Abstract
Natural killer (NK) cells, which are innate lymphocytes endowed with potent cytotoxic activity, have recently attracted attention as potential anticancer therapeutics. While NK cells mediate encouraging responses in patients with leukemia, the therapeutic effects of NK cell infusion in patients with solid tumors are limited. Preclinical and clinical data suggest that the efficacy of NK cell infusion against solid malignancies is hampered by several factors including inadequate tumor infiltration and persistence/activation in the tumor microenvironment (TME). A number of metabolic features of the TME including hypoxia as well as elevated levels of adenosine, reactive oxygen species, and prostaglandins negatively affect NK cell activity. Moreover, cancer-associated fibroblasts, tumor-associated macrophages, myeloid-derived suppressor cells, and regulatory T cells actively suppress NK cell-dependent anticancer immunity. Here, we review the metabolic and cellular barriers that inhibit NK cells in solid neoplasms as we discuss potential strategies to circumvent such obstacles towards superior therapeutic activity.
Similar content being viewed by others
Introduction
Cancer immunotherapy has gained considerable momentum in the past decade, especially in the forms of immune checkpoint inhibition [1] and adoptive cell therapy (ACT), which consists in the infusion of autologous or allogeneic lymphocytes upon ex vivo expansion and (in some instances) genetic engineering [2]. However, while autologous chimeric antigen receptor (CAR)-expressing T cells have rapidly become a mainstay for the treatment of various hematological malignancies [3], such an ACT variant is not yet licensed for the treatment of solid tumors, and no other forms of ACT has yet received regulatory approval for routine clinical use in cancer patients. Moreover, while > 80% of patients with hematological tumors receiving CAR-expressing T cells experience (often profound) objective responses, a sizeable proportion thereof ultimately relapse, often (but not always) due to the loss of the antigenic CAR target [4, 5]. Thus, there is ample room for improvement in the ACT field.
In this context, natural killer (NK) cells have attracted considerable attention as a potential form of ACT, largely due to their antigen-independent, robust cytolytic activity against cells that display specific surface features (Box 1) [6]. Abundant preclinical data indicate that NK cells not only participate in cancer immunosurveillance (at least in some tumors) [7,8,9], but also support therapeutic responses as elicited by a variety of treatments, including chemotherapy [10, 11], radiation therapy (RT) [12, 13], targeted anticancer agents [14, 15], and peptide-mediated oncolysis [16]. Moreover, signs of NK cell activation have been associated with improved clinical outcome in various oncological settings, including acute myeloid leukemia (AML) [17,18,19], gastrointestinal stromal tumors [20, 21] and breast cancer [22, 23].
Early clinical studies demonstrate that alloreactive NK cells can efficiently eliminate leukemic blasts in subjects with AML during haploidentical hematopoietic stem cell transplantation (HSCT), de facto extending patient survival [24,25,26]. The adoptive transfer of alloreactive NK cells has also shown encouraging clinical responses in patients with AML, refractory lymphoma, and advanced multiple myeloma outside of the HSCT setting [27,28,29,30]. More recently, CAR-expressing NK cells have been shown to mediate objective responses in eight of eleven patients with B cell malignancies, including four complete remissions [31]. However, while no less than 40 clinical trials are currently open to investigate the safety and efficacy of adoptively infused NK cells (often combined with other therapeutic modality) in patients with solid tumors (Table 1), signals of efficacy remain sporadic, as in the case of two distinct clinical trials reporting clinical benefits in neuroblastoma patients receiving allogeneic NK cells in combination with ganglioside D2 (GD2)-targeting antibodies [32, 33].
Clinical findings suggest that two parameters are critical for adoptively transferred NK cells to mediate therapeutically relevant effects in patients with solid tumors: (1) intratumoral accumulation [34], and (2) persistence in an activated state [28]. Here, we review metabolic and immunological features of the tumor microenvironment (TME) that prevent adoptively transferred NK cells from successfully infiltrating, persisting within and mediating effector functions against solid tumors as we critically discuss strategies to circumvent such barriers in support of superior therapeutic activity.
Environmental obstacles for optimal NK cell anticancer activity
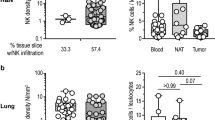
A number of environmental obstacles prevent the accumulation of adoptively transferred NK cells into the TME of solid neoplasms, limit their persistence therein and/or inhibit their cytotoxic functions, including (but not limited to) impaired NK cell trafficking as well as metabolic TME features with potent immunosuppressive effects [35, 36] (Fig. 1).
Environmental obstacles against optimal NK cell activity in solid tumors. For optimal anticancer effects, adoptively transferred natural killer (NK) cells must (1) access and abundantly infiltrate the tumor microenvironment, (2) persist and proliferate therein in the context of preserved NK cell-activating receptor expression and limited NK cell-inhibiting receptor expression, and (3) ultimately mediate potent secretory and cytotoxic functions. Moreover, malignant cells must retain expression of NK cell-activating ligands and sensitivity to the cytotoxic activity of NK cells. Defects in NK cell trafficking as well as environmental parameters including (but not limited to) hypoxia, reactive oxygen species (ROS), prostaglandin E2 (PGE2) secretion and extracellular adenosine abundance interfere with one or several of these sine qua non, ultimately limiting (and hence representing valid targets to improve) the therapeutic effects of adoptively transferred NK cells against solid tumors. CCL5, C-C motif chemokine ligand 5; CXCL, C-X-C motif chemokine ligand; CX3CL1, C-X3-C motif chemokine ligand 1; DC, dendritic cell; MDSC, myeloid-derived suppressor cell; TAM, tumor-associated macrophage; TEFF, effector T; TREG, regulatory T
Impaired NK cell trafficking. NK cell trafficking and homing are regulated by various factors including integrins, selectins, and chemokine receptors [37]. Specifically, C-X-C motif chemokine receptor 3 (CXCR3) appears to play a major role in NK cell recruitment to solid tumors. In line with this notion, > 60% of NK cells infiltrating human breast cancer has been reported to express CXCR3 [38]. Moreover, the CXCR3 ligands C-X-C motif chemokine ligand 9 (CXCL9), CXCL10 and CXCL11, which are secreted in response to type I interferon (IFN) and interferon gamma (IFNG) signaling, have been mechanistically implicated in NK cell infiltration of experimental lung adenocarcinomas [39] lymphomas [40] and melanomas [41]. Thus, strategies aimed at enhancing CXCR3 expression by NK cells and/or secretion of CXCR3 ligands in the TME may result in increased NK cell recruitment to the TME of solid tumors. On the one hand, CXCR3 expression by NK cells has been shown to increase during ex vivo expansion in the presence of interleukin 2 (IL2) [41], but rather drop in the context of short-term IL2 stimulation [42]. Along with the considerable drawbacks of recombinant IL2 administration – including a non-negligible toxicity and the expansion of immunosuppressive CD3+CD4+CD25+FOXP3+ regulatory T (TREG) cells [43] – these observations delineate a benefit for ex vivo expansion prior to ACT over in vivo NK cell stimulation. On the other hand, a variety of strategies other than type I IFN or IFNG administration (which are also associated with considerable side effects and hence have been mostly abandoned) [44, 45] has been shown to promote the secretion of CXCR3 ligands in the TME. Specifically, the administration of dipeptidyl peptidase inhibitors has been reported to drive CXCL9 and CXCL10 secretion in experimental models of hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (PDAC), culminating with CXCR3+ NK cell recruitment and (at least in some setting) synergy with immune checkpoint inhibitors (ICIs) [46, 47]. Similar results have been observed in models of colorectal carcinomas treated with curaxins (small molecules that interfere with DNA-histone interactions) [48], as well as in models of HPV-driven tumors responding to RT plus ATR serine/threonine kinase (ATR) inhibitors [49].
A number of genotoxic agents including RT are indeed able to drive a type I IFN response culminating with CXCL10 secretion via the accumulation of nuclear and mitochondrial DNA in the cytoplasm and consequent activation of cyclic GMP-AMP synthase (CGAS) [50,51,52]. Moreover, it has recently been reported both CXCL10 and C-C motif chemokine ligand 5 (CCL5) are abundantly secreted by glioblastoma (GBM) cells upon exposure to the lysosomal inhibitor chloroquine, resulting in accrued accumulation of adoptively transferred CAR-expressing NK cells in support of superior therapeutic activity [53]. Similar findings have been documented in preclinical models of melanoma [54] and non-small cell lung carcinoma (NSCLC) [55] receiving pharmacological inhibitors of autophagy or genetically engineered to become autophagy-deficient [56], culminating with accrued infiltration of endogenous NK cells and hence inhibited tumor growth [55]. Of note, the ability of autophagy inhibitors to promote NK cell chemotaxis downstream of CXCL10 and CCL5 hypersecretion has been linked (at least some settings) with superior CGAS signaling [57]. In line with this notion, pharmacological agonism of the CGAS signal transducer stimulator of interferon response cGAMP interactor 1 (STING1) has recently been shown to drive an abundant recruitment of CAR-expressing NK cells to mesothelioma organoids, culminating with potent tumor killing [58]. A similar improvement in tumor infiltration by natural or adoptively transferred NK cells has been documented in preclinical melanoma models secreting CCL5 downstream of viral infection [59], as well as in models of HCC receiving a CCL5-coding adenoviral vector [60].
Additional chemokine receptors that have been shown to promote NK cell chemotaxis and recruitment to the TME of solid tumors (at least in mice) include: (1) CXCR4, whose overexpression endowed adoptively transferred NK cells with superior homing capacities to GBM xenografts [61]; (2) C-C motif chemokine receptor 7 (CCR7), which upon acquisition via trogocytosis promotes lymph node homing [62, 63], and (3) chemokine (C-X3-C motif) receptor 1 (CX3CR1), which has been involved in superior NK cell responses driven in experimental melanomas and CRCs by the transgene-driven expression of its cognate ligand C-X3-C motif chemokine ligand 1 (CX3CL1) [64]. However, clinically viable strategies to drive the expression of CXCR4, CCR7 and CX3CR1 ligands in the TME remain to be identified. Moreover, at least in some oncological indications, high intratumoral levels of CX3CL1 have been associated with dismal prognosis [65], calling for at least some caution on strategies that would increase the intratumoral levels of this cytokine.
On the contrary, RT has been successfully employed to drive NK cell infiltration in experimental mammary tumors [66, 67] and PDACs [68], via a mechanism that involved CXCL16 and CXCL8 secretion, respectively. However, CXCL8 has been associated with immunoevasion, tumor progression and resistance to (immuno)therapy in a variety of oncological settings [69, 70]. Thus, promoting CXCL8 secretion by cancer cells might not only favor the recruitment of NK cells but also promote tumor infiltration by immunosuppressive cells that may offset therapeutic efficacy. Conversely, many tumors express high levels of CXCL8 at baseline [71], pointing to the transgene-driven overexpression of CXCR1 and CXCR2 (the main CXCL8 receptors) as a feasible approach to action the CXCL8 axis in support of NK cell recruitment to the TME of solid tumors. Preclinical data in support of this possibility have already been obtained in models of renal cell carcinoma (RCC) [72] and ovarian cancer [73].
Taken together, these data suggest that NK cell trafficking to solid malignancies can be ameliorated by various strategies that prime the TME to secrete increased amounts of NK cell-targeting chemokines or by genetically engineering NK cells to overexpress relevant chemokine receptors.
Hypoxia. The TME of solid tumors is frequently hypoxic owing to defects in vasculature coupled to increased local oxygen demand [74, 75], which is toxic for tumor-infiltrating lymphocytes, particularly NK cells [76, 77]. Indeed, hypoxia has a variety of detrimental effects on NK cells, including a transcriptional rewiring accompanied by the downregulation of multiple NK cell-activating receptors and effector molecules, but less so NK cell-inhibitory receptors, cytokine receptors or the receptors that mediate antibody-dependent cellular cytotoxicity (ADCC) (Box 1) [78,79,80,81]. At least in part, this originates from the ability of hypoxia to potently inhibit mitogen-activated protein kinase 1 (MAPK1, best known as ERK) signaling in NK cells, resulting in reduced transcription of signal transducer and activator of transcription 3 (STAT3) target genes [79] coupled with limited sensitivity to NK cell-activating stimuli including phorbol 12-myristate 13-acetate (PMA) plus ionomycin, as well as IL-15 and IL-18 [81]. Moreover, hypoxia promotes the expression of various surface proteins that impair NK cell functions via killer immunoglobulin-like receptors (KIRs) and other co-inhibitor receptors (Box 1), such as major histocompatibility complex, class I, G (HLA-G) and CD274 (best known as PD-L1) [82, 83].
Finally, at least in some setting, hypoxia causes mitochondrial fragmentation in NK cells via a pathway involving constitutive mechanistic target of rapamycin (MTOR) signaling and consequent activation of dynamin 1 like (DNM1L, best known as DRP1), resulting in a shift from oxidative phosphorylation to glycolysis and compromised tumor control [84]. On the contrary, glycolysis appears to be critical for NK cells to properly control viral infections [85]. Whether this apparent discrepancy reflects the particularly disadvantageous conditions of the solid TME remains to be elucidated. Interestingly, despite being poorly cytotoxic, NK cells lacking hypoxia inducible factor 1 subunit alpha (HIF1A), a master transcription factor for hypoxia adaptation [86], have been shown to mediate anticancer effects in vivo by antagonizing vascular endothelial growth factor A (VEGFA)-driven vascularization [87]. That said, the molecular alterations driven by hypoxia via HIF1A in NK cells remain to be elucidated.
Importantly, hypoxia also reduces cancer cell sensitivity to NK cell killing, via numerous mechanisms. For example, hypoxic breast cancer cells exhibit an increased autophagic flux resulting in superior granzyme B (GZMB) (Box 1) degradation [88]. A similar mechanism has been shown to originate by pseudohypoxia as driven by VHL mutations in RCC cells, resulting in accrued autophagic flux via the endothelial PAS domain protein 1 (EPAS1)-dependent upregulation of inositol 1,4,5-trisphosphate receptor type 1 (ITPR1) [89]. Moreover, hypoxia has been shown to downregulate the expression of NK cell-activating ligands (Box 1) on the surface on NSCLC and prostate cancer cells, at least in some settings via a HIF1A-dependent mechanism, culminating in limited NK cell activation [90, 91]. Tumor-derived microvesicles (TD-MVs) produced under hypoxic conditions are enriched in transforming growth factor beta 1 (TGFB1) and miR-23a, and hence suppress NK cell activity upon uptake by downregulating NK cell-activating receptors (Box 1) as well as the effector molecule lysosomal-associated membrane protein 1 (LAMP1, best known as CD107a) [92].
Finally, hypoxic tumor regions are enriched in a variety of immunosuppressive cells that interfere with NK cell functions (see below), including (but not limited to): TREG cells [93,94,95], M2-like tumor-associated macrophages (TAMs) [96, 97] and myeloid-derived suppressor cells (MDSCs) [98,99,100].
Reversing hypoxia stands out as a potential strategy to restore NK cell functions in the TME of solid tumors. Some studies have suggested that physical exercise may improve oxygenation in the TME and hence reverse, at least partially, hypoxia [101]. However, the ability of exercise training to restore NK cell functions in cancer patients remain to be formally demonstrated [102]. Conversely, myo-inositol-trispyrophosphate (ITPP), which increases oxygen liberation by hemoglobin, has been shown to increase NK cell abundance while decreasing TREG cell numbers in the TME of experimental melanomas [103]. Similar results have been obtained with human breast cancer spheroids treated with manganese dioxide nanoparticles encapsulated into polylactic-co-glycolic acid, which degrade tumor-derived hydrogen peroxide into molecular oxygen [104]. Of note, in this latter setting, HIF1A downregulation was accompanied by other favorable alterations of the TME, including reactive oxygen species (ROS), lactate and adenosine reductions [104].
An alternative approach to circumvent the detrimental effects of hypoxia on NK cells consists in rendering the latter more tolerant to low oxygen levels. At least in some setting, IL2 priming has been shown to prevent the hypoxia driven downregulation of killer cell lectin like receptor K1 (KLRK1, best known as NKG2D) [80]. Along similar lines, NK cells engineered to overexpress CD16 (Box 1) and IL2 preserve their ability to mediate ADCC and antibody-independent cytotoxicity in hypoxic microenvironments [105]. Finally, pharmacological inhibition of the ERK phosphatase protein tyrosine phosphatase non-receptor type 6 (PTPN6, best known as SHP-1) has been shown to efficiently prevent hypoxia-driven ERK-STAT3 silencing and consequent NK cell dysfunction [79]. The latter approach, however, may result in the compensatory expression of PD-L1, which in models of prostate cancer has been shown to support (rather than prevent) NK cell dysfunction while providing a therapeutic target for ICIs [91]. Finally, autophagy inhibition has been shown to restore the sensitivity of hypoxic cancer cells to NK cell-dependent cytotoxicity [88]. However, clinically viable pharmacological autophagy inhibitors remain elusive [106]. Moreover, NK cells are critically dependent on autophagy for their development and activity [107], in thus far resembling most other immune effector cells [108], overall casting doubts on non-targeted autophagy inhibition as a viable strategy to restore cancer cell sensitivity to lysis by NK cells.
In summary, increasing oxygen availability in the TME or rendering NK cells resistant to hypoxia stand out as the most promising strategies to circumvent the detrimental effects of poor oxygen availability on NK cell functions.
Reactive oxygen species. ROS are abundant in the TME of solid tumors and promote disease progression via a variety of mechanisms including immunoevasion [109,110,111]. Indeed, while malignant cells as well as immunosuppressive M2-like TAMs are generally endowed with efficient mechanisms for ROS detoxification [112, 113], non-transformed cells as well as immune effector cells including NK cells are particularly sensitive to the genotoxic and cytotoxic effects of ROS [114].
Besides overt cytotoxicity, which only emerges in the presence of high ROS levels, one of the mechanisms through which ROS impair the anticancer activity of NK cells involve alterations in NK cell membrane properties. Specifically, ROS promote the accumulation of anionic charges on the surface of NK cells, limiting their ability to adhere to similarly charged target cancer cells, a defect that can be prevented by antioxidant molecules including superoxide dismutase (SOD) mimetics and catalase (CAT) [115]. Moreover, ROS species produced by cytochrome b-245, beta polypeptide (CYBB, best known as NOX2) have been mechanistically implicated in the ability of experimental melanomas to form metastasis via a mechanism that (1) is manifest only in immunocompetent (but not IFGN deficient) hosts, and (2) involves NK cell dysfunction [116]. At least in part, this may reflect the ability of ROS to downregulate CD16 on the surface of (and hence impair ADCC by) NK cells [117]. Finally, ROS have also been shown to promote the accumulation of M2-like macrophages, which also limit NK cell activation [118].
Increasing the tolerance of NK cells to ROS stands out as a promising approach to improve their anticancer effects in the TME of solid tumors beyond ROS scavenging [115] and inhibition of ROS-producing systems [116]. For example, IL15 – which is a potent NK cell activator [119] – has been reported to upregulate thioredoxin (TXN) in NK cells via an MTOR-dependent mechanism that increases the availability of reducing thiols on the cell surface, culminating with preserved cytotoxic functions despite environmental oxidative stress [120]. Similar results have been obtained with an activator of NFE2 like bZIP transcription factor 2 (NFE2L2, a master regulator of antioxidant responses best known as NRF2) [121] in NK cells from healthy donors [122], as well as by engineering CAR-expressing T cells specific for erb-b2 receptor tyrosine kinase 2 (ERBB2, best known as HER2) to overexpress CAT, which resulted not only in superior cytotoxicity against HER2-expressing mammary tumors, but also in preserved bystander cytotoxicity by otherwise ROS-sensitive NK cells [123].
These observations exemplify strategies that might be employed to limit the detrimental effects of ROS on NK cells adoptively transferred for the treatment of solid tumors.
Prostaglandin E2. A variety of tumors emerge and progress in the context of a chronic, indolent inflammatory response that ultimately promote immunoevasion, which is commonly known as tumor-promoting inflammation (TPI) [124]. Prostaglandin-endoperoxide synthase 1 (PTGS1, best known as COX1) and PTGS2 (best known as COX2) are key contributors to TPI as they secrete the mitogenic and immunosuppressive factor eicosanoid prostaglandin (prostaglandin E2) [125,126,127].
While mouse splenic NK cells express all four main PGE2 receptors, it appears that prostaglandin E receptor 2 (PTGER2, best known as EP2) and even more so PTGER4 (EP4) are the main transducers of PGE2-elicited immunosuppression [128, 129]. Indeed, selective EP4 agonists have been shown to efficiently inhibit both IFNG production and chemotactic responses to serum chemokines by mouse NK cells, while EP2 agonists only had partial suppressive activity [128]. Moreover, pharmacological inhibition of EP4 reportedly protects NK cells from the immunosuppressive effects of PGE2 in preclinical models of mammary carcinomas, resulting in preserved effector functions and antimetastatic activity [129]. Similar findings have been obtained in models of CRC [130].
Mechanistically, PGE2 produced by HCC cells has been shown to cooperate with products of the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1) at inducing the downregulation of NKG2D and other NK cell-activating receptors (Box 1) in tumor-infiltrating human NK cells, resulting in profound dysfunction [131]. Along similar lines, PGE2 secretion by BRAF-mutant melanoma cells considerably reduces the viability of mouse NK cells recruited to the TME, as well as their capacity to secrete CCL5 and X-C motif chemokine ligand 1 (XCL1) [132], which is critical for the recruitment of cross-presenting dendritic cells (DCs) and hence for the initiation of tumor-targeting adaptive immune responses that can be therapeutically actioned with ICIs [133]. Finally, tumor-derived PGE2 mediates indirect immunosuppressive effects by an EP2- and EP4-elicited, ERK-dependent mechanism whereby tumor-infiltrating MDSCs are activated to produce increase levels of TGFB1, culminating with NK cell dysfunction in both human and mouse experimental systems [134, 135].
EP4 inhibitors stand out as a potential strategy to prevent NK cell dysfunction driven by PGE2 [129]. Alongside, IL15 has been shown to endow NK cells with resistance to PGE2 and preserved anticancer activity, both in vitro and in vivo, as a function of sustained MTOR signaling coupled with phosphodiesterase 4 A (PDE4A) expression and CD25/CD54 co-expression [136]. Whether any of these proteins can be directly targeted to limit NK cell suppression by PGE2, however, remains to be elucidated.
Adenosine. Adenosine is a ubiquitous metabolite generated as the terminal product of ATP degradation [137]. Adenosine accumulates in the extracellular milieu in the context of immunogenic cell stress and death as imposed by the (natural or treatment-driven) adverse microenvironmental conditions of the TME [138, 139], which is coupled to abundant ATP release, thanks to the sequential activity of two nucleotidases: (1) ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39), which degrades ATP into ADP and AMP, and (2) 5’-nucleotidase ecto (NT5E, best known as CD73), which degrades AMP into adenosine [140, 141]. CD39 and CD73 are overexpressed by malignant cells as well both myeloid [142, 143] and lymphoid [144, 145] components of the TME in a variety of solid tumors, resulting in constitutively high extracellular adenosine levels [146, 147].
Adenosine mediates broad immunosuppressive effects upon binding to adenosine A2a receptor (ADORA2A, also known as A2AR) or ADORA2B (also known as A2BR) on the surface of immune effector cells [148]. Specifically, adenosine has been shown to suppress both cytokine release [149] and cytotoxic functions [150, 151] in activated NK cells via an A2AR-initiated, cyclic AMP (cAMP)-dependent signaling cascade resulting in protein kinase A (PKA) engagement [149,150,151]. At least in some setting, such a dose-dependent inhibitory effect [152] manifest with decreased expression of CD56 [153], Fas ligand (FASL) and perforin 1 (PRF1) (Box 1) [150], as well as with limited IFNG and TNF secretion [149].
Of note, tumor-infiltrating lymphocytes including NK cells generally express higher levels of CD39, CD73 and/or CD38 (yet another adenosine-producing enzyme) [154, 155] as compared to their circulating counterparts [153, 156, 157], which (at least in some settings) results in the acquisition of immunosuppressive properties. Specifically, CD73+ NK cells infiltrating breast carcinomas and sarcomas have been shown to express a number of immunosuppressive molecules including (but not limited to) PD-L1, PD-1, lymphocyte activating 3 (LAG3), hepatitis A virus cellular receptor 2 (HAVCR2, best known as TIM-3) and T cell immunoreceptor with Ig and ITIM domains (best known as TIGIT) [158], correlating with abundant secretion of immunosuppressive cytokines such as IL10 and TGFB1 [156]. Along similar lines, CD56brightCD16− NK cells (but not their CD56dim counterparts) express not only CD38 but also ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1, which is required for adenosine synthesis downstream of CD38), and hence can exert potent immunosuppressive effects on other immune effectors – including CD4+ helper T lymphocytes – as a consequence of abundant adenosine production [159]. At least in part, the detrimental effects of adenosine on NK cells also involve the activation of tumor-resident immunosuppressive cells including M2-like TAMs, MDSCs and TREG cells [160, 161].
Corroborating the potent immunosuppressive activity of adenosine on NK cells, both pharmacological and genetic strategies aimed at interrupting A2AR and/or A2BR activation have been shown to prevent adenosine-driven NK cell dysfunction in a variety of experimental settings [149,150,151,152]. Along similar lines, Entpd1 or Nte5 deletion as well as pharmacological or antibody-mediated inhibition of CD39 and/or CD73 have been consistently associated with restored NK cell activity and improved tumor control in numerous preclinical models of malignancy, including melanoma [162], sarcoma [163], glioblastoma [53], as well as prostate [163], breast [164], and colorectal cancer [165, 166]. In the context of ACT, CD73 blockage has been shown to promote the recruitment of CAR-expressing NK cells engineered to display increased levels of NKG2D to NSCLC xenografts [167]. Moreover, IL15 appears to be superior to IL2 to render NK cells expanded ex vivo resistant to adenosine [168]. That said, while cytokine administration in the context of ACT promotes NK cell expansion and persistence, this approach is associated with various problems including (but not limited to) non-negligible toxicity and the expansion of (adenosine-producing) TREG cells [43, 169].
Taken together, these observations suggest that inhibiting adenosine receptors and/or adenosine-producing enzymes stands out as a promising strategy to improve NK cell activation in the TME of solid tumors.
Immunological and stromal barriers against NK cell activity
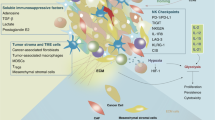
A number of immune and stromal cellular compartments of the TME potently inhibit the effector functions of NK cells. Thus, targeting these cell populations – which include TREG cells, TAMs, MDSCs and cancer-associated fibroblasts (CAFs) – represents a promising approach to endow tumor-infiltrating NK cells with superior effector functions (Fig. 2).
Immunological and stromal barriers against optimal NK cell activity in solid tumors. Tumor-infiltrating natural killer (NK) cells engage in contact-dependent and independent interactions with a variety of cells that ultimately inhibit their anticancer activity. Such cells include not only regulatory T (TREG) cells, M2-like tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), but also cancer-associated fibroblasts (CAFs). ARG1, arginase 1; IDO1, indoleamine 2,3-dioxygenase 1; IL, interleukin; MMP, metalloprotease; NO, nitric oxide; PGE2, prostaglandin E2; ROS, reactive oxygen species; TGFB1, transforming growth factor beta 1
Regulatory T cells. Tumor-infiltrating TREG cells mediate potent immunosuppressive effects via direct, contact-dependent pathways, as well as through direct and indirect humoral mechanisms [170]. For instance, TREG cells have been shown to kill effector T (TEFF) cells upon GZMA and GZMB secretion, compete with TEFF cells for IL2 availability (because TREG cells express high levels of the high affinity IL2 receptor CD25) and secrete immunosuppressive cytokines including IL10 and TGFB1 [170]. In line with this notion, high intratumoral levels of TREG cells have been associated with poor disease outcome in numerous cohorts of patients with cancer [171]. Moreover, a number of pharmacological and genetic strategies for TREG cell depletion, including CD25-targeting antibodies [172, 173] as well as the expression of the diphtheria toxin (DT) receptor under the control of the Foxp3 promote coupled to DT administration [174, 175], have been shown to improve the efficacy of various anticancer regimens in mice, generally in the absence of overt autoimmune reactions.
Both human and mouse canonical NK cells are highly sensitive to TREG cell-mediated immunosuppression, generally resulting in decreased expression of NK cell-activating receptors such as NKG2D (Box 1), upregulation of co-inhibitory receptors such as PD-1 and interleukin 1 receptor accessory protein like 1 (IL1RAPL1, best known as IL1R8), coupled to limited proliferative and cytotoxic responses upon activation [176,177,178]. This has been shown to translate into limited control of primary tumor growth and metastatic dissemination in models of NSCLC and PDAC, through a mechanism that relies on STAT3 signaling in TREG cells and TGFB1 secretion [176, 179]. At least in some settings, NK cells can also be made resistant to the immunosuppressive effects of TREG cells upon exposure to IL2, IL7 or IL12 as well as neutralization of the IL1R8 ligand IL37 [176, 178, 180]. Moreover, adaptive NK cells that develop in the context of cytomegalovirus infection appear to be naturally insensitivity to TREG cell-mediated immunosuppression [178]. At least in part, this phenomenon results from stable epigenetic modifications resulting in the expression of multiple NK cell-activating receptors and (paradoxically) TIM-3 coupled to the loss of NK cell-inhibiting receptors (Box 1), ultimately endowing adaptive NK cells with potent effector functions despite their terminally differentiated state [181,182,183]. Finally, a NK cell line engineered to express a chimeric receptor encompassing the extracellular domain of transforming growth factor beta receptor 2 (TGFBR2) fused to the intracellular domain of NKG2D has recently been shown to mediate superior therapeutic efficacy in preclinical HCC models, reflecting not only improved cytotoxic responses (which could further be ameliorated by TGFB1, at least in vitro), but also (1) enhanced recruitment to the solid TME, and (2) suppressed TREG cell differentiation [184].
In summary, although therapeutically inhibiting or depleting TREG cells in patients remain challenging [185], these immunosuppressive components of the TME stand out as promising targets to improve the efficacy of adoptively transferred NK cells against solid tumors.
Tumor-associated macrophages. Human solid tumors are abundantly infiltrated by TAMs, often (but not always) driven by the CCL2-dependent recruitment of circulating monocytes [97, 186]. TAMs are a very plastic component of the TME that can adapt a spectrum of phenotypic and functional features ranging from a predominantly pro-inflammatory (M1-like) state (which is promoted by IFNG and TNF) to a prominently anti-inflammatory (M2-like) state (which is promoted by IL4, TGFB1 and PGE2) [97]. M2-like macrophages not only mediate robust immunosuppressive effects via contact-dependent (e.g., PD-L1 expression) and independent (e.g., TGFB1 and IL10 secretion; arginine depletion) mechanisms, but also promote neo-angiogenesis and metastatic tumor dissemination, de facto supporting disease progression and resistance to treatment in variety of oncological settings [187]. Accordingly, abundant tumor infiltration by TAMs as well as elevated circulating levels of TAM-relevant cytokines including CCL2, CCL8 and colony stimulating factor 1 (CSF1) have been linked with poor disease outcome in multiple cohorts of cancer patients [171, 187, 188]. Moreover, an abundant preclinical literature demonstrates that depleting M2-like TAMs or inhibiting their immunosuppressive functions mediates robust anticancer effects in mice, either as a standalone therapeutic strategy or combined with other antineoplastic regimens [187, 189].
M2-like TAMs isolated from spontaneous mouse mammary carcinomas as well as differentiated ex vivo from the peritoneum or bone marrow of healthy mice have been shown to potently inhibit NK cell cytotoxicity coupled to the acquisition of an exhausted CD27lowCD11bhigh phenotype via a TGFB1-dependent mechanism [190]. At least in preclinical CRC models, such an immunosuppressive pathway is initiated by the CAF-driven, CXCL8-dependent polarization of TAMs towards an M2-like phenotype [191]. M2-like TAMs collected from the ascites of patients with ovarian cancer and exposed to Toll-like receptor (TLR) ligands appear to undergo repolarization towards an M1-like state, resulting in IL12 secretion and acquisition of cytolytic functions by co-cultured NK cells [192, 193]. Finally, monoclonal antibodies targeting scavenger receptors on M2-like TAMs have been shown to limit their immunosuppressive effects and efficiently derepress the cytolytic functions of NK cells in human and mouse models of melanoma [194].
Despite the scarcity of studies directly investigating the interactions between TAMs and NK cells, these observations suggest that M2-like TAMs may also offer targets to improve the activity of adoptively transferred NK cells against solid tumors. That said, no agent conceived to deplete M2-like TAMs or repolarize them into their M1-like counterparts is available for clinical use yet.
Myeloid-derived suppressor cells. MDSCs are a heterogenous population of immature bone marrow-derived myeloid cells with prominent immunosuppressive effects [195]. Human MDSCs are generally subdivided into CD11b+CD14+CD33+HLA-DRlow/neg monocytic (M)-MDSCs or CD11b+CD15+HLA-DRlowCD66b+ granulocytic (G)- or polymorphonuclear (PMN)-MDSCs [196]. MDSCs expand peripherally in both cancer patients and tumor-bearing mice, at least in part driven by the systemic effects of cancer cell-derived cytokines that influence hematopoiesis in the bone marrow, including IL6 and CSF2 [197]. Moreover, MDSCs can accumulate in the TME of solid tumors upon recruitment via chemokines including (but not limited to) CCL2 [99, 195].
In tumor-bearing mice, the frequency of CD11b+Gr1+ MDSCs inversely correlates with the expression of NK cell-activating receptors including NKG2D and natural cytotoxicity triggering receptor 3 (NCR3, best known as NKp30) on the NK cell surface, as well as with IFNG and PRF1 production [198, 199]. At least in preclinical models, the ability of MDSCs to suppress NK cell functions requires physical contact, which is facilitated by membrane-bound TGFB1 [200]. In line with this notion, depleting MDSCs (but not TREG cells) has been shown to restore NK cell-dependent tumor control in an orthotopic model of HCC [199]. Additional mechanisms through which MDSCs inhibit NK cells (as well as TEFF cells) include the production of ROS and reactive nitrogen species (see above), as well as the depletion of essential amino acids such as arginine, reflecting the elevated expression of arginase 1 (ARG1) [195].
Several strategies to inhibit MDSCs in support of superior NK cell activity have been explored. For example, inhibiting MDSC trafficking by targeting CXCR1 and CXCR2 has been shown to efficiently limit MDSC recruitment in preclinical models of head and neck cancer (HNC), resulting in superior efficacy from adoptively transferred NK cells [198]. Along similar lines, inhibition of nitric oxide (NO) production by MDSCs with a nitric oxide synthase 2 (NOS2) inhibitor reportedly restores NK-cell mediated ADCC in preclinical models of breast cancer, resulting in superior tumor control in vivo [117]. Similar benefits have been documented with pharmacological inhibitors of ARG1 in preclinical models of CRC treated with adoptively transferred NK cells [201]. Additional strategies that efficiently inhibit MDSCs resulting in derepressed NK cell activity include specific chemotherapeutic agents (especially, doxorubicin, gemcitabine and low-dose cyclophosphamide) [202,203,204,205,206], approaches to prevent PGE2 secretion by MDSCs [134], as well as all-trans retinoic acid (ATRA), which is known to promote MDSC differentiation and hence limit their immunosuppressive activity [207]. Interestingly, ATRA can also promote the expression of NK cell-activating ligands including MHC class I polypeptide-related sequence A (MICA) and MICB on malignant cell, de facto rendering them more susceptible to NK cells [208].
Taken together, these observations delineate multiple, clinically viable strategies for targeting MDSCs to improve the activity of NK cell-based ACT in patients with solid tumors.
Cancer-associated fibroblasts. Solid tumors are abundantly infiltrated by a highly plastic and functionally heterogeneous population of CAFs, which can originate from a variety of tissue-resident cells as well as from circulating precursors [209,210,211]. Several factors have been shown to promote the accumulation of CAFs in the TME of solid tumors including not only cytokines such as TGFB1 [212], CXCL12 [213] and platelet derived growth factor (PDGF) [214], but also oxidative stress coupled to mitochondrial dysfunction [215]. In multiple settings, CAFs have been shown to express fibroblast activation protein alpha (FAP), actin alpha 2, smooth muscle (ACTA2, best known as αSMA), S100 calcium binding protein A4 (S100A4, best known as FSP1), vimentin (VIM), and both subunits of the heterodimeric PDGF receptor [209].
Early studies demonstrated that CAFs promote tumor progression and resistance to treatment by a number of mechanisms, such as (1) favoring neoangiogenesis, (2) generating a dense stromal reactions that hinder tumor infiltration by drugs and immune effector cells, as well as (3) directly inhibiting the activity of the latter, notably NK cells [209, 216]. Specifically, CAFs produce abundant TGFB1 [217], which is known to potently suppress NK cell functions [218]. Moreover, fibroblasts exposed to melanoma, CRC or HCC cells secrete PGE2 and express IDO1, hence acquiring the ability to promote NKG2D and NKp30 downregulation on NK cells, coupled with suppressed cytotoxic activity [131, 219, 220]. Along similar lines, CAFs isolated from patients with endometrial cancer have been shown to potently suppress NK cell activity along with the downregulation of PVR cell adhesion molecule (PVR), yet another NK cell-activating receptor [221]. Finally, melanoma-associated CAFs have been reported to secrete metalloproteases that efficiently shed MICA and MICB from the surface of malignant cells, thus rendering them less prone to activate NK cells upon contact [222, 223].
Targeting FAP-expressing CAFs or TGFB1 signaling has been shown to mediate potent anticancer effects in a variety of preclinical tumor models [212, 224,225,226]. At least in models of PDAC, the beneficial effects of CAF-targeting strategies have been shown to originate from NK cell (rather than TEFF cell) reactivation [227]. Moreover, NK cells can contribute to CAF inhibition when ADCC-competent CAF-targeting monoclonal antibodies are employed, as demonstrated in models of CRC where CAFs express abundant epidermal growth factor receptor (EGFR) levels [228].
In summary, while CAFs may represent valid targets to improve the activity of NK cells in the solid TME, the lack of specific and reliable CAF markers poses a non-negligible obstacle to this approach.
Concluding remarks and future perspectives
While NK cells are attracting considerable interest as potential anticancer therapeutics and encouraging data have been documented in patients with hematological malignancies, the use of NK cells for the treatment of solid tumors remains hindered by a number of obstacles, as amply discussed herein [229,230,231]. Indeed, while numerous strategies aimed at improving the recruitment of NK cells to the TME as well as their persistence and activation have been proven effective in preclinical tumor models (see above), some of these approaches are complex to translate into clinically viable procedures (e.g., systemic infusion of NK cell-activating cytokines). On the contrary, priming the TME with (ideally FDA-approved) agents, such as low-dose chemotherapy to deplete TREG cells and MDSCs [232] or RT to jumpstart anticancer immunity [233], stands out as a powerful and clinically valid approach to provide adoptively transferred NK cells with a relatively more permissive microenvironment. Alongside, incorporating specific agents (e.g., difference cytokines as well as modifications or combinations thereor) in ex vivo expansion protocols may offer a safe and yet powerful approach to create NK cell populations with increased resistance to the adverse metabolic and immunological conditions of the TME, such as cytokine-induced memory-like NK cells, in support of superior treatment efficacy. Finally, NK cells resemble TEFF cells in expressing a number of co-inhibitory receptors that may targeted for therapeutic purposes, including (but not limited to) PD-1 and killer cell lectin like receptor C1 (KLRC1, best known as NKG2A). While only PD-1/PD-L1- and cytotoxic T lymphocyte-associated protein 4 (CTLA4)-targeting agents are approved for use in patients with cancer nowadays [234], several other ICIs are currently in clinical development including NKG2A-, LAG3- and TIM-3 blockers [234]. These agents may also offer a safe and convenient approach to boost the activity of adoptively transferred NK cells against solid tumors [235, 236].
In conclusion, while additional work is required, we surmise that combinatorial strategies aimed at enabling robust tumor infiltration and protecting NK cells from the metabolic and immunological conditions of the TME are key to unlock the therapeutic potential of adoptively transferred NK cells for human solid tumors.
Box 1 - principles of NK cell biology
Human natural killer (NK) cells are CD45+CD3CD56+ cells that originate in the bone marrow and mature in peripheral lymphoid and non-lymphoid organs [237]. The ability of NK cells to proliferate, secrete cytokines and mediate cytotoxic effects is not regulated by an antigen-specific receptor as in the case of T and B lymphocytes, but is rather controlled by a balance between activating and inhibitory signals elicited by antigen-independent interactions with other cells [238]. Such signals are dispatched to NK cells by a variety of surface receptors including: (1) killer immunoglobulin-like receptors (KIRs), which generally deliver inhibitory cues via immunoreceptor tyrosine-based inhibitory motifs (ITIMs); (2) c-type lectin receptors, such as the immunosuppressive receptor killer cell lectin like receptor C1 (KLRC1, best known as NKG2A) and the immunostimulatory receptor KLRK1 (best known as NKG2D); (3) leukocyte immunoglobulin-like receptors (LIRs), such as the immunosuppressive molecule leukocyte immunoglobulin like receptor B1 (LILRB1), and (4) natural cytotoxicity receptors, such as natural cytotoxicity triggering receptor 1 (NCR1, best known as NKp46) and NCR2 (best known as NKp44), which deliver activating stimuli via immunoreceptor tyrosine-based activation motifs (ITAMs) [239].
NK cells also express (1) various cytokine receptors, notably the receptors for interleukin 12 (IL12), IL15 and IL21, which deliver mitogenic signals [240]; (2) at least some of the co-inhibitory receptors that suppress effector T cell activation, such as programmed cell death 1 (PDCD1, best known as PD-1) and T cell immunoreceptor with Ig and ITIM domains (best known as TIGIT) [239]; as well as (3) high affinity receptors for immunoglobulins, notably Fc gamma receptor IIIa (FCGR3A) and FCGR3B (the heterodimeric CD16 receptor), which underlie their ability to mediate antibody-dependent cellular cytotoxicity (ADCC) against opsonized cells [241, 242]. Besides ADCC, NK cells can harness at least three additional mechanisms to mediate cytotoxic effects: (1) the exocytic release of granules containing perforin 1 (PRF1) and various members of the granzyme protease family; (2) the secretion of interferon gamma (IFNG) and tumor necrosis factor (TNF); and (3) the engagement of death receptors including Fas cell surface death receptor (FAS) and TNF receptor superfamily member 10b (TNFRSF10B, best known as TRAIL-R2) on the surface of target cells [243]. Moreover, at least in some settings, NK cells secrete chemotactic factors for dendritic cells (DCs), such as X-C motif chemokine ligand 1 (XCL1) and C-C motif chemokine ligand 5 (CCL5), ultimately promoting the initiation of antigen-specific immune responses downstream of T-cell cross-priming [132]. Importantly, all these functions exhibit considerable degree of heterogeneity across diverse NK cell subsets, reflecting not only different maturation stages, but also (at least some degree) of tissue specificity [243].
Data availability
Not applicable.
References
Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–37.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8.
Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. 2021;18:71–84.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85.
Huo CD, Yang J, Gu YM, Wang DJ, Zhang XX, Li YM. Overcome tumor relapse in CAR T cell therapy. Clin Transl Oncol. 2022;24:1833–43.
Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56.
Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–69.
Buque A, Bloy N, Perez-Lanzon M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 2020;11:3819.
Knab VM, Gotthardt D, Klein K, Grausenburger R, Heller G, Menzl I, Prinz D, Trifinopoulos J, List J, Fux D, et al. Triple-negative breast cancer cells rely on kinase-independent functions of CDK8 to evade NK-cell-mediated tumor surveillance. Cell Death Dis. 2021;12:991.
Shaked Y, Pham E, Hariharan S, Magidey K, Beyar-Katz O, Xu P, Man S, Wu FT, Miller V, Andrews D, Kerbel RS. Evidence Implicating Immunological Host Effects in the Efficacy of Metronomic Low-Dose Chemotherapy. Cancer Res. 2016;76:5983–93.
Wu L, Yun Z, Tagawa T, De la Maza L, Wu MO, Yu J, Zhao Y, de Perrot M. Activation of CD1d-restricted natural killer T cells can inhibit cancer cell proliferation during chemotherapy by promoting the immune responses in murine mesothelioma. Cancer Immunol Immunother. 2014;63:1285–96.
Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606.
Pilones KA, Charpentier M, Garcia-Martinez E, Daviaud C, Kraynak J, Aryankalayil J, Formenti SC, Demaria S. Radiotherapy Cooperates with IL15 to Induce Antitumor Immune Responses. Cancer Immunol Res. 2020;8:1054–63.
Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–61.
Borg C, Terme M, Taïeb J, Ménard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–88.
Yamazaki T, Wennerberg E, Hensler M, Buque A, Kraynak J, Fucikova J, Zhou XK, Sveinbjornsson B, Rekdal O, Demaria S, Galluzzi L. LTX-315-enabled, radiotherapy-boosted immunotherapeutic control of breast cancer by NK cells. Oncoimmunology. 2021;10:1962592.
Truxova I, Kasikova L, Salek C, Hensler M, Lysak D, Holicek P, Bilkova P, Holubova M, Chen X, Mikyskova R, et al. Calreticulin exposure on malignant blasts correlates with improved natural killer cell-mediated cytotoxicity in acute myeloid leukemia patients. Haematologica. 2020;105:1868–78.
Liu G, Zhang Q, Yang J, Li X, Xian L, Li W, Lin T, Cheng J, Lin Q, Xu X, et al. Increased TIGIT expressing NK cells with dysfunctional phenotype in AML patients correlated with poor prognosis. Cancer Immunol Immunother. 2022;71:277–87.
Rakova J, Truxova I, Holicek P, Salek C, Hensler M, Kasikova L, Pasulka J, Holubova M, Kovar M, Lysak D, et al. TIM-3 levels correlate with enhanced NK cell cytotoxicity and improved clinical outcome in AML patients. Oncoimmunology. 2021;10:1889822.
Ménard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taïeb J, Delahaye NF, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–9.
Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–7.
Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang XY, Dumur CI, Bedognetti D, Tomei S, Ascierto PA, et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med. 2013;11:145.
Muntasell A, Servitja S, Cabo M, Bermejo B, Pérez-Buira S, Rojo F, Costa-García M, Arpí O, Moraru M, Serrano L, et al. High Numbers of Circulating CD57(+) NK Cells Associate with Resistance to HER2-Specific Therapeutic Antibodies in HER2(+) Primary Breast Cancer. Cancer Immunol Res. 2019;7:1280–92.
Suen WC, Lee WY, Leung KT, Pan XH, Li G. Natural Killer Cell-Based Cancer Immunotherapy: A Review on 10 Years Completed Clinical Trials. Cancer Invest. 2018;36:431–57.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100.
Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9.
Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, Moreno A, Dupont B, Hsu KC, Baxter-Lowe LA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–53.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7.
Björklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, Cooley S, Miller JS, Klimkowska M, Schaffer M, et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin Cancer Res. 2018;24:1834–44.
Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382:545–53.
Modak S, Le Luduec JB, Cheung IY, Goldman DA, Ostrovnaya I, Doubrovina E, Basu E, Kushner BH, Kramer K, Roberts SS, et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology. 2018;7:e1461305.
Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, Meagher M, Shafer A, Ng CY, Leung W, et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin Cancer Res. 2017;23:6441–9.
Zhang S, Liu W, Hu B, Wang P, Lv X, Chen S, Shao Z. Prognostic Significance of Tumor-Infiltrating Natural Killer Cells in Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol. 2020;11:1242.
Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK Cell Metabolism and Tumor Microenvironment. Front Immunol. 2019;10:2278.
Domagala J, Lachota M, Klopotowska M, Graczyk-Jarzynka A, Domagala A, Zhylko A, Soroczynska K, Winiarska M: The Tumor Microenvironment-A Metabolic Obstacle to NK Cells’ Activity. Cancers (Basel) 2020, 12.
Bernardini G, Gismondi A, Santoni A. Chemokines and NK cells: regulators of development, trafficking and functions. Immunol Lett. 2012;145:39–46.
Rezaeifard S, Talei A, Shariat M, Erfani N. Tumor infiltrating NK cell (TINK) subsets and functional molecules in patients with breast cancer. Mol Immunol. 2021;136:161–7.
Yamamoto Y, Miyazato K, Takahashi K, Yoshimura N, Tahara H, Hayakawa Y. Lung-resident natural killer cells control pulmonary tumor growth in mice. Cancer Sci. 2018;109:2670–6.
Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–45.
Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. 2015;64:225–35.
Hodge DL, Schill WB, Wang JM, Blanca I, Reynolds DA, Ortaldo JR, Young HA. IL-2 and IL-12 alter NK cell responsiveness to IFN-gamma-inducible protein 10 by down-regulating CXCR3 expression. J Immunol. 2002;168:6090–8.
Overwijk WW, Tagliaferri MA, Zalevsky J. Engineering IL-2 to Give New Life to T Cell Immunotherapy. Annu Rev Med. 2021;72:281–311.
Jett JR, Maksymiuk AW, Su JQ, Mailliard JA, Krook JE, Tschetter LK, Kardinal CG, Twito DI, Levitt R, Gerstner JB. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12:2321–6.
Jones GJ, Itri LM. Safety and tolerance of recombinant interferon alfa-2a (Roferon-A) in cancer patients. Cancer. 1986;57:1709–15.
Nishina S, Yamauchi A, Kawaguchi T, Kaku K, Goto M, Sasaki K, Hara Y, Tomiyama Y, Kuribayashi F, Torimura T, Hino K. Dipeptidyl Peptidase 4 Inhibitors Reduce Hepatocellular Carcinoma by Activating Lymphocyte Chemotaxis in Mice. Cell Mol Gastroenterol Hepatol. 2019;7:115–34.
Fitzgerald AA, Wang S, Agarwal V, Marcisak EF, Zuo A, Jablonski SA, Loth M, Fertig EJ, MacDougall J, Zhukovsky E, et al: DPP inhibition alters the CXCR3 axis and enhances NK and CD8 + T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma. J Immunother Cancer 2021, 9.
Chen M, Brackett CM, Burdelya LG, Punnanitinont A, Patnaik SK, Matsuzaki J, Odunsi AO, Gudkov AV, Singh AK, Repasky EA, Gurova KV. Stimulation of an anti-tumor immune response with “chromatin-damaging” therapy. Cancer Immunol Immunother. 2021;70:2073–86.
Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, et al. ATR Inhibition Potentiates the Radiation-induced Inflammatory Tumor Microenvironment. Clin Cancer Res. 2019;25:3392–403.
Vanpouille-Box C, Demaria S, Formenti SC, Galluzzi L. Cytosolic DNA Sensing in Organismal Tumor Control. Cancer Cell. 2018;34:361–78.
Yamazaki T, Kirchmair A, Sato A, Buque A, Rybstein M, Petroni G, Bloy N, Finotello F, Stafford L, Navarro Manzano E, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–71.
Klapp V, Alvarez-Abril B, Leucci G, Ciccia A, Galluzzi L: The DNA damage response in inflammation and cancer. Cancer Cell 2022:in press.
Wang J, Toregrosa-Allen S, Elzey BD, Utturkar S, Lanman NA, Bernal-Crespo V, Behymer MM, Knipp GT, Yun Y, Veronesi MC, et al: Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc Natl Acad Sci U S A 2021, 118.
Mgrditchian T, Arakelian T, Paggetti J, Noman MZ, Viry E, Moussay E, Van Moer K, Kreis S, Guerin C, Buart S, et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc Natl Acad Sci U S A. 2017;114:E9271–9.
Yao C, Ni Z, Gong C, Zhu X, Wang L, Xu Z, Zhou C, Li S, Zhou W, Zou C, Zhu S. Rocaglamide enhances NK cell-mediated killing of non-small cell lung cancer cells by inhibiting autophagy. Autophagy. 2018;14:1831–44.
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K, Cecconi F, Choi AMK, et al. Autophagy in major human diseases. Embo j. 2021;40:e108863.
Yan X, Yao C, Fang C, Han M, Gong C, Hu D, Shen W, Wang L, Li S, Zhu S. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci. 2022;18:585–98.
Knelson EH, Ivanova EV, Tarannum M, Campisi M, Lizotte PH, Booker MA, Ozgenc I, Noureddine M, Meisenheimer B, Chen M, et al. Activation of Tumor-Cell STING Primes NK-Cell Therapy. Cancer Immunol Res. 2022;10:947–61.
Bhat H, Zaun G, Hamdan TA, Lang J, Adomati T, Schmitz R, Friedrich SK, Bergerhausen M, Cham LB, Li F, et al. Arenavirus Induced CCL5 Expression Causes NK Cell-Mediated Melanoma Regression. Front Immunol. 2020;11:1849.
Li J, Liu H, Li L, Wu H, Wang C, Yan Z, Wang Y, Su C, Jin H, Zhou F, et al. The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma. Oncol Rep. 2013;29:895–902.
Müller N, Michen S, Tietze S, Töpfer K, Schulte A, Lamszus K, Schmitz M, Schackert G, Pastan I, Temme A. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-secreting Glioblastoma. J Immunother. 2015;38:197–210.
Somanchi SS, Somanchi A, Cooper LJ, Lee DA. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood. 2012;119:5164–72.
Carlsten M, Levy E, Karambelkar A, Li L, Reger R, Berg M, Peshwa MV, Childs RW. Efficient mRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front Immunol. 2016;7:105.
Xin H, Kikuchi T, Andarini S, Ohkouchi S, Suzuki T, Nukiwa T, Huqun, Hagiwara K, Honjo T, Saijo Y. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35:1371–80.
Jiang G, Wang H, Huang D, Wu Y, Ding W, Zhou Q, Ding Q, Zhang N, Na R, Xu K. The Clinical Implications and Molecular Mechanism of CX3CL1 Expression in Urothelial Bladder Cancer. Front Oncol. 2021;11:752860.
Yoon MS, Pham CT, Phan MT, Shin DJ, Jang YY, Park MH, Kim SK, Kim S, Cho D. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy. 2016;18:1532–42.
Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107.
Walle T, Kraske JA, Liao B, Lenoir B, Timke C, von Bohlen Und Halbach E, Tran F, Griebel P, Albrecht D, Ahmed A, et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci Adv. 2022;8:eabh4050.
Olivera I, Sanz-Pamplona R, Bolanos E, Rodriguez I, Etxeberria I, Cirella A, Egea J, Garasa S, Migueliz I, Eguren-Santamaria I, et al. A Therapeutically Actionable Protumoral Axis of Cytokines Involving IL-8, TNFalpha, and IL-1beta. Cancer Discov. 2022;12:2140–57.
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92.
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, Ponz M, Schalper KA, Pérez-Gracia JL, Melero I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31.
Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, Colón E, Scherman-Plogell AH, Lundqvist A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer. 2017;5:73.
Ng YY, Tay JCK, Wang S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol Ther Oncolytics. 2020;16:75–85.
Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;18:751–72.
Hassan Venkatesh G, Abou Khouzam R, Shaaban Moustafa Elsayed W, Ahmed Zeinelabdin N, Terry S, Chouaib S. Tumor hypoxia: an important regulator of tumor progression or a potential modulator of tumor immunogenicity? Oncoimmunology 2021, 10:1974233.
DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21:785–97.
Garcés-Lázaro I, Kotzur R, Cerwenka A, Mandelboim O. NK Cells Under Hypoxia: The Two Faces of Vascularization in Tumor and Pregnancy. Front Immunol. 2022;13:924775.
Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756–64.
Teng R, Wang Y, Lv N, Zhang D, Williamson RA, Lei L, Chen P, Lei L, Wang B, Fu J, et al. Hypoxia Impairs NK Cell Cytotoxicity through SHP-1-Mediated Attenuation of STAT3 and ERK Signaling Pathways. J Immunol Res. 2020;2020:4598476.
Sarkar S, Germeraad WT, Rouschop KM, Steeghs EM, van Gelder M, Bos GM, Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS ONE. 2013;8:e64835.
Parodi M, Raggi F, Cangelosi D, Manzini C, Balsamo M, Blengio F, Eva A, Varesio L, Pietra G, Moretta L, et al. Hypoxia Modifies the Transcriptome of Human NK Cells, Modulates Their Immunoregulatory Profile, and Influences NK Cell Subset Migration. Front Immunol. 2018;9:2358.
Mouillot G, Marcou C, Zidi I, Guillard C, Sangrouber D, Carosella ED, Moreau P. Hypoxia modulates HLA-G gene expression in tumor cells. Hum Immunol. 2007;68:277–85.
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90.
Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, Shen Y, Zhang H, Sun R, Tian Z, Wei H. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol. 2019;20:1656–67.
Mah AY, Rashidi A, Keppel MP, Saucier N, Moore EK, Alinger JB, Tripathy SK, Agarwal SK, Jeng EK, Wong HC, et al: Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight 2017, 2.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–83.
Krzywinska E, Kantari-Mimoun C, Kerdiles Y, Sobecki M, Isagawa T, Gotthardt D, Castells M, Haubold J, Millien C, Viel T, et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun. 2017;8:1597.
Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 2013;110:17450–5.
Messai Y, Noman MZ, Janji B, Hasmim M, Escudier B, Chouaib S. The autophagy sensor ITPR1 protects renal carcinoma cells from NK-mediated killing. Autophagy 2015:0.
Schilling D, Tetzlaff F, Konrad S, Li W, Multhoff G. A hypoxia-induced decrease of either MICA/B or Hsp70 on the membrane of tumor cells mediates immune escape from NK cells. Cell Stress Chaperones. 2015;20:139–47.
Xu LJ, Ma Q, Zhu J, Li J, Xue BX, Gao J, Sun CY, Zang YC, Zhou YB, Yang DR, Shan YX. Combined inhibition of JAK1,2/Stat3–PD–L1 signaling pathway suppresses the immune escape of castration–resistant prostate cancer to NK cells in hypoxia. Mol Med Rep. 2018;17:8111–20.
Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, Chouaib S. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology. 2016;5:e1062968.
Suthen S, Lim CJ, Nguyen PHD, Dutertre CA, Lai HLH, Wasser M, Chua C, Lim TKH, Leow WQ, Loh TJ, et al: Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology 2022.
Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–93.
Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1α to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42:1062–74.
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019;30:36–50.
Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020;30:R246-r248.
Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, Moller A. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G + immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–11.
Jimenez-Cortegana C, Galassi C, Klapp V, Gabrilovich DI, Galluzzi L. Myeloid-Derived Suppressor Cells and Radiotherapy. Cancer Immunol Res. 2022;10:545–57.
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53.
Idorn M, Hojman P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol Med. 2016;22:565–77.
Pal A, Zimmer P, Schmidt ME, Hummel M, Ulrich CM, Wiskemann J, Steindorf K. No Evidence for Effect of Exercise on Transcriptome of NK Cells in Breast Cancer Patients Undergoing Adjuvant Therapy: Results From a Pilot Study. Front Physiol. 2019;10:959.
El Hafny-Rahbi B, Brodaczewska K, Collet G, Majewska A, Klimkiewicz K, Delalande A, Grillon C, Kieda C. Tumour angiogenesis normalized by myo-inositol trispyrophosphate alleviates hypoxia in the microenvironment and promotes antitumor immune response. J Cell Mol Med. 2021;25:3284–99.
Murphy DA, Cheng H, Yang T, Yan X, Adjei IM. Reversing Hypoxia with PLGA-Encapsulated Manganese Dioxide Nanoparticles Improves Natural Killer Cell Response to Tumor Spheroids. Mol Pharm. 2021;18:2935–46.
Solocinski K, Padget MR, Fabian KP, Wolfson B, Cecchi F, Hembrough T, Benz SC, Rabizadeh S, Soon-Shiong P, Schlom J, Hodge JW. Overcoming hypoxia-induced functional suppression of NK cells. J Immunother Cancer 2020, 8.
Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511.
López-Soto A, Bravo-San Pedro JM, Kroemer G, Galluzzi L, Gonzalez S. Involvement of autophagy in NK cell development and function. Autophagy. 2017;13:633–6.
Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19:170–83.
Renaudin X. Reactive oxygen species and DNA damage response in cancer. Int Rev Cell Mol Biol. 2021;364:139–61.
Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22:280–97.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–83.
Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253.
Chong SJF, Marchi S, Petroni G, Kroemer G, Galluzzi L, Pervaiz S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020;30:537–55.
Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21:363–81.
Nakamura K, Matsunaga K. Susceptibility of natural killer (NK) cells to reactive oxygen species (ROS) and their restoration by the mimics of superoxide dismutase (SOD). Cancer Biother Radiopharm. 1998;13:275–90.
Aydin E, Johansson J, Nazir FH, Hellstrand K, Martner A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol Res. 2017;5:804–11.
Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S, Abood D, et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin Cancer Res. 2018;24:1891–904.
Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914.
Xu H, Buhtoiarov IN, Guo H, Cheung NV. A novel multimeric IL15/IL15Ralpha-Fc complex to enhance cancer immunotherapy. Oncoimmunology. 2021;10:1893500.
Yang Y, Neo SY, Chen Z, Cui W, Chen Y, Guo M, Wang Y, Xu H, Kurzay A, Alici E, et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J Clin Invest. 2020;130:5508–22.
Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295–317.
Renken S, Nakajima T, Magalhaes I, Mattsson J, Lundqvist A, Arner ESJ, Kiessling R, Wickstrom SL. Targeting of Nrf2 improves antitumoral responses by human NK cells, TIL and CAR T cells during oxidative stress. J Immunother Cancer 2022, 10.
Ligtenberg MA, Mougiakakos D, Mukhopadhyay M, Witt K, Lladser A, Chmielewski M, Riet T, Abken H, Kiessling R. Coexpressed Catalase Protects Chimeric Antigen Receptor-Redirected T Cells as well as Bystander Cells from Oxidative Stress-Induced Loss of Antitumor Activity. J Immunol. 2016;196:759–66.
Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18:261–79.
Hangai S, Ao T, Kimura Y, Matsuki K, Kawamura T, Negishi H, Nishio J, Kodama T, Taniguchi T, Yanai H. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc Natl Acad Sci U S A. 2016;113:3844–9.
Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–70.
Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93.
Holt DM, Ma X, Kundu N, Collin PD, Fulton AM. Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. J Immunother. 2012;35:179–88.
Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, Fulton AM. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE(2)-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2:e22647.
Wang Y, Cui L, Georgiev P, Singh L, Zheng Y, Yu Y, Grein J, Zhang C, Muise ES, Sloman DL, et al. Combination of EP4 antagonist MF-766 and anti-PD-1 promotes anti-tumor efficacy by modulating both lymphocytes and myeloid cells. Oncoimmunology. 2021;10:1896643.
Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–61.
Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S. Reis e Sousa C: NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172:1022–37.e1014.
Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, Mensurado S, Moeini A, Flanagan E, Bell CR, et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity. 2020;53:1215–29 e1218.
Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20:4096–106.
Bernardo M, Tolstykh T, Zhang YA, Bangari DS, Cao H, Heyl KA, Lee JS, Malkova NV, Malley K, Marquez E, et al. An experimental model of anti-PD-1 resistance exhibits activation of TGFss and Notch pathways and is sensitive to local mRNA immunotherapy. Oncoimmunology. 2021;10:1881268.
Chen Z, Yang Y, Neo SY, Shi H, Chen Y, Wagner AK, Larsson K, Tong L, Jakobsson PJ, Alici E, et al. Phosphodiesterase 4A confers resistance to PGE2-mediated suppression in CD25(+) /CD54(+) NK cells. EMBO Rep. 2021;22:e51329.
Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17:611–29.
Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23:487–500.
Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120–34.
Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, Galluzzi L. ATP and cancer immunosurveillance. Embo j. 2021;40:e108130.
Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601–18.
Zanin RF, Braganhol E, Bergamin LS, Campesato LF, Filho AZ, Moreira JC, Morrone FB, Sévigny J, Schetinger MR, de Souza Wyse AT, Battastini AM. Differential macrophage activation alters the expression profile of NTPDase and ecto-5’-nucleotidase. PLoS ONE. 2012;7:e31205.
Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology. 2017;6:e1320011.
Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4 + Foxp3 + regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–40.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41.
Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–5.
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7.
Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19:37–50.
Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–65.
Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–91.
Raskovalova T, Lokshin A, Huang X, Jackson EK, Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: involvement of protein kinase A isozyme I (PKA I). Immunol Res. 2006;36:91–9.
Tay AHM, Prieto-Diaz R, Neo S, Tong L, Chen X, Carannante V, Onfelt B, Hartman J, Haglund F, Majellaro M, et al: A2B adenosine receptor antagonists rescue lymphocyte activity in adenosine-producing patient-derived cancer models. J Immunother Cancer 2022, 10.
Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018;78:1003–16.
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–41.
Wennerberg E, Mukherjee S, Sainz RM, Stiles BM. The ART of tumor immune escape. Oncoimmunology. 2022;11:2076310.
Neo SY, Yang Y, Record J, Ma R, Chen X, Chen Z, Tobin NP, Blake E, Seitz C, Thomas R, et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J Clin Invest. 2020;130:1185–98.
Chambers AM, Wang J, Dao TN, Lupo KB, Veenhuis P, Ayers MG, Slivova V, Cohen-Gadol AA, Matosevic S. Functional expression of CD73 on human natural killer cells. Cancer Immunol Immunother 2022.
Yu L, Liu X, Wang X, Yan F, Wang P, Jiang Y, Du J, Yang Z. TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virusrelated hepatocellular carcinoma. Oncoimmunology. 2021;10:1942673.
Morandi F, Horenstein AL, Chillemi A, Quarona V, Chiesa S, Imperatori A, Zanellato S, Mortara L, Gattorno M, Pistoia V, Malavasi F. CD56brightCD16- NK Cells Produce Adenosine through a CD38-Mediated Pathway and Act as Regulatory Cells Inhibiting Autologous CD4 + T Cell Proliferation. J Immunol. 2015;195:965–72.
Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–9.
Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190.
Zhang H, Vijayan D, Li XY, Robson SC, Geetha N, Teng MWL, Smyth MJ. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology. 2019;8:e1593809.
Stagg J, Beavis PA, Divisekera U, Liu MC, Möller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–6.
Wennerberg E, Spada S, Rudqvist NP, Lhuillier C, Gruber S, Chen Q, Zhang F, Zhou XK, Gross SS, Formenti SC, Demaria S. CD73 Blockade Promotes Dendritic Cell Infiltration of Irradiated Tumors and Tumor Rejection. Cancer Immunol Res. 2020;8:465–78.
Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41.
Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7.
Wang J, Lupo KB, Chambers AM, Matosevic S. Purinergic targeting enhances immunotherapy of CD73(+) solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer. 2018;6:136.
Chambers AM, Wang J, Lupo KB, Yu H, Atallah Lanman NM, Matosevic S. Adenosinergic Signaling Alters Natural Killer Cell Functional Responses. Front Immunol. 2018;9:2533.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65.
Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20:680–93.
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34.
Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577–86.
Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, Henry JY, Ghorani E, Akarca AU, Marafioti T, et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. 2020;1:1153–66.
Kohno M, Murakami J, Wu L, Chan ML, Yun Z, Cho BCJ, de Perrot M. Foxp3(+) Regulatory T Cell Depletion after Nonablative Oligofractionated Irradiation Boosts the Abscopal Effects in Murine Malignant Mesothelioma. J Immunol. 2020;205:2519–31.
Knitz MW, Bickett TE, Darragh LB, Oweida AJ, Bhatia S, Van Court B, Bhuvane S, Piper M, Gadwa J, Mueller AC, et al: Targeting resistance to radiation-immunotherapy in cold HNSCCs by modulating the Treg-dendritic cell axis. J Immunother Cancer 2021, 9.
Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4 + CD25 + T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7.
Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A. CD4 + CD25 + T regulatory cells inhibit cytotoxic activity of T CD8 + and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–67.
Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res. 2018;6:766–75.
Piper M, Van Court B, Mueller A, Watanabe S, Bickett T, Bhatia S, Darragh LB, Mayeda M, Nguyen D, Gadwa J, et al. Targeting Treg-Expressed STAT3 Enhances NK-Mediated Surveillance of Metastasis and Improves Therapeutic Response in Pancreatic Adenocarcinoma. Clin Cancer Res. 2022;28:1013–26.
Dean JW, Peters LD, Fuhrman CA, Seay HR, Posgai AL, Stimpson SE, Brusko MA, Perry DJ, Yeh WI, Newby BN, et al. Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun. 2020;108:102417.
Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–56.
Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–42.
Della Chiesa M, De Maria A, Muccio L, Bozzano F, Sivori S, Moretta L. Human NK Cells and Herpesviruses: Mechanisms of Recognition, Response and Adaptation. Front Microbiol. 2019;10:2297.
Wang Z, Guo L, Song Y, Zhang Y, Lin D, Hu B, Mei Y, Sandikin D, Liu H. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-βR II and NKG2D. Cancer Immunol Immunother. 2017;66:537–48.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–71.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904.
Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. 2022;19:402–21.
Goswami S, Anandhan S, Raychaudhuri D, Sharma P. Myeloid cell-targeted therapies for solid tumours. Nat Rev Immunol 2022.
Krneta T, Gillgrass A, Poznanski S, Chew M, Lee AJ, Kolb M, Ashkar AA. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J Leukoc Biol. 2017;101:285–95.
Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273.
Bellora F, Castriconi R, Dondero A, Pessino A, Nencioni A, Liggieri G, Moretta L, Mantovani A, Moretta A, Bottino C. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur J Immunol. 2014;44:1814–22.
Oyarce C, Vizcaino-Castro A, Chen S, Boerma A, Daemen T. Re-polarization of immunosuppressive macrophages to tumor-cytotoxic macrophages by repurposed metabolic drugs. Oncoimmunology. 2021;10:1898753.
Eisinger S, Sarhan D, Boura VF, Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, Arsenian-Henriksson M, Lane D, Wikström SL, Kiessling R, et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci U S A. 2020;117:32005–16.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98.
Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–84.
Umansky V, Blattner C, Fleming V, Hu X, Gebhardt C, Altevogt P, Utikal J. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. 2017;39:295–305.
Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, Horn LA, Palena C, Schlom J, Maeda DY, et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020;26:1420–31.
Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9.
Li Z, Pang Y, Gara SK, Achyut BR, Heger C, Goldsmith PK, Lonning S, Yang L. Gr-1 + CD11b + cells are responsible for tumor promoting effect of TGF-β in breast cancer progression. Int J Cancer. 2012;131:2584–95.
Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5:101.
Van der Meer JMR, de Jonge P, van der Waart AB, Geerlings AC, Moonen JP, Brummelman J, de Klein J, Vermeulen MC, Maas RJA, Schaap NPM, et al. CD34(+) progenitor-derived NK cell and gemcitabine combination therapy increases killing of ovarian cancer cells in NOD/SCID/IL2Rg(null) mice. Oncoimmunology. 2021;10:1981049.
Gürlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, Yevsa T, Woller N, Kloos A, Ostroumov D, et al. Administration of Gemcitabine After Pancreatic Tumor Resection in Mice Induces an Antitumor Immune Response Mediated by Natural Killer Cells. Gastroenterology. 2016;151:338–50.e337.
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18.
Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21.
Van Wigcheren GF, De Haas N, Mulder TA, Horrevorts SK, Bloemendal M, Hins-Debree S, Mao Y, Kiessling R, van Herpen CML, Florez-Grau G, et al. Cisplatin inhibits frequency and suppressive activity of monocytic myeloid-derived suppressor cells in cancer patients. Oncoimmunology. 2021;10:1935557.
Heine A, Flores C, Gevensleben H, Diehl L, Heikenwalder M, Ringelhan M, Janssen KP, Nitsche U, Garbi N, Brossart P, et al. Targeting myeloid derived suppressor cells with all-trans retinoic acid is highly time-dependent in therapeutic tumor vaccination. Oncoimmunology. 2017;6:e1338995.
Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, Hayashi N. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–61.
Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804.
Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP, Weichselbaum RR, Schreiber H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc Natl Acad Sci U S A. 2016;113:7551–6.
Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72.
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–43.
Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–14.
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34.
Taddei ML, Giannoni E, Raugei G, Scacco S, Sardanelli AM, Papa S, Chiarugi P. Mitochondrial Oxidative Stress due to Complex I Dysfunction Promotes Fibroblast Activation and Melanoma Cell Invasiveness. J Signal Transduct. 2012;2012:684592.
Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44.
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–32.
Souza-Fonseca-Guimaraes F, Rossi GR, Dagley LF, Foroutan M, McCulloch TR, Yousef J, Park HY, Gunter JH, Beavis PA, Lin CY, et al. TGFbeta and CIS Inhibition Overcomes NK-cell Suppression to Restore Antitumor Immunity. Cancer Immunol Res. 2022;10:1047–54.
Li T, Yi S, Liu W, Jia C, Wang G, Hua X, Tai Y, Zhang Q, Chen G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013;30:663.
Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:20847–52.
Inoue T, Adachi K, Kawana K, Taguchi A, Nagamatsu T, Fujimoto A, Tomio K, Yamashita A, Eguchi S, Nishida H, et al. Cancer-associated fibroblast suppresses killing activity of natural killer cells through downregulation of poliovirus receptor (PVR/CD155), a ligand of activating NK receptor. Int J Oncol. 2016;49:1297–304.
Ziani L, Safta-Saadoun TB, Gourbeix J, Cavalcanti A, Robert C, Favre G, Chouaib S, Thiery J. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget. 2017;8:19780–94.
Ziani L, Buart S, Chouaib S, Thiery J. Hypoxia increases melanoma-associated fibroblasts immunosuppressive potential and inhibitory effect on T cell-mediated cytotoxicity. Oncoimmunology. 2021;10:1950953.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115.
Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8.
Ireland L, Luckett T, Schmid MC, Mielgo A. Blockade of Stromal Gas6 Alters Cancer Cell Plasticity, Activates NK Cells, and Inhibits Pancreatic Cancer Metastasis. Front Immunol. 2020;11:297.
Costa D, Venè R, Benelli R, Romairone E, Scabini S, Catellani S, Rebesco B, Mastracci L, Grillo F, Minghelli S, et al. Targeting the Epidermal Growth Factor Receptor Can Counteract the Inhibition of Natural Killer Cell Function Exerted by Colorectal Tumor-Associated Fibroblasts. Front Immunol. 2018;9:1150.
Laskowski TJ, Biederstadt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 2022.
Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol 2022.
Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov. 2022;21:559–77.
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41.
Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22:124–38.
Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21:509–28.
Tata A, Dodard G, Fugere C, Leget C, Ors M, Rossi B, Vivier E, Brossay L. Combination blockade of KLRG1 and PD-1 promotes immune control of local and disseminated cancers. Oncoimmunology. 2021;10:1933808.
André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731–43.e1713.
Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–57.
Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–44.
Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–54.
Gaggero S, Witt K, Carlsten M, Mitra S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front Immunol. 2020;11:621225.
Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inoges S, Melero I, Berraondo P. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95:347–55.
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–66.
Björkström NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112–23.
Funding
LG is/has been supported (as a PI unless otherwise indicated) by two Breakthrough Level 2 grants from the US DoD BCRP (#BC180476P1; #BC210945), by a Transformative Breast Cancer Consortium Grant from the US DoD BCRP (#W81XWH2120034, PI: Formenti), by a U54 grant from NIH/NCI (#CA274291, PI: Deasy, Formenti, Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by startup funds from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by industrial collaborations with Lytix Biopharma (Oslo, Norway), Promontory (New York, US) and Onxeo (Paris, France), as well as by donations from Promontory (New York, US), the Luke Heller TECPR2 Foundation (Boston, US), Sotio a.s. (Prague, Czech Republic), Lytix Biopharma (Oslo, Norway), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy), and Noxopharm (Chatswood, Australia). AL is supported by grants from the Swedish Cancer Society (#21 1524 Pj), The Swedish Childhood Cancer Foundation (#PR2021-0039), and The Cancer Research Foundations of Radiumhemmet (#211253).
Author information
Authors and Affiliations
Contributions
LG and AL conceived the article. LT wrote the first version of the manuscript with constructive input from CJC, AT and SW, under supervision from LG and AL. CJC prepared display items under supervision from LG and AL. All authors approved the final version of the article.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
LG is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Sotio, Promontory, Noxopharm, EduCom, and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. All other authors have no conflicts to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tong, L., Jiménez-Cortegana, C., Tay, A.H. et al. NK cells and solid tumors: therapeutic potential and persisting obstacles. Mol Cancer 21, 206 (2022). https://doi.org/10.1186/s12943-022-01672-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-022-01672-z