Abstract
The malignant tumor is a multi-etiological, systemic and complex disease characterized by uncontrolled cell proliferation and distant metastasis. Anticancer treatments including adjuvant therapies and targeted therapies are effective in eliminating cancer cells but in a limited number of patients. Increasing evidence suggests that the extracellular matrix (ECM) plays an important role in tumor development through changes in macromolecule components, degradation enzymes and stiffness. These variations are under the control of cellular components in tumor tissue via the aberrant activation of signaling pathways, the interaction of the ECM components to multiple surface receptors, and mechanical impact. Additionally, the ECM shaped by cancer regulates immune cells which results in an immune suppressive microenvironment and hinders the efficacy of immunotherapies. Thus, the ECM acts as a barrier to protect cancer from treatments and supports tumor progression. Nevertheless, the profound regulatory network of the ECM remodeling hampers the design of individualized antitumor treatment. Here, we elaborate on the composition of the malignant ECM, and discuss the specific mechanisms of the ECM remodeling. Precisely, we highlight the impact of the ECM remodeling on tumor development, including proliferation, anoikis, metastasis, angiogenesis, lymphangiogenesis, and immune escape. Finally, we emphasize ECM "normalization" as a potential strategy for anti-malignant treatment.
Similar content being viewed by others
Background
Tumor cells and the tumor microenvironment (TME) constitute the main part of solid tumors. TME is composed of multiple cellular components, including fibroblasts, endothelial cells (ECs), immunocytes, adipocytes, and acellular component: the extracellular matrix (ECM) [1]. The occurrence and the development of malignant tumors depend on the extracellular signals and is not a completely autonomous process of tumor cells [2, 3]. Besides of intercellular contact, signaling transductions are mostly dependent on acellular components, which contains not only bioactive agents but also mechano-transductal properties. As an acellular component in the TME, the ECM attracts more attention from scientists as a key factor in cancer progression.
The ECM mainly consists of proteoglycans, glycoproteins, matricellular proteins (including secreted proteins acidic and rich in cysteine (SPARC/osteonectin), osteopontin (OPN/SPP-1), thrombospondin (THBS/TSP)) and structural proteins such as tenascin, collagen and laminin [4,5,6]. The ECM remodeling, which featured by the changes on the content, activity and crosslinking of these proteins trigger the variations of signal transduction. In most tumor tissues, the ECM remodeling is characterized by increased collagen synthesis and deposition, usually accompanied by the expression of remodeling enzymes such as matrix metalloproteinases (MMPs), lysyl oxidase (LOX), lysyl oxidase-like proteins (LOXLs), WNT1-inducible signaling pathway proteins (WISPs) and others [7]. These enzymes can treat specific ECM components as substrates and catalyze them to control tissues stiffness and cell–matrix interactions through their unique biochemical and physical properties [8]. Some enzymes process matrix components, such as collagen, resulting in the production and release of bioactive fragments [9, 10]. Amount of changes in the expression level of MMPs in the tumor microenvironment represent the malignant degree of the tumor, reflecting the structural remodeling function of MMPs in the progression of many epithelial cancers, such as lung cancer, breast cancer, and pancreatic cancer [11, 12].
Meanwhile, cell-to-cell activity residing in the TME is constantly reshaping ECM, and these cells are affected by the signals provide by ECM itself [13]. Cytotoxic immune cells, which mainly conduct immune elimination, are incapable of stifling tumor cells in the remodeled ECM because of the formation of an immunosuppressive environment. Thus, ECM acts as a rock-solid shield protecting tumor survival and progression. However, there is no shield that cannot be destroyed. ECM can be a double-edged sword; while it stimulates tumor progression, it can also be a sally port to antitumor therapy. In this paper, we discuss the key role of ECM remodeling in the TME and its interaction with tumor cells, including the molecular composition of ECM and the impact of ECM remodeling on the occurrence and development of malignant tumors. We also highlight the impact of ECM remodeling on therapeutic resistance and potential therapeutic targets.
Molecular composition of ECM
The ECM is described as a collection of exocrine molecules that provide structural and biochemical support for the surrounding cells [14]. Similar to the significance of soil composition to plants, the ECM is the basic condition to provide adherable environment for cell proliferation and survival. From the perspective of molecular composition, the ECM mainly includes structural proteins (such as collagen and elastin), glycosaminoglycan, proteoglycan, and adhesion proteins (such as fibronectin and laminin). At the structural level, the ECM contains interstitial connective tissue matrix and basement membrane [15, 16]. The basic functions of the ECM include sustaining cell proliferation, differentiation and maintenance of tissue homeostasis [17]. Whereas, different kinds of organs or tissues have their own specific composition of ECMs. For example, loose connective tissue ECM is made up of reticular fibers and ground substances, and bone ECM contains collagen fibers and bone minerals, and the ECM for circulating blood cells is plasma. ECMs with different characteristics and compositions can play a role in many mechanisms. Various carbohydrate-rich polysaccharides and protein-rich fibers play an important role in maintaining tissue homeostasis and hydration [18]. The physical pressure on the ECM is controlled by the interspace composed of fibrils and the compression and buffering activities of polysaccharide gel [19, 20]. In normal tissues, the sequentially regulated ECM acts as a signal library and provides anchorage points and architectural definitions for mechanical sensing [21]. Whereas in the TME, ECM structures are reconstituted and intercellular signals are disrupted [22]. Therefore, better understanding the composition and structural characteristics of ECM remodeling in cancer is critical for discovering therapeutic targets and diagnostic markers [23].
Multiple factors mediate ECM remodeling
Hypoxia and CAFs
Hypoxia is a common feature of TME. Indeed, hypoxia is the result of unlimited expanding of tumor tissue and increasing requirement of nutrients including oxygen. It can weaken the function of cytotoxic T lymphocytes (CTLs) and attract regulatory T cells (Tregs), thereby reducing the immunogenicity of tumors and enhancing the invasive clonal expansion of heterogeneous tumor cells [24]. With the advancing of tumor progression, the establishment of hypoxic microenvironment can promote hypoxia-inducible factor 1 (HIF-1) activation-dependent signal transduction and help tumor cells and stromal cells adapting to surrounding hypoxia conditions, thus supporting tumor progression [25]. Hypoxia-induced increased expression level of several ECM remodeling enzymes such as LOX and collagen prolyl 4-hydroxylase (C-P4H) has been reported to mediate modification on collagens and promote tumor progression [26,27,28].
Endogenous fibroblasts overexpressing HIF-1α can promote tumor growth both in vivo and in vitro [29]. Nevertheless, another research suggested that chronic hypoxia plays dual role on cancer-associated fibroblasts (CAFs) function in a HIF-1α dependent manner [30]. On the one hand, HIF-1α can promote the carcinogenic effects of CAFs, such as promoting tumor growth, while block other carcinogenic functions, such as tumor invasion and metastasis [30]. Significantly, CAFs are main “architects” to mediate ECM remodeling and cause ECM stiffness and degradation [31]. Rising evidence emphasize different role of specific subtypes of CAFs in pancreatic cancer [32, 33]. Even a proinflammation role of hypoxia-induced CAFs has been reported recently [34]. Therefore, the role of Hypoxia-mediated CAFs in the whole process of tumor development needs to be further revealed.
ECM remodeling enzymes
In the development of various organs, the LOX family of enzymes trigger extracellular collagen crosslinking, which also contributes to the formation of ECM stiffness in malignancy, in a hypoxic environment [35, 36]. During hypoxia, key target genes regulated by HIF transcription factors include LOX, LOXL2 and LOXL4. These transcription factors are intricate in collagen crosslinking and are one of the key factors leading to tumor fibrosis [37]. In general, hypoxic signal transduction may be involved throughout tumor progression and contribute to ECM remodeling in the tumor microenvironment. Besides of LOX and LOXLs, WISPs can also mediate collagen I linearization to control cancer metastasis. WISP1 fuels the linearization and metastasis and is overexpressed in cancer cells, while WISP2 against the process but its expression level is suppressed [38]. However, a recent report highlights that CAFs-derived WISP1 can hampers lung metastasis [39]. It is considered that the derivation of WISP1 may play the critical role.
MMPs are a group of zinc-dependent endopeptidases that bind to various ECM proteins and are one of the key enzymes for connective tissue remodeling [40, 41]. A big family contains more than 30 kinds of MMP have been identified since the MMPs’ role as collagen hydrolase was unveiled [42, 43]. MMP-2, -3, -9 and -14 are overexpressed and associated with ECM remodeling in a variety of malignant tumors [44, 45]. In the process of tumor progression, MMP-2 and MMP-9 can mediate the invasion of tumor cells into the basement membrane through the degradation of collagen IV, thus resulting in tumor metastasis and diffusion [46]. Besides, collagen degradation is an important mechanism for remodeling the biomechanical properties of ECM. Immunocytes recruited by LOX at the pre-transfer site degraded collagen IV through high expression of MMP-2, then promote the formation of pre-transfer niche by MMPs [47]. Conversely, reduced activity of MMPs inhibits pulmonary vascular permeability and limits the infiltration of immunocytes in the lung prior to metastasis [48]. Similarly, MMP-14-induced collagen dissolution around tumor cells is also one of the critical factors for cell invasion and migration [49]. MMP-14 produced by the tip cells of polarized multicellular masses degrades interstitial collagen, resulting in a locus that is a key pathway for cell invasion [50, 51]. Besides of ECM degradation, the binding of MMP-9 to α4β1 integrin induces several intracellular signaling to promote anti-apoptotic pathway and metastatic pathway in cancer cells, suggesting another pivotal mechanism of MMPs-induced tumor progression [52, 53].
Heparan sulfate proteoglycans (HSPGs) are one of major component of ECM and can regulate cell behavior and maintain stromal structure stability by binding and releasing many signaling molecules, such as interleukin-8 (IL-8), fibroblast growth factors (FGFs) and vascular endothelial growth factor (VEGF) [54, 55]. Heparinase is an essentially endo-β-D-glucuronidase, which can degrade HSPGs to produce low molecular weight fragments [56]. High expression of heparinase has been detected in a variety of tumor patients and is significantly associated with poor prognosis in patients with head and neck squamous cell carcinoma [57], breast cancer [58], and gastric cancer [59]. Physiologically, heparinase is produced by keratinocytes, platelets, placental trophoblast cells, and white blood cells (including mast cells) [60]. In the tumor microenvironment, heparinase drives the cleavage of HSPGs to enhance the availability of various secretory factors, leading to tumor angiogenesis, and promoting cell invasion and migration [61,62,63]. Heparinase can also degrade perlecan and syndecan-1 (Sdc-1/CD138) to mediate tumor cell growth and invasion [64]. Interestingly, heparinase plays a crucial role in the invasion of natural killer (NK) cells into dense tumor ECM, thereby resisting tumor progression and metastasis, emphasizing that the ECM is a barrier for both tumor cells and immunocytes [65].
Besides of enzymes discussed above, more tumor-associated ECM remodeling enzymes have been unveiled. These enzymes show significant role in boosting tumor progression and can be considered as potential candidates for anticancer therapy.
Myeloid cells
Myeloid cells are the main hematopoietic cells in the human body, which are differentiated from hematopoietic stem cells [66]. Myeloid cells are involved in ECM remodeling in varying degrees, and produce both ECM remodeling enzymes/mediators and ECM molecules directly. In the tumor microenvironment, MMPs are primarily derived from myeloid cells and are involved in ECM collagen remodeling [67]. Stromal cell protein SPARC is a matrix regulator and collagen chaperone [68]. SPARC-deficient microenvironment in breast cancer reduced primary tumor growth and lung metastasis, possibly due to the macrophages with SPARC-deficiency and unable to support stroma formation and collagen deposition [69, 70]. These studies suggest that macrophages may be the source of ECM-related proteins. Transcriptomic and proteomic analyses of these cells have demonstrated that tumor-associated macrophages (TAMs) are one of the sources of ECM molecules and process collagen synthesis, stability, assembly and cross-linking [71, 72]. Abnormal collagen fiber deposition was found in colorectal cancer grown in macrophage-deficient mice [73]. Precisely, TAMs can regulate collagen production by stimulating CAFs [73, 74]. In pancreatic cancer, TAM-derived C-X-C motif chemokine ligand 3 (CXCL3) targets CAFs’ C-X-C motif chemokine receptor 2 (CCR2) to mediate CAF-myofibroblasts (myCAF) transition, subsequent type III collagen generation and tumor metastasis [75]. Intriguingly, single cell analysis uncovered a specific TAM-CAFs transition in lung cancer, highlights the consistent interaction between TAMs and CAFs [76]. The precise introduction of TAMs in ECM remodeling will be discussed later.
Microbiome
With the in-depth study of tumor microenvironment, the role of microbiome in tumor ECM remodeling cannot be ignored [77, 78]. There are several sets of evidence that the microbiome is actively involved in creating the tumor microenvironment and interacting with multiple elements [79, 80]. Representative research found out that subcutaneous injection of M. hyorhinis in mice can develop resistance of pancreatic ductal adenocarcinoma (PDAC) to gemcitabine, and further investigation unveiled microbiome prevalence (especially γ-proteobacteria) in approximately 75% of human PDAC clinical samples [81]. Similarly, other researchers have reported the presence of different types of microbiotas in samples from multiple malignancies [82, 83]. Clinical trials about chemotherapeutic agents, cyclophosphamide and oxaliplatin, have shown that reactive oxygen species (ROS) produced by the microbiome in the tumor microenvironment contributes to better chemotherapeutic efficacy [84]. The efficacy of these drugs was significantly reduced in immunocompetent mice compared to germ-free mice, along with significantly reduced tumor clearance and immune activation, highlighting the critical role of symbiotic bacteria in the regulation of the host immune system [85].
Positive interactions between tumor cells and the microbiome increase the likelihood of ECM remodeling leading to cancer niche formation, tumor progression, and drug resistance. A variety of bacterial enzymes (such as collagenase, elastase and hyaluronidase) are known to degrade host ECM [86, 87]. Studies have shown that in bladder cancer, the interaction between the host bacterial population and ECM components regulates the main composition of the microenvironment, thus determining tumor growth and metastasis [88]. In addition, the microbiome can induce intestinal fibrosis by triggering host immune cells [89]. Taken together, all these results suggest that the microbiome can influence host ECM and its homeostasis, but the details of these interactions have not been elucidated and extensive further studies are needed to accurately elucidate the molecular and signal cascades involved.
ECM remodeling and cancer proliferation
Unlimited expanding of tumor tissues results in increasing risk of heterogeneity and severe local vicinity which abrogates the infiltration of anti-tumor agent and cells in TME. It’s worth noting that the sum of bulk acellular and cellular components in TME, specifically cells with accelerated cell cycle and/or suppressed cell death regulated by ECM, leads to the uncontrolled expanding of cancer. However, different composition of ECM determines various fates of cell proliferation. Phenomena from the radiation-induced ECM regulation on cell cycle arrest/progression of both malignancy [90, 91] and fibroblasts [92] underpins the opinion. Nevertheless, most studies indicated that acellular components secreted by depositional cells show the potential to participate in oncogenic progression, or suppress cancer growth to transform tumor cells into a quiescent state, which endowing cells with stem-like characteristics and proliferative potential, for against stresses during growth and metastasis. For instance, the role of type III collagen in maintaining tumor dormancy to form a metastatic niche has been unveiled [93]. Therefore, ECM seems like a specific reaction pool filled with signals of mechano-transduction and bioactive molecules transduced from cancer and stromal cells to regulate tumor tissue proliferation, and it is emergency to unveil the program of ECM remodeling and identify specific therapeutic target for cancer elimination [94].
Receptors and signaling pathways: eyes blinded by mirage
The activation of cell cycle signals is critical for tumor proliferation. ECM can activate or inhibit intracellular signal transduction process, thus regulating cell biological behavior. The most prominent transduction pathway downstream of ECM signals is direct transduction, which occurs via classical transmembrane gated proteins such as integrins [95]. Dimerization of integrin subunits (including α chain and β chain) triggers phosphorylation of the focal adhesion kinase (FAK)/Src pathway, leading to increased cell adhesion and migration [96]. Substantial transformation of cell behavior occurs due to activation of intermediary pathways downstream of FAK/Src activation, such as the extracellular signal-regulated kinase 2 (ERK2)/mitogen-activated protein kinase (MAPK) cascade, and small guanosine triphosphatase (GTPase) (such as β-catenin pathway, Rac and Rho) (Fig. 1) [97,98,99]. Attentionally, specific integrin β subunits are critical for cancer cell proliferation in different aspects. For instance, β1 integrin alteration physiologically maintains mammary gland proliferation and is associated with accelerated cell cycle and temsirolimus resistance in bladder cancer [100, 101]. By contrast, β3 integrin is necessary for stem-like tumor-repopulating cell (TRC) dormancy [102], and CD90-inhibited anchorage-independent growth in cancer stem cells (CSCs) [103]. Thus, β1 integrin inclines to accelerate cell cycle, whereas β3 integrin prefer to stemness.
Receptors for cell-ECM interaction. Matrix changes modulate intracellular signaling in cancer, changes in the extracellular matrix regulate many intracellular signaling pathways. However, the illustration only summarizes familiar receptors in cell-ECM interaction, such as integrins, DDRs, CD44 and syndecans. Other receptors and regulatory networks are precisely introduced in the context
Hyaluronan/hyaluronic acid (HA) is a member of glycosaminoglycans and prevalently overproduced by cancer and stromal cells. The interaction of HA and membrane receptor CD44 or Toll-like receptor 4 (TLR-4) sustains proliferation of cancer and the formation of fibrosis [104,105,106]. HA/CD44 stimulates epidermal growth factor receptor (EGFR) singling pathway [107], whereas HA/TLR4 is associated with the activation of nuclear factor-kappaB (NF-κB) pathway [106, 108]. Receptor for hyaluronan-mediated motility (RHAMM, or CD168) is another HA-specific receptor that mediates inflammation and tumor progression [109]. Physiologically, hyaluronan synthase 2 (HAS2)-dependent HA/CD44/RHAMM pathway is critical for mammary gland morphogenesis [110]. In glioblastoma, the negative association between the concentration of extracellular HA and the efficacy of EGFR inhibitor indicates that HA is a main signal factor to stimulate cell proliferation [111]. Rho-GTPase is the primary downstream pathway to response HA/CD44 activation, and inhibition of Rho-associated coiled-coil containing kinase (ROCK) results in elevated CD44 expression and maintaining of CSCs [112, 113]. Both Rho-GTPase and EGFR could stimulate phosphoinositide 3-kinase (PI3K)/Akt pathway to sustain proliferation in cancer cells [114]. Attentionally, the function of HA in pro- or anti-oncogenesis is determined by its’ molecular mass [115,116,117]. Thus, the identification of HA molecular mass in cancer is critical.
Syndecans are cell-surface heparan sulfate proteoglycans known to play a role in cell adhesion, migration, and binding of growth factors. A canonical signaling about syndecans in cancer is heparinase/syndecan-1 pathway [118]. Heparinase stimulates syndecan-1 expression, cleavage and shedding to promote fibrillar collagen deposition and tumor growth [119,120,121]. Shedding syndecan-1 could combine with VEGF and adhere to ECM, then induce invasion and angiogenesis in melanoma [121]. While depletion of syndecan-1 in colon cancer cell line in turn stimulates heparinase expression and retain cells into a stem-like state [122]. Furthermore, previous explorations have identified that tenascin-C (TNC) interacts with syndecan-4 and blocks integrin/syndecan complex to mediates cell-fibronectin adhesion, which sustains proliferation and induces angiogenesis in cancer [123,124,125]. After that, more subtypes of syndecans have been identified to be associated with progression of cancer [126, 127].
Stromal cells in ECM such as CAFs play a significant role in supporting cancer cell proliferation. A colorectal patient-derived organoid (PDO) model shows that solely CAFs are sufficient to support cell proliferation with the absence of conventional PDO-associated growth factor, highlight the CAFs’ pro-oncogenic role in cancer culture maintenance [128]. In a 3D model of PDAC, pancreatic stellate cells (PSCs) could play the same role [129]. A 3D-bioprinting culture of glioblastoma which contains cancer cells, endothelial cells, and hyaluronic acid derivatives has proved the significance of ECM stiffness on the variation of gene transcription program and interaction between cancer and endothelial cells [130]. Moreover, high expression of syndecan-1 in CAFs correlated with tumor progression in specific cancer types from breast, colon, prostate, ovary and lung [131,132,133,134]. Matrix enzymes and ECM components secreted from stromal cell are critical for cancer cell proliferation, while cancer secrets soluble growth factors sustaining stromal cells survival and ECM generation in turn [135, 136]. Therefore, the interaction between cancer cells and stromal cells controls ECM remodeling leading to the proliferation of cancer, whereas the expanding tumor tissue will face some troubles, especially under the overseeing of pathways controlling organ size.
Escape from tissue size surveillance
Hippo signaling pathway is a key regulator of tissue growth and organ size control when facing mechanosensory pressure and increasing cell adhesion. Though crowded space caused by tumor proliferation and desmoplasia may result in enhanced mechano-transduction and intercellular contact, cancer cells present the tenacity to adapt the pressure from ECM and maintain proliferation. Abnormal activation of Hippo core factors Yes1 associated transcriptional regulator (YAP) and WW domain containing transcription regulator (TAZ, or WWTR1) sustain cell proliferation and is prevalently detected in various cancers, implying the success adaption of cancer to crowed environment [137, 138]. Integrins and downstream pathways are main signal traducers for YAP/TAZ activation [139]. Among them, canonical Rho GTPases senses signals from ECM and regulates YAP/TAZ activation, while dual role of Rho GTPases in tumor progression is dependent on their expression and mutation states [140].
Other receptors identified recently such as CXCR4 in hepatoma also senses stiffness of ECM and activates YAP/TAZ activation [141]. Moreover, dysregulation of YAP/TAZ co-transcriptional function triggers and impairs autophagy sensitivity to contact inhibition via absent F-actin expression [142], while ECM stiffness stimulates YAP/TAZ activation and exosome secretion to enhance cell mobility [143]. Besides manchanotransduction, Wnt ligand-linked collagen culture is sufficient to induce YAP/TAZ activation and support cellular reprogramming [144], in accord with previous discovery of Wnt-YAP/TAZ pathway [145, 146]. It’s worth noting that roles of YAP and TAZ in cell cycle regulation are considered different. In non-small cell lung cancer (NSCLC), YAP favors cell cycle progression while TAZ is preferentially associated with ECM organization [147]. Role of TAZ in ECM remodeling has been described in fibroblasts and adipocytes [148, 149]. Moreover, YAP increases nucleic accumulation of P27, a cell cycle suppressor by acetylating and nucleic-exporting S-phase kinase associated protein 2 (SKP2) via Akt activation to sustain cytoplasm retention of SKP2 and to inhibit mitosis of cancer cells, which causes polyploidy formation [150]. While accumulation of cytoplasmic SKP2 then triggers ubiquitination of forkhead box protein O1 (FOXO1) and then stimulates cell proliferation [150]. Nevertheless, another research found out that nuclear YAP inhibits P27 expression in a transcriptional pattern [151]. These results indicate that YAP activation is a primary factor to induce cell proliferation under the surveillance of Hippo pathway (Fig. 2A). Compared with malignant cells, YAP activation in PSCs triggers the secretion of SPARC, an ECM protein, to suppress tumor growth [152]. Importantly, ECM is essential for reprogramming of normal cells into premalignancy, and YAP/TAZ is responsible for the transformation induced by ECM [153]. The crosstalk of YAP/TAZ pathway between cancer cells and stromal cells also provides novel view in ECM-induced tumor progression [154]. Thus, it’s critical to unveil the precise function of YAP and TAZ in ECM-induced tumor progression.
ECM role in proliferation, cell cycle arrest and anoikis resistance. The changes in ECM proteins, especially the crosslink of collagen, enhance the stiffness of ECM. Receptors sense the mechanic signal form ECM and activates downstream pathways including FAK and YAP/TAZ to induce cell cycle progression and cell-ECM adhesion (A). Moreover, ECM support detachment cell survival, even induce cell cycle arrest in a quiescent state to decrease energy consumption and resist to anoikis (B). Several components support the acquirement of anoikis resistance such as tenascin-C, collagen, HA and PDGFB (C)
Cell cycle progression and quiescent: the golden mean
With tumor progression, epithelial-mesenchymal transition (EMT) transforms cells into heterogeneity which favors invasion and metastasis. Some cells lose their intercellular adhesion and detached from tumor tissue in a timepoint. However, cell adhesion is still necessary for survival, especially for detached cancer cells with insufficient preparation. Intracellular contact provides basal signal for cell survival and loss of cell adhesion triggers cells death. Physiological tissue stiffness, which causes decreased cell adhesion, suppresses cell cycle via inhibiting FAK signal responding and downstream Rac-dependent Cyclin D1 expression [155, 156]. However, high matrix density caused by cancer provides components for cell adhesion and stimulates vital survival signaling against detachment. Pathologic ECM induces FAK and ERK signaling pathway activation to promote cell proliferation in breast cancer [157] and gastric cancer [158]. Within ECM, collagen I sustains CSCs renewal and tumor initiation via the activation of FAK in pancreatic cancer [159]. Moreover, epithelial cell transforming sequence 2 (ECT2)/FAK and collagen type X alpha 1(COL10A1)/discoid protein domain receptor 2 (DDR2)/FAK pathways contribute to the proliferation and adhesion on ECM of lung adenocarcinoma cells [160, 161]. Thus, ECM components provide signals to activate FAK pathway and sustain cell proliferation under the situation of detachment, which is critical for pre-metastasis state formation of cancer (Fig. 2B).
Some therapies are introduced to target ECM-induced proliferation which may hamper cancer cell transformation into pre-metastasis state. Homologous esophageal ECM treatment hinders malignant proliferation and suggests a suppression of cell cycle in neoplastic esophageal cells [162]. Moreover, synthetic materials are considered to mimic the variation of ECM and observe changes in cell cycle, such as C60 nanofilm [163]. Identification of critical cell cycle factors may also contribute to the discovery of ECM-based anti-tumor treatment. Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor, which targets the combination of cyclin D1 and CDK4/6 to suppress cell cycle, shows extensive influence in inhibiting ECM deposition and oncogenic properties [164]. Besides Cyclin D1, CDK1 recently has been recognized as a pivotal cell cycle regulator which response to cell adhesion and has a profound association with cancer progression [165,166,167]. However, the precise mechanism of CDK1 in responding to pro-oncogenic ECM is still unclear.
By the way, cell cycle arrest doesn’t present a low risk of tumor progression. The state of quiescence featured by reversible proliferative arrest reserves the capacity to reenter the cell cycle upon receiving an appropriate stimulus [168]. When facing therapeutic treatment or disseminated from the primary tumor tissue during metastasis, cancer cells with quiescent state are able to survival under these pressures. Before obvious formation of distant metastasis, systemic dissemination of quiescent tumor cells has been existed depending on normal ECM-cell adhesion-induced syndecan-1 activation [169]. Quiescent cancer cells with the potential of recurrence form a comfortable environment of fibrillar fibronectin matrix via integrin, ROCK and transforming growth factor β2 (TGF-β2) pathways, whereas an outgrowth would be started by MMP-2-mediated ECM degradation in breast cancer [170]. In melanoma, ECM stiffness triggers translocation of Cdc42, a transcription factor belongs to Rho-GTPase, then upregulating ten-eleven translocation 2 (Tet2) expression which resulting in induction of quiescent via decreasing expression of p21 and p27 [102]. A gene signature analysis based on glioblastoma PDO model supports the correlation between quiescent state and hypoxia/TGF-β-dependent ECM [171]. Hydrogel models cultured by cancer cell lines from different organ demonstrate various quiescent dynamic, which implying a significant of organ specific ECM in the maintenance of quiescent state [172]. Changes in chemical and mechanical properties in hydrogel culture determines balance between quiescence and reactivation [173]. These phenomena highlight that stiffness and biomolecules in ECM are both critical for quiescent state sustaining. Herein, tumor with enhanced proliferation and necessary quiescence triggered by ECM becomes invasive and dangerous.
Anoikis resistance: survival from detachment
Anoikis is a property of programmed cell death responding to the detachment between cell and ECM. Anoikis is pivotal in normal development and tissue homeostasis, whereas anoikis resistance is essential in cancer invasiveness and metastasis [174]. As described previously, the activation of FAK signaling pathway plays the pivotal role in sustaining cancer cell proliferation from detachment. Signal receptors such as integrins are critical for FAK activation. While inhibition of integrin can mimic the situation of cell-ECM detachment and blocking integrin suggests a reasonable treatment to trigger anoikis and tumor suppression. Arg-Gly-Asp (RGD) peptide is designed to specific target and block integrins, and shows the stimulation of anoikis in glioma cancer stem cells [175]. Expressing β3 integrin in cancer cells is essential for distant metastasis in breast cancer [176]. Specific RGD targeting β3 integrin could inhibit adhesion and tumor-derived small extracellular vesicles absorption in normal breast cells [177], it also favors anti-apoptosis and autophagy in breast cancer cells [178]. Whereas in esophageal squamous cell carcinoma, indomethacin driving β3 integrin blocking results in reduced tumor growth and the potential of metastasis [179]. The paradoxical phenomenon may due to the anti-tumor immune response triggered by indomethacin.
EGFR pathway is strong associated with detachment induced anoikis. Targeting EGFR is effective for anoikis suppression [180]. For instance, α2β1/α5β1 integrin/EGFR pathway supports cell survival and stimulates anoikis resistance in colon carcinoma [181]. Pre-mRNA splicing factor 4 kinase (PRP4K), a pre-mRNA splicing enzyme, decreases degradation of EGFR during detachment and induces anoikis resistance [182]. Moreover, when PDAC treated with ECM depletion agent FAK inhibitor, the abnormal expression of signal transducer and activator of transcription 3 (STAT3), downstream of EGFR, hampers the function of FAK inhibitor and leads to drug resistance [183]. Furthermore, syndecan binding protein melanoma differentiation associated gene-9 (MDA-9, or syntenin), sustains EGFR signal and protective autophagy against anoikis in glioma CSCs [184]. Lung fibroblast expressed syndecan-1 promotes breast cancer lung metastasis, implying the pro-oncogenic role of this glycoprotein in ECM [185]. Syndecan-4 also participates in the progression of anoikis resistance [186, 187]. Intriguingly. inhibition of EGFR triggers suppression of EMT, Notch pathway and syndecan-1 expression via ERβ pathway, suggests the pivotal role of EGFR in anoikis resistance [188].
Components within ECM sustain cell survival against detachment induced anoikis. Collagens such as collagen I/β1 integrin in gastric cancer [189], collagen XIII/β1 integrin in breast cancer [190], COL11A1/Akt/Cyclic AMP response-element binding protein (CREB) in PDAC [191], collagen IV/integrin in hepatoma [192], support anoikis resistance via B-cell lymphoma (BCL) family proteins and downstream pathways. Attentionally, acid stress in local environment is associated with the function of collagens on tumor cells [193]. Suppression of phosphatases also contributes to the inhibition of collagen generation, highlights the critical role of posttranslational modification of collagen [194]. HA/CD44 also contributes to the activation of Akt pathway, which is responsible to collagen-induced anoikis resistance [195, 196]. Compared to the activation of AMP-activated protein kinase (AMPK) pathway in circulating cancer cells, Akt activation is prevalently detected in primary and distant metastatic cancer cells, and the negative feedback loop between AMPK and Akt implying the ability of adaptation of cancer cells to ECM [197]. Besides, platelet-derived growth factor-BB (PDGFB) secretion from anoikis resistant gastric cancer cells constructs a C/EBPβ-PDGFB-PDGFRβ-MAPK feedback loop with vascular ECs, which supports angiogenesis and metastasis in gastric cancer [198]. Tumor-secreted PDGF also stimulates CAFs to facilitate ECM stiffness [199]. Additionally, α11 integrin/PDGFRβ+ CAFs respond to the stimulation and promotes invasion and metastasis via c-Jun N-terminal kinase (JNK)/TNC in breast cancer [200]. Under the chemotherapy, JNK pathway activation triggers osteopotin (or SPP-1) and TNC secretion, then induces chemoresistance and metastasis in breast cancer [201]. Intriguingly, TNC-derived peptide TNIIIA2 confers anoikis resistance and PDGFRβ activation in an α5β1 integrin-dependent manner, suggests a possible positive feedback loop between cancer and CAFs [202, 203]. With the stimulating of ECM components, cancer cells are advantaged to survival against anoikis (Fig. 2C).
Indeed, intercellular and intracellular elements take together trigger anoikis resistance. P53 is a pivotal factor to mediate cell apoptosis, and the existence of gene mutation of P53 is prevalent in most cancer cells [204]. Physiologically, losing of cell adhesion would trigger P53-mediated apoptosis to sustain tissue homeostasis. ECM components provide the loci for cell adhesion and overcome the P53 mediated apoptosis in glioblastoma [205]. While the treatment of FAK inhibitor, which results in ECM depletion, in overcoming chemoresistance depends on the activation of P53 signaling pathway [206]. Nevertheless, P53 mutation plays a pivotal role in anoikis resistance against losing adhesion signals [207]. When β1 integrin is blocked, mutation of P53 in cancer fails to response to the absence of β1 integrin signal therefore bypasses apoptosis [208]. Moreover, P53 mutation itself links to ECM remodeling and mediates cancer progression. For example, P53 mutation is necessary for HIF-1 constructed ECM [209]. It also increases podocalyxin sorting into exosomes and induce ECM deposition [210]. Mechanically, mut-p53/HIF1α/miR-30d axis triggers tubulo-vesiculation of the Golgi apparatus and promotes ECM deposition and remodeling [211]. Thus, adequate preparation for the distant metastasis of malignancy has been prepared under the circumstances of ECM remodeling (Fig. 2). These variations link anoikis with metastasis of cancer cells, suggest that anoikis is prerequisite to cancer invasion.
ECM remodeling and metastasis in cancer
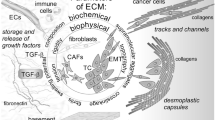
ECM remodeling constructs the architectural and bioactive environment to support cancer invasion and metastasis. Tumor surrounded by collagen-rich circumstances not only faces the pressure from ECM stiffness, but also receives stimulators from other components within ECM. When tumor cells overcome the detachment-induced anoikis, further variations on the phenotypes of cancer are needed for distant metastasis. These characterizations including EMT for basic ability to induce invasion, collagen orientation for metastatic architectural construction, matrix-related enzymes to facilitates ECM remodeling, metabolism preparation to support cell metastasis, secretion of ECM components to provide cell-ECM adhesion and drivers mediates metastasis from primary loci to intravasation. All features are essential for ECM-mediated tumor invasion and metastasis, and the understanding of this profound network may contribute to the anti-tumor therapy targeting ECM. Some ECM components promote cancer progression doesn’t mentioned below are listed in Table 1.
ECM-induced EMT: ready for invasion
For epithelial cells, EMT is a critical process for tumor progression and metastasis [237]. EMT is featured by decreased expression of epithelial markers such as E-cadherin, and the upregulation of mesenchymal factors including N-cadherin, snail family transcriptional repressor (SNAIL), twist family basic helix-loop-helix transcription factor (TWIST), and other factors. These variations trigger mobility and invasion of cancer cells. Increasing number of researches focus on the function of ECM components on EMT in cancer cells. For instance, co-culture of high-metastatic breast cancer-derived ECM stimulates EMT in breast cancer [238]. When cellular components are eliminated, acellular scaffolds are sufficient to promote EMT and invasion of breast cancer [239, 240]. Specific chondroitin sulfate scaffold also stimulates EMT in prostate cancer [241]. A logical analysis based on EMT cellular regulatory network unveils ECM stiffness is a prerequisite for FAK/Src activation to stimulate SNAIL expression and intracellular adhesion via stimulating receptor-type tyrosine-protein phosphatases kappa (PTPRK) expression [242]. YAP may also play a significant role in mechano-regulated EMT. Wilms Tumor-1/YAP pathway suppresses E-cadherin expression and mediates cell–cell detachment, while YAP/Trio Rho guanine nucleotide exchange factor (TRIO)/Merlin mediated regulation of Rho GTPases, which promotes cell migration in renal cancer [243]. Gene-deficiency also confers cancer cells with EMT and metastasis via ECM generation, such as SET domain containing 2 (Setd2) in pancreatic cancer [244].
TGF-β is a primary inducer of EMT in cells whereas TGF-β signaling pathway plays dual function to promote tumor progression, or inhibits oncogenesis [245]. TGF-β promotes SRY-Box transcription factor 4 (SOX4) expression to mediate the cooperation between SOX4 and Kruppel-like factor 5 (KLF5) and induce subsequent pro-oncogenic EMT [246]. By contrast, small mothers against decapentaplegic protein 4 (SMAD4)-dependent EMT hinders the function and causes death in PDAC [246]. However, ECM-associated aberrant TGF-β-induced-EMT is prevalent observed in various cancers. RAS-responsive element binding protein 1 (RREB1) is a critical partner for TGF-β/SMAD-induced EMT, and fibrosis via downstream activating of SNAIL and other mesenchymal genes [247]. When treated with additional TGF-β, ovarian cancer cells demonstrate stimulated EMT and ECM remodeling [248]. In PDAC, TGF-β treatment triggers fibronectin and collagen I deposition, and metabolic variation which is responsible to metastasis [249]. ECM components such as the binding between tenascins and TGF-β isoforms [250] and EGF-domain of fibronectin fibrils [251] stimulate TGF-β signaling pathway and EMT process in normal cells, may potentially influence EMT in cancer cells (Fig. 3A).
The process and regulatory network during tumor invasion and metastasis within ECM. The metastatic process of cancer cells is similar to a distant journey. EMT, which triggers decreased cell adhesion, confers the initiation of invasion (A). Then cancer cells with anoikis resistance show multiple changes in metabolic process to support energy requirement for cell survival and invasion (B). Thus, a gradient directionality constructed by several ECM components guides them to penetrate through the tissue for hematogenous and/or lymphatic metastasis (C), the activation of which has been stimulated by ECM components (D)
In normal cells, TGF-β activation physiologically transforms myofibroblasts into fibroblast for pro-healing homeostasis sustaining [252]. However, the balance of this homeostasis will be destroyed under the regulation of cancer [32]. For instance, COL11A1 stimulates NK-κB/insulin-like growth factor binding protein 2 (IGFBP2) to induce TGF-β3 activation in ovarian CAFs to promote tumor growth [253]. PDAC cells induce TGF-β/SMAD5 activation in CAFs for branched-chain amino acids supplementation, which supports cancer metastasis [254]. In gastric cancer, activation of TGF-β1 in CAFs promotes mobility and invasion to lymphatic vessel in vivo [255]. In ovarian cancer, the activation of TGF-β2/SMAD upregulates the expression of CD44, MMP-9, and RHAMM via VCAN expression (encoding the chondroitin sulfate proteoglycan Versican) in CAFs to promote invasion and metastasis [256].
Additionally, transforming growth factor beta-induced protein (TGFBI, or βig-H3) is a matrix protein determined by TGFβ1 and secreted into ECM by various cancers [257]. Previous researches unveil that TGFBI stabilizes microtubules via FAK and Rho pathways [258]. Some researchers consider that TGFBI is a tumor suppressor because of the deficiency of TGFBI favors tumor formation [259], and high expression of TGFBI in tumor is associated with optimal chemotherapy sensitivity [260, 261]. However, TGFBI is a trouble maker to push cancer far away from primary location. In colorectal cancer, TGFBI promotes metastasis via stimulating β5 integrin/Src and disconnection of VE-cadherin junctions between epithelial cells [262]. In melanoma, TGFBI hinders cell adhesion to fibronectin, collagen-I, and laminin whereas co-localized with fibrillar fibronectin/TNC/periostin structures which favors invasive state of cancer [263]. In ovarian cancer, β3 integrin, against β1 integrin and syndecan-1, facilitates cell adhesion to TGFBI and induces migration and paclitaxel resistance [264]. Moreover, TGFBI sustains tumor cell survival after radiotherapy via stimulating FAK pathway activation in gastric cancer [265]. TGFBI also induces androgen deprivation therapy resistance, EMT, and bone metastasis in prostate cancer [266]. A research finds out that TGFBI causes decreased vessel perfusion and sever hypoxia, which favors metastasis in breast cancer [267]. Nevertheless, another research considers that TGFBI induce angiogenesis in colorectal cancer, indicating the organ-specific function of TGFBI in angiogenesis [268]. Intriguingly, TGFBI derived from non-cancer cells such as TAM in ovarian cancer also promotes cell invasion and metastasis [269, 270]. And the positive feedback loop of TGFBI secretion among TAMs/CAFs/TAMs constructs immunosuppressive TME [270], implying that the concentration of TGFBI in ECM is critical for metastasis but ignores its’ derivation (Fig. 3A).
By the way, ECM stiffness transduces mechanical signal to TWIST1, then destroys the interaction between TWIST1 and Ras-GTPase activating protein SH3 domain-binding protein 2 (G3BP2), which induces nuclear import of TWIST1 and EMT in tumor [271]. Mechanosensory receptor such as Ephrin type-A receptor 2 (EPHA2)/LYN protein also stimulates TWIST1 to induce EMT and invasion in responding ECM stiffness [272]. Other mechanosensors promoting EMT via several pathways such as transient receptor potential melastatin 7 (TRPM7)/SOX4 in breast cancer [273], CXCR4/ubiquitin domain containing 1 (UBTD1)/YAP in hepatoma [141] and EGFR in glioblastoma [274] have been reported. Attentionally, different tumor types rely on discrete EMT effectors [275]. A pan-genomic analysis links EMT phenotype with gene-coding proteins involved in the degradation of the ECM, supporting the oponin [276]. Because of different ECM program (generating acellular components in varying proportions) favors specific cancer progression, targeting characterized EMT biomarker is necessary.
ECM-induced metabolism reprogramming: fueling the metastasis
Metabolic reprogramming facilitates the basic requirement for cancer cell survival and progression by providing energy and specific products to synthesize proteins [277]. Warburg effect, a phenomenon marked by overwhelming glucose uptake and a disproportionate utilization of glycolysis for energy production, could rapidly generates adenosine triphosphates (ATPs) and nicotinamide adenine dinucleotide phosphate (NADPH) to support malignant biosynthesis [278]. Aberrant metabolism pattern in cancer also correlated with ECM remodeling. Glycolytic enzyme uridine diphosphate (UDP)-glucose 6-dehydrogenase in cancer promotes the secretion of HA during the EMT process [279]. Targeting rate-limiting enzymes, such as phosphofructokinase-2/fructose-2, 6-bisphosphatase 3 (PFKFB3) of glycolysis is sufficient to suppress tumor migration and fibrosis [280, 281]. Additionally, ECM can shape metabolic reprogramming of cancer cells, thus enhance their aggressiveness. HA digestion stimulates glycolytic metabolism and glucose transporter protein type 1 (GLUT-1) translocation on the membrane of various cancer cell lines in vitro and in vivo [282]. To respond to HA degradation, zinc finger protein 36 (ZFP36) is rapidly upregulated and induces the degradation of thioredoxin-interacting protein (TXNIP), followed by enrichment of GLUT1 at the plasma membrane and enhanced tumor migration [282]. Signals of mechano-transduction from ECM stiffness to hepatoma triggers activation of MAPK/YAP pathway and accelerated aerobic glycolysis [283]. Intriguingly, increased collagen density sheds metabolic program of triple negative breast cancer (TNBC) cells from glycolysis to oxidative phosphorylation (OXPHOS), the metabolic pathway relies on mitochondria [284]. Another research investigates the impact of ECM stiffness on cancer cells in a 3D culture, demonstrating that softer ECM favors YAP pathway activation, glycolytic metabolism and proliferation [285]. By contrast, stiffer ECM induces upregulation of fibronectin 1 and MMP-9 expression, OXPHOS and lipid metabolism, and invasion [285]. Though some reviews focus on the crosstalk between ECM and metabolism [286], the complex relationship between ECM and tumor metabolism is still mysterious, further explorations are needed.
Though Warburg effect decreases the requirement for oxygen and relies on glycolysis, acid products such as lactate secreted into ECM may hamper tumor cell function. However, high concentration of lactate facilitates immunosuppression and sustains tumor progression via stimulating stromal cells [287]. For instance, collagen production in ECM dependents on CAFs, and the activation of pyruvate carboxylase (PC), a rate-limiting enzyme of aerobic glycolysis, of CAFs can be detected in TME [288]. Lactates fulfilling TME are ideal substrates of PC and sustaining non-essential amino acid biosynthesis [288]. Thus, secreted amino acids promote cancer progression in turn. Indeed, mechanical signal from ECM stiffness triggers glycolysis and glutamine metastasis in CAFs, then CAFs provide aspartate to support cancer proliferation and induce ECM remodeling [289]. Intriguingly, an acid concentration gradient from the center to the outside of the tumor tissue, which naturally forms the metastatic directionality, mediates cell mobility and invasion [290, 291]. Besides of CAFs, the crosstalk between mitochondria and ECM stiffness in cancer has been unveiled recently. Softer ECM of distant metastatic niche can enhance therapeutic resistance of cancer cells [292]. Precisely, cancer cells generate mitochondrial fission, which is the state that mitochondria are split into smaller components, and triggers the activation of nuclear factor E2-related factor 2 (NRF2) to enhance glutathione metabolism [292]. Therefore, the crosstalk between metabolism and mechanotransduction has called more attention recently and considered as a potential target for anti-cancer therapy (Fig. 3B).
When cancer cells are detached from ECM, increasing production of ROS is lethal for tumor cell survival [293]. Thus, elimination of ROS is critical for cancer metastasis. A systemic review has discussed metabolic mechanisms suppressing ROS generation, including the activation of pentose phosphate pathway (PPP), NRF2-induced catalase and superoxide dismutase 2 (SOD2), glutamine metabolism, and AMPK pathway-induced autophagy [294]. Warburg effect could stimulate PPP pathway and NADPH production, which suppress ROS generation.
Intriguingly, several researches of pro-metastatic role of glutamine in ECM remodeling have been reported. Physiologically, HIF-1α controls chondrocyte proliferation under hypoxia during endochondral ossification whereas prolonged HIF-1α stimulation replaces aerobic glycolysis by glutamine metabolism. During the process, chondrocytes alter their function from collagen production into collagen proline and lysine hydroxylation, which leads to skeletal dysplasia [295]. In cancer, the sustaining of proliferation of detached cells depends on AMPK/Nrf2-induced glutamine metabolism when detached from ECM [296]. Tumor cells also enhance their aggressiveness via several glutamine-associated mechanisms such as upregulation of glutamine receptor [297], tissue transglutaminase [298] and glutaminolysis [299]. Besides, cancer cell clustering triggers mitophagy activation, which can decrease the generation of ROS, to support tumor survival during metastasis [300]. The activation of mitophagy in cancer during the detachment from ECM depends on the expression of receptor-interacting protein kinase 1 (RIPK-1) [301], and secretion of ECM protein decorin [302, 303].
Collagen orientation and CAFs: the fingerposts
Here, cancer cells are prepared for a long distant journey. The question is, where should they go? During normal development of organs, programs of cells have been well set from embryonic period to differentiation. Within a differentiated organ facing injury, cells just need to perform their duty to proliferation and migration which limited under a range of ECM [304]. The program will be shut off when everything becomes normal or cells meet their death. Nevertheless, cancer cells generating ECM create a gradient from primary loci of cancer to extravascluation site, then guide themselves’ migration in a certain direction. The organization of collagen, the most prevalent component in ECM, is critical for cancer progression [305, 306]. Clinical examinations have detected a profound association of collagen fiber orientation disorder to poor prognosis in breast cancer [307,308,309], Gastric cancer [310], and salivary gland cancer [311]. Pathological examination also detects prevalent disordered collagen orientation in various cancers [312,313,314]. A laboratory study demonstrates that high-density collagen co-culture transforms normal breast tissue into premalignancy [315]. Aged ECM transplantation also stimulates oncogenesis in breast cancer [316].
Importantly, collagen I-Matrigel composite extracellular matrix featured collagen orientation or less waveforms in ECM provides a contact guidance to typically induce directional migration of cancer cells [317, 318]. Several studies indicate that tumors prefer to invade and disseminate along radially aligned fibers rather than circumferentially oriented fibers [319, 320]. Moreover, collagen-rich environment mostly stimulates integrin signaling pathway and favors pre-metastasis niche [321]. In breast cancer, the balance between intracellular and cell–matrix adhesion determines ECM dynamics and the invasion of cancer cells [322]. Collagen I/α2β1 integrin triggers PI3K/Akt/SNAIL pathway to promotes metastasis in colorectal cancer [323]. In squamous cell carcinoma, ECM stiffness triggers collective invasion via mechanical sensing EGFR pathway-induced calcium absorption [324]. Additionally, when cancer cell invasion on aligned collagen fibers, the structure will lead cancer cell to invasion by sensing focal adhesion-mediated contact guidance [325]. In a 3D culture demonstrates that glycation enhances ECM stiffness and reduces directionality in aligned collagen gel, highlights the significance of posttranslational modification of collagen on tumor invasion [326]. These features together push cancer cells invasion, migration and metastasis in a certain direction.
CAFs are the primary derivation of acellular components including collagens in ECM [327]. Cancer stimulates CAFs proliferation and activation to facilitate ECM remodeling [328]. A ROCK-dependent paracrine axis has been recently identified and is responsible to breast cancer stimulated reprogramming of CAFs [329]. In PDAC, ECM stiffness induced by CAFs activates ERK pathway and promotes cancer metastasis [330]. In squamous cell carcinoma, IL-6 plays the same role by targeting STAT3/ERK pathway in CAFs [331]. Components derived from CAFs also provides bioactive signals for cancer progression. For instance, CAFs derived lumican promotes progression of gastric cancer via stimulating β1 integrin/FAK signaling pathway [228]. In breast cancer, CAFs expressing Hic-5 stimulates ECM remodeling and induces lung metastasis [332]. Matrix components laminin-5γ2 (LN-5γ2) triggers tumor budding, which defined as isolated single cancer cells or clusters of up to four cancer cells located at the invasive tumour front, of colorectal cancer cells [333, 334]. Mechanistically, LN-5γ2 interacts with β1 integrin to stimulate FAK and YAP activation in colorectal cancer [333], while increases density of stromal myofibroblasts in oral squamous cell carcinoma [335]. By contrast, decreased hyaluronan cross-linking mediated by CAFs favors invasion in breast cancer [336]. Attentionally, by using single cell high-throughput sequencing, specific CAF clones and their distinguished functions are precisely identified in PDAC [337, 338]. Intriguingly, a specific clone of CAFs associated with ECM (eCAFs) is identified in pancreatic cancer [339] and gastric cancer [340], and recently identified Endo180 and paired related homeobox 1 (Prrx1) may be significant genes associated with the function and plasticity of eCAFs [341, 342]. However, the precise role of eCAFs in cancer progression have yet to be elucidated.
Intriguingly, the orientation of fibers also contributes to the long-range distribution of CAFs [343]. Radially aligned ECM fibril constructed by cancer cells stimulates exosome secretion from CAFs to normal fibroblasts, therefore induces pro-oncogenic transformation of fibroblasts and tumor metastasis [344,345,346]. Moreover, activation of TWIST in CAFs at the tumor invasive front is associated with the expression of palladin and collagen α1, which facilitates arrangement of CAFs [347]. The concentration gradient of oxygen may also contribute to guide cancer cell metastasis. For instance, Hypoxia-induced Malic enzyme 1 expression and stimulates tumor budding, lactate production and YAP activation in oral squamous cell carcinoma [348]. Stress relaxion triggers procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) expression and results in migration according to oxygen concentration [349]. In tumor tissue, hypoxia suppresses collagen I deposition, while a gradient deposition of collagen I may be associated to the oxygen supplement, which needs further studies (Fig. 3C) [350].
Other factors point out the direction of metastasis
To adapt signals from ECM, changes on membrane-receptor in cancer cells are necessary to invade through the direction. DDRs are receptor tyrosine kinases that bind with collagen in an integrin-independent way [351, 352], and DDR1 can supplement the collagen-induced tumor progression when β1 integrin was eliminated [353]. Physiologically, DDR1 controls Mammary morphogenesis [354]. By contrast, DDRs play dual role to cancer cells. Downregulation of DDR1 triggers TGFBI secretion and tumor progression [355]. Collagen XV-mediated DDR1 suppression inhibits tumor invasion in PDAC [356, 357]. While activation of DDR1a/MMP2 promotes cell invasion in glioblastoma [358]. Collagen IV binds with DDR1 and mediates Src-dependent MMP2/9 secretion in TNBC invasion [359] and EMT in epithelial breast cell line [360]. Attentionally, the regulatory feedback between DDR1 and MMP secretion contributes to sustain the homeostasis in local ECM, highlights the significant role of DDR1 in ECM remodeling [361, 362]. For invasive directionality regulation, DDR1-induced Rho-GTPase member Cdc42 and its specific guanine nucleotide-exchange factor (GEF), Tuba is needed for ECM protein degradation [363]. Furthermore, inhibition of DDR2 induces decreased invasion of murine melanoma via ERK/NF-κB-mediated MMP secretion [364]. K14+ breast cancer cells expression DDR2 and CXCR4 and CAFs expressing DDR2 guides metastasis from primary tumor organoids to polarize to the leading edge and direct migration [365, 366]. Intriguingly, DDR2 mediates cell cycle arrest under the treatment of collagen, whereas deglycosylation of collagen can overcome the suppression in melanoma, implying the significance of collagen glycosylation [367].
Besides expression of ECM signaling receptors such as integrin and DDRs, expression of HA/CD44-mediated motility receptor RHAMM at the invasive front of colorectal cancer cells is necessary for invasion and metastasis [368]. It also mediates chemoresistance via activating TGF-β/Smad2 pathway [369]. Previous research demonstrates that HA/CD44 is essential for cancer cell bone metastasis [370]. After radiotherapy, a HA-rich environment constructed by activation of IL-1α/NF-κB/HAS2 pathway in cancer cells promotes glioblastoma metastasis [371]. These reports emphasize that the distribution of CAFs, together with cancer cells, is depends on the mechanical and bioactive signaling pathways (Fig. 3C).
Non-CAFs stromal cells also pave the way to invade ECM and guide cancer cells to metastasis. For instance, PSCs change the alignment of collagen fibers and induce ECM remodeling via Endo180 [372] and SPARC-dependent TGF-β/ROCK activation [129] to support cancer metastasis. In the omental environment of ovarian cancer, omental adipocytes, mesothelial cells and CAFs provides signals for cancer progression [373]. TAM polarization induces microtubules coherent within cancer cell and TAM and enhance tumor cell elongation [374]. Moreover, TAM-derived granulin stimulates hepatic stellate cells (HSCs) to secret periostin resulting in fibrotic microenvironment [375]. Bone-derived mesenchymal stromal cells (MSCs) secreted ECM components contribute to the metastasis [376]. The deficiency of stromal cell-secreted decorin stimulates colorectal cancer initiation and triggers ECM remodeling [377]. Other factor such as blood derived neural Wiskott-Aldrich syndrome protein (N-WASP) regulates lysophosphatidic acid recycling in a self-generated gradient and promotes PDAC metastasis [378]. Though a profound regulatory network within various cell types makes trouble to unveil precise therapeutic target, the program of ECM is the sally port for treatment designation, especially the identification of expression of ECM-associated enzymes.
Matrix-related enzymes: crossing the red sea
LOX family, including LOX and LOXLs, plays a significant role in cancer-associated ECM stiffness by crosslinking extracellular matrix proteins, collagen and elastin [379]. LOX increases stiffness and stimulates FAK/Src pathway in colorectal cancer [380]. Hypoxia stimulates LOX expression and increase collagen crosslinking and tumor invasion in ovarian cancer [381]. Among LOX family, the profound association between LOXL2 and cancer progression has been reported. High expression of LOXL2 in CAFs is correlated with poor prognosis in colorectal cancer [382], prostate cancer [383]. And High expression of LOXL2 in tumor tissues predicts poor survival in cervical cancer [384], neck squamous cell carcinoma [385], hepatoma carcinoma [386] and other cancer patients [387].
Mechanistically, HIF-1α stimulates LOXL2 expression and tumor progression in hepatoma [388]. Additional LOXL2 treatment increases PI3K/Akt-dependent fibronectin deposition and lung metastasis in hepatoma [389]. A positive feedback loop between stiffness-induced zinc finger E-box binding homeobox 1 (ZEB1) expression and ZEB1/LOXL2-induced ECM stiffness remains tumor cells in a mesenchymal state [390]. Moreover, the competition between miR-200 and ZEB1 regulates collagen deposition and crosslinking, which stimulates β1 integrin/FAK/Src pathway, to promote invasion and metastasis [391]. Proinflammatory cytokines oncostatin M stimulates LOXL2 expression and collagen I crosslinking and invasion [390]. While overexpression of LOXL2 increases lung metastasis possibly via SNAIL1 upregulation in breast cancer [392]. Anti-LOXL2 treatment is sufficient to suppress tumor progression in breast cancer [393]. However, inhibition of LOXL2, which causes decreased matrix context and stiffness, promotes cancer progression in PDAC, suggests the organ specificity of ECM stiffness in oncogenesis [394]. Other LOX family proteins also participate in tumor progression whereas less results have been reported. For instance, LOXL1 supports intraductal xenograft of lobular breast carcinoma cells survival [395]. LOXL4 stimulates activation of programmed death ligand 1 (PD-L1), which induces immunosuppressive phenotype in macrophage, and facilitates an immunosuppressive microenvironment to promote hepatocarcinogenesis [396].
MMPs, the matrix-proteinases mediate generation of fragments from acellular components, and have long been associated with cancer invasion, metastasis and angiogenesis [397]. The role of MMPs in cancer progression and inhibitors of MMPs have been well reviewed recently [398]. Heat shock protein (HSP) family participates protein folding and maturation intracellular and associated with tumor progression [399]. Intriguingly, some researchers suggest that extracellular HSPs are critical to assist MMPs and promote tumor progression via ECM remodeling [400]. For instance, extracellular HSP70 and HSP90 are critical to interact with MMP2 and enhance migration in breast cancer [401]. The function of HSP90 and MMP2 interaction is under controlled by tissue inhibitors of metalloproteinase 2 (TIMP2) and ATPase homolog 1 (AHA1) co-chaperones [402]. AUY922, an inhibitor of HSP90, decreases fibronectin secretion into ECM and hampers invasion in prostate cancer [403]. Interestingly, inhibition of HSP90 facilitates decreased contractility and increased TGF-β2 expression of CAFs in prostate cancer [404]. Moreover, HSP47 regulates TGF-β to mediate ECM remodeling in glioblastoma [405]. In breast cancer, HSP47 interacts with non-muscle myosin IIA (NMIIAH) to promote contractile force of actin filaments [406]. HSP47 also promotes cell-platelet adhesion to mediate tumor invasion and metastasis via collagen I [407, 408]. Extracellular HSPs have demonstrate the potential to act as a therapeutic target for ECM-associated cancer treatment, whereas lacking enough evidences.
ECM remodeling and angiogenesis in cancer
Unlimited expanding of cancer and increasing generation of ECM finally results in insufficient nutrients and oxygen, even impedes metastasis of cancer cells, theoretically. Angiogenesis is the process to form new blood vessels from pre-existing vessels, and it is essential to provide nutrients and oxygen for promoting tumor growth and hematogenous metastasis [409]. Besides, fibroblasts are critical in angiogenesis, while their function can be seriously controlled by cancer cells. A thorough review has concluded the role of CAFs and extracellular components generated from CAFs in tumor angiogenesis [410]. Briefly, ECM is essential for vascular integrity whereas sustaining pro-angiogenic signaling in tumors impairs the subsequent steps of vascular morphogenesis, namely the acquisition of a quiescent EC phenotype and the development of an intact and selectively permeable vascular barrier. In another word, in cancer, vascular basement membrane and ECM is destroyed and become less conjunctive with ECs. These features facilitate the advantage for cancer metastasis whereas enhance a potential permeability of immune cells, such as TAM (Fig. 3D) [410]. The influence of immune cells on ECM will be discussed latter.
Integrin signaling facilitates the basic stimulation of ECM-induced angiogenesis. For instance, β3 integrin induces VEGFR2 in ECs [411]. α6β1 integrin stimulated by VEGFA in ECs is essential for the formation of endothelial podosome rosettes, which promotes new vessel formation in tumor tissue, while laminin of the vessel basement membrane hampers the fucntion of α6β1 integrin [412]. Additionally, syndecan-1 is essential for new blood vessel maturation in cancer [413]. Chitinase-3-like protein 1 (CHI3L1/YKL-40), a glycoprotein secreted by various cancer cells and stromal cells, enhances the synergic effect of syndecan-1 and β3 integrin and activation of FAK/ERK pathway in ECs to promote angiogenesis in breast and colon cancer [414, 415]. Moreover, in glioblastoma, YKL-40 induces the synergic effect of syndecan-1 and β5 integrin and FAK/ERK-dependent VEGF secretion and VEGFR2 expression [416, 417]. In TNBC, β5 integrin facilitates cancer angiogenesis in vivo [418]. Syndecan-4 may also participate in the YKL-40-induced vessel formation [419]. Furthermore, tumor-derived ECM transforms normal endothelial cell and stimulate VEGF2 expression via β3 integrin/FAK/Src pathway in melanoma [420]. Low expression of β3 integrin in mural cells of blood vessel is associated with cancer progression [421]. Mechanically, losing β3 integrin in mural cells triggers phosphorylate FAK/HGFR/p65 and upregulation of CXCL1, C–C motif chemokine ligand 2 (CCL2) and TIMP-1, while CCL2 stimulates tumor progression via MAP/ERK kinase 1 (MEK1)-ERK1/2-ROCK2 pathway [421].
Recently, basement membrane multidomain heme peroxidase human peroxidasin 1 (hsPxd01) has been identified as a pivotal regulator to promotes angiogenesis via ERK, Akt and FAK pathway [422]. Peroxidasin crosslinks collagen IV and releases bromide to sustain ECs survival [423, 424]. Nevertheless, high expression of peroxidasin could be detected in invasive melanoma cells rather than non-invasive cells and generates hypobromous acid, suggests the additional reaction with other biomolecules which have yet to be elucidated [425]. ECM component elastin microfibrillar interface protein 2 (EMILIN2) binds on EGFR and stimulates angiogenesis, mediates sensitivity to chemotherapy [426]. The absence of Multimerin-2 (MMRN2), a member of EMILIN family expressed by ECs, hampers VEGFA/VEGFR2 activation and angiogenesis in xenograft model [427, 428]. The expression of matrix protein CLEC14A combines with MMRN2 and reactivate angiogenesis in cancer [429, 430]. MMP-9 mediates MMRN2 degradation and enhances tumor angiogenesis [431]. Intriguingly, blocking CD93-MMRN2 interaction in ECs favors angiogenesis and metastasis in tumor [432]. MMRN2 combines with and stabilizes CD93, therefore stimulates downstream pathways including β1 integrin, FAK and fibronectin expression [433]. Thus, MMRN2 is a potential biomarker for anti-angiogenesis therapy.
ECM remodeling and lymphangiogenesis in cancer
The lymphatic system is indispensable for the collection and cycling of tissue-extravasated fluids, macromolecules and immune cells into the bloodstream, especially for tumor metastasis through lymphatic vessel [434]. The canonical pathway for lymphangiogenesis in cancer is the combination of VEGF-C/D and VEGFR-3. The interaction would trigger activation of downstream pathways including Akt phosphorylation [435]. Factors and signaling pathways associated with lymphatic formation have been introduced whereas ECM role in lymphangiogenesis is unclear [436].
During wounding healing and inflammation, a fibrin-binding variant of VEGF-C induces lymphangiogenesis and ECM deposition [437]. ECM stiffness stimulates GATA binding protein 2 (GATA2) and GATA2-dependent VEGFR-3 expression, which mediates lymphatic endothelial cell growth and migration in vivo [438]. HA increases expression of VEGF-C and VEGF-D in tumor-stromal interfaces to mediate lymphangiogenesis [439, 440]. Soluble factors such as heparanase stimulates VEGF-C expression in cancer [441, 442], while TGFBI induces activation of FAK signaling pathway in lymphatic endothelial cells (LECs), and increased expression of CCL21 on the surface of LECs to induce the dissociation of VE-cadherin junctions between LECs [443, 444]. Additionally, expression of membrane glycoprotein podoplanin increases lymphatic vessel formation in oral squamous cell carcinoma [445]. Podoplanin also expressed on the membrane surface of TAM, links TAM and LECs to mediate ECM deposition and lymphangiogenesis via β1 integrin activation in breast cancer [446].
Another potential target associated with malignant lymphatic formation is a collagen-associated binding protein: collagen- and calcium-binding EGF domains 1 (CCBE1), an indispensable regulator for embryonic lymphangiogenesis [447]. Physiologically, CCBE1 promotes VEGF-C expression in a posttranslational layer to transform inactive form of VEGF-C into a mature form [448]. Further research unveils that the activation of CCBE1-induced VEGF-C is depend on the EGF domain of CCBE1, whereas collagen domain is essential for CCBE1 activation [449]. Precise mechanism of CCBE1-induced VEGF-C activation is the enhancing of cleavage activity of a disintegrin and metalloproteinase with thrombospondin motifs 3 (ADAMTS3) and the facilitating of the colocalization of VEGF-C and ADAMTS3 [450]. Indeed, both cancer- and CAF-secreted CCBE1 mediates activation of VEGF-C within ECM and induce lymphangiogenesis and tumor progression [451]. Expression of TGF-β in cancer cells and CAFs transcriptionally suppresses CCEB1 expression by activating downstream SMAD [451]. Besides, high expression of CCBE1 is associated with tumor progression and drug resistance in gastrointestinal stromal tumor [452] and colorectal cancer [453]. Suppression of CCBE1 by miR-330-3p hampers breast cancer metastasis [454]. However, the downregulation of CCBE1 expression could be detected in ovarian cancer and associated with metastasis, implying a potential organ specific role of CCBE1 (Fig. 3D) [455].
Generally, tumor lymphatic metastasis can benefit from enhanced lymphatic vessel formation, whereas the process can also provide more chances for immunocytes to migrate through the lymphatic vessel. Nevertheless, immunosuppressive environment commonly identified to be accompanied with abnormal ECM remodeling, which hampers the function of immune cells to recognize and eliminate cancer cells. Thus, it is crucial to uncover the precise mechanism of ECM-mediated immunosuppressive microenvironment.
ECM remodeling and immunosuppression in cancer
A pro-oncogenic ECM featured by collagen crosslink and glycoprotein-presented bioactivators transduce signals to regulate functions of immune cells. These factors not only mediate cytoplasmic signaling to promote tumor progression and suppress immunoreaction, an impenetrable shield constructed by remodeled ECM also hampers immuno-infiltration. Collagen density and tissue stiffness is pivotal for the infiltration of immunocytes. For instance, a 3D culture constituted by various density of collagen unveils the changes in for T cell composition, while high collagen favors high ratio of CD4/CD8 T cells and lower activity of CD8 T cells [456]. Different colonies of infiltrating T lymphocytes are changed in number, surface markers, subsets and gene expression under the stress from ECM stiffness [457]. Additionally, tumor-modified arrangement of collagen, which also known as collagen orientation, hinders the infiltration of immunocytes. A real-time microscopy motoring the trace of CD8 T in ovarian tumor tissue demonstrates that the mechano-gradient formed by peritumoral collagens guides CD8 T cells moving lingering in a certain direction whereas waken their infiltration to intrude the tumor islets [458]. Therefore, similar to cancer cells, previous discoveries suggest that migration of immune cells in ECM depends on the distribution and interaction among ECM components, especially among fibril collagen network [459]. Additionally, the influence of ECM stiffness on other immune cells such as dendric cells maturation [460] and macrophages [461, 462] have been recently reported. These remodeled ECM composite a maze, with impenetrable wall and winding roads, makes immune cells lost their way. To precisely explore the variation in immune cells surrounded by ECM is essential for further understanding of immune-oncology. Here, the relationship between ECM stiffness or ECM components with immune cells will be discussed.
ECM remodeling and immune cell migration
Collagen crosslink and orientation constructs a stiff and parallel fibril alignment around tumor cells. It’s need to be emphasized that the architecture of collagen fibril shows less impact on immune cell infiltration within tumor tissue, but hamper immune cell migration into the inside, or the islets in another word, of tumor. The phenomenon could be observed in several results [313, 463, 464]. A possible hypothesis is that the proliferation of tumor cell is unlimited, matrix stiffness derived from collagen crosslink contributes to retain the pressure inside the tumor tissue and constructs a pressure gradient, which push not only immune cell but also agents away from cancer cells. While at least, new vascular and lymphatic vessels induced by tumor would not be collapsed by interstitial fluid pressure, but by ECM remodeling [465]. Thus, matrix stiffness conducts both mechanical and contact signaling to mediate immune cell migration (Fig. 4).
ECM hampers activation and migration of immune cells. ECM constructed immunosuppressive environment demonstrate various mechanisms to induce immune escape in TME. Firstly, increasing density and modification of ECM components strikingly enhance the stiffness, then hamper immune cell migration into tumor islets by composing a physical barrier or directly cell-ECM surface inhibition (via interaction or receptors such as DDRs and LAIR-1). Moreover, tenascin-C immobilizes T cells with immunosuppressive cells and stromal cell, thus immune cells are fixed in the ECM. Finally, OPN stimulates PD-1/PD-L1 expression in immune cells, thus induce immunosuppression
In ovarian cancer, ECM stiffness hampers migration of T cell from peritumoral stroma into tumor islets, while degradation of matrix overcomes the situation [313]. This research, together with a discovery from PDAC, which demonstrates that expression of T-cell-active chemokines and β-integrin pathway is irresponsible to the intertumoral T cell-infiltration, highlights the pro-migration role of collagen density on T lymphocytes [466]. Moreover, matrix stiffness increases the CD4/CD8 T cell ratio, and decreases their activation [456, 467]. Mechanistically, CD4 T cell can form a mechanically complex with stiff matrix surface, this interaction modulates T cell cytoskeletal organization which may suppress T cell activation [468]. Another research using hydrogel-integrated culture unveils that stiffer ECM triggers IL-2 secretion whereas reduced proliferation of Jurket T cell (a human T lymphocyte cell line) [469]. Therefore, ECM remodeled by tumor shows immunosuppressive function on T lymphocytes, not only by suppressing their activation, but also by changing their composition.
For antigen-presenting cells, such as macrophages and dendritic cells (DCs), ECM stiffness acts as a disparate role. Increasing stiffness of ECM enhances M2 polarization and HIF-1α-induced LOXL2 expression via β5 integrin/FAK/MEK/ERK pathway of macrophage in hepatoma [470]. Intriguingly, soft matrix physiologically induces mitochondrial ROS generation in a Rho-GTPase-depend way and M1 polarization in macrophage [471]. These results suggest that matrix stiffness may enhance the activation and M2-like polarization of macrophage, which tends to support tumor progression. For DCs, matrix stiffness enhances expression of C-type lectin, which mediates antigen internalization [472]. Stiff matrix stimulates glycolytic metabolism and YAP/TAZ pathway to enhance proliferation and activation of DC [460]. By the way, HA, another widely expressed protein to construct ECM stiffness, also transforms macrophages into M2-like polarization via targeting CD44, and induces apoptosis of neutrophil even activation of DCs via binding with TLR-4 [473]. Thus, unlike T cell, antigen-presenting cells respond positively to ECM stiffness, whereas the function is advantageous for tumor progression.
Additionally, though less research targets the role of ECM stiffness in regulating Treg, some clues are worthy of reference. For example, activation of α4β1 integrin, an ECM-associated receptor, enhances immunosuppressive function of Treg [474]. High expression of leukocyte-associated Ig-like receptor 2 (LAIR-2), an inhibitor of immune inhibition receptor LAIR-1, in Treg is correlated with poor outcome in lung adenocarcinoma [475]. A significant observation demonstrates that losing of hyaluronan and proteoglycan link protein 1 (HAPLN1), a hyaluronic and proteoglycan link protein, in skin could trigger collagen alignment in melanoma, which hampers CD8 T cell migration whereas enhances infiltration of myeloid-derived suppressor cells (MDSCs) and Treg in melanoma [476]. Besides, Treg influence local immunoreaction via interaction with CAFs. In breast cancer, CAFs secrets IL-6 and enhances adenosine generation from CD73 + γδT cells by stimulating STAT3 activation. Then, adenosine stimulates IL-6 secretion by CAFs via adenosine/ adenosine A2 receptor (A2BR)/p38MAPK pathway and constructs a positive feedback loop between Treg and CAFs [477]. A single-cell RNA sequencing about the heterogeneity of fibroblasts in TNBC also indicates the significant correlation between Treg and CAFs [478]. It’s worth to further explore the relationship between Treg and CAFs in various cancers.
However, in PDAC, collagen seems as a trouble maker for cancer survival. The breakthrough of stromal cells role in pancreatic cancer immunosuppression is the identification of different subtypes of CAFs, which preliminarily classified into αSMA-expressing myCAFs, associated with desmoplastic stroma generation, and IL-6/leukemia inhibitory factor (LIF)-expressing inflammatory fibroblasts (iCAFs), associated with inflammation [337]. myCAFs located adjacent to tumor cells whereas iCAFs located more distantly. After that, MHC class II-expressing antigen presenting CAFs (apCAFs) have been identified, and their role in stimulating activation of CD4 T cells via antigen-specific manner has been uncovered [338]. Further investigation finds out that IL-1 and TGF-β oppositely regulate IL-1R/JAK/STAT activation and induce differentiation of CAFs into iCAFs (IL-1), or myCAFs (TGF-β) [32]. Importantly, when generation of collagen I from spinal muscular atrophy (SMA)+ CAFs is reduced, spontaneous PDAC formation mice incline to meet poor survival and immunosuppressive environment [33]. Collagen I depletion in tumor tissue results in enhanced oncogenesis and increased expression of Cxcl5, which causes recruitment of myeloid-derived suppressor cells and suppression of CD8 + T cells in a CXCR2/CCR2-dependent manner [33]. Further investigation using 3D matrices and time-lapse microscopy unveils the distribution of T cell within pancreatic tumor tissue doesn’t correlate to the orientation of collagen alignment [479]. These results depict a distinguish role of collagen in PDAC compared to other cancers.
DDRs and LAIRs: collagens’ best friends
As previously discussed, DDRs are critical receptors sensing collagen and mediates tumor progression. Expression of DDRs in immune cells is also pivotal for cell migration and cell-ECM adhesion. Under the stimulation of collagen, in renal cancer, high expression of DDR1a in monocytes mediates infiltration and migration in ECM [480], while DDR1b triggers MAPK/NF-κB pathway to activate macrophage during inflammation [481]. In a 3D collagen culture, DDR2 expression in neutrophils induces MMP secretion and local generation of collagen-derived chemotactic peptide gradients to regulates directionality [482]. In addition, suppressing DDRs in cancer contributes to the immunoactivity. A pan-cancer in vivo model verifies the efficiency of suppression on DDR2, which significantly enhances the efficacy of anti-PD-1 immunotherapy [483]. Recently, a novel discovery unveils that DDR1 extracellular domain (DDR1-ECD) mechanically binds with collagen and mediates collagen fiber alignment, which hampers T cell infiltration, suggests as a potent therapeutic target for immunotherapy [484].
LAIR-1 (or CD305) is an immune inhibitory receptor prevalently expressed by almost all types of immune cells and its’ activation triggers immunosuppression. In chronic lymphocytic leukemia, oral squamous cell carcinoma, breast cancer and hepatoma, expression of LAIR-1 is negatively correlated with severity of illness [485,486,487,488,489]. In ovarian cancer, CD11c+CD11b−CD103+ DCs could be observed in tumor tissues, while PD-1 expression is positively correlated with LAIR-1high in DC [490]. Collagen is the ligand of LAIR-1 in ECM. In melanoma, collagen mediates CD8 + T cell exhaustion through LAIR1/Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP1) pathway while combination of programmed death 1 (PD-1) blockades with overexpression of LAIR2, a soluble homologue of LAIR-1 with higher affinity for collagen and thereby acts as a decoy receptor, significantly overcome tumor growth and lung metastasis [491, 492]. MMP1/9-mediated collagen I fragment production targets LAIR-1 to suppress CD3 pathway and interferon gamma (IFN-γ) secretion in T cells [493]. A LAIR-2-Fc recombinant protein also suppresses tumor progression and re-activate anti-tumor immune response [491, 493, 494]. Especially, double blockage of LAIR-1 and TGF-β can support totally tumor elimination by PD-L1 [495]. However, A research reports that overexpression of LAIR-1 suppresses ovarian tumor growth via inhibiting PI3K/Akt/mTOR pathway [496]. While the in vivo experiments of this research are based on nude mice, which lacks specific immunity. Thus, LAIR-1 primarily acts as an immunosuppressor rather than a tumor inhibitor.
TNC and Osteopotin: immune cells shall not pass!
Proteins secreted by cancer cells and stroma cells are seductive traps to immune cells. Some are neighborly while some are vicious. The role of collagen [497] and other ECM components [498] in immunoreaction in cancer has been well reviewed. Among them, TNC and osteopotin act as the rising stars for immunotherapy.
TNC
Overexpression of TNC could be specifically detected and associated with immunosuppression and sever progression in various cancers, such as NSCLC [499], breast cancer [500] and oral tongue squamous cell carcinoma [501]. ECM from glioblastoma cell line culture expressing TNC shows suppressive function on Jurkat T cell migration [502]. During the formation of PDAC in vivo model with normal immune function, the existence of TNC promotes oncogenesis and activation of Wnt singling pathway in cancer cells [503]. Further investigation finds out that TNC interacts with α5β1 integrin and suppresses cytoskeleton reorganization of T cells, then supports the survival of lymph node metastatic prostate cancer [504]. In glioblastoma, cancer cells secrets TNC-expressing exosome, then TNC targets α5β1 and αvβ6 integrins to suppresses mTOR singling and activation of T cells [505].
Intriguingly, another research suggests that CD47, which mediates immune evasion via hampering phagocytosis, negatively controls TNC secretion in glioblastoma cells [506]. While the increasing expression of TNC enhances tumor necrosis factor alpha (TNFα) secretion from TAMs via TLR-4/STAT3 pathway [506]. The similar function on TLR-4 could be observed in microglial, the specific type of macrophage in neuro system [507]. It seems like TNC enhances immunoactivities of macrophage. However, more studies demonstrate that TNC triggers immunosuppression via regulating macrophage in the TME.
Breast cancer-derived TNC transforms TAMs into M2-like polarization and triggers immune-suppressive phenotype whereas inhibition of TLR-4 enhances PD-L1 immunotherapy [508]. In oral squamous cell carcinoma, TNC/TLR-4 increases the expression of CCR7 in CD11c+ myeloid cells [509]. Meanwhile, TNC/α9β1 integrin induces CCL21 expression and binds on CCL21 in LECs [509]. Thus, CD11c + myeloid cells and LECs are co-located and hijacked by TNC and construct a lymphoid immune-suppressive stromal environment [509]. The similar function of the anchoring effect of TNC has been reported recently. In TNBC, TNC/TLR-4 induces CXCL12 expression in CD8 T cells and retains CD8 T cells within the ECM and decreases immunoreaction via CXCR4/CXCL12 (Fig. 4) [510]. The two reports hightlight the novel role of TNC in limiting immune cell within peritumoral stoma, and the function further hampers immune cell migration and provides a immunosuppressive environment. For the upstream factors of TNC, JNK role in stimulating TNC expression has been discussed. In addition, TNBC with autophagy-deficiency could ubiquitinate TNC and sustain TNC expression by SKP2, therefore induce immunosuppression [511]. TNC may also combine with secreted TGF-β and stimulate activation of TGF-β, whereas the precise mechanism is still unclear [250].
Osteopotin (OPN, or SPP-1)
Previous research has unveiled that OPN is the ligand to CD44 and integrin, and contributes to the anoikis resistance and promoting adhesion in ECM [512]. Some reports targeting the role of intracellular OPN in immune regulation. For instance, expression of intracellular OPN is controlled by T-bet signaling pathway and regulates migration of T cells [513], as well as induces IFN-α expression via TLR-9 in plasmacytoid DCs [514]. In macrophage, intracellular OPN interacts with MyD88 to suppress TLR singling pathway, therefore inhibits inflammation-stimulated formation of hepatoma [515]. Further investigation demonstrates that both intracellular and secreted isoforms of OPN are dispensable to stimulate activation of DCs [516]. Intriguingly, DCs under hypoxia, which is commonly detected in TME, shows increasing secretion of OPN and impaired immune activation to support tumor immune escape [517]. Another research in a spontaneous breast metastasis model suggests that secreted OPN is mostly derived from cancer cells and supports tumor survival during metastasis, while high expression of intracellular OPN in MDSCs induces immunosuppression around metastatic site [518].
Indeed, additional investigations highlight the role of secreted OPN in immunosuppression. Secreted OPN form tumor cells contributes to the accumulation of MDSCs via targeting CD44 and stimulating downstream ERK/MAPK pathway to induce extramedullary myelopoiesis and immune escape [519]. Similar result could be detected in helicobacter pylori-induced gastric cancer, another inflammation-associated cance [520]. Precisely, high expression of intracellular OPN results in increased expression of PD-1 in macrophage and lung cancer cells via NK-κB pathway [521, 522]. Whereas in glioblastoma, tumor secreted OPN targets αvβ5 integrin to recruit and induce M2-like polarization of macrophage [523]. Interferon regulatory factor 8 (IRF8) transcriptionally suppress secreted OPN expression in CD11b+Ly6ClowLy6G+ myeloid cells, which caused decreased interaction between OPN and CD44 receptor in T cell and suppresses immune escape in colon cancer [524]. High expression of secreted OPN in hepatoma stimulates colony-stimulating factor 1 (CSF1)/CSF1R activation in macrophage which further increases PD-1 expression in hepatoma cells [525]. While in PDAC, WD repeat domain 5 (WDR5), a histone methyltransferase, mediates H3K4me3 on promoters of OPN and CD44 and enhances their expression to promote PD-L1 expression in tumor cells and MDSCs [526]. Thus, OPN links expression of PD-1/PD-L1 expression in both cancer cells and immune cells to induce immunosuppression.
By the way, specific transcripts from secreted phosphoprotein 1 (SPP-1) are critical for distinguished function of OPN. Overexpressed three different transcripts of OPN in breast cancer unveils similar oncogenic role but different immunosuppressive role in three trascripts [527]. Importantly, orally OPN administration suppresses tumor growth in mice with normal immune function, highlights the significance of endogenous OPN in promoting tumor progression [528]. Indeed, some results have proven the hypothesis. The isoform of secreted OPN from glioblastoma could not be observed from normal human astrocytes [529]. This isoform triggers macrophage M2-like polarization and tumor progression [529]. Another research suggests that the specific fragment of OPN cleaved by MMP-9 is sufficient to trigger MDSC infiltration and immune suppression [530]. The expression of membrane-anchored protein a disintegrin and metalloproteinase domain 8 (ADAM8) in glioblastoma cells and macrophages mediates OPN secretion and suppresses angiogenesis via JAK/STAT3 pathway, may also contributes to the formation of the specific isoform of OPN in ECM [531]. Thus, antibody targeting specific OPN in cancer could be an optimal option [532].
TAM-induced ECM remodeling: the betrayer
TAMs are accompanied with tumor cells to composite pro-oncogenic environment. Under the stimulation from cancer cells or ECM environment, TAMs mediate matrix remodeling and contributes to the migration of cancer cells in a certain direction. TAM shapes ECM remodeling by high-rate degradation of the matrix and generation of ECM proteins [533]. In colorectal cancer, depletion of TAM results in a huge variation of expression of ECM components. ECM fragments from TAM-sufficient tumors triggers tumorigenesis compared with TAM-deficient tumors [73]. Deficiency of TAM strikingly decreases density and crosslink of collagen, especially reduces collagen I and XIV expression in CAFs [73].
Additionally, the secretion of MMPs from TAMs acts as a pivotal role in carving out cancer cells’ way to metastasis. High expression of MMP-11 in TAMs is associated with poor outcomes in breast cancer patients [534]. Overexpression of MMP-11 in macrophages, but not in cancer cells, increase monocyte recruitment and migration of Her-2+ breast cancer cells via CCL2/CCR2/MAPK pathway [534]. Furthermore, aberrant expression of genes in TAMs controls ECM remodeling to provide the advantage for tumor migration. Overexpression of B7-H3 in TAM shows fewer collagen fibers and enhanced angiogenesis in breast cancer [71]. In TNBC, high podoplanin expressing TAMs has been identified to adhere to LECs [446]. The combination of glycosylated podoplanin from TAMs with galectin 8 on LECs stimulates the activation of β1 integrin. This function facilitates angiogenesis and lymphangiogenesis via localized ECM remodeling [446]. Disabled homolog 2 mitogen-responsive phosphoprotein (DAB2) positive TAMs, which localized at the tumor-invasive front, promotes metastasis via matrix remodeling [535]. Precisely, knockdown of DAB2 impairs integrins’ surface distribution and their ability to internalize ECM fragments, under the expression of α5β1 integrin and stiffer ECM [535]. By contrast, ECM stiffness stimulates DAB expression via mechanically upregulating YAP/TAZ pathway, triggers ECM remodeling and integrin-dependent migration, which assists tumor invasion [535]. Therefore, TAMs act as tour guides, pointing out the way out for them from primary tumor tissue to distant metastatic sites.
Other immune cells also participate to the formation of ECM remodeling. Increasing stimulation from tobacco will sustain a lung inflammation, neutrophils response to the inflammation and secrets neutrophil elastase and MMP-9 [536]. Unfortunately, laminin would be cleaved by MMPs and remodeled laminin then awakens quiescent cancer cells via stimulating α3β1 integrin signaling pathway [536]. Besides, BMDC derived ECM miR-92a induces activation of HSCs, subsequently increasing ECM deposition [537]. Roles of other immune cells, such as NKp46-expressing NK cells [538], LOX-expressing T cells [539] and TGF-β/CD73/adenosine-dependent Myeloid cells [540] in ECM remodeling and tumor progression are recently reported. After all, the profound regulatory network between ECM and immune system is the treasure, which provides targets to us to validate their potential in anti-tumor treatment.
Anti-cancer therapy targeting ECM
Raising morbidity of cancer worldwide demonstrates the emergency of precise treatment strategy design and the discovery of novel therapeutic targets [541]. Canonical therapeutic strategies including chemotherapy, radiotherapy, as well as biomarker-based therapies such as endocrinotherapy and targeted therapy significantly increase cancer patients’ prognosis. Nevertheless, aberrant gene expression and disordered signaling pathway of tumor cells shape pro-tumorigenic ECM remodeling, which forms the tough environment to impede infiltration of anti-tumor immune cells and agents, as well as sustaining cancer cell survival under the stress from anti-tumor agents [542].
ECM shaped by cancer cells links multiple drug resistance. Hydrogel constructed matrix mimics ECM stiffness and unveils enhanced chemoresistance in breast cancer [543, 544], and glioblastoma [545]. A reasonable model constructed by breast cancer patient derived scaffold which perfectly mimic acellular structure of ECM and unveiled enhanced drug resistance in cultured cancer cells [546]. Cancer cells 3D culture of HA also shows severe chemoresistance, especially for glioblastoma cells [547, 548]. On the other hand, chemotherapy can push remodeling of acellular matrix components, stiffness and matrix adhesion, which results in chemoresistance. For instance, after chemotherapy treatment, a positive feedback loop between THBS2-deficient CD133+ liver CSCs and local soft ECM induces cell metastasis in hepatoma [549]. In ovarian high-grade serous carcinoma, cells with high upregulated expression of collagen VI strikingly stiffen ECM and induce chemoresistance via enhancing integrin-based cancer cell adhesion on stiff collagen VI substrate after cisplatin-based chemotherapy [550]. Intriguingly, soluble laminin-322 secreted by HSCs in conditional medium of hepatoma can induce sorafenib resistance by sustaining activation of α3 integrin/FAK pathway, implying is that the components of ECM that matter, not their location [551]. Another research finds out that a short-time contact between placenta ECM and Erα+ breast cancer cells stimulate α5 integrin-dependent autophagy and paclitaxel resistance in cancer cells, highlights the critical role of ECM in chemoresistance [552]. Thus, targeting ECM is an optimal method assisting chemotherapy, even enhancing the efficacy of other therapies, and several strategies will be discussed here (Fig. 5).
ECM signature as predictive biomarker
Each tumor tissue featured by unique gene signature contains particular ECM composition. ECM gene signature may help to identify the sensitivity of cancer to therapy. Several attempts have been made by previous studies. For example, specific stromal-related gene signature is correlated with prognosis of large-B-cell lymphoma patients receiving chemotherapy [553]. Similar association between ECM gene signature and outcomes of patients could be observed in other cancer [554,555,556,557]. Besides gene signature, proteomic analysis of ECM proteins in cancer also contributes to identify cancer and normal tissues [558]. Recently, a study demonstrates that matrix components released in blood after anti-PD-1 treatment in melanoma is associated with prognosis of patients [559]. Among them, collagen III and vimentin contribute to the prediction of the efficacy of PD-1 inhibitor [559].
Multiple predictive signatures may provide a method to analyze patient outcomes. While the designation of treatment based on these results is difficult to decide. Fortunately, ECM gene expression is commonly correlated with genes that has been well studied. Detection of these genes would contribute to the designation of treatment. For instance, ECM signature genes are identified under the control of TGF-β1, and expression of COL11A1, one of the signature genes, is upregulated during ovarian cancer progression [560]. Moreover, B-Raf proto-oncogene (BRAF) mutation (BRAFV600) in melanoma favors collagen-rich environment generated by CAFs, while dual targeting DDR1 and DDR2 contributes to the efficacy of imatinib in vivo [561]. Thus, identification of pivotal gene in ECM remodeling is critical for therapeutic designation.
ECM degradation
A reasonable treatment to increase migration of anti-tumor immune cells and agents is normalizing ECM remodeling. Matrix-associated enzymes, including MMPs, LOX and LOXLs, are primary factors modify ECM structure. Targeting these enzymes to mediate mechanical signals and enhance the efficacy of immunotherapy in cancer shows great potential [562]. Though suppressing of MMPs could suppress cancer cell migration and invasion, it also hampers infiltration of other cells and agents. Thus, other factors inducing ECM degradation such as nitric oxide (NO) [563] and HSP70 [564] are potential targets. A recent study indicates that even through an intertumoral injection, ECM stiffness strikingly hampers drug delivery compared with soft one [565]. Thus, LOX and LOXs, which mediates collagen crosslink in tumor cell, are optimal targets to alleviate ECM stiffness. Suppression of LOXL2 is effective for tumor suppression. Beta-aminopropionitrile (BAPN) is the pan LOX inhibitor. Several research has proved that BANP treatment can suppress proliferation and invasion of tumor cells [395, 566, 567]. In osteosarcoma, a positive feedback loop between c-Fos/AP-1/Wnt pathway and LOXL2-induced matrix crosslink, while BAPN destroys the feedback loop [568]. A monoclonal antibody (AB0023) targeting LOXL2 inhibits stoma generation and TGF-β activation in cancer and shows more efficacy and safety than BANP [569]. Moreover, aminomethylenethiophenes (AMTs) is a potent oral bioavailable anti-tumor agent targeting dual LOX and LOXL2 and exhibit improved pharmacokinetic properties and excellent antitumor efficacy in vivo [570].
Besides, HA is one of the most prevalent ECM components and contributes to the formation and progression of cancer. 4-methylumbelliferone (4-MU), the HA synthesis inhibitor, significantly decreases HA expression and tumor progression in ER-negative breast cancer [571] and hepatoma [572], it also overcomes chemoresistance in ovarian cancer [573]. Oral dietary supplement of 4-MU is effective to suppress prostate cancer progression via abrogating HA signaling in vivo [574]. Intriguingly, targeting metabolic metabolism associated with HA synthesis may be another selection. A small molecule glutamine analog targeting glutamine-fructose amidotransferase 1 (GFAT1), the rate-limiting enzyme of HA synthesis, decreases renewal and metastatic ability of tumor cell, and increases sensitivity to anti-PD-1 treatment [575]. The combination of 4-MU and anti-metabolic therapy (dichloroacetate, the pyruvate dehydrogenase kinase inhibitor) strikingly suppresses esophageal cancer progression in vitro and in vivo [576]. Moreover, the combination treatment of 4-MU and trametinib, the MEK inhibitor, significantly inhibits ERK phosphorylation and PD-1/PD-L1 expression in malignant pleural mesothelioma [577]. Thus, 4-MU is a promising anti-cancer agent.
Other treatments such as normalization of ECM stiffness by photothermal depletion of cancer-associated fibroblasts in desmoplastic cholangiocarcinoma [578], and stiffness-based anti-angiogenic therapy in hepatoma [579] suggest that soften ECM stiffness is critical. Additionally, TNFα-CSG fusion protein, which interacts with laminin-nidogen complexes, decreases ECM stiffness and upregulates immuoinfiltration [580]. However, cholesterol depletion treatment could induce cancer stiffness and enhance T-cell immunotherapy [581]. Another research also demonstrates that softer ECM favors chemosensitivity in TNBC (via elevating NF-κB activity and compromising JNK activity) [582]. Thus, different strategies targeting ECM stiffness should be considered.
Receptor surface domain blockage and drug delivery
Antibodies targeting ECM-associated receptors on the membrane of cancer cells can shut down the signal transduction from ECM to cancer cells, thus inhibit tumor progression. As previous introduced, RGDs are designed to recognize specific integrin and abrogates the function of integrin [175]. Besides, RGD-motif is an ideal guide to link anti-tumor agents, thus transport agents to target cells overexpressing specific integrins [583]. Moreover, RGDs can be used as developing agent such as a positron-emission tomography (PET) tracer to demonstrate the edge and volume of tumor, suggesting a potential role of ECM-associated monoclonal antibodies in oncogenic imaging [584, 585].
Similar to integrin, other receptors such as DDRs, CD44 [586] and syndecans are potential target for anti-cancer therapy. The ECD in DDRs interacts with collagen and stimulates activation of downstream signaling pathways. In DDR2, ECD domain suppress collagen I expression and mediates fibrillogenesis [587, 588]. Soluble DDR-ECD plays the same role [589]. Recently, a critical discovery unveils the role of DDR1-ECD in immunosuppression [484]. Precisely, ECD domain of DDR1 mediates collagen fibril alignment by interacting with collagen, then impede immune cell infiltration [484]. Other research indicates that methyl (CH3) and amino (NH2) mechanisms facilitated surface modification induce apoptosis in breast cancer [590]. Poly peptide of NH2-terminal fragment of zinc finger FYVE-type containing 21 (ZF21), which is the binding region of FAK, reduces metastasis progression [591]. These studies emphasize the strategy of domain blockage for impeding cell-ECM interaction.
For secreted proteins in ECM, blocking interaction domain of proteins to receptors by monoclonal antibody is an optimal selection. Fibrinogen-like globe (FBG) is a conserved domain in tenascins and mediates cytoskeletal re-organization by binding to fibronectin [592]. Monoclonal antibody targeting FBG can suppress tumor progression and enhance immunotherapy [508]. By the way, FBG domain on tenascin-X, a member of tenascins, supports its mechanic interaction with TGF-β, then induce maturation of TGF-β, highlights the potential role of FBG domain on other tenascins in TGF-β maturation [250, 593]. Additionally, extra-domain B (EDB) of fibronectin is an ideal target for drug delivery [594, 595]. A nanovehicle with a peptide bi-targeting EDB on fibronectin and FBG on TNC strikingly suppress tumor growth and increase survival of mice in vivo [596]. Thus, domains on ECM proteins are also optimal targets for drug delivery.
Though ECM are rich in proteins promoting tumor progression and immunosuppression in cancer, these proteins are indeed ideal targets for drug delivery. Among them, drug delivery system based on fibronectin has been concluded [597]. Nanoparticles featured by targeting ECM components are optimal to penetrate tumor tissue and deliver drugs. For instance, Collagenase IV modified nanoparticles loading doxorubicin can increase penetration of drug and with weaker side effects compared to nanoparticles without collagenase IV [598]. Overexpression of RHAMM, the specific receptor of HA, in cancer cells also provides a perfect target for HA nanogels [599]. Additionally, overexpression of ECM-associated enzymes in TME provides another strategy. For instance, a novel nanovehicle model with potential of hydrogen (pH)/ROS/MMP-2 triple-responsive will gradually release anti-cancer drug when meets MMP-2-rich, low pH and high density of intracellular ROS [600]. Then high concentration of intertumoral ROS induced by photothermal and acidic pH induces precisely delivery of sorafenib in vivo [600]. The similar strategy has been reported in a lipase-rich TME, which enhance the penetration of doxorubicin/monostearin constructed nanoparticles [601]. Importantly, to deal with the complex environment containing mechanic signals in ECM, novel nano materials automatically regulating mechanosensing according to ECM in cancer are on the way [602].
CAR-T
Chimeric antigen receptor redirected T cells (CAR-T cells) are reengineered T cells from patients and shows significant therapeutic effects on cancers, especially on hematologic malignancies. Compared to monoclonal antibody, CAR-T shows the advantage of infiltration. For instance, human epidermal growth factor receptor 2 (Her-2) specific CAR-T shows a perfect penetration in tumor matrix better than Her-2 monoclonal antibodies [603]. However, the low therapeutic effects of CAT-T in solid tumor still suggests the emergency to enhance the ability of penetration of CAR-T. Thus, reengineered CAR-T cell with overexpression of heparinase, which can degrade ECM components and increases T-cell infiltration, strikingly inhibits tumor growth [604]. A therapeutic model with the combination of oncolytic adenovirus carried decorin with a CAR-T targeting carbonic anhydrase IX (CAIX) has been reported to induce ECM remodeling and immunoreaction in cancer recently [605]. Moreover, targeting stromal cells which are the primary cells generating ECM components, may be another selection. Fibroblast activation protein (FAD) is overexpressed in CAFs and associated with ECM remodeling in tumors and wound healing, and it is a potential target for ECM degradation and tumor imaging [606]. A study has demonstrated that FAP specific CAR-T significantly suppresses tumor progression and induce ECM degradation in PDAC [607]. Recently, an enhanced-affinity ligand for FAP has been developed and shows the potential for clinical application via CAR-T [608]. Additionally, CAR-147 modified macrophage increases T cell-infiltration by targeting MMPs in matrix [609]. Though less studies focus on the role of CAR-T in ECM remodeling, yet the model has the potential.
Vitamin D
The role of activated Vitamin D (1,25-dihydroxy vitamin D3, or 1,25(OH)2 D3) in ECM remodeling has been reported that 1,25(OH)2 D3 stimulates generation of bone-associated proteins [610], as well as for OPN in bone [611] and TNC in mammary cells [612]. Most research targets the relationship between 1,25(OH)2 D3 and uterine leiomyomas. For instance, 1,25(OH)2 D3 level in plasma is negatively correlated with the risk of uterine leiomyomas [613]. TGF-β induces generation of fibronectin and collagen I whereas 1,25(OH)2 D3 overcomes the function in vitro [614, 615]. Long-term treatment of 1,25(OH)2 D3 shows the similar result in vivo [616]. It also inhibits the activation of Wnt4/β-catenin/mTOR pathway to suppress ECM generation [617], especially for mediator complex subunit 12 (MED12) mutation uterine leiomyomas [618]. Thus, 1,25(OH)2 D3 is a potential fibrosis inhibitor which may play a role in anti-malignant therapy.
Intriguingly, in vitro experiments suggest that 1,25(OH)2 D3 induce apoptosis in malignancy [619, 620] and differentiation [621] in malignancies. Moreover, generation of MMP-9 and MMP-13 is controlled by 1,25(OH)2 D3 in squamous cell carcinoma [622]. Recently, several studies focus on the function of 1,25(OH)2 D3 in ECM remodeling to suppress cancer progression. The interaction between PDAC cells and PSCs, and ECM remodeling induced by PSCs can be inhibited by treatment of the combination of Vitamin D receptor modulators and gemcitabine in vitro and in vivo [623]. Moreover, treatment of gemini-72, a vitamin D analog, normalizes ECM remodeling and induce apoptosis of prostatic precancerous cells in vivo [624]. However, the precise mechanism of Vitamin D-induced ECM remodeling in cancer is still unclear. To unveil the regulatory network may contribute to the ECM-based anti-cancer therapy.
Future prospective and conclusion
Solid tumor is a high heterogeneity tissue composited by various cells and acellular components. This heterogeneity is not limited in the different composition of cancer cells and stromal cells, numerous ECM proteins with structural and/or bioactive role are critical to contribute to the formation of heterogeneity. Especially, multiple studies have indicated that the location, crosslink and modification of ECM proteins play an increasing role in ECM remodeling. To improve the therapeutic effects and outcomes of cancer patients, precise treatments targeting heterogeneity is the inevitable chosen. Nevertheless, almost all agents or cells selected to kill tumor cells are bound to face the resistance from ECM, the fenced wall around malignancy. The combination of anti-cancer therapy and anti-ECM treatment will be the next generation of ideal anti-malignancy strategy. This review has summarized the interaction between ECM and cancer, especially introduced the role of ECM in multiple hallmarks of cancer, including proliferation, anoikis, invasion, metastasis, angiogenesis, lymphangiogenesis, and immune escape. According to these mechanisms, we emphasize the importance of clinical applications targeting ECM to increase therapeutic effects and overcome drug resistance. ECM-targeting therapy has been proved to be effective in supporting anti-cancer treatment,and it is also a treasure which needs further exploration.
Availability of data and materials
Not applicable.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- ATP:
-
Adenosine triphosphate
- AHA1:
-
ATPase homolog 1
- ADAMTS3:
-
A disintegrin and metalloproteinase with thrombospondin motifs 3
- A2BR:
-
Adenosine A2 receptor
- apCAF:
-
Antigen presenting cancer-associated fibroblast
- ADAM8:
-
A disintegrin and metalloproteinase domain 8
- AMT:
-
Aminomethylenethiophene
- BCL:
-
B-cell lymphoma
- BRAF:
-
B-Raf proto-oncogene
- BAPN:
-
Beta-aminopropionitrile
- CTL:
-
Cytotoxic T lymphocyte
- C-P4H:
-
Collagen prolyl 4-hydroxylase
- CAF:
-
Cancer-associated fibroblast
- CD138/Sdc-1:
-
Syndecan-1
- CXCL3:
-
C-X-C motif chemokine ligand 3
- CCR2:
-
C-X-C motif chemokine receptor 2
- CSC:
-
Cancer stem cell
- COL10A1:
-
Collagen type X alpha 1
- CDK4/6:
-
Cyclin-dependent kinases 4 and 6
- CHI3L1/YKL-40:
-
Chitinase-3-like protein 1
- CCL2:
-
C–C motif chemokine ligand 2
- CCBE1:
-
Collagen- and calcium-binding EGF domains 1
- CSF1:
-
Colony-stimulating factor 1
- CAR-T:
-
Chimeric antigen receptor redirected T cell
- CAIX:
-
Carbonic anhydrase IX
- DDR2:
-
Discoid protein domain receptor
- DC:
-
Dendritic cell
- DAB2:
-
Disabled homolog 2 mitogen-responsive phosphoprotein
- ECM:
-
Extracellular matrix
- EC:
-
Endothelial cell
- ERK2:
-
Extracellular signal-regulated kinase 2
- EGFR:
-
Epidermal growth factor receptor
- ECT2:
-
Epithelial cell transforming sequence 2
- EMT:
-
Epithelial-mesenchymal transition
- EPHA2:
-
Ephrin type-A receptor 2
- EMILIN2:
-
Elastin microfibrillar interface protein 2
- ECD:
-
Extracellular domain
- EDB:
-
Extra-domain B
- FGF:
-
Fibroblast growth factor
- FAK:
-
Focal adhesion kinase
- FOXO1:
-
Forkhead box protein O1
- FBG:
-
Fibrinogen-like globe
- FAD:
-
Fibroblast activation protein
- GTPase:
-
Guanosine triphosphatase
- G3BP2:
-
Ras-GTPase activating protein SH3 domain-binding protein 2
- GLUT-1:
-
Glucose transporter protein type 1
- GEF:
-
Guanine nucleotide-exchange factor
- GATA2:
-
GATA binding protein 2
- GFAT1:
-
Glutamine-fructose amidotransferase 1
- HIF-1:
-
Hypoxia-inducible factor 1
- HSPG:
-
Heparan sulfate proteoglycan
- HA:
-
Hyaluronan/hyaluronic acid
- HAS2:
-
Hyaluronan synthase 2
- HSC:
-
Hepatic stellate cell
- HSP:
-
Heat shock protein
- hsPxd01:
-
Heme peroxidase human peroxidasin 1
- HAPLN1:
-
Hyaluronan and proteoglycan link protein 1
- Her-2:
-
Human epidermal growth factor receptor 2
- IL-8:
-
Interleukin-8
- IGFBP2:
-
Insulin-like growth factor binding protein 2
- iCAF:
-
Inflammatory cancer-associated fibroblast
- IFN-γ:
-
Interferon gamma
- JNK:
-
C-Jun N-terminal kinase
- KLF5:
-
Kruppel-like factor 5
- LOX:
-
Lysyl oxidase
- LOXL:
-
Lysyl oxidase-like proteins
- LN-5γ2:
-
Laminin-5γ2
- LAIR:
-
Leukocyte-associated Ig-like receptor
- LEC:
-
Lymphatic endothelial cell
- LIF:
-
Leukemia inhibitory factor
- MMP:
-
Matrix metalloproteinase
- MAPK:
-
Mitogen-activated protein kinase
- MDA-9:
-
Melanoma differentiation associated gene-9
- mTOR:
-
Mechanistic target of rapamycin
- MSC:
-
Mesenchymal stromal cell
- MMRN2:
-
Multimerin-2
- myCAF:
-
Cancer-associated myofibroblasts
- MDSC:
-
Myeloid-derived suppressor cell
- MED12:
-
Mediator complex subunit 12
- NK:
-
Natural killer
- NF-κB:
-
Nuclear factor-kappaB
- NSCLC:
-
Non-small cell lung cancer
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NRF2:
-
Nuclear factor E2-related factor
- N-WASP:
-
Neural Wiskott-Aldrich syndrome protein
- NMIIAH:
-
Non-muscle myosin IIA
- NO:
-
Nitric oxide
- OPN/SPP-1:
-
Osteopotin
- OXPHOS:
-
Oxidative phosphorylation
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PI3K:
-
Phosphoinositide 3-kinase
- PDO:
-
Patient-derived organoid
- PSC:
-
Pancreatic stellate cell
- PRP4K:
-
Pre-mRNA splicing factor 4 kinase
- PDGFB:
-
Platelet-derived growth factor-BB
- PTPRK:
-
Receptor-type tyrosine-protein phosphatases kappa
- PFKFB3:
-
Phosphofructokinase-2/fructose-2, 6-bisphosphatase 3
- PPP:
-
Pentose phosphate pathway
- Prrx1:
-
Paired related homeobox 1
- PLOD2:
-
Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2
- PD-L1:
-
Programmed death ligand 1
- PD-1:
-
Programmed death 1
- ROS:
-
Reactive oxygen species
- RHAMM/CD168:
-
Receptor for hyaluronan-mediated motility
- ROCK:
-
Rho-associated coiled-coil containing kinase
- RGD:
-
Arg-Gly-Asp
- RREB1:
-
RAS-responsive element binding protein 1
- PC:
-
Pyruvate carboxylase
- RIPK-1:
-
Receptor-interacting protein kinase 1
- SPARC:
-
Secreted proteins acidic and rich in cysteine/osteonectin
- SKP2:
-
S-phase kinase associated protein 2
- STAT3:
-
Signal transducer and activator of transcription 3
- SNAIL:
-
Snail family transcriptional repressor
- Setd2:
-
SET domain containing 2
- SOX4:
-
SRY-Box transcription factor 4
- SMAD4:
-
Small mothers against decapentaplegic protein 4
- SOD2:
-
Superoxide dismutase 2
- SHP-1:
-
Src homology 2 domain-containing protein tyrosine phosphatase 1
- SMA:
-
Spinal muscular atrophy
- TME:
-
Tumor microenvironment
- THBS/TSP:
-
Thrombospondin
- Treg:
-
Regulatory T cell
- TAM:
-
Tumor-associated macrophage
- TRC:
-
Tumor-repopulating cell
- TLR-4:
-
Toll-like receptor 4
- TNC:
-
Tenascin-C
- TAZ/WWTR1:
-
WW domain containing transcription regulator
- TGF-β-2:
-
Transforming growth factor β2
- Tet2:
-
Ten-eleven translocation 2
- TWIST:
-
Twist family basic helix-loop-helix transcription factor
- TRIO:
-
Trio Rho guanine nucleotide exchange factor
- TGFBI/βig-H3:
-
Transforming growth factor beta-induced protein
- TRPM7:
-
Transient receptor potential melastatin 7
- TXNIP:
-
Thioredoxin-interacting protein
- TNBC:
-
Triple negative breast cancer
- TIMP2:
-
Tissue inhibitors of metalloproteinase 2
- TNFα:
-
Tumor necrosis factor alpha
- UBTD1:
-
Ubiquitin domain containing 1
- UDP:
-
Uridine diphosphate
- VEGF:
-
Vascular endothelial growth factor
- Vitamin D:
-
1,25-dihydroxy vitamin D3
- WISP:
-
WNT1-inducible signaling pathway protein
- WDR5:
-
WD repeat domain 5
- YAP:
-
Yes1 associated transcriptional regulator
- ZFP36:
-
Zinc finger protein 36
- ZEB1:
-
Zinc finger E-box binding homeobox 1
- ZF21:
-
Zinc finger FYVE-type containing 21
- 4-MU:
-
4-methylumbelliferone
References
Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front Oncol. 2018;8:431.
Resovi A, Bani MR, Porcu L, Anastasia A, Minoli L, Allavena P, et al. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol Med. 2018;10(8):e8741.
Boch T, Kohler J, Janning M, Loges S. Targeting the EGF receptor family in non-small cell lung cancer-increased complexity and future perspectives. Cancer Biol Med. 2022;19(11):1543–64.
Soliman H, Theret M, Scott W, Hill L, Underhill TM, Hinz B, et al. Multipotent stromal cells: One name, multiple identities. Cell Stem Cell. 2021;28(10):1690–707.
Tuleta I, Frangogiannis NG. Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities. Adv Drug Deliv Rev. 2021;176:113904.
Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14.
Sangaletti S, Chiodoni C, Tripodo C, Colombo MP. The good and bad of targeting cancer-associated extracellular matrix. Curr Opin Pharmacol. 2017;35:75–82.
Long Y, Niu Y, Liang K, Du Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022;32(1):70–90.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Karamanos NK, Theocharis AD, Neill T, Iozzo RV. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75–76:1–11.
Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol. 2016;240(4):397–409.
Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5(4):309–20.
Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406.
Barallobre-Barreiro J, Loeys B, Mayr M, Rienks M, Verstraeten A, Kovacic JC. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(17):2189–203.
Ambade AS, Hassoun PM, Damico RL. Basement Membrane Extracellular Matrix Proteins in Pulmonary Vascular and Right Ventricular Remodeling in Pulmonary Hypertension. Am J Respir Cell Mol Biol. 2021;65(3):245–58.
Manou D, Caon I, Bouris P, Triantaphyllidou IE, Giaroni C, Passi A, et al. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. Methods Mol Biol. 2019;1952:1–20.
Chakraborty A, Edkins AL. HSP90 as a regulator of extracellular matrix dynamics. Biochem Soc Trans. 2021;49(6):2611–25.
Fu R, Jiang X, Li G, Zhu Y, Zhang H. Junctional complexes in epithelial cells: sentinels for extracellular insults and intracellular homeostasis. FEBS J. 2022;289(23):7314–33.
Hagiwara M, Maruyama H, Akiyama M, Koh I, Arai F. Weakening of resistance force by cell-ECM interactions regulate cell migration directionality and pattern formation. Commun Biol. 2021;4(1):808.
Nam S, Hu KH, Butte MJ, Chaudhuri O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc Natl Acad Sci U S A. 2016;113(20):5492–7.
Haas AJ, Zihni C, Ruppel A, Hartmann C, Ebnet K, Tada M, et al. Interplay between Extracellular Matrix Stiffness and JAM-A Regulates Mechanical Load on ZO-1 and Tight Junction Assembly. Cell Rep. 2020;32(3):107924.
Selman M, Pardo A. Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res Rev. 2021;70:101393.
Iozzo RV, Gubbiotti MA. Extracellular matrix: The driving force of mammalian diseases. Matrix Biol. 2018;71–72:1–9.
Mortezaee K, Majidpoor J. The impact of hypoxia on immune state in cancer. Life Sci. 2021;286:120057.
Ban HS, Uto Y, Nakamura H. Hypoxia-inducible factor (HIF) inhibitors: a patent survey (2016–2020). Expert Opin Ther Pat. 2021;31(5):387–97.
Aro E, Khatri R, Gerard-O’Riley R, Mangiavini L, Myllyharju J, Schipani E. Hypoxia-inducible factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction of collagen prolyl 4-hydroxylases in primary newborn mouse epiphyseal growth plate chondrocytes. J Biol Chem. 2012;287(44):37134–44.
Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM, et al. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1alpha stabilization and TNBC chemoresistance. Nat Commun. 2018;9(1):4456.
Saatci O, Kaymak A, Raza U, Ersan PG, Akbulut O, Banister CE, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11(1):2416.
Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9(17):3534–51.
Madsen CD, Pedersen JT, Venning FA, Singh LB, Moeendarbary E, Charras G, et al. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep. 2015;16(10):1394–408.
Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120(3):2782–90.
Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9(2):282–301.
Chen Y, Kim J, Yang S, Wang H, Wu CJ, Sugimoto H, et al. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39(4):548-656 e6.
Mello AM, Ngodup T, Lee Y, Donahue KL, Li J, Rao A, et al. Hypoxia promotes an inflammatory phenotype of fibroblasts in pancreatic cancer. Oncogenesis. 2022;11(1):56.
Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A. 2014;111(45):E4832–41.
Makris EA, Hu JC, Athanasiou KA. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage. 2013;21(4):634–41.
Murdocca M, De Masi C, Pucci S, Mango R, Novelli G, Di Natale C, et al. LOX-1 and cancer: an indissoluble liaison. Cancer Gene Ther. 2021;28(10–11):1088–98.
Janjanam J, Pano G, Wang R, Minden-Birkenmaier BA, Breeze-Jones H, Baker E, et al. Matricellular Protein WISP2 Is an Endogenous Inhibitor of Collagen Linearization and Cancer Metastasis. Cancer Res. 2021;81(22):5666–77.
Kim HJ, Yang K, Kim K, Lee YJ, Lee S, Ahn SY, et al. Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling. Cell Mol Immunol. 2022;19(12):1373–91.
Laghezza A, Luisi G, Caradonna A, Di Pizio A, Piemontese L, Loiodice F, et al. Virtual screening identification and chemical optimization of substituted 2-arylbenzimidazoles as new non-zinc-binding MMP-2 inhibitors. Bioorg Med Chem. 2020;28(3):115257.
Chan ZC, Oentaryo MJ, Lee CW. MMP-mediated modulation of ECM environment during axonal growth and NMJ development. Neurosci Lett. 2020;724:134822.
Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol. 2018;81:241–330.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516.
Luo L, Yang JX, Luo T, Liu D, Wu GH, He JM. A study on the mechanism of PP2A in the recovery of SCI in rats through downregulation of MMP-9 via MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(23):7195–203.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824(1):68–88.
Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147(10):4950–9.
Shi X, Huang H, Zhou M, Liu Y, Wu H, Dai M. Paeonol Attenuated Vascular Fibrosis Through Regulating Treg/Th17 Balance in a Gut Microbiota-Dependent Manner. Front Pharmacol. 2021;12:765482.
Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69(19):7529–37.
Chen N, Zhang G, Fu J, Wu Q. Matrix metalloproteinase-14 (MMP-14) downregulation inhibits esophageal squamous cell carcinoma cell migration, invasion, and proliferation. Thorac Cancer. 2020;11(11):3168–74.
Snyman C, Niesler CU. MMP-14 in skeletal muscle repair. J Muscle Res Cell Motil. 2015;36(3):215–25.
Vos MC, van der Wurff AAM, van Kuppevelt TH, Massuger L. The role of MMP-14 in ovarian cancer: a systematic review. J Ovarian Res. 2021;14(1):101.
Redondo-Munoz J, Ugarte-Berzal E, Terol MJ, Van den Steen PE, Hernandez del Cerro M, Roderfeld M, et al. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell. 2010;17(2):160–72.
Aguilera-Montilla N, Bailon E, Uceda-Castro R, Ugarte-Berzal E, Santos A, Gutierrez-Gonzalez A, et al. MMP-9 affects gene expression in chronic lymphocytic leukemia revealing CD99 as an MMP-9 target and a novel partner in malignant cell migration/arrest. Oncogene. 2019;38(23):4605–19.
Marques C, Reis CA, Vives RR, Magalhaes A. Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression. Front Oncol. 2021;11:778752.
Hassan N, Greve B, Espinoza-Sanchez NA, Gotte M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell Signal. 2021;77:109822.
Mayfosh AJ, Nguyen TK, Hulett MD. The Heparanase Regulatory Network in Health and Disease. Int J Mol Sci. 2021;22(20):11096.
Doweck I, Feibish N. Opposing Effects of Heparanase and Heparanase-2 in Head & Neck Cancer. Adv Exp Med Biol. 2020;1221:847–56.
Yang WJ, Shi L, Wang XM, Yang GW. Heparanase is a novel biomarker for immune infiltration and prognosis in breast cancer. Aging (Albany NY). 2021;13(16):20836–52.
Liu LP, Sheng XP, Shuai TK, Zhao YX, Li B, Li YM. Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression. World J Gastroenterol. 2018;24(40):4565–77.
Jayatilleke KM, Hulett MD. Heparanase and the hallmarks of cancer. J Transl Med. 2020;18(1):453.
Piperigkou Z, Kyriakopoulou K, Koutsakis C, Mastronikolis S, Karamanos NK. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers (Basel). 2021;13(6):1441.
Barash U, Cohen-Kaplan V, Dowek I, Sanderson RD, Ilan N, Vlodavsky I. Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis. FEBS J. 2010;277(19):3890–903.
Wei RR, Sun DN, Yang H, Yan J, Zhang X, Zheng XL, et al. CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacol Sin. 2018;39(8):1326–37.
Teixeira F, Gotte M. Involvement of Syndecan-1 and Heparanase in Cancer and Inflammation. Adv Exp Med Biol. 2020;1221:97–135.
Putz EM, Mayfosh AJ, Kos K, Barkauskas DS, Nakamura K, Town L, et al. NK cell heparanase controls tumor invasion and immune surveillance. J Clin Invest. 2017;127(7):2777–88.
Schupp J, Krebs FK, Zimmer N, Trzeciak E, Schuppan D, Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol. 2019;343:103713.
Lynch CC. Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone. 2011;48(1):44–53.
Kos K, Wilding JP. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol. 2010;6(4):225–35.
Bawazeer S, Sabry D, Mahmoud RH, Elhanbuli HM, Yassen NN, Abdelhafez MN. Association of SPARC gene polymorphisms rs3210714 and rs7719521 with VEGF expression and utility of Nottingham Prognostic Index scoring in breast cancer in a sample of Egyptian women. Mol Biol Rep. 2018;45(6):2313–24.
Bradshaw AD. The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes? J Mol Cell Cardiol. 2016;93:156–61.
Cheng N, Bei Y, Song Y, Zhang W, Xu L, Zhang W, et al. B7–H3 augments the pro-angiogenic function of tumor-associated macrophages and acts as a novel adjuvant target for triple-negative breast cancer therapy. Biochem Pharmacol. 2021;183:114298.
Muliaditan T, Caron J, Okesola M, Opzoomer JW, Kosti P, Georgouli M, et al. Macrophages are exploited from an innate wound healing response to facilitate cancer metastasis. Nat Commun. 2018;9(1):2951.
Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213(11):2315–31.
Miki Y, Yashiro M, Moyano-Galceran L, Sugimoto A, Ohira M, Lehti K. Crosstalk Between Cancer Associated Fibroblasts and Cancer Cells in Scirrhous Type Gastric Cancer. Front Oncol. 2020;10:568557.
Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut. 2022;71(1):129–47.
Tang PC, Chung JY, Xue VW, Xiao J, Meng XM, Huang XR, et al. Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition. Adv Sci (Weinh). 2022;9(1):e2101235.
Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200.
Martinez-Vidal L, Murdica V, Venegoni C, Pederzoli F, Bandini M, Necchi A, et al. Causal contributors to tissue stiffness and clinical relevance in urology. Commun Biol. 2021;4(1):1011.
Rao Malla R, Marni R, Kumari S, Chakraborty A, Lalitha P. Microbiome Assisted Tumor Microenvironment: Emerging Target of Breast Cancer. Clin Breast Cancer. 2022;22(3):200–11.
Qiu Q, Lin Y, Ma Y, Li X, Liang J, Chen Z, et al. Exploring the Emerging Role of the Gut Microbiota and Tumor Microenvironment in Cancer Immunotherapy. Front Immunol. 2020;11:612202.
Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–60.
Sugimura N, Li Q, Chu ESH, Lau HCH, Fong W, Liu W, et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut. 2021;71(10):2011–21.
Matson V, Chervin CS, Gajewski TF. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology. 2021;160(2):600–13.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6(1):92.
McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012;20(2):125–36.
Wu S, Baum MM, Kerwin J, Guerrero D, Webster S, Schaudinn C, et al. Biofilm-specific extracellular matrix proteins of nontypeable Haemophilus influenzae. Pathog Dis. 2014;72(3):143–60.
Alfano M, Canducci F, Nebuloni M, Clementi M, Montorsi F, Salonia A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat Rev Urol. 2016;13(2):77–90.
Aragon IM, Herrera-Imbroda B, Queipo-Ortuno MI, Castillo E, Del Moral JS, Gomez-Millan J, et al. The Urinary Tract Microbiome in Health and Disease. Eur Urol Focus. 2018;4(1):128–38.
Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro. Br J Cancer. 2003;88(9):1470–9.
Hodkinson PS, Elliott T, Wong WS, Rintoul RC, Mackinnon AC, Haslett C, et al. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13(10):1776–88.
Dimitrijevic-Bussod M, Balzaretti-Maggi VS, Gadbois DM. Extracellular matrix and radiation G1 cell cycle arrest in human fibroblasts. Cancer Res. 1999;59(19):4843–7.
Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022;3(1):90–107.
Koohestani F, Braundmeier AG, Mahdian A, Seo J, Bi J, Nowak RA. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells. PLoS ONE. 2013;8(9):e75844.
Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3(3):a004994.
Tuguzbaeva G, Yue E, Chen X, He L, Li X, Ju J, et al. PEP06 polypeptide 30 is a novel cluster-dissociating agent inhibiting alpha v integrin/FAK/Src signaling in oral squamous cell carcinoma cells. Acta Pharm Sin B. 2019;9(6):1163–73.
Yeh CM, Hsieh MJ, Yang JS, Yang SF, Chuang YT, Su SC, et al. Geraniin inhibits oral cancer cell migration by suppressing matrix metalloproteinase-2 activation through the FAK/Src and ERK pathways. Environ Toxicol. 2019;34(10):1085–93.
Yu MH, Yang TY, Ho HH, Huang HP, Chan KC, Wang CJ. Mulberry Polyphenol Extract Inhibits FAK/Src/PI3K Complex and Related Signaling To Regulate the Migration in A7r5 Cells. J Agric Food Chem. 2018;66(15):3860–9.
Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014;34:144–53.
Juengel E, Natsheh I, Najafi R, Rutz J, Tsaur I, Haferkamp A, et al. Mechanisms behind Temsirolimus Resistance Causing Reactivated Growth and Invasive Behavior of Bladder Cancer Cells In Vitro. Cancers (Basel). 2019;11(6):777.
Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005;24(11):1942–53.
Liu Y, Lv J, Liang X, Yin X, Zhang L, Chen D, et al. Fibrin Stiffness Mediates Dormancy of Tumor-Repopulating Cells via a Cdc42-Driven Tet2 Epigenetic Program. Cancer Res. 2018;78(14):3926–37.
Chen WC, Hsu HP, Li CY, Yang YJ, Hung YH, Cho CY, et al. Cancer stem cell marker CD90 inhibits ovarian cancer formation via beta3 integrin. Int J Oncol. 2016;49(5):1881–9.
Song JM, Im J, Nho RS, Han YH, Upadhyaya P, Kassie F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol Carcinog. 2019;58(3):321–33.
Makkar S, Riehl TE, Chen B, Yan Y, Alvarado DM, Ciorba MA, et al. Hyaluronic Acid Binding to TLR4 Promotes Proliferation and Blocks Apoptosis in Colon Cancer. Mol Cancer Ther. 2019;18(12):2446–56.
Yang YM, Noureddin M, Liu C, Ohashi K, Kim SY, Ramnath D, et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci Transl Med. 2019;11(496):eaat9284.
Compagnone M, Gatti V, Presutti D, Ruberti G, Fierro C, Markert EK, et al. DeltaNp63-mediated regulation of hyaluronic acid metabolism and signaling supports HNSCC tumorigenesis. Proc Natl Acad Sci U S A. 2017;114(50):13254–9.
Ferrandez E, Gutierrez O, Segundo DS, Fernandez-Luna JL. NFkappaB activation in differentiating glioblastoma stem-like cells is promoted by hyaluronic acid signaling through TLR4. Sci Rep. 2018;8(1):6341.
Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front Immunol. 2015;6:201.
Tolg C, Yuan H, Flynn SM, Basu K, Ma J, Tse KCK, et al. Hyaluronan modulates growth factor induced mammary gland branching in a size dependent manner. Matrix Biol. 2017;63:117–32.
Pedron S, Hanselman JS, Schroeder MA, Sarkaria JN, Harley BAC. Extracellular Hyaluronic Acid Influences the Efficacy of EGFR Tyrosine Kinase Inhibitors in a Biomaterial Model of Glioblastoma. Adv Healthc Mater. 2017;6(21):10.1002/adhm.201700529.
Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18(4):251–9.
Ohata H, Ishiguro T, Aihara Y, Sato A, Sakai H, Sekine S, et al. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72(19):5101–10.
Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH, et al. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol Cell. 2012;47(2):281–90.
Bohaumilitzky L, Huber AK, Stork EM, Wengert S, Woelfl F, Boehm H. A Trickster in Disguise: Hyaluronan’s Ambivalent Roles in the Matrix. Front Oncol. 2017;7:242.
Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286(15):2883–908.
Tavianatou AG, Piperigkou Z, Koutsakis C, Barbera C, Beninatto R, Franchi M, et al. The action of hyaluronan in functional properties, morphology and expression of matrix effectors in mammary cancer cells depends on its molecular size. FEBS J. 2021;288(14):4291–310.
Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, et al. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013;280(10):2294–306.
Huang X, Reye G, Momot KI, Blick T, Lloyd T, Tilley WD, et al. Heparanase Promotes Syndecan-1 Expression to Mediate Fibrillar Collagen and Mammographic Density in Human Breast Tissue Cultured ex vivo. Front Cell Dev Biol. 2020;8:599.
Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD Jr, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282(18):13326–33.
Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115(12):2449–57.
Katakam SK, Pelucchi P, Cocola C, Reinbold R, Vlodavsky I, Greve B, et al. Syndecan-1-Dependent Regulation of Heparanase Affects Invasiveness, Stem Cell Properties, and Therapeutic Resistance of Caco2 Colon Cancer Cells. Front Oncol. 2020;10:774.
Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61(23):8586–94.
Lange K, Kammerer M, Hegi ME, Grotegut S, Dittmann A, Huang W, et al. Endothelin receptor type B counteracts tenascin-C-induced endothelin receptor type A-dependent focal adhesion and actin stress fiber disorganization. Cancer Res. 2007;67(13):6163–73.
Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206(3):691–705.
Park H, Kim Y, Lim Y, Han I, Oh ES. Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem. 2002;277(33):29730–6.
Sun M, Gomes S, Chen P, Frankenberger CA, Sankarasharma D, Chung CH, et al. RKIP and HMGA2 regulate breast tumor survival and metastasis through lysyl oxidase and syndecan-2. Oncogene. 2014;33(27):3528–37.
Luo X, Fong ELS, Zhu C, Lin QXX, Xiong M, Li A, et al. Hydrogel-based colorectal cancer organoid co-culture models. Acta Biomater. 2021;132:461–72.
Tanaka HY, Kitahara K, Sasaki N, Nakao N, Sato K, Narita H, et al. Pancreatic stellate cells derived from human pancreatic cancer demonstrate aberrant SPARC-dependent ECM remodeling in 3D engineered fibrotic tissue of clinically relevant thickness. Biomaterials. 2019;192:355–67.
Tang M, Tiwari SK, Agrawal K, Tan M, Dang J, Tam T, et al. Rapid 3D Bioprinting of Glioblastoma Model Mimicking Native Biophysical Heterogeneity. Small. 2021;17(15):e2006050.
Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004;64(2):612–21.
Mennerich D, Vogel A, Klaman I, Dahl E, Lichtner RB, Rosenthal A, et al. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumours. Eur J Cancer. 2004;40(9):1373–82.
Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res. 2004;10(15):5178–86.
Yang N, Mosher R, Seo S, Beebe D, Friedl A. Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. Am J Pathol. 2011;178(1):325–35.
Pach E, Kumper M, Fromme JE, Zamek J, Metzen F, Koch M, et al. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. Int J Mol Sci. 2021;22(22):12276.
Chen YT, Chen FW, Chang TH, Wang TW, Hsu TP, Chi JY, et al. Hepatoma-derived growth factor supports the antiapoptosis and profibrosis of pancreatic stellate cells. Cancer Lett. 2019;457:180–90.
Ma S, Meng Z, Chen R, Guan KL. The Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem. 2019;88:577–604.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83.
Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20(8):888–99.
Crosas-Molist E, Samain R, Kohlhammer L, Orgaz JL, George SL, Maiques O, et al. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102(1):455–510.
Yang N, Chen T, Wang L, Liu R, Niu Y, Sun L, et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10(13):5790–801.
Pavel M, Renna M, Park SJ, Menzies FM, Ricketts T, Fullgrabe J, et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun. 2018;9(1):2961.
Patwardhan S, Mahadik P, Shetty O, Sen S. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials. 2021;279:121185.
Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22(1):35-49 e7.
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780–94.
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70.
Shreberk-Shaked M, Dassa B, Sinha S, Di Agostino S, Azuri I, Mukherjee S, et al. A Division of Labor between YAP and TAZ in Non-Small Cell Lung Cancer. Cancer Res. 2020;80(19):4145–57.
Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, et al. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol. 2017;312(3):C277–85.
Kuroda M, Wada H, Kimura Y, Ueda K, Kioka N. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J Cell Sci. 2017;130(5):989–1002.
Zhang S, Chen Q, Liu Q, Li Y, Sun X, Hong L, et al. Hippo Signaling Suppresses Cell Ploidy and Tumorigenesis through Skp2. Cancer Cell. 2017;31(5):669-84 e7.
Hoxha S, Shepard A, Troutman S, Diao H, Doherty JR, Janiszewska M, et al. YAP-Mediated Recruitment of YY1 and EZH2 Represses Transcription of Key Cell-Cycle Regulators. Cancer Res. 2020;80(12):2512–22.
Xiao Y, Zhang H, Ma Q, Huang R, Lu J, Liang X, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett. 2019;462:51–60.
Panciera T, Citron A, Di Biagio D, Battilana G, Gandin A, Giulitti S, et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat Mater. 2020;19(7):797–806.
Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19(8):454–64.
Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19(18):1511–8.
Gerard C, Goldbeter A. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus. 2014;4(3):20130075.
Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28(49):4326–43.
Jang M, Koh I, Lee JE, Lim JY, Cheong JH, Kim P. Increased extracellular matrix density disrupts E-cadherin/beta-catenin complex in gastric cancer cells. Biomater Sci. 2018;6(10):2704–13.
Begum A, Ewachiw T, Jung C, Huang A, Norberg KJ, Marchionni L, et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12(7):e0180181.
Kosibaty Z, Murata Y, Minami Y, Noguchi M, Sakamoto N. ECT2 promotes lung adenocarcinoma progression through extracellular matrix dynamics and focal adhesion signaling. Cancer Sci. 2021;112(2):703–14.
Liang Y, Xia W, Zhang T, Chen B, Wang H, Song X, et al. Upregulated Collagen COL10A1 Remodels the Extracellular Matrix and Promotes Malignant Progression in Lung Adenocarcinoma. Front Oncol. 2020;10:573534.
Saldin LT, Patel S, Zhang L, Huleihel L, Hussey GS, Nascari DG, et al. Extracellular Matrix Degradation Products Downregulate Neoplastic Esophageal Cell Phenotype. Tissue Eng Part A. 2019;25(5–6):487–98.
Sosnowska M, Kutwin M, Jaworski S, Strojny B, Wierzbicki M, Szczepaniak J, et al. Mechano-signalling, induced by fullerene C(60) nanofilms, arrests the cell cycle in the G2/M phase and decreases proliferation of liver cancer cells. Int J Nanomedicine. 2019;14:6197–215.
Chou A, Froio D, Nagrial AM, Parkin A, Murphy KJ, Chin VT, et al. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2018;67(12):2142–55.
Jones MC, Askari JA, Humphries JD, Humphries MJ. Cell adhesion is regulated by CDK1 during the cell cycle. J Cell Biol. 2018;217(9):3203–18.
Yasukawa M, Ando Y, Yamashita T, Matsuda Y, Shoji S, Morioka MS, et al. CDK1 dependent phosphorylation of hTERT contributes to cancer progression. Nat Commun. 2020;11(1):1557.
Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70(5):890–9.
Marescal O, Cheeseman IM. Cellular Mechanisms and Regulation of Quiescence. Dev Cell. 2020;55(3):259–71.
Shibue T, Reinhardt F, Weinberg RA. Syndecan-Mediated Ligation of ECM Proteins Triggers Proliferative Arrest of Disseminated Tumor Cells. Cancer Res. 2019;79(23):5944–57.
Barney LE, Hall CL, Schwartz AD, Parks AN, Sparages C, Galarza S, et al. Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci Adv. 2020;6(11):eaaz4157.
Tejero R, Huang Y, Katsyv I, Kluge M, Lin JY, Tome-Garcia J, et al. Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment. EBioMedicine. 2019;42:252–69.
Zhang M, Xu C, Wang HZ, Peng YN, Li HO, Zhou YJ, et al. Soft fibrin matrix downregulates DAB2IP to promote Nanog-dependent growth of colon tumor-repopulating cells. Cell Death Dis. 2019;10(3):151.
Pradhan S, Slater JH. Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials. 2019;215:119177.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–93.
Paolillo M, Galiazzo MC, Daga A, Ciusani E, Serra M, Colombo L, et al. An RGD small-molecule integrin antagonist induces detachment-mediated anoikis in glioma cancer stem cells. Int J Oncol. 2018;53(6):2683–94.
Carter RZ, Micocci KC, Natoli A, Redvers RP, Paquet-Fifield S, Martin AC, et al. Tumour but not stromal expression of beta3 integrin is essential, and is required early, for spontaneous dissemination of bone-metastatic breast cancer. J Pathol. 2015;235(5):760–72.
Altei WF, Pachane BC, Dos Santos PK, Ribeiro LNM, Sung BH, Weaver AM, et al. Inhibition of alphavbeta3 integrin impairs adhesion and uptake of tumor-derived small extracellular vesicles. Cell Commun Signal. 2020;18(1):158.
Lino RLB, Dos Santos PK, Pisani GFD, Altei WF, Cominetti MR, Selistre-de-Araujo HS. Alphavbeta3 integrin blocking inhibits apoptosis and induces autophagy in murine breast tumor cells. Biochim Biophys Acta Mol Cell Res. 2019;1866(12):118536.
Liu F, Wu Q, Han W, Laster K, Hu Y, Ma F, et al. Targeting integrin alphavbeta3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin Transl Med. 2021;11(10):e548.
Iradyan M, Iradyan N, Hulin P, Hambardzumyan A, Gyulkhandanyan A, Alves de Sousa R, et al. Targeting Degradation of EGFR through the Allosteric Site Leads to Cancer Cell Detachment-Promoted Death. Cancers (Basel). 2019;11(8):1094.
Guha D, Saha T, Bose S, Chakraborty S, Dhar S, Khan P, et al. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis. 2019;24(11–12):958–71.
Corkery DP, Clarke LE, Gebremeskel S, Salsman J, Pinder J, Le Page C, et al. Loss of PRP4K drives anoikis resistance in part by dysregulation of epidermal growth factor receptor endosomal trafficking. Oncogene. 2018;37(2):174–84.
Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;69(1):122–32.
Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci U S A. 2018;115(22):5768–73.
Chute C, Yang X, Meyer K, Yang N, O’Neil K, Kasza I, et al. Syndecan-1 induction in lung microenvironment supports the establishment of breast tumor metastases. Breast Cancer Res. 2018;20(1):66.
Onyeisi JOS, Pernambuco Filho PCA, Mesquita APS, Azevedo LC, Nader HB, Lopes CC. Effects of syndecan-4 gene silencing by micro RNA interference in anoikis resistant endothelial cells: Syndecan-4 silencing and anoikis resistance. Int J Biochem Cell Biol. 2020;128:105848.
Carneiro BR, Pernambuco Filho PC, Mesquita AP, da Silva DS, Pinhal MA, Nader HB, et al. Acquisition of anoikis resistance up-regulates syndecan-4 expression in endothelial cells. PLoS ONE. 2014;9(12):e116001.
Kyriakopoulou K, Kefali E, Piperigkou Z, Riethmuller C, Greve B, Franchi M, et al. EGFR is a pivotal player of the E2/ERbeta - mediated functional properties, aggressiveness, and stemness in triple-negative breast cancer cells. FEBS J. 2022;289(6):1552–74.
Lv Y, Shan Y, Song L, Zhao Y, Lai R, Su J, et al. Type I collagen promotes tumor progression of integrin beta1 positive gastric cancer through a BCL9L/beta-catenin signaling pathway. Aging (Albany NY). 2021;13(14):19064–76.
Zhang H, Fredericks T, Xiong G, Qi Y, Rychahou PG, Li JD, et al. Membrane associated collagen XIII promotes cancer metastasis and enhances anoikis resistance. Breast Cancer Res. 2018;20(1):116.
Wang H, Ren R, Yang Z, Cai J, Du S, Shen X. The COL11A1/Akt/CREB signaling axis enables mitochondrial-mediated apoptotic evasion to promote chemoresistance in pancreatic cancer cells through modulating BAX/BCL-2 function. J Cancer. 2021;12(5):1406–20.
Burnier JV, Wang N, Michel RP, Hassanain M, Li S, Lu Y, et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene. 2011;30(35):3766–83.
Damaghi M, Mori H, Byrne S, Xu L, Chen T, Johnson J, et al. Collagen production and niche engineering: A novel strategy for cancer cells to survive acidosis in DCIS and evolve. Evol Appl. 2020;13(10):2689–703.
Liu CC, Lin SP, Hsu HS, Yang SH, Lin CH, Yang MH, et al. Suspension survival mediated by PP2A-STAT3-Col XVII determines tumour initiation and metastasis in cancer stem cells. Nat Commun. 2016;7:11798.
Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70(16):6670–81.
Cieply B, Koontz C, Frisch SM. CD44S-hyaluronan interactions protect cells resulting from EMT against anoikis. Matrix Biol. 2015;48:55–65.
Saha M, Kumar S, Bukhari S, Balaji SA, Kumar P, Hindupur SK, et al. AMPK-Akt Double-Negative Feedback Loop in Breast Cancer Cells Regulates Their Adaptation to Matrix Deprivation. Cancer Res. 2018;78(6):1497–510.
Du S, Yang Z, Lu X, Yousuf S, Zhao M, Li W, et al. Anoikis resistant gastric cancer cells promote angiogenesis and peritoneal metastasis through C/EBPbeta-mediated PDGFB autocrine and paracrine signaling. Oncogene. 2021;40(38):5764–79.
Saini H, Rahmani Eliato K, Veldhuizen J, Zare A, Allam M, Silva C, et al. The role of tumor-stroma interactions on desmoplasia and tumorigenicity within a microengineered 3D platform. Biomaterials. 2020;247:119975.
Primac I, Maquoi E, Blacher S, Heljasvaara R, Van Deun J, Smeland HY, et al. Stromal integrin alpha11 regulates PDGFR-beta signaling and promotes breast cancer progression. J Clin Invest. 2019;129(11):4609–28.
Insua-Rodriguez J, Pein M, Hongu T, Meier J, Descot A, Lowy CM, et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol Med. 2018;10(10):e9003.
Fujita M, Sasada M, Iyoda T, Nagai R, Kudo C, Yamamoto T, et al. Anoikis resistance conferred by tenascin-C-derived peptide TNIIIA2 and its disruption by integrin inactivation. Biochem Biophys Res Commun. 2021;536:14–9.
Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, et al. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin alpha5beta1. J Biol Chem. 2014;289(25):17699–708.
Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9(2):95–107.
Yu Q, Xiao W, Sun S, Sohrabi A, Liang J, Seidlits SK. Extracellular Matrix Proteins Confer Cell Adhesion-Mediated Drug Resistance Through Integrin alpha v in Glioblastoma Cells. Front Cell Dev Biol. 2021;9:616580.
Hou J, Tan Y, Su C, Wang T, Gao Z, Song D, et al. Inhibition of protein FAK enhances 5-FU chemosensitivity to gastric carcinoma via p53 signaling pathways. Comput Struct Biotechnol J. 2020;18:125–36.
Tang Q, Su Z, Gu W, Rustgi AK. Mutant p53 on the Path to Metastasis. Trends Cancer. 2020;6(1):62–73.
Bui T, Gu Y, Ancot F, Sanguin-Gendreau V, Zuo D, Muller WJ. Emergence of beta1 integrin-deficient breast tumours from dormancy involves both inactivation of p53 and generation of a permissive tumour microenvironment. Oncogene. 2022;41(4):527–37.
Amelio I, Mancini M, Petrova V, Cairns RA, Vikhreva P, Nicolai S, et al. p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc Natl Acad Sci U S A. 2018;115(46):E10869–78.
Novo D, Heath N, Mitchell L, Caligiuri G, MacFarlane A, Reijmer D, et al. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat Commun. 2018;9(1):5069.
Capaci V, Bascetta L, Fantuz M, Beznoussenko GV, Sommaggio R, Cancila V, et al. Mutant p53 induces Golgi tubulo-vesiculation driving a prometastatic secretome. Nat Commun. 2020;11(1):3945.
Munasinghe A, Malik K, Mohamedi F, Moaraf S, Kocher H, Jones L, et al. Fibronectin acts as a molecular switch to determine SPARC function in pancreatic cancer. Cancer Lett. 2020;477:88–96.
Buckup M, Rice MA, Hsu EC, Garcia-Marques F, Liu S, Aslan M, et al. Plectin is a regulator of prostate cancer growth and metastasis. Oncogene. 2021;40(3):663–76.
DiGiacomo JW, Godet I, Trautmann-Rodriguez M, Gilkes DM. Extracellular Matrix-Bound FGF2 Mediates Estrogen Receptor Signaling and Therapeutic Response in Breast Cancer. Mol Cancer Res. 2021;19(1):136–49.
Hayashido Y, Lucas A, Rougeot C, Godyna S, Argraves WS, Rochefort H. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer. 1998;75(4):654–8.
Zhong C, Tao B, Tang F, Yang X, Peng T, You J, et al. Remodeling cancer stemness by collagen/fibronectin via the AKT and CDC42 signaling pathway crosstalk in glioma. Theranostics. 2021;11(4):1991–2005.
Nandhu MS, Behera P, Bhaskaran V, Longo SL, Barrera-Arenas LM, Sengupta S, et al. Development of a Function-Blocking Antibody Against Fibulin-3 as a Targeted Reagent for Glioblastoma. Clin Cancer Res. 2018;24(4):821–33.
Xiao H, Zhang Y, Li Z, Liu B, Cui D, Liu F, et al. Periostin deficiency reduces diethylnitrosamine-induced liver cancer in mice by decreasing hepatic stellate cell activation and cancer cell proliferation. J Pathol. 2021;255(2):212–23.
Jia YL, Shi L, Zhou JN, Fu CJ, Chen L, Yuan HF, et al. Epimorphin promotes human hepatocellular carcinoma invasion and metastasis through activation of focal adhesion kinase/extracellular signal-regulated kinase/matrix metalloproteinase-9 axis. Hepatology. 2011;54(5):1808–18.
Salo S, Boutaud A, Hansen AJ, He L, Sun Y, Morales S, et al. Antibodies blocking adhesion and matrix binding domains of laminin-332 inhibit tumor growth and metastasis in vivo. Int J Cancer. 2009;125(8):1814–25.
Govaere O, Wouters J, Petz M, Vandewynckel YP, Van den Eynde K, Van den Broeck A, et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J Hepatol. 2016;64(3):609–17.
Huang C, Wang M, Wang J, Wu D, Gao Y, Huang K, et al. Suppression MGP inhibits tumor proliferation and reverses oxaliplatin resistance in colorectal cancer. Biochem Pharmacol. 2021;189:114390.
Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D, et al. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020;30(3):793-806 e6.
Chen Y, Li J, Jin L, Lei K, Liu H, Yang Y. Fibulin-5 contributes to colorectal cancer cell apoptosis via the ROS/MAPK and Akt signal pathways by downregulating transient receptor potential cation channel subfamily V member 1. J Cell Biochem. 2019;120(10):17838–46.
Sharma BK, Mureb D, Murab S, Rosenfeldt L, Francisco B, Cantrell R, et al. Fibrinogen activates focal adhesion kinase (FAK) promoting colorectal adenocarcinoma growth. J Thromb Haemost. 2021;19(10):2480–94.
Wang Y, Xu X, Marshall JE, Gong M, Zhao Y, Dua K, et al. Loss of Hyaluronan and Proteoglycan Link Protein-1 Induces Tumorigenesis in Colorectal Cancer. Front Oncol. 2021;11:754240.
Gan L, Meng J, Xu M, Liu M, Qi Y, Tan C, et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin beta4/FAK/SOX2/HIF-1alpha signaling pathway in gastric cancer. Oncogene. 2018;37(6):744–55.
Wang X, Zhou Q, Yu Z, Wu X, Chen X, Li J, et al. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin beta1-FAK signaling pathway. Int J Cancer. 2017;141(5):998–1010.
Ma Y, Nenkov M, Schroder DC, Abubrig M, Gassler N, Chen Y. Fibulin 2 Is Hypermethylated and Suppresses Tumor Cell Proliferation through Inhibition of Cell Adhesion and Extracellular Matrix Genes in Non-Small Cell Lung Cancer. Int J Mol Sci. 2021;22(21):11834.
Kodama K, Ishii G, Miyamoto S, Goya M, Zhang SC, Sangai T, et al. Laminin 5 expression protects against anoikis at aerogenous spread and lepidic growth of human lung adenocarcinoma. Int J Cancer. 2005;116(6):876–84.
Qin X, Lin L, Cao L, Zhang X, Song X, Hao J, et al. Extracellular matrix protein Reelin promotes myeloma progression by facilitating tumor cell proliferation and glycolysis. Sci Rep. 2017;7:45305.
Banerji A, Das S, Chatterjee A. Culture of human A375 melanoma cells in the presence of fibronectin causes expression of MMP-9 and activation of MMP-2 in culture supernatants. J Environ Pathol Toxicol Oncol. 2008;27(2):135–45.
Rada M, Nallanthighal S, Cha J, Ryan K, Sage J, Eldred C, et al. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene. 2018;37(35):4809–20.
Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, et al. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol. 2009;221(1):98–108.
Wang X, Zhang Q, Li C, Qu X, Yang P, Jia J, et al. Fibulin-3 Has Anti-Tumorigenic Activities in Cutaneous Squamous Cell Carcinoma. J Invest Dermatol. 2019;139(8):1798-808 e5.
Li Q, Zhang Y, Jiang Q. MFAP5 suppression inhibits migration/invasion, regulates cell cycle and induces apoptosis via promoting ROS production in cervical cancer. Biochem Biophys Res Commun. 2018;507(1–4):51–8.
Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29(3):212–26.
R MB-C, Helal-Neto E, A MV, Barcellos-de-Souza P, Morgado-Diaz J, Barja-Fidalgo C. Extracellular Matrix Derived from High Metastatic Human Breast Cancer Triggers Epithelial-Mesenchymal Transition in Epithelial Breast Cancer Cells through alphavbeta3 Integrin. Int J Mol Sci. 2020;21(8):2995.
Liu G, Wang B, Li S, Jin Q, Dai Y. Human breast cancer decellularized scaffolds promote epithelial-to-mesenchymal transitions and stemness of breast cancer cells in vitro. J Cell Physiol. 2019;234(6):9447–56.
Jin Q, Liu G, Li S, Yuan H, Yun Z, Zhang W, et al. Decellularized breast matrix as bioactive microenvironment for in vitro three-dimensional cancer culture. J Cell Physiol. 2019;234(4):3425–35.
Xu K, Wang Z, Copland JA, Chakrabarti R, Florczyk SJ. 3D porous chitosan-chondroitin sulfate scaffolds promote epithelial to mesenchymal transition in prostate cancer cells. Biomaterials. 2020;254:120126.
Selvaggio G, Canato S, Pawar A, Monteiro PT, Guerreiro PS, Bras MM, et al. Hybrid Epithelial-Mesenchymal Phenotypes Are Controlled by Microenvironmental Factors. Cancer Res. 2020;80(11):2407–20.
Park J, Kim DH, Shah SR, Kim HN, Kshitiz, Kim P, et al. Switch-like enhancement of epithelial-mesenchymal transition by YAP through feedback regulation of WT1 and Rho-family GTPases. Nat Commun. 2019;10(1):2797.
Niu N, Lu P, Yang Y, He R, Zhang L, Shi J, et al. Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia-mesenchymal transition during pancreatic carcinogenesis. Gut. 2020;69(4):715–26.
Colak S, Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends Cancer. 2017;3(1):56–71.
David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-beta Tumor Suppression through a Lethal EMT. Cell. 2016;164(5):1015–30.
Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, et al. TGF-beta orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577(7791):566–71.
Al Ameri W, Ahmed I, Al-Dasim FM, Ali Mohamoud Y, Al-Azwani IK, Malek JA, et al. Cell Type-Specific TGF-beta Mediated EMT in 3D and 2D Models and Its Reversal by TGF-beta Receptor Kinase Inhibitor in Ovarian Cancer Cell Lines. Int J Mol Sci. 2019;20(14):3568.
Rajagopal MU, Bansal S, Kaur P, Jain SK, Altadil T, Hinzman CP, et al. TGFbeta Drives Metabolic Perturbations during Epithelial Mesenchymal Transition in Pancreatic Cancer: TGFbeta Induced EMT in PDAC. Cancers (Basel). 2021;13(24):6204.
Aubert A, Mercier-Gouy P, Aguero S, Berthier L, Liot S, Prigent L, et al. Latent TGF-beta Activation Is a Hallmark of the Tenascin Family. Front Immunol. 2021;12:613438.
Griggs LA, Hassan NT, Malik RS, Griffin BP, Martinez BA, Elmore LW, et al. Fibronectin fibrils regulate TGF-beta1-induced Epithelial-Mesenchymal Transition. Matrix Biol. 2017;60–61:157–75.
Kollmannsberger P, Bidan CM, Dunlop JWC, Fratzl P, Vogel V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci Adv. 2018;4(1):eaao4881.
Wu YH, Huang YF, Chang TH, Chen CC, Wu PY, Huang SC, et al. COL11A1 activates cancer-associated fibroblasts by modulating TGF-beta3 through the NF-kappaB/IGFBP2 axis in ovarian cancer cells. Oncogene. 2021;40(26):4503–19.
Zhu Z, Achreja A, Meurs N, Animasahun O, Owen S, Mittal A, et al. Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat Metab. 2020;2(8):775–92.
Ishimoto T, Miyake K, Nandi T, Yashiro M, Onishi N, Huang KK, et al. Activation of Transforming Growth Factor Beta 1 Signaling in Gastric Cancer-associated Fibroblasts Increases Their Motility, via Expression of Rhomboid 5 Homolog 2, and Ability to Induce Invasiveness of Gastric Cancer Cells. Gastroenterology. 2017;153(1):191–204 e16.
Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, et al. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73(16):5016–28.
Chen Y, Zhao H, Feng Y, Ye Q, Hu J, Guo Y, et al. Pan-Cancer Analysis of the Associations of TGFBI Expression With Prognosis and Immune Characteristics. Front Mol Biosci. 2021;8:745649.
Ahmed AA, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M, et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12(6):514–27.
Zhang Y, Wen G, Shao G, Wang C, Lin C, Fang H, et al. TGFBI deficiency predisposes mice to spontaneous tumor development. Cancer Res. 2009;69(1):37–44.
Irigoyen M, Pajares MJ, Agorreta J, Ponz-Sarvise M, Salvo E, Lozano MD, et al. TGFBI expression is associated with a better response to chemotherapy in NSCLC. Mol Cancer. 2010;9:130.
Ahmed AA, Wang X, Lu Z, Goldsmith J, Le XF, Grandjean G, et al. Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel. Cancer Res. 2011;71(17):5806–17.
Ma C, Rong Y, Radiloff DR, Datto MB, Centeno B, Bao S, et al. Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev. 2008;22(3):308–21.
Nummela P, Lammi J, Soikkeli J, Saksela O, Laakkonen P, Holtta E. Transforming growth factor beta-induced (TGFBI) is an anti-adhesive protein regulating the invasive growth of melanoma cells. Am J Pathol. 2012;180(4):1663–74.
Tumbarello DA, Temple J, Brenton JD. ss3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells. Mol Cancer. 2012;11:36.
Han B, Cai H, Chen Y, Hu B, Luo H, Wu Y, et al. The role of TGFBI (betaig-H3) in gastrointestinal tract tumorigenesis. Mol Cancer. 2015;14:64.
Chen WY, Tsai YC, Yeh HL, Suau F, Jiang KC, Shao AN, et al. Loss of SPDEF and gain of TGFBI activity after androgen deprivation therapy promote EMT and bone metastasis of prostate cancer. Sci Signal. 2017;10(492):eaam6826.
Fico F, Santamaria-Martinez A. TGFBI modulates tumour hypoxia and promotes breast cancer metastasis. Mol Oncol. 2020;14(12):3198–210.
Chiavarina B, Costanza B, Ronca R, Blomme A, Rezzola S, Chiodelli P, et al. Metastatic colorectal cancer cells maintain the TGFbeta program and use TGFBI to fuel angiogenesis. Theranostics. 2021;11(4):1626–40.
Steitz AM, Steffes A, Finkernagel F, Unger A, Sommerfeld L, Jansen JM, et al. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis. 2020;11(4):249.
Lecker LSM, Berlato C, Maniati E, Delaine-Smith R, Pearce OMT, Heath O, et al. TGFBI Production by Macrophages Contributes to an Immunosuppressive Microenvironment in Ovarian Cancer. Cancer Res. 2021;81(22):5706–19.
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17(5):678–88.
Fattet L, Jung HY, Matsumoto MW, Aubol BE, Kumar A, Adams JA, et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev Cell. 2020;54(3):302-16 e7.
Kuipers AJ, Middelbeek J, Vrenken K, Perez-Gonzalez C, Poelmans G, Klarenbeek J, et al. TRPM7 controls mesenchymal features of breast cancer cells by tensional regulation of SOX4. Biochim Biophys Acta Mol Basis Dis. 2018;1864(7):2409–19.
Basilico B, Palama IE, D’Amone S, Lauro C, Rosito M, Grieco M, et al. Substrate stiffness effect on molecular crosstalk of epithelial-mesenchymal transition mediators of human glioblastoma cells. Front Oncol. 2022;12:983507.
Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016–26.
Peixoto P, Etcheverry A, Aubry M, Missey A, Lachat C, Perrard J, et al. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019;10(3):205.
Wissfeld J, Werner A, Yan X, Ten Bosch N, Cui G. Metabolic regulation of immune responses to cancer. Cancer Biol Med. 2022;19(11):1528–42.
Garde A, Sherwood DR. Fueling Cell Invasion through Extracellular Matrix. Trends Cell Biol. 2021;31(6):445–56.
Arnold JM, Gu F, Ambati CR, Rasaily U, Ramirez-Pena E, Joseph R, et al. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene. 2020;39(15):3089–101.
Li HM, Yang JG, Liu ZJ, Wang WM, Yu ZL, Ren JG, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):7.
Mejias M, Gallego J, Naranjo-Suarez S, Ramirez M, Pell N, Manzano A, et al. CPEB4 Increases Expression of PFKFB3 to Induce Glycolysis and Activate Mouse and Human Hepatic Stellate Cells Promoting Liver Fibrosis. Gastroenterology. 2020;159(1):273–88.
Sullivan WJ, Mullen PJ, Schmid EW, Flores A, Momcilovic M, Sharpley MS, et al. Extracellular Matrix Remodeling Regulates Glucose Metabolism through TXNIP Destabilization. Cell. 2018;175(1):117-32 e21.
Liu QP, Luo Q, Deng B, Ju Y, Song GB. Stiffer Matrix Accelerates Migration of Hepatocellular Carcinoma Cells through Enhanced Aerobic Glycolysis Via the MAPK-YAP Signaling. Cancers (Basel). 2020;12(2):490.
Mah EJ, Lefebvre A, McGahey GE, Yee AF, Digman MA. Collagen density modulates triple-negative breast cancer cell metabolism through adhesion-mediated contractility. Sci Rep. 2018;8(1):17094.
Liu C, Li M, Dong ZX, Jiang D, Li X, Lin S, et al. Heterogeneous microenvironmental stiffness regulates pro-metastatic functions of breast cancer cells. Acta Biomater. 2021;131:326–40.
Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S. Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol. 2021;22(1):22–38.
Somarribas Patterson LF, Vardhana SA. Metabolic regulation of the cancer-immunity cycle. Trends Immunol. 2021;42(11):975–93.
Schworer S, Pavlova NN, Cimino FV, King B, Cai X, Sizemore GM, et al. Fibroblast pyruvate carboxylase is required for collagen production in the tumour microenvironment. Nat Metab. 2021;3(11):1484–99.
Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29(1):124-40 e10.
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66(10):5216–23.
Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66(13):6699–707.
Romani P, Nirchio N, Arboit M, Barbieri V, Tosi A, Michielin F, et al. Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. Nat Cell Biol. 2022;24(2):168–80.
Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90.
Mason JA, Hagel KR, Hawk MA, Schafer ZT. Metabolism during ECM Detachment: Achilles Heel of Cancer Cells? Trends Cancer. 2017;3(7):475–81.
Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1alpha metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–5.
Endo H, Owada S, Inagaki Y, Shida Y, Tatemichi M. Metabolic reprogramming sustains cancer cell survival following extracellular matrix detachment. Redox Biol. 2020;36:101643.
Piao Y, Lu L, de Groot J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol. 2009;11(3):260–73.
Condello S, Sima L, Ivan C, Cardenas H, Schiltz G, Mishra RK, et al. Tissue Tranglutaminase Regulates Interactions between Ovarian Cancer Stem Cells and the Tumor Niche. Cancer Res. 2018;78(11):2990–3001.
Dornier E, Rabas N, Mitchell L, Novo D, Dhayade S, Marco S, et al. Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nat Commun. 2017;8(1):2255.
Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab. 2019;30(4):720-34 e5.
Hawk MA, Gorsuch CL, Fagan P, Lee C, Kim SE, Hamann JC, et al. RIPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells. Nat Cell Biol. 2018;20(3):272–84.
Gubbiotti MA, Neill T, Frey H, Schaefer L, Iozzo RV. Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 2015;48:14–25.
Neill T, Torres A, Buraschi S, Owens RT, Hoek JB, Baffa R, et al. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) and mitostatin. J Biol Chem. 2014;289(8):4952–68.
Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22(8):407-8. 10-12.
Zunder SM, Gelderblom H, Tollenaar RA, Mesker WE. The significance of stromal collagen organization in cancer tissue: An in-depth discussion of literature. Crit Rev Oncol Hematol. 2020;151:102907.
Xi G, Qiu L, Xu S, Guo W, Fu F, Kang D, et al. Computer-assisted quantification of tumor-associated collagen signatures to improve the prognosis prediction of breast cancer. BMC Med. 2021;19(1):273.
Conklin MW, Gangnon RE, Sprague BL, Van Gemert L, Hampton JM, Eliceiri KW, et al. Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma In Situ. Cancer Epidemiol Biomarkers Prev. 2018;27(2):138–45.
Li H, Bera K, Toro P, Fu P, Zhang Z, Lu C, et al. Collagen fiber orientation disorder from H&E images is prognostic for early stage breast cancer: clinical trial validation. NPJ Breast Cancer. 2021;7(1):104.
Bodelon C, Mullooly M, Pfeiffer RM, Fan S, Abubakar M, Lenz P, et al. Mammary collagen architecture and its association with mammographic density and lesion severity among women undergoing image-guided breast biopsy. Breast Cancer Res. 2021;23(1):105.
Chen D, Liu Z, Liu W, Fu M, Jiang W, Xu S, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. 2021;12(1):179.
Angenendt L, Mikesch JH, Gorlich D, Busch A, Arnhold I, Rudack C, et al. Stromal collagen type VI associates with features of malignancy and predicts poor prognosis in salivary gland cancer. Cell Oncol (Dordr). 2018;41(5):517–25.
Drifka CR, Tod J, Loeffler AG, Liu Y, Thomas GJ, Eliceiri KW, et al. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod Pathol. 2015;28(11):1470–80.
Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910.
Wen BL, Brewer MA, Nadiarnykh O, Hocker J, Singh V, Mackie TR, et al. Texture analysis applied to second harmonic generation image data for ovarian cancer classification. J Biomed Opt. 2014;19(9):096007.
Northey JJ, Barrett AS, Acerbi I, Hayward MK, Talamantes S, Dean IS, et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J Clin Invest. 2020;130(11):5721–37.
Bahcecioglu G, Yue X, Howe E, Guldner I, Stack MS, Nakshatri H, et al. Aged Breast Extracellular Matrix Drives Mammary Epithelial Cells to an Invasive and Cancer-Like Phenotype. Adv Sci (Weinh). 2021;8(22):e2100128.
Fischer RS, Sun X, Baird MA, Hourwitz MJ, Seo BR, Pasapera AM, et al. Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates. Proc Natl Acad Sci USA. 2021;118(22):e2021135118.
Han W, Chen S, Yuan W, Fan Q, Tian J, Wang X, et al. Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci U S A. 2016;113(40):11208–13.
Su CY, Burchett A, Dunworth M, Choi JS, Ewald AJ, Ahn EH, et al. Engineering a 3D collective cancer invasion model with control over collagen fiber alignment. Biomaterials. 2021;275:120922.
Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, et al. Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature. 2020;585(7825):433–9.
Huang YL, Liang CY, Ritz D, Coelho R, Septiadi D, Estermann M, et al. Collagen-rich omentum is a premetastatic niche for integrin alpha2-mediated peritoneal metastasis. Elife. 2020;9:e59442.
Pally D, Pramanik D, Bhat R. An Interplay Between Reaction-Diffusion and Cell-Matrix Adhesion Regulates Multiscale Invasion in Early Breast Carcinomatosis. Front Physiol. 2019;10:790.
Wu X, Cai J, Zuo Z, Li J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother. 2019;114:108708.
Grasset EM, Bertero T, Bozec A, Friard J, Bourget I, Pisano S, et al. Matrix Stiffening and EGFR Cooperate to Promote the Collective Invasion of Cancer Cells. Cancer Res. 2018;78(18):5229–42.
Ray A, Lee O, Win Z, Edwards RM, Alford PW, Kim DH, et al. Anisotropic forces from spatially constrained focal adhesions mediate contact guidance directed cell migration. Nat Commun. 2017;8:14923.
Nuhn JAM, Perez AM, Schneider IC. Contact guidance diversity in rotationally aligned collagen matrices. Acta Biomater. 2018;66:248–57.
Kay EJ, Koulouras G, Zanivan S. Regulation of Extracellular Matrix Production in Activated Fibroblasts: Roles of Amino Acid Metabolism in Collagen Synthesis. Front Oncol. 2021;11:719922.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218.
Boyle ST, Poltavets V, Kular J, Pyne NT, Sandow JJ, Lewis AC, et al. ROCK-mediated selective activation of PERK signalling causes fibroblast reprogramming and tumour progression through a CRELD2-dependent mechanism. Nat Cell Biol. 2020;22(7):882–95.
Malik R, Luong T, Cao X, Han B, Shah N, Franco-Barraza J, et al. Rigidity controls human desmoplastic matrix anisotropy to enable pancreatic cancer cell spread via extracellular signal-regulated kinase 2. Matrix Biol. 2019;81:50–69.
Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018;78(17):4957–70.
Goreczny GJ, Ouderkirk-Pecone JL, Olson EC, Krendel M, Turner CE. Hic-5 remodeling of the stromal matrix promotes breast tumor progression. Oncogene. 2017;36(19):2693–703.
Zhou B, Zong S, Zhong W, Tian Y, Wang L, Zhang Q, et al. Interaction between laminin-5gamma2 and integrin beta1 promotes the tumor budding of colorectal cancer via the activation of Yes-associated proteins. Oncogene. 2020;39(7):1527–42.
Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18(2):101–15.
Marangon Junior H, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43(3):199–204.
Zhang G, He Y, Liu Y, Du Y, Yang C, Gao F. Reduced hyaluronan cross-linking induces breast cancer malignancy in a CAF-dependent manner. Cell Death Dis. 2021;12(6):586.
Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–96.
Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9(8):1102–23.
Helms EJ, Berry MW, Chaw RC, DuFort CC, Sun D, Onate MK, et al. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022;12(2):484–501.
Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022;12(2):620–38.
Jungwirth U, van Weverwijk A, Evans RJ, Jenkins L, Vicente D, Alexander J, et al. Impairment of a distinct cancer-associated fibroblast population limits tumour growth and metastasis. Nat Commun. 2021;12(1):3516.
Feldmann K, Maurer C, Peschke K, Teller S, Schuck K, Steiger K, et al. Mesenchymal Plasticity Regulated by Prrx1 Drives Aggressive Pancreatic Cancer Biology. Gastroenterology. 2021;160(1):346-61 e24.
Li X, Balagam R, He TF, Lee PP, Igoshin OA, Levine H. On the mechanism of long-range orientational order of fibroblasts. Proc Natl Acad Sci USA. 2017;114(34):8974–9.
Jung WH, Yam N, Chen CC, Elawad K, Hu B, Chen Y. Force-dependent extracellular matrix remodeling by early-stage cancer cells alters diffusion and induces carcinoma-associated fibroblasts. Biomaterials. 2020;234:119756.
Kong J, Tian H, Zhang F, Zhang Z, Li J, Liu X, et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer. 2019;18(1):175.
Dourado MR, Korvala J, Astrom P, De Oliveira CE, Cervigne NK, Mofatto LS, et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. J Extracell Vesicles. 2019;8(1):1578525.
Garcia-Palmero I, Torres S, Bartolome RA, Pelaez-Garcia A, Larriba MJ, Lopez-Lucendo M, et al. Twist1-induced activation of human fibroblasts promotes matrix stiffness by upregulating palladin and collagen alpha1(VI). Oncogene. 2016;35(40):5224–36.
Nakashima C, Kirita T, Yamamoto K, Mori S, Luo Y, Sasaki T, et al. Malic Enzyme 1 Is Associated with Tumor Budding in Oral Squamous Cell Carcinomas. Int J Mol Sci. 2020;21(19):7149.
Lewis DM, Pruitt H, Jain N, Ciccaglione M, McCaffery JM, Xia Z, et al. A Feedback Loop between Hypoxia and Matrix Stress Relaxation Increases Oxygen-Axis Migration and Metastasis in Sarcoma. Cancer Res. 2019;79(8):1981–95.
Goggins E, Kakkad S, Mironchik Y, Jacob D, Wildes F, Krishnamachary B, et al. Hypoxia Inducible Factors Modify Collagen I Fibers in MDA-MB-231 Triple Negative Breast Cancer Xenografts. Neoplasia. 2018;20(2):131–9.
Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23.
Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34.
Baltes F, Caspers J, Henze S, Schlesinger M, Bendas G. Targeting Discoidin Domain Receptor 1 (DDR1) Signaling and Its Crosstalk with beta(1)-integrin Emerges as a Key Factor for Breast Cancer Chemosensitization upon Collagen Type 1 Binding. Int J Mol Sci. 2020;21(14):4956.
Rauner G, Jin DX, Miller DH, Gierahn TM, Li CM, Sokol ES, et al. Breast tissue regeneration is driven by cell-matrix interactions coordinating multi-lineage stem cell differentiation through DDR1. Nat Commun. 2021;12(1):7116.
Rudra-Ganguly N, Lowe C, Mattie M, Chang MS, Satpayev D, Verlinsky A, et al. Discoidin domain receptor 1 contributes to tumorigenesis through modulation of TGFBI expression. PLoS ONE. 2014;9(11):e111515.
Clementz AG, Mutolo MJ, Leir SH, Morris KJ, Kucybala K, Harris H, et al. Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS ONE. 2013;8(8):e72250.
Aguilera KY, Huang H, Du W, Hagopian MM, Wang Z, Hinz S, et al. Inhibition of Discoidin Domain Receptor 1 Reduces Collagen-mediated Tumorigenicity in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2017;16(11):2473–85.
Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–48.
Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, Cortes-Reynosa P, Salazar EP. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 2011;28(5):463–77.
Espinosa Neira R, Salazar EP. Native type IV collagen induces an epithelial to mesenchymal transition-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol. 2012;44(12):2194–203.
Fu HL, Sohail A, Valiathan RR, Wasinski BD, Kumarasiri M, Mahasenan KV, et al. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J Biol Chem. 2013;288(17):12114–29.
Ruggeri JM, Franco-Barraza J, Sohail A, Zhang Y, Long D, Pasca di Magliano M, et al. Discoidin Domain Receptor 1 (DDR1) Is Necessary for Tissue Homeostasis in Pancreatic Injury and Pathogenesis of Pancreatic Ductal Adenocarcinoma. Am J Pathol. 2020;190(8):1735–51.
Juin A, Di Martino J, Leitinger B, Henriet E, Gary AS, Paysan L, et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207(4):517–33.
Poudel B, Lee YM, Kim DK. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-kappaB pathway. Acta Biochim Biophys Sin (Shanghai). 2015;47(4):292–8.
Corsa CA, Brenot A, Grither WR, Van Hove S, Loza AJ, Zhang K, et al. The Action of Discoidin Domain Receptor 2 in Basal Tumor Cells and Stromal Cancer-Associated Fibroblasts Is Critical for Breast Cancer Metastasis. Cell Rep. 2016;15(11):2510–23.
Hwang PY, Brenot A, King AC, Longmore GD, George SC. Randomly Distributed K14(+) Breast Tumor Cells Polarize to the Leading Edge and Guide Collective Migration in Response to Chemical and Mechanical Environmental Cues. Cancer Res. 2019;79(8):1899–912.
Wall SJ, Werner E, Werb Z, DeClerck YA. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280(48):40187–94.
Koelzer VH, Huber B, Mele V, Iezzi G, Trippel M, Karamitopoulou E, et al. Expression of the hyaluronan-mediated motility receptor RHAMM in tumor budding cells identifies aggressive colorectal cancers. Hum Pathol. 2015;46(11):1573–81.
Zhang H, Ren L, Ding Y, Li F, Chen X, Ouyang Y, et al. Hyaluronan-mediated motility receptor confers resistance to chemotherapy via TGFbeta/Smad2-induced epithelial-mesenchymal transition in gastric cancer. FASEB J. 2019;33(5):6365–77.
Hiraga T, Ito S, Nakamura H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013;73(13):4112–22.
Yoo KC, Suh Y, An Y, Lee HJ, Jeong YJ, Uddin N, et al. Proinvasive extracellular matrix remodeling in tumor microenvironment in response to radiation. Oncogene. 2018;37(24):3317–28.
Koikawa K, Ohuchida K, Takesue S, Ando Y, Kibe S, Nakayama H, et al. Pancreatic stellate cells reorganize matrix components and lead pancreatic cancer invasion via the function of Endo180. Cancer Lett. 2018;412:143–54.
Sommerfeld L, Finkernagel F, Jansen JM, Wagner U, Nist A, Stiewe T, et al. The multicellular signalling network of ovarian cancer metastases. Clin Transl Med. 2021;11(11):e633.
Luthria G, Li R, Wang S, Prytyskach M, Kohler RH, Lauffenburger DA, et al. In vivo microscopy reveals macrophage polarization locally promotes coherent microtubule dynamics in migrating cancer cells. Nat Commun. 2020;11(1):3521.
Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18(5):549–60.
Graf F, Horn P, Ho AD, Boutros M, Maercker C. The extracellular matrix proteins type I collagen, type III collagen, fibronectin, and laminin 421 stimulate migration of cancer cells. FASEB J. 2021;35(7):e21692.
Mao L, Yang J, Yue J, Chen Y, Zhou H, Fan D, et al. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol. 2021;95:1–14.
Juin A, Spence HJ, Martin KJ, McGhee E, Neilson M, Cutiongco MFA, et al. N-WASP Control of LPAR1 Trafficking Establishes Response to Self-Generated LPA Gradients to Promote Pancreatic Cancer Cell Metastasis. Dev Cell. 2019;51(4):431-45 e7.
Ye M, Song Y, Pan S, Chu M, Wang ZW, Zhu X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol Ther. 2020;215:107633.
Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32(14):1863–8.
Natarajan S, Foreman KM, Soriano MI, Rossen NS, Shehade H, Fregoso DR, et al. Collagen Remodeling in the Hypoxic Tumor-Mesothelial Niche Promotes Ovarian Cancer Metastasis. Cancer Res. 2019;79(9):2271–84.
Torres S, Garcia-Palmero I, Herrera M, Bartolome RA, Pena C, Fernandez-Acenero MJ, et al. LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival. Clin Cancer Res. 2015;21(21):4892–902.
Nguyen EV, Pereira BA, Lawrence MG, Ma X, Rebello RJ, Chan H, et al. Proteomic Profiling of Human Prostate Cancer-associated Fibroblasts (CAF) Reveals LOXL2-dependent Regulation of the Tumor Microenvironment. Mol Cell Proteomics. 2019;18(7):1410–27.
Cao C, Lin S, Zhi W, Lazare C, Meng Y, Wu P, et al. LOXL2 Expression Status Is Correlated With Molecular Characterizations of Cervical Carcinoma and Associated With Poor Cancer Survival via Epithelial-Mesenchymal Transition (EMT) Phenotype. Front Oncol. 2020;10:284.
Sanada T, Islam A, Kaminota T, Kirino Y, Tanimoto R, Yoshimitsu H, et al. Elevated exosomal lysyl oxidase like 2 is a potential biomarker for head and neck squamous cell carcinoma. Laryngoscope. 2020;130(5):E327–34.
Choi J, Chung T, Rhee H, Kim YJ, Jeon Y, Yoo JE, et al. Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis. Gut Liver. 2019;13(1):83–92.
Zhang X, Huang J, You F, Li W, Zou Z. Prognostic and clinicopathological significance of LOXL2 in cancers: A systematic review and meta-analysis. J Cell Physiol. 2019;234(11):21369–79.
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, et al. HIF-1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60.
Wu S, Xing X, Wang Y, Zhang X, Li M, Wang M, et al. The pathological significance of LOXL2 in pre-metastatic niche formation of HCC and its related molecular mechanism. Eur J Cancer. 2021;147:63–73.
Deng Y, Chakraborty P, Jolly MK, Levine H. A Theoretical Approach to Coupling the Epithelial-Mesenchymal Transition (EMT) to Extracellular Matrix (ECM) Stiffness via LOXL2. Cancers (Basel). 2021;13(7):1609.
Peng DH, Ungewiss C, Tong P, Byers LA, Wang J, Canales JR, et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene. 2017;36(14):1925–38.
Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A, et al. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017;77(21):5846–59.
Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, et al. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular Matrix. Cancer Res. 2016;76(14):4249–58.
Jiang H, Torphy RJ, Steiger K, Hongo H, Ritchie AJ, Kriegsmann M, et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest. 2020;130(9):4704–9.
Sflomos G, Battista L, Aouad P, De Martino F, Scabia V, Stravodimou A, et al. Intraductal xenografts show lobular carcinoma cells rely on their own extracellular matrix and LOXL1. EMBO Mol Med. 2021;13(3):e13180.
Tan HY, Wang N, Zhang C, Chan YT, Yuen MF, Feng Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology. 2021;73(6):2326–41.
Das S, Amin SA, Jha T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur J Med Chem. 2021;223:113623.
Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci. 2020;21(24):9739.
Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat Shock Proteins and Cancer. Trends Pharmacol Sci. 2017;38(3):226–56.
Secli L, Fusella F, Avalle L, Brancaccio M. The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol Life Sci. 2021;78(9):4069–83.
Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90alpha assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS ONE. 2011;6(4):e18848.
Baker-Williams AJ, Hashmi F, Budzynski MA, Woodford MR, Gleicher S, Himanen SV, et al. Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90: Client MMP2 Activity and Matrix Proteolysis. Cell Rep. 2019;28(7):1894-906 e6.
Armstrong HK, Gillis JL, Johnson IRD, Nassar ZD, Moldovan M, Levrier C, et al. Dysregulated fibronectin trafficking by Hsp90 inhibition restricts prostate cancer cell invasion. Sci Rep. 2018;8(1):2090.
Henke A, Franco OE, Stewart GD, Riddick AC, Katz E, Hayward SW, et al. Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90. Cancers (Basel). 2016;8(9):77.
Jiang X, Zhou T, Wang Z, Qi B, Xia H. HSP47 Promotes Glioblastoma Stemlike Cell Survival by Modulating Tumor Microenvironment Extracellular Matrix through TGF-beta Pathway. ACS Chem Neurosci. 2017;8(1):128–34.
Yoneda A, Minomi K, Tamura Y. HSP47 promotes metastasis of breast cancer by interacting with myosin IIA via the unfolded protein response transducer IRE1alpha. Oncogene. 2020;39(23):4519–37.
Xiong G, Chen J, Zhang G, Wang S, Kawasaki K, Zhu J, et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proc Natl Acad Sci U S A. 2020;117(7):3748–58.
Miyamura T, Sakamoto N, Kakugawa T, Taniguchi H, Akiyama Y, Okuno D, et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem Biophys Res Commun. 2020;530(3):561–5.
Bordeau BM, Balthasar JP. Strategies to enhance monoclonal antibody uptake and distribution in solid tumors. Cancer Biol Med. 2021;18(3):649–64.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74.
Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18(4):882–92.
Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Puliafito A, Bouvard C, et al. Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nat Cell Biol. 2014;16(10):931–41, 1-8.
Orecchia P, Conte R, Balza E, Petretto A, Mauri P, Mingari MC, et al. A novel human anti-syndecan-1 antibody inhibits vascular maturation and tumour growth in melanoma. Eur J Cancer. 2013;49(8):2022–33.
Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28(50):4456–68.
Bockelmann LC, Felix T, Calabro S, Schumacher U. YKL-40 protein expression in human tumor samples and human tumor cell line xenografts: implications for its use in tumor models. Cell Oncol (Dordr). 2021;44(5):1183–95.
Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286(17):15332–43.
Chen HY, Zhou ZY, Luo YL, Luo Q, Fan JT. Knockdown of YKL-40 inhibits angiogenesis through regulation of VEGF/VEGFR2 and ERK1/2 signaling in endometrial cancer. Cell Biol Int. 2021;45(12):2557–66.
Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin beta5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2013;32(25):3049–58.
Sun W, Xue Q, Zhao Y, Zheng J. The effects of YKL-40 on angiogenic potential of HUVECs are partly mediated by syndecan-4. Int J Med Sci. 2021;18(16):3759–67.
Helal-Neto E, Brandao-Costa RM, Saldanha-Gama R, Ribeiro-Pereira C, Midlej V, Benchimol M, et al. Priming Endothelial Cells With a Melanoma-Derived Extracellular Matrix Triggers the Activation of alphavbeta3/VEGFR2 Axis. J Cell Physiol. 2016;231(11):2464–73.
Wong PP, Munoz-Felix JM, Hijazi M, Kim H, Robinson SD, De Luxan-Delgado B, et al. Cancer Burden Is Controlled by Mural Cell-beta3-Integrin Regulated Crosstalk with Tumor Cells. Cell. 2020;181(6):1346-63 e21.
Medfai H, Khalil A, Rousseau A, Nuyens V, Paumann-Page M, Sevcnikar B, et al. Human peroxidasin 1 promotes angiogenesis through ERK1/2, Akt, and FAK pathways. Cardiovasc Res. 2019;115(2):463–75.
Lee SW, Kim HK, Naidansuren P, Ham KA, Choi HS, Ahn HY, et al. Peroxidasin is essential for endothelial cell survival and growth signaling by sulfilimine crosslink-dependent matrix assembly. FASEB J. 2020;34(8):10228–41.
He C, Song W, Weston TA, Tran C, Kurtz I, Zuckerman JE, et al. Peroxidasin-mediated bromine enrichment of basement membranes. Proc Natl Acad Sci U S A. 2020;117(27):15827–36.
Paumann-Page M, Kienzl NF, Motwani J, Bathish B, Paton LN, Magon NJ, et al. Peroxidasin protein expression and enzymatic activity in metastatic melanoma cell lines are associated with invasive potential. Redox Biol. 2021;46:102090.
Paulitti A, Andreuzzi E, Bizzotto D, Pellicani R, Tarticchio G, Marastoni S, et al. The ablation of the matricellular protein EMILIN2 causes defective vascularization due to impaired EGFR-dependent IL-8 production affecting tumor growth. Oncogene. 2018;37(25):3399–414.
Pellicani R, Poletto E, Andreuzzi E, Paulitti A, Doliana R, Bizzotto D, et al. Multimerin-2 maintains vascular stability and permeability. Matrix Biol. 2020;87:11–25.
Lorenzon E, Colladel R, Andreuzzi E, Marastoni S, Todaro F, Schiappacassi M, et al. MULTIMERIN2 impairs tumor angiogenesis and growth by interfering with VEGF-A/VEGFR2 pathway. Oncogene. 2012;31(26):3136–47.
Zanivan S, Maione F, Hein MY, Hernandez-Fernaud JR, Ostasiewicz P, Giraudo E, et al. SILAC-based proteomics of human primary endothelial cell morphogenesis unveils tumor angiogenic markers. Mol Cell Proteomics. 2013;12(12):3599–611.
Noy PJ, Lodhia P, Khan K, Zhuang X, Ward DG, Verissimo AR, et al. Blocking CLEC14A-MMRN2 binding inhibits sprouting angiogenesis and tumour growth. Oncogene. 2015;34(47):5821–31.
Andreuzzi E, Colladel R, Pellicani R, Tarticchio G, Cannizzaro R, Spessotto P, et al. The angiostatic molecule Multimerin 2 is processed by MMP-9 to allow sprouting angiogenesis. Matrix Biol. 2017;64:40–53.
Galvagni F, Nardi F, Spiga O, Trezza A, Tarticchio G, Pellicani R, et al. Dissecting the CD93-Multimerin 2 interaction involved in cell adhesion and migration of the activated endothelium. Matrix Biol. 2017;64:112–27.
Lugano R, Vemuri K, Yu D, Bergqvist M, Smits A, Essand M, et al. CD93 promotes beta1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J Clin Invest. 2018;128(8):3280–97.
Marchio S, Astanina E, Bussolino F. Emerging lymphae for the fountain of life. EMBO J. 2013;32(5):609–11.
Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73.
Rezzola S, Sigmund EC, Halin C, Ronca R. The lymphatic vasculature: An active and dynamic player in cancer progression. Med Res Rev. 2022;42(1):576–614.
Guc E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, et al. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials. 2017;131:160–75.
Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsater H, et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun. 2018;9(1):1511.
Guo LX, Zou K, Ju JH, Xie H. Hyaluronan promotes tumor lymphangiogenesis and intralymphantic tumor growth in xenografts. Acta Biochim Biophys Sin (Shanghai). 2005;37(9):601–6.
Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, et al. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol. 2008;172(1):179–93.
Cohen-Kaplan V, Naroditsky I, Zetser A, Ilan N, Vlodavsky I, Doweck I. Heparanase induces VEGF C and facilitates tumor lymphangiogenesis. Int J Cancer. 2008;123(11):2566–73.
Hunter KE, Palermo C, Kester JC, Simpson K, Li JP, Tang LH, et al. Heparanase promotes lymphangiogenesis and tumor invasion in pancreatic neuroendocrine tumors. Oncogene. 2014;33(14):1799–808.
Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene. 2016;35(2):196–205.
Maeng YS, Lee R, Lee B, Choi SI, Kim EK. Lithium inhibits tumor lymphangiogenesis and metastasis through the inhibition of TGFBIp expression in cancer cells. Sci Rep. 2016;6:20739.
Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi A, Gruber S, Kunstfeld R, et al. Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin. Am J Pathol. 2010;177(2):1004–16.
Bieniasz-Krzywiec P, Martin-Perez R, Ehling M, Garcia-Caballero M, Pinioti S, Pretto S, et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019;30(5):917-36 e10.
Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41(4):396–8.
Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppanen VM, Holopainen T, et al. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation. 2014;129(19):1962–71.
Roukens MG, Peterson-Maduro J, Padberg Y, Jeltsch M, Leppanen VM, Bos FL, et al. Functional Dissection of the CCBE1 Protein: A Crucial Requirement for the Collagen Repeat Domain. Circ Res. 2015;116(10):1660–9.
Jha SK, Rauniyar K, Karpanen T, Leppanen VM, Brouillard P, Vikkula M, et al. Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci Rep. 2017;7(1):4916.
Song J, Chen W, Cui X, Huang Z, Wen D, Yang Y, et al. CCBE1 promotes tumor lymphangiogenesis and is negatively regulated by TGFbeta signaling in colorectal cancer. Theranostics. 2020;10(5):2327–41.
Tian GA, Zhu CC, Zhang XX, Zhu L, Yang XM, Jiang SH, et al. CCBE1 promotes GIST development through enhancing angiogenesis and mediating resistance to imatinib. Sci Rep. 2016;6:31071.
Zhao YR, Liu H, Xiao LM, Jin CG, Zhang ZP, Yang CG. The clinical significance of CCBE1 expression in human colorectal cancer. Cancer Manag Res. 2018;10:6581–90.
Mesci A, Huang X, Taeb S, Jahangiri S, Kim Y, Fokas E, et al. Targeting of CCBE1 by miR-330-3p in human breast cancer promotes metastasis. Br J Cancer. 2017;116(10):1350–7.
Barton CA, Gloss BS, Qu W, Statham AL, Hacker NF, Sutherland RL, et al. Collagen and calcium-binding EGF domains 1 is frequently inactivated in ovarian cancer by aberrant promoter hypermethylation and modulates cell migration and survival. Br J Cancer. 2010;102(1):87–96.
Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbaek MS, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7(1):68.
Chirivi M, Maiullari F, Milan M, Presutti D, Cordiglieri C, Crosti M, et al. Tumor Extracellular Matrix Stiffness Promptly Modulates the Phenotype and Gene Expression of Infiltrating T Lymphocytes. Int J Mol Sci. 2021;22(11):5862.
Bougherara H, Mansuet-Lupo A, Alifano M, Ngo C, Damotte D, Le Frere-Belda MA, et al. Real-Time Imaging of Resident T Cells in Human Lung and Ovarian Carcinomas Reveals How Different Tumor Microenvironments Control T Lymphocyte Migration. Front Immunol. 2015;6:500.
Hallmann R, Zhang X, Di Russo J, Li L, Song J, Hannocks MJ, et al. The regulation of immune cell trafficking by the extracellular matrix. Curr Opin Cell Biol. 2015;36:54–61.
Chakraborty M, Chu K, Shrestha A, Revelo XS, Zhang X, Gold MJ, et al. Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell Rep. 2021;34(2):108609.
Liu XQ, Chen XT, Liu ZZ, Gu SS, He LJ, Wang KP, et al. Biomimetic Matrix Stiffness Modulates Hepatocellular Carcinoma Malignant Phenotypes and Macrophage Polarization through Multiple Modes of Mechanical Feedbacks. ACS Biomater Sci Eng. 2020;6(7):3994–4004.
Alonso-Nocelo M, Raimondo TM, Vining KH, Lopez-Lopez R, de la Fuente M, Mooney DJ. Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication. 2018;10(3):035004.
Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L, et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17(1):79.
Bordry N, Broggi MAS, de Jonge K, Schaeuble K, Gannon PO, Foukas PG, et al. Lymphatic vessel density is associated with CD8(+) T cell infiltration and immunosuppressive factors in human melanoma. Oncoimmunology. 2018;7(8):e1462878.
Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, Jain RK. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 2013;73(13):3833–41.
Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20(13):3422–33.
O’Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, et al. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189(3):1330–9.
Jin W, Tamzalit F, Chaudhuri PK, Black CT, Huse M, Kam LC. T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc Natl Acad Sci U S A. 2019;116(40):19835–40.
Chin MHW, Norman MDA, Gentleman E, Coppens MO, Day RM. A Hydrogel-Integrated Culture Device to Interrogate T Cell Activation with Physicochemical Cues. ACS Appl Mater Interfaces. 2020;12(42):47355–67.
Xing X, Wang Y, Zhang X, Gao X, Li M, Wu S, et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 2021;288(11):3465–77.
Chuang YC, Chang HM, Li CY, Cui Y, Lee CL, Chen CS. Reactive Oxygen Species and Inflammatory Responses of Macrophages to Substrates with Physiological Stiffness. ACS Appl Mater Interfaces. 2020;12(43):48432–41.
Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, van den Dries K. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci Rep. 2017;7(1):17511.
Caon I, Bartolini B, Parnigoni A, Carava E, Moretto P, Viola M, et al. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin Cancer Biol. 2020;62:9–19.
Klann JE, Kim SH, Remedios KA, He Z, Metz PJ, Lopez J, et al. Integrin Activation Controls Regulatory T Cell-Mediated Peripheral Tolerance. J Immunol. 2018;200(12):4012–23.
Ly D, Li Q, Navab R, Zeltz C, Fang L, Cabanero M, et al. Tumor-Associated Regulatory T Cell Expression of LAIR2 Is Prognostic in Lung Adenocarcinoma. Cancers (Basel). 2021;14(1):205.
Kaur A, Ecker BL, Douglass SM, Kugel CH 3rd, Webster MR, Almeida FV, et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019;9(1):64–81.
Hu G, Cheng P, Pan J, Wang S, Ding Q, Jiang Z, et al. An IL6-Adenosine Positive Feedback Loop between CD73(+) gammadeltaTregs and CAFs Promotes Tumor Progression in Human Breast Cancer. Cancer Immunol Res. 2020;8(10):1273–86.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33(3):463-79 e10.
Kamionka EM, Qian B, Gross W, Bergmann F, Hackert T, Beretta CA, et al. Collagen Organization Does Not Influence T-Cell Distribution in Stroma of Human Pancreatic Cancer. Cancers (Basel). 2021;13(15):3648.
Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15(14):2724–6.
Matsuyama W, Wang L, Farrar WL, Faure M, Yoshimura T. Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-kappa B. J Immunol. 2004;172(4):2332–40.
Afonso PV, McCann CP, Kapnick SM, Parent CA. Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood. 2013;121(9):1644–50.
Tu MM, Lee FYF, Jones RT, Kimball AK, Saravia E, Graziano RF, et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci Adv. 2019;5(2):eaav2437.
Sun X, Wu B, Chiang HC, Deng H, Zhang X, Xiong W, et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature. 2021;599(7886):673–8.
Poggi A, Catellani S, Bruzzone A, Caligaris-Cappio F, Gobbi M, Zocchi MR. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia. 2008;22(5):980–8.
Perbellini O, Falisi E, Giaretta I, Boscaro E, Novella E, Facco M, et al. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica. 2014;99(5):881–7.
Wu X, Zhang L, Zhou J, Liu L, Fu Q, Fu A, et al. Clinicopathologic significance of LAIR-1 expression in hepatocellular carcinoma. Curr Probl Cancer. 2019;43(1):18–26.
Yang LL, Zhang MJ, Wu L, Mao L, Chen L, Yu GT, et al. LAIR-1 overexpression and correlation with advanced pathological grade and immune suppressive status in oral squamous cell carcinoma. Head Neck. 2019;41(4):1080–6.
Joseph C, Alsaleem MA, Toss MS, Kariri YA, Althobiti M, Alsaeed S, et al. The ITIM-Containing Receptor: Leukocyte-Associated Immunoglobulin-Like Receptor-1 (LAIR-1) Modulates Immune Response and Confers Poor Prognosis in Invasive Breast Carcinoma. Cancers (Basel). 2020;13(1):80.
Flies DB, Higuchi T, Harris JC, Jha V, Gimotty PA, Adams SF. Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103(+) dendritic cells in late-stage ovarian cancer. Oncoimmunology. 2016;5(8):e1185583.
Ramos MIP, Tian L, de Ruiter EJ, Song C, Paucarmayta A, Singh A, et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. Elife. 2021;10:e62927.
Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat Commun. 2020;11(1):4520.
Vijver SV, Singh A, Mommers-Elshof E, Meeldijk J, Copeland R, Boon L, et al. Collagen Fragments Produced in Cancer Mediate T Cell Suppression Through Leukocyte-Associated Immunoglobulin-Like Receptor 1. Front Immunol. 2021;12:733561.
Xu L, Wang S, Li J, Li J, Li B. Cancer immunotherapy based on blocking immune suppression mediated by an immune modulator LAIR-1. Oncoimmunology. 2020;9(1):1740477.
Horn LA, Chariou PL, Gameiro SR, Qin H, Iida M, Fousek K, et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-beta signaling enables PD-L1-mediated tumor eradication. J Clin Invest. 2022;132(8):e155148.
Liu Y, Ma L, Shangguan F, Zhao X, Wang W, Gao Z, et al. LAIR-1 suppresses cell growth of ovarian cancer cell via the PI3K-AKT-mTOR pathway. Aging (Albany NY). 2020;12(16):16142–54.
Romer AMA, Thorseth ML, Madsen DH. Immune Modulatory Properties of Collagen in Cancer. Front Immunol. 2021;12:791453.
Gordon-Weeks A, Yuzhalin AE. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers (Basel). 2020;12(11):3331.
Parekh K, Ramachandran S, Cooper J, Bigner D, Patterson A, Mohanakumar T. Tenascin-C, over expressed in lung cancer down regulates effector functions of tumor infiltrating lymphocytes. Lung Cancer. 2005;47(1):17–29.
Orlando L, Renne G, Rocca A, Curigliano G, Colleoni M, Severi G, et al. Are all high-grade breast cancers with no steroid receptor hormone expression alike? The special case of the medullary phenotype. Ann Oncol. 2005;16(7):1094–9.
Sundquist E, Kauppila JH, Veijola J, Mroueh R, Lehenkari P, Laitinen S, et al. Tenascin-C and fibronectin expression divide early stage tongue cancer into low- and high-risk groups. Br J Cancer. 2017;116(5):640–8.
Huang JY, Cheng YJ, Lin YP, Lin HC, Su CC, Juliano R, et al. Extracellular matrix of glioblastoma inhibits polarization and transmigration of T cells: the role of tenascin-C in immune suppression. J Immunol. 2010;185(3):1450–9.
Saupe F, Schwenzer A, Jia Y, Gasser I, Spenle C, Langlois B, et al. Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep. 2013;5(2):482–92.
Jachetti E, Caputo S, Mazzoleni S, Brambillasca CS, Parigi SM, Grioni M, et al. Tenascin-C Protects Cancer Stem-like Cells from Immune Surveillance by Arresting T-cell Activation. Cancer Res. 2015;75(10):2095–108.
Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A, et al. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology. 2018;7(10):e1478647.
Ma D, Liu S, Lal B, Wei S, Wang S, Zhan D, et al. Extracellular Matrix Protein Tenascin C Increases Phagocytosis Mediated by CD47 Loss of Function in Glioblastoma. Cancer Res. 2019;79(10):2697–708.
Haage V, Elmadany N, Roll L, Faissner A, Gutmann DH, Semtner M, et al. Tenascin C regulates multiple microglial functions involving TLR4 signaling and HDAC1. Brain Behav Immun. 2019;81:470–83.
Deligne C, Murdamoothoo D, Gammage AN, Gschwandtner M, Erne W, Loustau T, et al. Matrix-Targeting Immunotherapy Controls Tumor Growth and Spread by Switching Macrophage Phenotype. Cancer Immunol Res. 2020;8(3):368–82.
Spenle C, Loustau T, Murdamoothoo D, Erne W, Beghelli-de la Forest Divonne S, Veber R, et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol Res. 2020;8(9):1122–38.
Murdamoothoo D, Sun Z, Yilmaz A, Riegel G, Abou-Faycal C, Deligne C, et al. Tenascin-C immobilizes infiltrating T lymphocytes through CXCL12 promoting breast cancer progression. EMBO Mol Med. 2021;13(6):e13270.
Li ZL, Zhang HL, Huang Y, Huang JH, Sun P, Zhou NN, et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat Commun. 2020;11(1):3806.
He B, Mirza M, Weber GF. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene. 2006;25(15):2192–202.
Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102(47):17101–6.
Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7(5):498–506.
Fan X, He C, Jing W, Zhou X, Chen R, Cao L, et al. Intracellular Osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res. 2015;75(1):86–97.
Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008;105(20):7235–9.
Yang M, Ma C, Liu S, Sun J, Shao Q, Gao W, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128(1 Suppl):e237–49.
Sangaletti S, Tripodo C, Sandri S, Torselli I, Vitali C, Ratti C, et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res. 2014;74(17):4706–19.
Kim EK, Jeon I, Seo H, Park YJ, Song B, Lee KA, et al. Tumor-derived osteopontin suppresses antitumor immunity by promoting extramedullary myelopoiesis. Cancer Res. 2014;74(22):6705–16.
Lee SH, Park JW, Go DM, Kim HK, Kwon HJ, Han SU, et al. Ablation of osteopontin suppresses N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric cancer development in mice. Carcinogenesis. 2015;36(12):1550–60.
Zhang Y, Du W, Chen Z, Xiang C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp Cell Res. 2017;359(2):449–57.
Li Y, Liu H, Zhao Y, Yue D, Chen C, Li C, et al. Tumor-associated macrophages (TAMs)-derived osteopontin (OPN) upregulates PD-L1 expression and predicts poor prognosis in non-small cell lung cancer (NSCLC). Thorac Cancer. 2021;12(20):2698–709.
Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129(1):137–49.
Klement JD, Paschall AV, Redd PS, Ibrahim ML, Lu C, Yang D, et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 2018;128(12):5549–60.
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68(9):1653–66.
Lu C, Liu Z, Klement JD, Yang D, Merting AD, Poschel D, et al. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J Immunother Cancer. 2021;9(7):e002624.
Sun J, Feng A, Chen S, Zhang Y, Xie Q, Yang M, et al. Osteopontin splice variants expressed by breast tumors regulate monocyte activation via MCP-1 and TGF-beta1. Cell Mol Immunol. 2013;10(2):176–82.
Rittling SR, Wejse PL, Yagiz K, Warot GA, Hui T. Suppression of tumour growth by orally administered osteopontin is accompanied by alterations in tumour blood vessels. Br J Cancer. 2014;110(5):1269–77.
Ellert-Miklaszewska A, Wisniewski P, Kijewska M, Gajdanowicz P, Pszczolkowska D, Przanowski P, et al. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene. 2016;35(50):6366–77.
Shao L, Zhang B, Wang L, Wu L, Kan Q, Fan K. MMP-9-cleaved osteopontin isoform mediates tumor immune escape by inducing expansion of myeloid-derived suppressor cells. Biochem Biophys Res Commun. 2017;493(4):1478–84.
Li Y, Guo S, Zhao K, Conrad C, Driescher C, Rothbart V, et al. ADAM8 affects glioblastoma progression by regulating osteopontin-mediated angiogenesis. Biol Chem. 2021;402(2):195–206.
Klement JD, Poschel DB, Lu C, Merting AD, Yang D, Redd PS, et al. Osteopontin Blockade Immunotherapy Increases Cytotoxic T Lymphocyte Lytic Activity and Suppresses Colon Tumor Progression. Cancers (Basel). 2021;13(5):1006.
Liguori M, Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers (Basel). 2011;3(4):3740–61.
Kang SU, Cho SY, Jeong H, Han J, Chae HY, Yang H, et al. Matrix metalloproteinase 11 (MMP11) in macrophages promotes the migration of HER2-positive breast cancer cells and monocyte recruitment through CCL2-CCR2 signaling. Lab Invest. 2022;102(4):376-90.
Marigo I, Trovato R, Hofer F, Ingangi V, Desantis G, Leone K, et al. Disabled Homolog 2 Controls Prometastatic Activity of Tumor-Associated Macrophages. Cancer Discov. 2020;10(11):1758–73.
Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227.
Hsu YL, Huang MS, Hung JY, Chang WA, Tsai YM, Pan YC, et al. Bone-marrow-derived cell-released extracellular vesicle miR-92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene. 2020;39(4):739–53.
Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, et al. NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity. 2018;48(1):107-19 e4.
Haj-Shomaly J, Vorontsova A, Barenholz-Cohen T, Levi-Galibov O, Devarasetty M, Timaner M, et al. T cells promote metastasis by regulating extracellular matrix remodeling following chemotherapy. Cancer Res. 2022;82(2):278-91.
Vasiukov G, Novitskaya T, Zijlstra A, Owens P, Ye F, Zhao Z, et al. Myeloid Cell-Derived TGFbeta Signaling Regulates ECM Deposition in Mammary Carcinoma via Adenosine-Dependent Mechanisms. Cancer Res. 2020;80(12):2628–38.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21(4):217–38.
Li Y, Khuu N, Prince E, Tao H, Zhang N, Chen Z, et al. Matrix Stiffness-Regulated Growth of Breast Tumor Spheroids and Their Response to Chemotherapy. Biomacromol. 2021;22(2):419–29.
Byrne CE, Decombe JB, Bingham GC, Remont J, Miller LG, Khalif L, et al. Evaluation of Extracellular Matrix Composition to Improve Breast Cancer Modeling. Tissue Eng Part A. 2021;27(7–8):500–11.
Hill L, Bruns J, Zustiak SP. Hydrogel matrix presence and composition influence drug responses of encapsulated glioblastoma spheroids. Acta Biomater. 2021;132:437–47.
Leiva MC, Garre E, Gustafsson A, Svanstrom A, Bogestal Y, Hakansson J, et al. Breast cancer patient-derived scaffolds as a tool to monitor chemotherapy responses in human tumor microenvironments. J Cell Physiol. 2021;236(6):4709–24.
Xiao W, Wang S, Zhang R, Sohrabi A, Yu Q, Liu S, et al. Bioengineered scaffolds for 3D culture demonstrate extracellular matrix-mediated mechanisms of chemotherapy resistance in glioblastoma. Matrix Biol. 2020;85–86:128–46.
Xiao W, Zhang R, Sohrabi A, Ehsanipour A, Sun S, Liang J, et al. Brain-Mimetic 3D Culture Platforms Allow Investigation of Cooperative Effects of Extracellular Matrix Features on Therapeutic Resistance in Glioblastoma. Cancer Res. 2018;78(5):1358–70.
Ng KY, Shea QT, Wong TL, Luk ST, Tong M, Lo CM, et al. Chemotherapy-Enriched THBS2-Deficient Cancer Stem Cells Drive Hepatocarcinogenesis through Matrix Softness Induced Histone H3 Modifications. Adv Sci (Weinh). 2021;8(5):2002483.
Pietila EA, Gonzalez-Molina J, Moyano-Galceran L, Jamalzadeh S, Zhang K, Lehtinen L, et al. Co-evolution of matrisome and adaptive adhesion dynamics drives ovarian cancer chemoresistance. Nat Commun. 2021;12(1):3904.
Azzariti A, Mancarella S, Porcelli L, Quatrale AE, Caligiuri A, Lupo L, et al. Hepatic stellate cells induce hepatocellular carcinoma cell resistance to sorafenib through the laminin-332/alpha3 integrin axis recovery of focal adhesion kinase ubiquitination. Hepatology. 2016;64(6):2103–17.
Komemi O, Shochet GE, Pomeranz M, Fishman A, Pasmanik-Chor M, Drucker L, et al. Placenta-conditioned extracellular matrix (ECM) activates breast cancer cell survival mechanisms: A key for future distant metastases. Int J Cancer. 2019;144(7):1633–44.
Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–23.
Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214(3):357–67.
Yang X, Chen L, Mao Y, Hu Z, He M. Progressive and Prognostic Performance of an Extracellular Matrix-Receptor Interaction Signature in Gastric Cancer. Dis Markers. 2020;2020:8816070.
Busuttil RA, George J, Tothill RW, Ioculano K, Kowalczyk A, Mitchell C, et al. A signature predicting poor prognosis in gastric and ovarian cancer represents a coordinated macrophage and stromal response. Clin Cancer Res. 2014;20(10):2761–72.
Lecchi M, Verderio P, Cappelletti V, De Santis F, Paolini B, Monica M, et al. A combination of extracellular matrix- and interferon-associated signatures identifies high-grade breast cancers with poor prognosis. Mol Oncol. 2021;15(5):1345–57.
Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11(4):M111014647.
Hurkmans DP, Jensen C, Koolen SLW, Aerts J, Karsdal MA, Mathijssen RHJ, et al. Blood-based extracellular matrix biomarkers are correlated with clinical outcome after PD-1 inhibition in patients with metastatic melanoma. J Immunother Cancer. 2020;8(2):e001193.
Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res. 2014;20(3):711–23.
Berestjuk I, Lecacheur M, Carminati A, Diazzi S, Rovera C, Prod’homme V, et al. Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma. EMBO Mol Med. 2022;14(2):e11814.
Zhu P, Lu H, Wang M, Chen K, Chen Z, Yang L. Targeted mechanical forces enhance the effects of tumor immunotherapy by regulating immune cells in the tumor microenvironment. Cancer Biol Med. 2023;20(1):44–55.
Huang HC, Sung YC, Li CP, Wan D, Chao PH, Tseng YT, et al. Reversal of pancreatic desmoplasia by a tumour stroma-targeted nitric oxide nanogel overcomes TRAIL resistance in pancreatic tumours. Gut. 2022;71(9):1843–55.
Zhou A, Du J, Jiao M, Xie D, Wang Q, Xue L, et al. Co-delivery of TRAIL and siHSP70 using hierarchically modular assembly formulations achieves enhanced TRAIL-resistant cancer therapy. J Control Release. 2019;304:111–24.
Munoz NM, Williams M, Dixon K, Dupuis C, McWatters A, Avritscher R, et al. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J Immunother Cancer. 2021;9(2):e001800.
Wuest M, Kuchar M, Sharma SK, Richter S, Hamann I, Wang M, et al. Targeting lysyl oxidase for molecular imaging in breast cancer. Breast Cancer Res. 2015;17(1):107.
Nilsson M, Adamo H, Bergh A, Halin BS. Inhibition of Lysyl Oxidase and Lysyl Oxidase-Like Enzymes Has Tumour-Promoting and Tumour-Suppressing Roles in Experimental Prostate Cancer. Sci Rep. 2016;6:19608.
Matsuoka K, Bakiri L, Wolff LI, Linder M, Mikels-Vigdal A, Patino-Garcia A, et al. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020;30(10):885–901.
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17.
Smithen DA, Leung LMH, Challinor M, Lawrence R, Tang H, Niculescu-Duvaz D, et al. 2-Aminomethylene-5-sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth. J Med Chem. 2020;63(5):2308–24.
Karalis TT, Heldin P, Vynios DH, Neill T, Buraschi S, Iozzo RV, et al. Tumor-suppressive functions of 4-MU on breast cancer cells of different ER status: Regulation of hyaluronan/HAS2/CD44 and specific matrix effectors. Matrix Biol. 2019;78–79:118–38.
Sukowati CHC, Anfuso B, Fiore E, Ie SI, Raseni A, Vascotto F, et al. Hyaluronic acid inhibition by 4-methylumbelliferone reduces the expression of cancer stem cells markers during hepatocarcinogenesis. Sci Rep. 2019;9(1):4026.
Lokman NA, Price ZK, Hawkins EK, Macpherson AM, Oehler MK, Ricciardelli C. 4-Methylumbelliferone Inhibits Cancer Stem Cell Activation and Overcomes Chemoresistance in Ovarian Cancer. Cancers (Basel). 2019;11(8):1187.
Yates TJ, Lopez LE, Lokeshwar SD, Ortiz N, Kallifatidis G, Jordan A, et al. Dietary supplement 4-methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst. 2015;107(7):djv085.
Sharma NS, Gupta VK, Garrido VT, Hadad R, Durden BC, Kesh K, et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J Clin Invest. 2020;130(1):451–65.
Twarock S, Reichert C, Bach K, Reiners O, Kretschmer I, Gorski DJ, et al. Inhibition of the hyaluronan matrix enhances metabolic anticancer therapy by dichloroacetate in vitro and in vivo. Br J Pharmacol. 2019;176(23):4474–90.
Cho H, Matsumoto S, Fujita Y, Kuroda A, Menju T, Sonobe M, et al. Trametinib plus 4-Methylumbelliferone Exhibits Antitumor Effects by ERK Blockade and CD44 Downregulation and Affects PD-1 and PD-L1 in Malignant Pleural Mesothelioma. J Thorac Oncol. 2017;12(3):477–90.
Nicolas-Boluda A, Vaquero J, Laurent G, Renault G, Bazzi R, Donnadieu E, et al. Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano. 2020;14(5):5738–53.
Shen Y, Wang X, Lu J, Salfenmoser M, Wirsik NM, Schleussner N, et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell. 2020;37(6):800-17 e7.
Yeow YL, Kotamraju VR, Wang X, Chopra M, Azme N, Wu J, et al. Immune-mediated ECM depletion improves tumour perfusion and payload delivery. EMBO Mol Med. 2019;11(12):e10923.
Lei K, Kurum A, Kaynak M, Bonati L, Han Y, Cencen V, et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat Biomed Eng. 2021;5(12):1411–25.
Drain AP, Zahir N, Northey JJ, Zhang H, Huang PJ, Maller O, et al. Matrix compliance permits NF-kappaB activation to drive therapy resistance in breast cancer. J Exp Med. 2021;218(5):e20191360.
Dayan A, Fleminger G, Ashur-Fabian O. Targeting the Achilles’ heel of cancer cells via integrin-mediated delivery of ROS-generating dihydrolipoamide dehydrogenase. Oncogene. 2019;38(25):5050–61.
Chen SH, Wang HM, Lin CY, Chang JT, Hsieh CH, Liao CT, et al. RGD-K5 PET/CT in patients with advanced head and neck cancer treated with concurrent chemoradiotherapy: Results from a pilot study. Eur J Nucl Med Mol Imaging. 2016;43(9):1621–9.
Kurz FT, Schlemmer HP. Imaging in translational cancer research. Cancer Biol Med. 2022;19(11):1565–85.
Bourguignon LYW. Matrix Hyaluronan-CD44 Interaction Activates MicroRNA and LncRNA Signaling Associated With Chemoresistance, Invasion, and Tumor Progression. Front Oncol. 2019;9:492.
Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006;361(5):864–76.
Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. J Mol Biol. 2009;385(3):902–11.
Flynn LA, Blissett AR, Calomeni EP, Agarwal G. Inhibition of collagen fibrillogenesis by cells expressing soluble extracellular domains of DDR1 and DDR2. J Mol Biol. 2010;395(3):533–43.
Zhang J, Li L, Peng Y, Chen Y, Lv X, Li S, et al. Surface chemistry induces mitochondria-mediated apoptosis of breast cancer cells via PTEN/PI3K/AKT signaling pathway. Biochim Biophys Acta Mol Cell Res. 2018;1865(1):172–85.
Nagano M, Hoshino D, Toshima J, Seiki M, Koshikawa N. NH(2) -terminal fragment of ZF21 protein suppresses tumor invasion via inhibiting the interaction of ZF21 with FAK. Cancer Sci. 2020;111(12):4393–404.
To WS, Midwood KS. Cryptic domains of tenascin-C differentially control fibronectin fibrillogenesis. Matrix Biol. 2010;29(7):573–85.
Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, et al. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol. 2014;205(3):409–28.
Saw PE, Xu X, Kang BR, Lee J, Lee YS, Kim C, et al. Extra-domain B of fibronectin as an alternative target for drug delivery and a cancer diagnostic and prognostic biomarker for malignant glioma. Theranostics. 2021;11(2):941–57.
Han Z, Zhou Z, Shi X, Wang J, Wu X, Sun D, et al. EDB Fibronectin Specific Peptide for Prostate Cancer Targeting. Bioconjug Chem. 2015;26(5):830–8.
Lingasamy P, Tobi A, Haugas M, Hunt H, Paiste P, Asser T, et al. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials. 2019;219:119373.
Kumra H, Reinhardt DP. Fibronectin-targeted drug delivery in cancer. Adv Drug Deliv Rev. 2016;97:101–10.
Huang HY, Chen LQ, Sun W, Du HH, Dong S, Ahmed AMQ, et al. Collagenase IV and clusterin-modified polycaprolactone-polyethylene glycol nanoparticles for penetrating dense tumor tissues. Theranostics. 2021;11(2):906–24.
Yang C, Li C, Zhang P, Wu W, Jiang X. Redox Responsive Hyaluronic Acid Nanogels for Treating RHAMM (CD168) Over-expressive Cancer, both Primary and Metastatic Tumors. Theranostics. 2017;7(6):1719–34.
Shu M, Tang J, Chen L, Zeng Q, Li C, Xiao S, et al. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials. 2021;268:120574.
Wang C, Chen S, Wang Y, Liu X, Hu F, Sun J, et al. Lipase-Triggered Water-Responsive “Pandora’s Box” for Cancer Therapy: Toward Induced Neighboring Effect and Enhanced Drug Penetration. Adv Mater. 2018;30(14):e1706407.
Min S, Ko MJ, Jung HJ, Kim W, Han SB, Kim Y, et al. Remote Control of Time-Regulated Stretching of Ligand-Presenting Nanocoils In Situ Regulates the Cyclic Adhesion and Differentiation of Stem Cells. Adv Mater. 2021;33(11):e2008353.
Szoor A, Toth G, Zsebik B, Szabo V, Eshhar Z, Abken H, et al. Trastuzumab derived HER2-specific CARs for the treatment of trastuzumab-resistant breast cancer: CAR T cells penetrate and eradicate tumors that are not accessible to antibodies. Cancer Lett. 2020;484:1–8.
Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–9.
Zhang C, Fang L, Wang X, Yuan S, Li W, Tian W, et al. Oncolytic adenovirus-mediated expression of decorin facilitates CAIX-targeting CAR-T therapy against renal cell carcinoma. Mol Ther Oncolytics. 2022;24:14–25.
Dendl K, Koerber SA, Kratochwil C, Cardinale J, Finck R, Dabir M, et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers (Basel). 2021;13(19):4946.
Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75(14):2800–10.
Millul J, Bassi G, Mock J, Elsayed A, Pellegrino C, Zana A, et al. An ultra-high-affinity small organic ligand of fibroblast activation protein for tumor-targeting applications. Proc Natl Acad Sci U S A. 2021;118(16):e2101852118.
Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–45.
Fraser JD, Otawara Y, Price PA. 1,25-Dihydroxyvitamin D3 stimulates the synthesis of matrix gamma-carboxyglutamic acid protein by osteosarcoma cells. Mutually exclusive expression of vitamin K-dependent bone proteins by clonal osteoblastic cell lines. J Biol Chem. 1988;263(2):911–6.
Noda M, Rodan GA. Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone. J Cell Biol. 1989;108(2):713–8.
Gonzalez-Sancho JM, Alvarez-Dolado M, Munoz A. 1,25-Dihydroxyvitamin D3 inhibits tenascin-C expression in mammary epithelial cells. FEBS Lett. 1998;426(2):225–8.
Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–53.
Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754–62.
Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89(6):150.
Corachan A, Ferrero H, Escrig J, Monleon J, Faus A, Cervello I, et al. Long-term vitamin D treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil Steril. 2020;113(1):205-16 e4.
Al-Hendy A, Diamond MP, Boyer TG, Halder SK. Vitamin D3 Inhibits Wnt/beta-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells. J Clin Endocrinol Metab. 2016;101(4):1542–51.
Corachan A, Trejo MG, Carbajo-Garcia MC, Monleon J, Escrig J, Faus A, et al. Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation. Fertil Steril. 2021;115(2):512–21.
Fife RS, Sledge GW Jr, Proctor C. Effects of vitamin D3 on proliferation of cancer cells in vitro. Cancer Lett. 1997;120(1):65–9.
Baudet C, Perret E, Delpech B, Kaghad M, Brachet P, Wion D, et al. Differentially expressed genes in C6.9 glioma cells during vitamin D-induced cell death program. Cell Death Differ. 1998;5(1):116–25.
Thompson L, Wang S, Tawfik O, Templeton K, Tancabelic J, Pinson D, et al. Effect of 25-hydroxyvitamin D3 and 1 alpha,25 dihydroxyvitamin D3 on differentiation and apoptosis of human osteosarcoma cell lines. J Orthop Res. 2012;30(5):831–44.
Meephansan J, Komine M, Tsuda H, Ohtsuki M. Suppressive effect of calcipotriol on the induction of matrix metalloproteinase (MMP)-9 and MMP-13 in a human squamous cell carcinoma cell line. Clin Exp Dermatol. 2012;37(8):889–96.
Kang Z, Wang C, Tong Y, Li Y, Gao Y, Hou S, et al. Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J Med Chem. 2021;64(1):629–43.
Abu El Maaty MA, Grelet E, Keime C, Rerra AI, Gantzer J, Emprou C, et al. Single-cell analyses unravel cell type-specific responses to a vitamin D analog in prostatic precancerous lesions. Sci Adv. 2021;7(31):eabg5982.
Acknowledgements
Not applicable.
Funding
This work was supported by the following grant: National Natural Science Foundation of China (No. 81872145, 81872460); Natural Science Foundation of Heilongjiang Province (LH2021H076).
Author information
Authors and Affiliations
Contributions
ZY, YL, SZ, and XW preliminarily constructed the conceptual framework and finished the manuscript. HD, XY and ZZ reviewed and edited the writing. SY and MX advised and supervised the work. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors read and approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, Z., Li, Y., Zhang, S. et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer 22, 48 (2023). https://doi.org/10.1186/s12943-023-01744-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-023-01744-8