Abstract
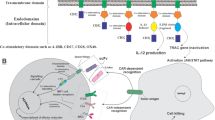
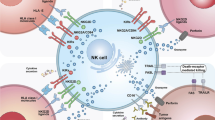
The capacity of natural killer (NK) cells to kill tumor cells without specific antigen recognition provides an advantage over T cells and makes them potential effectors for tumor immunotherapy. However, the efficacy of NK cell adoptive therapy can be limited by the immunosuppressive tumor microenvironment. Transforming growth factor-β (TGF-β) is a potent immunosuppressive cytokine that can suppress NK cell function. To convert the suppressive signal induced by TGF-β to an activating signal, we genetically modified NK-92 cells to express a chimeric receptor with TGF-β type II receptor extracellular and transmembrane domains and the intracellular domain of NK cell-activating receptor NKG2D (TN chimeric receptor). NK-92 cells expressing TN receptors were resistant to TGF-β-induced suppressive signaling and did not down-regulate NKG2D. These modified NK-92 cells had higher killing capacity and interferon γ (IFN-γ) production against tumor cells compared with the control cells and their cytotoxicity could be further enhanced by TGF-β. More interestingly, the NK-92 cells expressing TN receptors were better chemo-attracted to the tumor cells expressing TGF-β. The presence of these modified NK-92 cells significantly inhibited the differentiation of human naïve CD4+ T cells to regulatory T cells. NK-92-TN cells could also inhibit tumor growth in vivo in a hepatocellular carcinoma xenograft tumor model. Therefore, TN chimeric receptors can be a novel strategy to augment anti-tumor efficacy in NK cell adoptive therapy.






Similar content being viewed by others
Abbreviations
- α-MEM:
-
Minimum essential medium alpha
- BFA:
-
Brefeldin A
- DAP:
-
DNAX-activating protein
- DMEM:
-
Dulbecco-modified eagle medium
- FACS:
-
Fluorescence-activated cell sorter
- FBS:
-
Fetal bovine serum
- HLA:
-
Human leucocyte antigen
- HRP:
-
Horseradish Peroxidase
- IFN-γ:
-
Interferonγ
- IgA:
-
Immunoglobulin A
- IL-2:
-
Interleukin 2
- KIR:
-
Killer cell Ig-like Receptors
- LDH:
-
Lactic dehydrogenase
- mAb:
-
Monoclonal antibody
- MFI:
-
Mean fluorescent intensity
- MHC:
-
Major histocompatibility complex
- NK:
-
Natural killer
- NK-92-TN:
-
NK-92 expressing extracellular and transmembrane domains of TGF-β receptor II with intracellular domain of NKG2D
- NK-92-Vector:
-
NK-92 expressing vector
- NKG2C:
-
NK Group 2 member C
- NKG2D:
-
NK Group 2 member D
- PCR:
-
Polymerase chain reaction
- PE:
-
Phycoerythrin
- PMA:
-
Phorbol myristate acetate
- PVDF:
-
Polyvinylidene fluoride
- RPMI:
-
Roswell Park Memorial Institute
- RT-PCR:
-
Real-time PCR
- SDS–PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TGF-β:
-
Transforming growth factor-β
- TGF-βR:
-
Transforming growth factor-β receptor
- Treg:
-
Regulatory T
- VSVG:
-
Vesicular stomatitis virus G
- YFP:
-
Yellow fluorescent protein
References
Ljunggren HG, Malmberg KJ (2007) Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 7:329–339. doi:10.1038/nri2073
Barkholt L, Alici E, Conrad R et al. (2009) Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy 1:753–764. doi:10.2217/imt.09.47
Leung W (2014) Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res 20:3390–3400. doi:10.1158/1078-0432.CCR-13-1766
Jung B, Staudacher JJ, Beauchamp D (2016) Transforming growth factor β superfamily signaling in development of colorectal cancer. Gastroenterology. doi:10.1053/j.gastro.2016.10.015
Bierie B, Moses HL (2006) Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6:506–520. doi:10.1038/nrc1926
Massagué J (2008) TGFβ in cancer. Cell 134:215–230. doi:10.1016/j.cell.2008.07.001
Drabsch Y, ten Dijke P (2012) TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 31:553–568. doi:10.1007/s10555-012-9375-7
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT (2010) TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol 12:7–13. doi:10.1093/neuonc/nop009
Kopp HG, Placke T, Salih HR (2009) Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69:7775–7783. doi:10.1158/0008-5472.CAN-09-2123
Yang B, Liu H, Shi W, Wang Z, Sun S, Zhang G, Hu Y, Liu T, Jiao S (2013) Blocking transforming growth factor-beta signaling pathway augments antitumor effect of adoptive NK-92 cell therapy. Int Immunopharmacol 17:198–204. doi:10.1016/j.intimp.2013.06.003
Gong JH, Maki G, Klingemann HG (1994) Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8:652–658
Wu L, Zhang C, Tian Z, Zhang J (2011) NK cell-based approach for screening novel functional immune genes. Int Immunopharmacol 11:274–279. doi:10.1016/j.intimp.2010.12.003
Maki G, Klingemann HG, Martinson JA, Tam YK (2001) Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother. Stem Cell Res 10:369–383. doi:10.1089/152581601750288975
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727–729
Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97:192–197
Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13:95–109
Rosenzweig SD, Holland SM (2005) Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev 203:38–47. doi:10.1111/j.0105-2896.2005.00227.x
Ghiringhelli F, Larmonier N, Schmitt E et al (2004) CD4+ CD25+ regulatory T†„cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344. doi:10.1002/eji.200324181
Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. doi:10.1126/science.1198687
Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA (2011) Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 17:6287–6297. doi:10.1158/1078-0432.ccr-11-1347
de Souza AP, Bonorino C (2009) Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther 9:1317–1332. doi:10.1586/era.09.88
Jones E, Pu H, Kyprianou N (2009) Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets 13:227–234. doi:10.1517/14728220802705696
Gajewski TF, Meng Y, Harlin H (2006) Immune suppression in the tumor microenvironment. J Immunother 29:233–240. doi:10.1097/01.cji.0000199193.29048.56
Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S, Zhang D (2015) Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther 8:1553–1559. doi:10.2147/OTT.S82616. eCollection 2015
Li Z, Zhang LJ, Zhang HR et al (2014) Tumor-derived transforming growth factor-beta is critical for tumor progression and evasion from immune surveillance. Asian Pac J Cancer Prev 15:5181–5186
Carambia A, Freund B, Schwinge D et al (2014) TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J Hepatol 61:594–599. doi:10.1016/j.jhep.2014.04.027
Rouce RH, Shaim H, Sekine T et al (2015) The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B acute lymphoblastic leukemia. Leukemia 30(4):800–811. doi:10.1038/leu.2015.327
Donatelli SS, Zhou JM, Gilvary DL et al (2014) TGF- -inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci 111:4203–4208. doi:10.1073/pnas.1319269111
Krneta T, Gillgrass A, Chew M, Ashkar AA (2015) The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol 13(5):628–639. doi:10.1038/cmi.2015.42
Alvarez M, Bouchlaka MN, Sckisel GD, Sungur CM, Chen M, Murphy WJ (2014) Increased antitumor effects using IL-2 with anti-TGF-beta reveals competition between mouse NK and CD8 T cells. J Immunol 193:1709–1716. doi:10.4049/jimmunol.1400034
Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y (2006) CD4 + CD25 + T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176:1582–1587
Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H (2012) TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 8:e1002594. doi:10.1371/journal.ppat.1002594
Laouar Y, Sutterwala FS, Gorelik L, Flavell RA (2005) Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 6:600–607. doi:10.1038/ni1197
Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T (2007) The trafficking of natural killer cells. Immunol Rev 220:169–182. doi:10.1111/j.1600-065X.2007.00563.x
Shi FD, Ljunggren HG, La Cava A, Van Kaer L (2011) Organ-specific features of natural killer cells. Nat Rev Immunol. 11(10):658–671. doi:10.1038/nri3065
Acknowledgements
We thank Dr. Feili Gong for providing NK-92 cells and Dr. Yun Zhao for providing lenti-virus vectors. This work has been supported by grants from the National Natural Science Foundation of China (81273268, 81471586), the project funding from Suzhou city (SWG0904), Priority Academic Program Development of Jiangsu Higher Education Institutions, and the start-up grant from the National University of Singapore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Z. Wang, L. Guo, and Y. Song contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Guo, L., Song, Y. et al. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-βR II and NKG2D. Cancer Immunol Immunother 66, 537–548 (2017). https://doi.org/10.1007/s00262-017-1959-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1959-1