Abstract
Background
Low-carbohydrate diet (LCD) is effective for weight loss and glycaemic control in humans. Here, the study aimed to explore the effects of LCD/high-fat diet (HFD) in both humans and mice.
Methods
Twenty-two overweight or obese participants received LCD for 3 weeks. Based on carbohydrate intake > 10% or ≤ 10% of calories, the participants were divided into moderate LCD (MLCD) and very LCD (VLCD) groups. The participants completed a 10-question food preference survey. Meanwhile, C57BL/6J mice were assigned to five groups: chow diet (CD, 10% fat), HFD with 60%, 70%, and 75% fat from cocoa butter (HFD-C), and HFD with 60% fat from lard (HFD-L) and fed for 24 weeks. Eight mice were acclimatised for the food-choice test.
Results
LCD decreased the total energy intake in humans. The VLCD group showed greater weight loss and better glycaemic control than the MLCD group. A food preference survey showed that 65% of participants tended to choose high-carbohydrate foods. In mice, HFD resulted in energy overconsumption, obesity, and metabolic disorders. When CD and HFD-L were administered simultaneously, mice rarely consumed CD. In the HFD-C groups, the energy intake and body weight increased with increasing dietary fat content. Compared with the HFD-C group, the HFD-L group consumed more energy and had poorer metabolism.
Conclusions
Lower carbohydrate intake contributed to lower energy intake and improved metabolism in humans. In mice, diets with a higher proportion of fat become more attractive and obesogenic by fixing the fat sources. Since the mice preferred lard to cocoa butter, lard induced excess energy intake and poorer metabolism. Different food preferences may be the underlying mechanism behind the opposite effects of the LCD/HFD in humans and mice.
Trial registration
The clinical trial was registered with the Chinese Clinical Trial Registry (www.chictr.org.cn). The registration number is ChiCTR1800016786. All participants provided written informed consent prior to enrolment.
Similar content being viewed by others
Background
The prevalence of obesity in adults has reached 12%, and approximately 2.3 billion individuals worldwide are overweight or obese [1, 2]. Obesity stands as a significant risk factor for chronic noncommunicable diseases, including cardiovascular diseases, type 2 diabetes, hypertension, and cancer, imposing substantial health and socioeconomic burdens on society [3]. Although sustaining a healthy lifestyle for a long time can indeed be challenging, lifestyle interventions, particularly dietary strategies, consistently considered as the primary approach to managing obesity and various metabolic disorders before pharmaceutical interventions. Low-carbohydrate diets (LCDs) have garnered substantial interest due to their benefits in weight loss, glycaemic control, and reduction in serum triglyceride (TG) [4,5,6,7]. High carbohydrate consumption leads to hyperinsulinemia, a condition that fosters fat accumulation and exacerbates adiposity, especially in individuals with metabolic disorders such as obesity and diabetes. LCDs, on the other hand, restrict carbohydrate intake, rely on fat as the primary energy source, alleviate water-sodium retention, reduce insulin secretion, and facilitate fat mobilisation from adipose tissue, thus contributing to weight loss and metabolic improvement [8, 9].
In our prior clinical trial, we observed that an LCD improved glucose homeostasis, alleviated fatty liver disease, and reduced body weight [10]. These findings suggested its superior efficacy in promoting weight loss and regulating glucose homeostasis compared to short-term exercise. In the present study, the authors reassembled and reanalysed the data from the trial by dividing the participants who followed LCDs into two groups based on their carbohydrate intake: the moderate LCD (MLCD) group and the very LCD (VLCD) group. Notably, the fat intake in the VLCD group was approximately 60% of the total daily energy intake. The lower the proportion of carbohydrates, the better the metabolism (details of the analysis are provided in the subsequent section).
In humans, LCDs generally refer to carbohydrate intake < 130 g/day or less than 26% of the 2000 kcal/day [11]. When the carbohydrate intake drops to < 10% of the total energy consumed, it is also called a VLCD or ketogenic diet. A reduction in carbohydrate consumption is usually accompanied by an increase in dietary fat and, to a lesser extent, protein [12]. The macronutrient composition of a high-fat diet (HFD) used in animal experiments is similar to that of an LCD. In HFD for mice, fat typically constitutes 40–60% of the total energy and may include various fat sources such as lard, coconut oil, cocoa butter, soybean oil, and more. It’s worth noting that, unlike LCDs in humans, HFDs in mice are primarily employed to induce obesity and mimic a range of human metabolic disorders. When C57BL/6J mice are provided with HFD ad libitum, they tend to develop metabolic abnormalities, including obesity, hyperlipidemia, and hyperglycemia [13, 14]. Inspired by the results of the aforementioned clinical trial, which demonstrated a positive correlation between reduced carbohydrate intake and improved metabolism but lacked an in-depth mechanistic exploration, we wanted to apply similar dietary strategies to mice, aiming to replicate the findings observed in humans and delve into the underlying mechanisms driving these outcomes.
Lard and cocoa butter are two of the most commonly used sources of animal- and plant-derived fats, respectively, in HFD designed for mice. Lard consists of 45% saturated fatty acids (SFAs) [15], 45% monounsaturated fatty acids (MUFAs) [16] and approximately 9–10% polyunsaturated fatty acids (PUFAs) [17]. Cocoa butter mainly comprises 62.0% SFAs, 33.6% MUFAs, and 2.7% PUFAs [18]. Unlike vegetable oils such as soybean oil, both lard and cocoa butter can be processed into rod-like solids, which have a geometric shape similar to that of the standard chow diet (CD), even when their proportion is increased to 60% or more (cocoa butter only). Additionally, cocoa butter maintains its stability as a solid at room temperature due to its short plastic range. In contrast, products containing lard transition into a paste-like consistency at room temperature when the lard proportion exceeds 60%. Therefore, diets with 60%, 70%, and 75% cocoa butter and 60% lard were finally chosen, which were all stable with similar geometric shapes, to feed the mice.
Hence, based on our prior clinical trial, a post hoc analysis and animal experiments are conducted in this study to investigate the impacts of an LCD/HFD on metabolism in humans and mice.
Methods
Human study: study subjects and protocol
Informed consent was obtained from all the participants. This trial was approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine, Affiliated Sixth People’s Hospital. The trial was registered with the Chinese Clinical Trial Registry (ChiCTR1800016786). The inclusion criteria were as follows: age 18–40 years, BMI ≥24 kg/m2 and waist circumference ≥90 cm in male and ≥85 cm in female [19]. The exclusion criteria included a diagnosis or history of diabetes mellitus and recent participation in any weight loss interventions within 1 month before the study. A more detailed methodology has been previously described [10, 20]. In brief, 22 participants were instructed to go on an LCD (carbohydrate intake < 50 g/day was suggested) for 3 weeks with no restrictions imposed on calorie, fat, or protein intake. During the intervention period, participants were required to document their daily dietary intake and submit photographs of their meals to the investigators via WeChat, a widely used mobile social application in China. Furthermore, participants were required not to modify their other usual lifestyle or engage in additional physical exercise throughout the intervention. Based on their carbohydrate intake, the 22 participants were categorized into two groups after the intervention. Carbohydrate intake was > 10% and ≤ 10% of the total energy intake in the MLCD group (n = 9) and in the VLCD group (n = 13), respectively.
The first day of the intervention was designated as day 1, and the final day was designated as day 21. Anthropometric parameters were measured on day − 3 (three days before the intervention commenced) and day 22 (the day following the completion of the intervention). The participants were instructed to wear flash glucose monitors continuously, recording glucose values from day − 3 to day 24 (utilizing two sensors throughout the entire intervention). The participants reverted to their prior diet between days 22 and 24. The mean sensor glucose (MSG), standard deviations of sensor glucose (SDSG), coefficient of variation (CV), and largest amplitude of glycaemic excursion (LAGE) were calculated to assess daily glycaemic levels and fluctuations [21]. MSG is the mean glucose value that represents a simple summary of overall glycaemic control. SDSG and CV quantify the relative variability of glucose levels in relation to the mean, and LAGE represents the difference between the highest and lowest glucose values within a defined period, highlighting the largest fluctuations in glucose levels.
The participants completed a 10-question food preference survey questionnaire to gain insights into their dietary habits and investigate whether food preferences had an impact on metabolism. For each question, the participants were asked to choose their preferred dish from the two or three listed dishes. The dishes were either high-carbohydrate or high-fat foods, with at least 50% of their calories derived from carbohydrates or fat, respectively. To ensure comparability, the dishes presented for the same question shared similar appearances and raw materials. Option for high-carbohydrate foods was scored as 1 and − 1 of option for high-fat foods. The cumulative score was then calculated by summing the scores for all the questions. A total score > 0 indicated a preference for high-carbohydrate foods, while a score < 0 indicated a preference for high-fat foods. A score of 0 denoted no preference for either food type. The details of the questionnaire are presented in Table S1.
Animals and treatments
The animal study was reviewed and approved by the Animal Ethical and Welfare Committee of the Shanghai Jiao Tong University School of Medicine, Affiliated Sixth People’s Hospital. Three-week-old male C57BL/6J mice, a strain sensitive to high fat feed and easily to be induced obesity, were obtained from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). All mice were housed under standard conditions with ad libitum access to water and food. After 3 weeks of acclimatisation, 50 mice were randomly divided into five groups (n = 10 per group): CD (10% kcal from fat); HFD with 60%, 70%, and 75% fat, mainly from cocoa butter (HFD-C, Jiangsu Synergetic Pharmaceutical Bioengineering Co., Ltd, Nanjing, China); and HFD with 60% fat, mainly from lard (HFD-L, Research Diets, Inc., New Brunswick, NJ, USA). The fat content in each diet was defined as the percentage of total calories obtained from fat and 20% of total calories were derived from proteins in all diets. The details of the diets are presented in Table S2.
All mice were fed for 24 weeks. Food intake was calculated every 2–3 days, based on the amount of food given and withdrawn. All mice were weighed weekly. Before euthanasia, the mice were evenly and randomly divided into two groups. One group was sacrificed after 15-hour fasting, and the other group was sacrificed on another day. Under isoflurane anaesthesia, mice were sacrificed by cervical dislocation. Plasma samples were collected separately from each orbit. Liver and brain tissues were extracted and immediately placed in liquid nitrogen. All samples were stored at -80 °C before use. Liver tissue samples were treated with 4% paraformaldehyde for Oil red and PAS staining.
Another eight mice were used for the food choice test after 2 weeks of acclimatisation. Mice were housed in four cages. Adequate amounts of CD and HFD-L were simultaneously administered to each cage for a week. Daily intake of the two diets was recorded.
Blood analysis
Blood glucose and β-hydroxybutyric acid (β-HB) were measured from the tail vein using a glucometer (Roche, Basel, Switzerland) and a blood glucose and ketone test meter (Abbott Laboratories, Chicago, IL, USA), respectively. Serum insulin concentrations were determined using an enzyme-linked immunosorbent assay kit (Crystal Chem; Cook, IL, USA). The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as: (fasting glucose in mmol/L × fasting insulin, µU/mL) / 22.5. Serum alanine transaminase (ALT), total cholesterol (TC), TG, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and free fatty acid (FFA) levels were determined using an autoanalyzer (Hitachi Inc., Tokyo, Japan). Liver glycogen levels were determined using quantitative colorimetric assay kits (Bioassay Systems, Hayward, CA, USA).
Histological examinations
For Oil Red O staining, liver tissue samples were fixed and embedded in optimal cutting temperature compounds after sucrose dehydration. Standard frozen sections, 8 μm in thickness, were obtained. Sections were stained with Oil Red O solution for 10 min, rinsed with distilled water, differentiated with 75% alcohol, and counterstained with haematoxylin. For periodic acid-Schiff (PAS) staining, the specimens were embedded after dehydration using an ethanol gradient. Liver tissues were cut into 4 μm thick sections. After dewaxing and oxidation, the sections were stained with Schiff reagent for 20–25 min and counterstained with haematoxylin.
Tolerance test
Intraperitoneal glucose tolerance test (IPGTT), pyruvate tolerance test (PTT), and insulin tolerance test (ITT) are classic tests used to assess overall glucose metabolism, gluconeogenesis, and insulin sensitivity, respectively. For the IPGTT and PTT, the mice were fasted for 12 h and then intraperitoneally injected with D-glucose or pyruvate at a dose of 2 g/kg. For the ITT, the mice were fasted for 6 h and then injected intraperitoneally with insulin (1 U/kg). Blood glucose levels were measured 0, 15, 30, 60, and 120 min after injection. The area under the curve (AUC) was calculated using a trapezoidal formula.
Quantitative real-time PCR (qPCR)
TRIzol reagent (Thermo Scientific, Waltham, MA, USA) was used to extract total RNA from the hypothalamic tissues. The sequences of the primers used for qPCR were as follows: Npy: forward 5’-ATGCTAGGTAACAAGCGAATGG-3’, reverse 5’-TGTCGCAGAGCGGAGTAGTAT-3’; Agrp: forward 5’-AGAGTTCCCAGGTCTAAGTCTG-3’, reverse 5’-GCGGTTCTGTGGATCTAGCA-3’; Pomc: forward 5’-ATGCCGAGATTCTGCTACAGT-3’, reverse 5’-CCACACATCTATGGAGGTCTGAA-3’; Cartpt: forward 5’-CCCGAGCCCTG-GACATCTA-3’, reverse 5’-GCTTCGATCTGCAACATAGCG-3’; and glyceraldehyde 3-phosphate dehydrogenase (Gapdh): forward 5’-AGGTCGGTGTGAACGGATTTG-3’, reverse 5’-GGGGTCGTTGATGGCAACA-3’.
Statistical analyses
All statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Results are presented as mean ± standard deviation (SD). Macronutrient content was expressed as a percentage of the total energy intake. The Shapiro–Wilk test was used to check for normality. Differences among groups were analysed using analysis of variance. Paired-samples t test was used to examine within-group differences and independent-samples t tests were used to explore between-group differences.
Results
VLCD group had greater weight loss and better glycaemic control
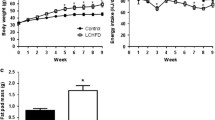
After the 3-week intervention, significant weight loss was evident in both the MLCD and VLCD groups (P < 0.001, Fig. 1A). Body weight decreased by 2.41 ± 1.20 kg (2.7%) in the MLCD group and 4.35 ± 1.60 kg (5.3%) in the VLCD group (Fig. 1B). The loss in the VLCD group was significantly greater than that in the MLCD group (P < 0.01). The mean glucose levels and glycaemic fluctuations before and during the intervention were compared, and the MLCD group showed a trend of reduction, but the difference was not statistically significant, whereas the VLCD group showed a significant decrease (Fig. 1C-F). The VLCD group had significantly lower values than the MLCD group for MSG (4.30 ± 0.06 vs. 3.89 ± 0.03 mmol/L), SDSG (0.64 ± 0.03 vs. 0.42 ± 0.01 mmol/L), CV (14.37 ± 0.65% vs. 10.87 ± 0.31%) and LAGE (3.11 ± 0.16 vs. 1.96 ± 0.06 mmol/L) (all P < 0.001). After day 21, the subjects returned to their former diet for 3 days. The favourable effects on glycaemic control disappeared. Blood glucose levels and glycaemic swings returned to baseline in both groups.
Body weight and blood glucose response to LCD in human study. (A) Body weight trend during the intervention. (B) Changes in body weight. Curves of (C) MSG, (D) SDSG, (E) CV, and (F) LAGE
Notes: Data are expressed as mean ± SD (n = 9 for the MLCD group and n = 13 for the VLCD group). Independent-samples t test was used. * P < 0.05; ** P < 0.01; *** P < 0.001
Abbreviations: MLCD: moderately low-carbohydrate diet; VLCD: very low-carbohydrate diet; Before: 3 days before the intervention; During: during the intervention (21 days); After: 3 days after the intervention; MSG: mean sensor glucose; SDSG: standard deviations of sensor glucose; CV: coefficient of variation; LAGE: largest amplitude of glycemic excursion
Dietary patterns and food preferences of the subjects
Before adopting the LCD, all the subjects consumed a normal diet. The percentage of fat, protein, and carbohydrate in the pre-intervention diet was 30.50 ± 3.07%, 19.68 ± 1.58%, 49.82 ± 4.08% in the MLCD group, and 31.27 ± 3.03%, 20.67 ± 3.33%, 48.05 ± 3.87% in the VLCD group, respectively. There were no statistically significant differences between the two groups. During the intervention, the macronutrient compositions of fat, protein, and carbohydrate changed to 55.15 ± 2.61%, 31.28 ± 3.38%, 13.57 ± 2.18% in the MLCD group, and 59.79 ± 5.33%, 32.89 ± 4.77%, 7.32 ± 2.36% in the VLCD group, respectively (Fig. 2A). Carbohydrate intake in the VLCD group was approximately 48% lower than that in the MLCD group (P < 0.001). During the intervention, daily calorie intake significantly decreased in both groups (all P < 0.05; Fig. 2B).
Macronutrient compositions of diets and surveyed food preferences in subjects. (A) Macronutrient compositions of diets. (B) Total energy intake. (C) Total scores of the subjects
Notes: Data are expressed as mean ± SD and the macronutrient content is expressed as a percentage of total energy intake (n = 9 for the MLCD group and n = 13 for the VLCD group). Independent-samples t test and paired t test were used. * P < 0.05; ** P < 0.01; *** P < 0.001. Figure 2C: Dots on the left side of the vertical axis indicate preference for high-fat foods. Dots on the right side of the vertical axis indicate preference for high-carbohydrate foods. Dots on the vertical axis indicate no preference for either
Abbreviations: MLCD: moderately low-carbohydrate diet; VLCD: very low-carbohydrate diet; Before: 3 days before the intervention; During: during the intervention (21 days)
Twenty participants completed the food preference survey. Of these, 13 preferred high-carbohydrate foods, three favoured high-fat foods, and four showed no preference (Fig. 2C). These findings revealed an overall preference for high-carbohydrate foods.
HFD-related metabolic disorders in mice
After 24 weeks of feeding, the HFD groups showed higher body and liver weights than the CD group (all P < 0.001, Fig. 3A and B). Fasting blood glucose, fasting insulin, and HOMA-IR were also higher in the HFD group than in the CD group, revealing impaired glucose metabolism and decreased insulin sensitivity in HFD-fed mice (all P < 0.05, Fig. 3C-E). The levels of blood β-HB did not differ significantly among the five groups (Fig. 3F). A significant increase in hepatic lipid deposition in HFD-fed mice was observed by Oil Red O staining compared to CD mice (Fig. 3M).
Effects of different diets on body weight and glucolipid metabolism in mice. Panels A-M respectively show values of body weight, liver weight, blood glucose, insulin, HOMA-IR, blood β-HB, fasting serum TC, LDL, HDL, TG, serum FFA, fasting serum ALT, and Oil red O staining of liver tissue
Notes: Data are expressed as mean ± SD (n = 10 for A & C-F and n = 5 for B & G-L). Analysis of Variance and independent-samples t test were used. If the letters on the top of bars are different it means there is statistical difference between the two groups (P < 0.05); NS, no statistical difference
Abbreviations: CD: chow diet; 60%HFD-C: HFD with 60% fat mainly from cocoa butter; 70%HFD-C: HFD with 70% fat mainly from cocoa butter; 75%HFD-C: HFD with 75% fat mainly from cocoa butter. 60%HFD-L: HFD with 60% fat mainly from lard; HOMA-IR: homeostatic model assessment for insulin resistance; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipo-protein; TG: triglyceride; FFA: free fatty acid; β-HB: β-hydroxybutyric acid; ALT: Serum alanine transaminase
Among the three HFD-C groups, increasing dietary fat content was associated with increased weight gain, higher fasting blood glucose levels, more severely decreased insulin sensitivity, and greater hepatic lipid accumulation in mice (Fig. 3A, C, E, and M). Body weight, blood glucose, HOMA-IR, TC, LDL, HDL, TG, FFA, and liver lipid deposition were higher in the HFD-L group than those in the HFD-C and CD groups (Fig. 3A, C, E, G-K, and M). The serum ALT concentration was also significantly elevated in the HFD-L group, indicating impaired liver function (Fig. 3L).
Impaired glucose tolerance, decreased insulin sensitivity, and hyper-gluconeogenesis in HFD-fed mice
In the IPGTT, the blood glucose levels of the HFD-L group were always significantly higher than those of the other groups (all P < 0.01; Fig. 4A). The AUC in the HFD-L group was also much higher, indicating impaired glucose tolerance (P < 0.001; Fig. 4B). In the ITT, after the administration of insulin, all HFD groups presented higher blood glucose levels at all time points and greater AUCs than the corresponding values in the CD group (P < 0.01; Fig. 4C and D). Sixty minutes after insulin injection, the blood glucose levels of lard-fed mice were significantly higher than those of cocoa butter-fed mice. Compared with the other groups, the HFD-L group displayed a higher AUC. This finding indicated that HFD reduced insulin sensitivity in mice, and lard aggravated this resistance compared to cocoa butter. In the PTT, compared with CD-fed mice, HFD-fed mice showed higher glycaemia in response to the administration of pyruvate, a substrate of gluconeogenesis (Fig. 4E). The AUCs were greater for mice in the HFD groups compared with that for mice in the CD group, with the highest value in the HFD-L group (P < 0.001, Fig. 4F). These findings indicated that HFD upregulated hepatic gluconeogenesis, with the effect being more significant in lard-fed mice.
Tolerance tests in mice fed with different diets. Curves and AUCs for (A) IPGTT, (B) ITT, and (C) PTT
Notes: Data are expressed as mean ± SD (n = 10). Analysis of Variance and independent-samples t test were used. If the letters on the top of or beside bars are different it means there is statistical difference between the two groups (P < 0.05)
Abbreviations: CD: chow diet; 60%HFD-C: HFD with 60% fat mainly from cocoa butter; 70%HFD-C: HFD with 70% fat mainly from cocoa butter; 75%HFD-C: HFD with 75% fat mainly from cocoa butter. 60%HFD-L: HFD with 60% fat mainly from lard; AUC: area under the curve; IPGTT: intraperitoneal glucose tolerance test; ITT: insulin tolerance test; PTT: pyruvate tolerance test
Preference for HFD by mice
The total food intake in the HFD-C group was lower than that in the CD and HFD-L groups (both P < 0.05; Fig. 5A). However, energy intake in the HFD group was significantly greater than that in the CD group (P < 0.001, Fig. 5B). Mice in the HFD-C group consumed more calories as the dietary fat content increased. Lard-fed mice consumed more energy than cocoa butter-fed mice. When provided with CD and HFD-L simultaneously, mice almost exclusively consumed HFD-L (0.15 ± 0.04 vs. 2.46 ± 0.29 g/day/mouse, P < 0.001, Fig. 5C). The mice clearly preferred HFD over CD. qPCR revealed decreased mRNA levels of Npy and Agrp (both P < 0.01) and increased mRNA levels of Pomc and Cartpt (both P < 0.001) in the hypothalamus of HFD-fed mice compared to CD-fed mice (Fig. 5D). Npy and Agrp are hypothalamic orexigenic genes, whereas Pomc and Cartpt are hypothalamic anorexigenic genes [22,23,24,25,26]. The HFD-fed mice were in a relatively satiated state after overnight fasting owing to excessive energy intake.
Food intake, energy intake, and mRNA levels of hypothalamic hormones in mice. (A) Food intake. (B) Energy intake. (C) Food intake of mice provided with CD and HFD-L simultaneously. (D) The mRNA levels of Agrp, Npy, Pomc, and Cartpt in hypothalamus
Notes: Data are expressed as mean ± SD (n = 10 for A& B, n = 8 for C and n = 5 for D). Analysis of Variance and independent-samples t test were used. If the letters on the top of bars are different mean there are statistical difference between the two groups (P < 0.05). * P < 0.05; ** P < 0.01; *** P < 0.001
Liver glycogen content is not decreased in HFD-fed mice during fasting
PAS staining and glycogen measurement revealed that the fasting liver glycogen content was approximately 36% lower than the postprandial level in the CD group (P < 0.05; Fig. 6A and B). However, there were no significant differences between the fasting and postprandial hepatic glycogen levels in the HFD groups. In the fasting state, hepatic glycogen levels were higher in the HFD groups than in the CD group (All P < 0.05). No differences were observed among the HFD groups. There were no differences in the postprandial state among the five groups.
Discussion
Previous studies on LCDs have primarily focused on comparing them to moderate carbohydrate diets (about 30–50% carbohydrate) [27,28,29] or low-fat diets [7, 30]. Few studies, however, have explored the differences between MLCD and VLCD. In particular, whether lower carbohydrate intake (e.g., < 10%) will bring about greater improvement in metabolism is controversial when carbohydrate intake is already < 20% [7, 31]. This study revealed the greater efficacy of VLCD in weight loss and glucose homeostasis. During the intervention, the VLCD group consumed approximately 52% less carbohydrates than the MLCD group, while no significant difference in total calorie intake was observed between the two groups. Although both groups experienced reductions in body weight, the VLCD group exhibited a roughly twofold greater decrease compared to the MLCD group, implying a promising effect in weight control. However, a meta-analysis that included 50 trials indicated a U-shaped effect of carbohydrate restriction on body weight, with the most significant reduction observed in the 35% carbohydrate diet [30]. When carbohydrate intake was < 35%, body weight slightly increased with decreasing proportions of carbohydrates in diets over a relatively long-term observation, probably due to the challenges of adhering to a strict LCD for a long time. Therefore, balance between the amount of daily carbohydrate intake and the possibility of adhering to it over an extended period is crucial to weight management. To the best of our knowledge, no studies have directly compared the effects of MLCD (10–20% carbohydrate) and VLCD (≤ 10% carbohydrate), hence, future studies are needed to provide a more comprehensive understanding of the difference between MLCD and VLCD.
Given the exclusion of individuals with diabetes from this study, significant reductions in blood glucose levels and fluctuations were only observed when carbohydrate intake was restricted to 10%, as seen in the VLCD group, rather than in the MLCD group. Reducing glucose fluctuation is important for patients with chronic conditions, such as diabetes, cardiovascular diseases, and obesity, because decreased glycaemic excursions are beneficial for improving insulin sensitivity, oxidative stress, and endothelial dysfunction [32, 33]. High glucose variability is a critical predictor of macrovascular and microvascular complications [34, 35]. In addition, severe glucose variability, usually in the form of glucose spikes, is also observed in individuals with normoglycemia and is associated with a high risk of developing diabetes [36]. VLCD can effectively lower glucose swings and may reduce the risk of diabetes and its vascular complications.
The original intention of this study was to replicate the effect of LCDs on mice and reveal the underlying mechanism. However, the effects of LCD/HFD appeared to diverge between humans and mice, and different food preference was considered as the critical factor. Specifically, in mice, dietary fat seemed to stimulate energy intake, whereas in humans, it had the opposite effect, suppressing appetite and promoting sense of fullness and satiety. Both the MLCD and VLCD groups exhibited a significant reduction in total energy intake, implying that the favourable metabolic outcomes associated with LCDs might be partially attributed to calorie reduction. In contrast, when food with different fat content was provided ad libitum, mice preferred the HFD and consumed more calories. In animals, food intake is influenced by a balance between homeostatic control and hedonic values. However, this equilibrium was disrupted in the mice on HFD. Dietary fat stimulates the hedonic systems that override internal homeostatic mechanisms and promote energy intake [37]. In the fasting state, mice in the HFD group showed lower expression of starvation-related genes and higher expression of satiety-related genes than mice in the CD group. This suggested that, even after an overnight fasting, HFD-fed mice remained relatively energy-replete due to their excess food consumption compared with CD-fed mice. This excessive calorie intake accounts for why the HFD-fed mice failed to replicate the effects induced by LCDs in humans.
The study further revealed that the dietary fat source and fat content could also influence energy intake in mice. Calorie intake in the HFD-L group was higher than that of the HFD-C group, and even exceeded that of the HFD-C group with 75% fat content. This preference for lard over cocoa butter by mice led to an accumulation of excess energy and subsequent metabolic deterioration. Preference for lard in mice has rarely been reported. In contrast, humans usually favour cocoa butter over lard. Furthermore, when the fat content varied for the same fat source, a distinct positive correlation between fat content and total energy intake in mice emerged. A similar result was observed that fat intake increased significantly with increasing fat content, thereby contributing to higher energy intake and, consequently, weight gain [37]. This phenomenon can be explained by hypothalamic hunger pathways, including 5-HT receptors, and the dopamine and opioid signalling pathways stimulated by fat instead of protein or sucrose [37].
It is also worth noting that the protein content in the HFD for mice differed from that in humans. The reasons for applying a 20% protein content in HFD of mice can be summarised in the following three aspects. Firstly, the widely adopted standard CD for mice comprises 20% protein of total calories. Secondly, a higher protein proportion, such as 30%, would have been unfeasible due to the study’s objective of investigating HFDs with a fat content exceeding 70%. Most importantly, despite the satiating effect of a high-protein diet, typically around 35% [38], a previous study indicated that protein content did not influence total energy intake in mice [37]. Another aspect worth mentioning regarding the design of animal experiment is the choice of the C57BL/6 strain. C57BL/6 mice are the most widely used strain in studies of metabolism worldwide, readily available and with relatively better cost performance. Other common stains, such as Kunming, ICR, and BALB/c, are much more commonly used in research related to immunology and oncology, even though they have shown similar responses to HFD as the C57BL/6 strain [39]. Another strain, ob/ob mice, is characterized by a mutation in the leptin gene, resulting in hyperphagic and overeating due to the absence of functional leptin [40]. Thus, they fail to represent the natural physiological status and are not suitable for our study as well.
In addition, liver glycogen content at both fasting and postprandial levels did not differ in HFD-fed mice in this study, which is consistent with the authors’ previous findings [41]. The elevated levels of fasting insulin in HFD-fed mice might be one of the reasons for this, as increased insulin levels are known to stimulate glycogen synthesis. Furthermore, hyperglycaemia inhibits glycogen breakdown. Combined with the PTT results, it can be speculated that fasting energy utilisation in HFD-fed mice predominantly relies on lipolysis and hypergluconeogenesis, rather than glycogenolysis. In CD-fed mice, the fasting liver glycogen content was much lower than the postprandial levels, suggesting that glycogen may serve as one of the primary energy sources during fasting.
Study strengths and limitations
Until now, few studies have investigated the differences in food preferences between humans and mice or analysed the mechanisms underlying this inconsistency. This study also had several limitations. First, the sample size of the clinical trial was small which might have augmented the potential for bias and could have influenced the robustness of the results. Second, the follow-up duration was relatively brief, and whether the VLCD-induced effects could be sustained for a long time remains unknown. Third, this study was a retrospective subgroup analysis of a previous clinical trial. Therefore, larger randomised trials with longer follow-up periods are needed to ascertain the long-term effects of MLCD and VLCD. In addition, the protein contents in the diets of human and animal study were not exactly the same as aforementioned, although a previous study has confirmed that protein content did not influence the total energy intake in mice. Future attention should be given to this issue, and more precise and well-matched animal studies are warranted for further mechanistic investigations.
Conclusion
This study indicates that lower carbohydrate intake (such as carbohydrate intake ≤ 10%) can contribute to decreased energy intake and better metabolic improvement in individuals with obesity. However, diets with lower carbohydrates and higher fat contents led to increased energy intake and deterioration in metabolism in mice. Different food preferences, in that human prefer high-carbohydrate foods and mice prefer high-fat foods might be the main reason for the contrasting results between humans and mice. This study raises awareness that it is not what you eat but how much you eat that matters in weight control. Preferred foods are easy to overindulge in, and restricting intake is crucial for energy balance and weight management.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine transaminase
- AUC:
-
Area under the curve
- β-HB:
-
β-hydroxybutyric acid
- CD:
-
Chow diet
- CV:
-
Coefficient of variance
- FFA:
-
Free fatty acid
- HDL:
-
High density lipoprotein
- HFD:
-
High-fat diet
- HFD-C:
-
High-fat diet cocoa butter
- HFD-L:
-
High-fat diet lard
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IPGTT:
-
Intraperitoneal glucose tolerance test
- ITT:
-
Insulin tolerance test
- LAGE:
-
Largest amplitude of glycaemic excursion
- LCD:
-
Low-carbohydrate diet
- LDL:
-
Low density lipoprotein
- MLCD:
-
Moderate low-carbohydrate diet
- MSG:
-
Mean sensor glucose
- MUFA:
-
Monounsaturated fatty acids
- PAS:
-
Perodic acid-Schiff
- PTT:
-
Pyruvate tolerance test
- PUFA:
-
Polyunsaturated fatty acids
- SD:
-
Standard deviation
- SDSG:
-
Standard deviations of sensor glucose
- SFA:
-
Saturated fatty acids
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- VLCD:
-
Very low-carbohydrate diet
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–40.
Health Effects of. Overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Thomsen MN, Skytte MJ, Samkani A, Carl MH, Weber P, Astrup A, et al. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 Diabetes: a randomised controlled trial. Diabetologia. 2022;65(3):506–17.
Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition Therapy for adults with Diabetes or Prediabetes: a Consensus Report. Diabetes Care. 2019;42:731–54.
Ebbeling CB, Knapp A, Johnson A, Wong JMW, Greco KF, Ma C, et al. Effects of a low-carbohydrate diet on insulin-resistant dyslipoproteinemia-a randomized controlled feeding trial. Am J Clin Nutr. 2022;115:154–62.
Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 Diabetes Mellitus. Eur J Epidemiol. 2018;33:157–70.
Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–9.
Rabast U, Vornberger KH, Ehl M. Loss of weight, sodium and water in obese persons consuming a high- or low-carbohydrate diet. Ann Nutr Metab. 1981;25:341–9.
Cai L, Yin J, Ma X, Mo Y, Li C, Lu W, et al. Low-carbohydrate diets lead to greater weight loss and better glucose homeostasis than exercise: a randomized clinical trial. Front Med. 2021;15:460–71.
Goldenberg JZ, Day A, Brinkworth GD, Sato J, Yamada S, Jönsson T, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 Diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743.
Brouns F. Overweight and Diabetes prevention: is a low-carbohydrate-high-fat diet recommendable? Eur J Nutr. 2018;57:1301–12.
Alimujiang M, Yu XY, Yu MY, Hou WL, Yan ZH, Yang Y, et al. Enhanced liver but not muscle OXPHOS in Diabetes and reduced glucose output by complex I inhibition. J Cell Mol Med. 2020;24:5758–71.
Yu M, Alimujiang M, Hu L, Liu F, Bao Y, Yin J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int J Biol Sci. 2021;17:1693–707.
Djohan YF, Camara-Cissé M, Fouret G, Bonafos B, Jover B, Cristol JP, et al. Diets Rich in Olive Oil, Palm Oil, or Lard alter mitochondrial Biogenesis and mitochondrial membrane composition in Rat Liver. Biochem Res Int. 2022;2022:9394356.
Matsuzaka H, Matsuyama H, Tanaka W, Tajiri H, Sakakibara H. Selective consumption of Fish Oil at End of the Day increases the physiological fatty acid compositions of Eicosapentaenoic Acid and Docosahexaenoic Acid in mice. Molecules. 2022;27(4):1271.
Li W, Zhang K, Yang H. Pectin alleviates high Fat (Lard) Diet-Induced nonalcoholic fatty Liver Disease in mice: possible role of short-chain fatty acids and gut microbiota regulated by Pectin. J Agric Food Chem. 2018;66(30):8015–25.
Konzock O, Matsushita Y, Zaghen S, Sako A, Norbeck J. Altering the fatty acid profile of Yarrowia Lipolytica to mimic cocoa butter by genetic engineering of desaturases. Microb Cell Fact. 2022;21(1):25.
Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 Diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158.
Cai L, Li C, Wang Y, Mo Y, Yin J, Ma X. Increased serum GDF15 related to improvement in metabolism by lifestyle intervention among young overweight and obese adults. Diabetes Metab Syndr Obes. 2021;14:1195–202.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose Monitoring Data Interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–603.
Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–47.
Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–72.
Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–97.
Yang SC, Shieh KR, Li HY. Cocaine- and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience. 2005;133:841–51.
Lynch WC, Hart P, Babcock AM. Neuropeptide Y attenuates satiety: evidence from a detailed analysis of patterns ingestion. Brain Res. 1994;636:28–34.
Johnstone AM, Lobley GE, Horgan GW, Bremner DM, Fyfe CL, Morrice PC, et al. Effects of a high-protein, low-carbohydrate v. high-protein, moderate-carbohydrate weight-loss diet on antioxidant status, endothelial markers and plasma indices of the cardiometabolic profile. Br J Nutr. 2011;106(2):282–91.
Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583.
Saslow LR, Daubenmier JJ, Moskowitz JT, Kim S, Murphy EJ, Phinney SD, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 Diabetes Mellitus or prediabetes. Nutr Diabetes. 2017;7(12):304.
Buga A, Welton GL, Scott KE, Atwell AD, Haley SJ, Esbenshade NJ, et al. The effects of Carbohydrate versus Fat Restriction on lipid profiles in highly trained, recreational Distance runners: a Randomized, Cross-over Trial. Nutrients. 2022;14:1135.
Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, Hosseini Y, Jibril AT, Shahinfar H, et al. Dose-dependent effect of carbohydrate restriction for type 2 Diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116:40–56.
Wronka M, Krzemińska J, Młynarska E, Rysz J, Franczyk B. The influence of lifestyle and treatment on oxidative stress and inflammation in Diabetes. Int J Mol Sci. 2022;23(24):15743.
Škrha J, Šoupal J, Škrha J Jr, Prázný M. Glucose variability, HbA1c and microvascular Complications. Rev Endocr Metab Disord. 2016;17(1):103–10.
Mo Y, Zhou J, Li M, Wang Y, Bao Y, Ma X, et al. Glycemic variability is associated with subclinical Atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc Diabetol. 2013;12:15.
Lu J, Ma X, Zhang L, Mo Y, Ying L, Lu W, et al. Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune Diabetes of the adult and type 2 Diabetes. J Diabetes Investig. 2019;10:753–59.
Hall H, Perelman D, Breschi A, Limcaoco P, Kellogg R, McLaughlin T, et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018;16:e2005143.
Hu S, Wang L, Yang D, Li L, Togo J, Wu Y, et al. Dietary Fat, but not protein or carbohydrate, Regulates Energy Intake and causes adiposity in mice. Cell Metab. 2018;28(3):415–31e4.
Cuenca-Sánchez M, Navas-Carrillo D, Orenes-Piñero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Adv Nutr. 2015;6(3):260–6.
Li J, Wu H, Liu Y, Yang L. High fat diet induced obesity model using four strainsof mice: Kunming, C57BL/6, BALB/c and ICR. Exp Anim. 2020;69(3):326–35.
Suriano F, Vieira-Silva S, Falony G, Roumain M, Paquot A, Pelicaen R, et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome. 2021;9(1):147.
Hu L, Xia X, Zong Y, Gu Y, Wei L, Yin J. Calorie restriction enhanced glycogen metabolism to compensate for lipid insufficiency. Mol Nutr Food Res. 2022;66:e2200182.
Acknowledgements
The authors are grateful to the participants for their time and effort in the study. The authors acknowledge Mrs. Junxi Lu and the other members of the institute for their excellent technical assistance. The authors thank the staff at the Department of Laboratory Medicine, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine for their help with blood analysis.
Funding
This work was supported by the National Natural Science Foundation of China (82070885), Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant (20172025), Shanghai Research Center for Endocrine and Metabolic Diseases (2022ZZ01002) and Shanghai Municipal Key Clinical Specialty.
Author information
Authors and Affiliations
Contributions
Jun Yin conceived the study design. All authors designed the study and reviewed versions of the protocol. Lingli Cai conducted the clinical trial and collected the data. Cheng Li assisted in conducting the clinical trials. Xinyi Xia completed the animal experiments. Lili Hu and Yunjie Gu assisted in conducting animal experiments. Lingli Cai, Xinyi Xia and Yunjie Gu analyzed the data. Lingli Cai and Yunjie Gu wrote the manuscript. Jun Yin and Xiaojing Ma reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study in human was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine Affiliated Sixth People’s Hospital. This trial was registered at www.chictr.org.cn with clinical trial registration number ChiCTR1800016786. All subjects provided written informed consent before enrollment. The animal study was reviewed and approved by the Animal Ethical and Welfare Committee of Shanghai Jiao Tong University School of Medicine Affiliated Sixth People’s Hospital and all the procedures were performed according to Shanghai Jiao Tong University Affiliated Sixth People’s Hospital Guidelines for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, L., Xia, X., Gu, Y. et al. Opposite effects of low-carbohydrate high-fat diet on metabolism in humans and mice. Lipids Health Dis 22, 191 (2023). https://doi.org/10.1186/s12944-023-01956-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01956-3