Abstract
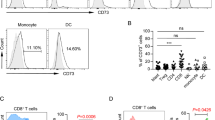
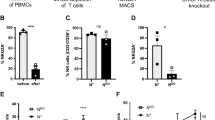
AML is the most common blood cancer in adults with a high relapse and an overall poor survival rate. NK cells have been demonstrated to have the capacity to eradicate AML blast, and an impaired NK cell function is involved in AML development and progression. Immune checkpoints are involved in immune escape in various cancers. Immune checkpoints blockade therapy mainly aimed to unleash CD8+T cells function, but NK cells have emerged as new target. However, immune checkpoints profile on NK cells has not been observed in AML patients. Here, we studied the immune checkpoints expression of NK cells from AML patients at initial diagnosis and found increased PD-1, TIGIT and TIM-3 expression compared to NK cells from healthy donors. Further analysis showed that TIGIT expressing NK cells from AML patients had a dysfunctional phenotype, as TIGIT+NK cells exhibit lower antileukemia effect, cytokine production and degranulation compared to TIGIT−NK cells. TIGIT blockade could significantly enhance the function of NK cells. Moreover, AML patients with high frequency of TIGIT+NK cells had higher frequency of poor prognosis risk. Further analysis found that IL-10 upregulated TIGIT expression on NK cells. Thus, TIGIT blockade alone or in combination with other therapy might be potential strategy to treat AML.







Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Change history
06 July 2021
Erxia Shen is the first corresponding author
08 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00262-023-03455-x
References
Prada-Arismendy J, Arroyave JC, Rothlisberger S (2017) Molecular biomarkers in acute myeloid leukemia. Blood Rev 31:63–76
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD, Diagnosis and management of AML in adults, (2017) ELN recommendations from an international expert panel. Blood 129:424–447
De Kouchkovsky I, Abdul-Hay M (2016) Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 6:e441
Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, Garcia-Manero G, Konopleva M, Ravandi F (2021) Acute myeloid leukemia: current progress and future directions. Blood Cancer J 11:41
Yang F, Wang R, Feng W, Chen C, Yang X, Wang L, Hu Y, Ren Q, Zheng G (2018) Characteristics of NK cells from leukemic microenvironment in MLL-AF9 induced acute myeloid leukemia. Mol Immunol 93:68–78
Miller JS, Lanier LL (2019) Natural killer cells in cancer immunotherapy. Annual Rev Cancer Biol 3:77–103
Baragano Raneros A, Lopez-Larrea C, Suarez-Alvarez B (2019) Acute myeloid leukemia and NK cells: two warriors confront each other. Oncoimmunology 8:e1539617
Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL (2012) Natural killer cell immune escape in acute myeloid leukemia. Leukemia 26:2019–2026
Dulphy N, Chretien AS, Khaznadar Z, Fauriat C, Nanbakhsh A, Caignard A, Chouaib S, Olive D, Toubert A (2016) Underground adaptation to a hostile environment: acute myeloid leukemia vs. Nat Killer Cells Front Immunol 7:94
Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R (2012) Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 90:109–115
Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT (2007) Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109:323–330
Khan M, Arooj S, Wang H (2020) NK cell-based immune checkpoint inhibition. Front Immunol 11:167
Guillerey C, Huntington ND, Smyth MJ (2016) Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17:1025–1036
Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011) Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89:216–224
Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S (2013) Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 31:227–258
Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z (2018) Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunol 19:723–732
Wang M, Bu J, Zhou M, Sido J, Lin Y, Liu G, Lin Q, Xu X, Leavenworth JW, Shen E (2018) CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin Immunol 190:64–73
Fu X, Liu Y, Li L, Li Q, Qiao D, Wang H, Lao S, Fan Y, Wu C (2011) Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO− natural killer cells following stimulation with interleukin-12. Immunology 134:41–49
Li Y, Jiang T, Zhou W, Li J, Li X, Wang Q, Jin X, Yin J, Chen L, Zhang Y, Xu J, Li X (2020) Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat Commun 11:1000
Izzi V, Lakkala J, Devarajan R, Savolainen ER, Koistinen P, Heljasvaara R, Pihlajaniemi T (2018) Vanin 1 (VNN1) levels predict poor outcome in acute myeloid leukemia. Am J Hematol 93:E4–E7
Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z (2015) TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol 45:2886–2897
Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Banas H, Morgado S, Casado JG, Solana R, Tarazona R (2013) Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine 61:885–891
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161:205–214
Stamm H, Klingler F, Grossjohann EM, Muschhammer J, Vettorazzi E, Heuser M, Mock U, Thol F, Vohwinkel G, Latuske E, Bokemeyer C, Kischel R, Dos Santos C, Stienen S, Friedrich M, Lutteropp M, Nagorsen D, Wellbrock J, Fiedler W (2018) Immune checkpoints PVR and PVRL2 are prognostic markers in AML and their blockade represents a new therapeutic option. Oncogene 37:5269–5280
Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H (2016) T-Cell immunoglobulin and ITIM Domain (TIGIT) associates with CD8+ T-Cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res : An Offic J Am Assoc Cancer Res 22:3057–3066
Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL (2009) The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 10:48–57
Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O (2009) The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 106:17858–17863
Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL (2014) The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26:923–937
Martínez-Sánchez MV, Fuster JL, Campillo JA, Galera AM, Bermúdez-Cortés M, Llinares ME, Ramos-Elbal E, Pascual-Gázquez JF, Fita AM, Martínez-Banaclocha H, Galián JA, Gimeno L, Muro M, Minguela A (2021) Expression of NK cell receptor ligands on leukemic cells is associated with the outcome of childhood acute leukemia. Cancers 13:2294
Mastaglio S, Wong E, Perera T, Ripley J, Blombery P, Smyth MJ, Koldej R, Ritchie D (2018) Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv 2:335–346
Hattori N, Kawaguchi Y, Sasaki Y, Shimada S, Murai S, Abe M, Baba Y, Watanuki M, Fujiwara S, Arai N, Kabasawa N, Tsukamoto H, Uto Y, Yanagisawa K, Saito B, Harada H, Nakamaki T (2019) Monitoring TIGIT/DNAM-1 and PVR/PVRL2 Immune checkpoint expression levels in allogeneic stem cell transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant 25:861–867
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M (2018) Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest 128:4654–4668
Zhang B, Zhao W, Li H, Chen Y, Tian H, Li L, Zhang L, Gao C, Zheng J (2016) Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol, Immunotherapy : CII 65:305–314
Giannopoulos K (2019) Targeting immune signaling checkpoints in acute myeloid leukemia. J Clin Med 8:236
da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N (2014) Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2:410–422
Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y (2015) Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol 29:635–641
Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao S, Qi Y, Zhang Y, Huang L (2019) TNF-alpha-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med 17:165
Goncalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R, Siligardi G, Ceccone G, Berger SM, Ushkaryov YA, Gibbs BF, Fasler-Kan E, Sumbayev VV (2017) The tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine 22:44–57
Dama P, Tang M, Fulton N, Kline J, Liu H (2019) Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer 7:175
Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, Akashi K (2010) TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 7:708–717
Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, Darley RL (2011) CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 25:792–799
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81971482, to ES), the Science and Technology Program of Guangzhou (No.007095050049 to ES), and the Science and Technology Planned Project of Bureau of Education of Guangzhou (No. 1201610221, to ES).
Author information
Authors and Affiliations
Contributions
ES, MZ and GL conceived the experiments. GL, ZQ and JY performed the study. ES, GL and ZQ analyzed the data. XL, JC, and QL assisted to perform the experiments. YL assisted to do the statistical analysis. LX, WL, TL, QL and XX collected the samples. ES and GL wrote the paper. All authors contributed to the manuscript review. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
YL was employed by Shenzhen Withsum Technology Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study was conducted in compliance with the Declaration of Helsinki and was approved by the ethics committee of Guangdong General Hospital (Guangzhou, China).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Erxia Shen is the first corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Zhang, Q., Yang, J. et al. Increased TIGIT expressing NK cells with dysfunctional phenotype in AML patients correlated with poor prognosis. Cancer Immunol Immunother 71, 277–287 (2022). https://doi.org/10.1007/s00262-021-02978-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02978-5