manuscript was written by Jin Ma, Qun Wang, Ling-Ling Wei, Si-Dan, Hong, Yu Zhao, Guo-Zhe Zhang, and all authors commented on previous versions of the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was provided by College of Landscape and Architecture, Zhejiang Agriculture & Forestry University. Prior written informed consent was obtained from all participants.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Schedule 1.
Significantly expressed differential proteins under high-temperaturestress.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, J., Wang, Q., Wei, LL. et al. Responses of the tree peony (Paeonia suffruticosa, Paeoniaceae) cultivar ‘Yu Hong’ to heat stress revealed by iTRAQ-based quantitative proteomics. Proteome Sci 20, 18 (2022). https://doi.org/10.1186/s12953-022-00202-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12953-022-00202-5