Abstract
Background
Modifying diet is crucial for diabetes and complication management. Numerous studies have shown that adjusting eating habits to align with the circadian rhythm may positively affect metabolic health. However, eating midpoint, eating duration, and their associations with diabetic kidney disease (DKD) are poorly understood.
Methods
The National Health and Nutrition Examination Survey (2013–2020) was examined for information on diabetes and dietary habits. From the beginning and ending times of each meal, we calculated the eating midpoint and eating duration. Urinary albumin-to-creatinine ratio (UACR) ≥ 30 mg/g and/or estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 were the specific diagnostic criteria for DKD.
Results
In total, details of 2194 subjects with diabetes were collected for analysis. The overall population were divided into four subgroups based on the eating midpoint quartiles. The prevalence of DKD varied noticeably (P = 0.037) across the four categories. When comparing subjects in the second and fourth quartiles of eating midpoint to those in the first one, the odds ratios (ORs) of DKD were 1.31 (95% CI, 1.03 to 1.67) and 1.33 (95% CI, 1.05 to 1.70), respectively. And after controlling for potential confounders, the corresponding ORs of DKD in the second and fourth quartiles were 1.42 (95% CI, 1.07 to 1.90) and 1.39 (95% CI, 1.04 to 1.85), respectively.
Conclusions
A strong correlation was found between an earlier eating midpoint and a reduced incidence of DKD. Eating early in the day may potentially improve renal outcomes in patients with diabetes.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) is a prevalent microvascular consequence of diabetes, with its prevalence rising in response to the global rise in diabetes incidence [1]. The adverse effects of DKD extend beyond the heightened risk of advancing to end-stage renal disease [2]. They also substantially elevate the occurrence of cardiovascular disease and death [3], posing a severe concern to public health. Moreover, despite efforts to manage several risk factors associated with DKD, its development and advancement may still occur in some individuals with diabetes [4]. Hence, it has immense therapeutic importance to undertake any endeavor aimed at improving renal damage in diabetic individuals.
Circadian rhythms regulate physiological and behavioral rhythms in a nearly 24-hour cycle. The central clock of the hypothalamus located in the suprachiasmatic nucleus (SCN) mainly receives light stimulation, and then down-regulates the circadian rhythms of multiple organ systems to coordinate physiological processes [5]. Furthermore, apart from the SCN, circadian rhythm genes are also expressed in several additional brain areas and peripheral organs. It is worth mentioning that the expression of circadian rhythm genes in the kidney is ranked second only to that seen in the liver, indicating the significant regulatory function of circadian rhythm in kidney cells [6]. Peripheral clocks can become disengaged from the regulation of the SCN as a consequence of alterations in eating behaviors. Research has shown that deviations from the typical meal cycle may lead to disturbances in the circadian rhythm [7]. Numerous studies have shown that persistent disruptions in circadian rhythm have been implicated in the development of obesity, hypertension, insulin resistance, inflammation, aberrant glucose and lipid metabolism, among other conditions [8,9,10]. These characteristics are well recognized as significant risk factors for DKD [8,9,10]. Subsequently, the regulation of nutrition may be advantageous in mitigating the risk of DKD via the modulation of circadian rhythm.
Dietary modification, an essential element of diabetes therapy and its associated problems, involves the strategic adjustment of nutritional consumption [11]. Caloric restriction (CR) is a common dietary modification, and has the potential to mitigate several chronic illnesses, including cancer, diabetes, and chronic kidney disease (CKD) [12]. However, the increased hunger caused by CR and low adherence limit the clinical use of CR, resulting in focusing on searching for other dietary modifications. Observational and interventional studies indicate that the timing of meals is essential for maintaining health. Two observational studies showed that the delay in the first meal was associated with increased blood pressure, C-reactive protein (CRP) level, glucose and insulin levels, and decreased high-density lipoprotein cholesterol (HDL-C) level [13, 14]. Wieth et al. also observed that a later last meal of the day was associated with a higher level of glycosylated hemoglobin (HbA1c) [14]. Likewise, a large prospective cohort study revealed that later times of first and last meals significantly contribute to increased risks of cardiovascular disease [15]. Time restricted eating (TRE) is a novel dietary strategy that seeks to maintain a resilient circadian rhythm by limiting the duration of eating to a relatively brief timeframe without imposing restrictions on the nutritional quality and caloric content of the consumed foods [16]. The efficacy of TRE in enhancing metabolic health has been shown by its ability to decrease body weight, enhance insulin resistance, and regulate metabolism [17, 18]. However, Sutton et al. compared the results of previous TRE clinical studies and concluded that the effects of TRE interventions might depend on the specific timing of the eating window throughout the day [19]. Based on this, Sutton and his colleagues proposed early time-restricted eating (eTRE), specifically, subjects receiving eTRF intervention needed to finish eating before 3 PM [19]. In their study, a 5-week intervention of eTRE in male individuals with prediabetes resulted in substantial improvements in insulin levels, insulin sensitivity, blood pressure, and oxidative stress levels [19]. Notably, these improvements were seen without any notable changes in the participants’ body mass. Similarly, a cross-sectional research using data from the National Health and Nutrition Examination Survey (NHANES) showed that consuming meals later in the day was associated with a notable rise in the likelihood of having raised fasting glucose levels [20]. However, the duration of eating window did not have a significant impact on this risk [20]. Hence, it was hypothesized that eating early in the day in individuals diagnosed with diabetes might potentially provide kidney protective benefits.
This observational research sought to investigate the potential correlation between eating midpoint, eating duration, and the incidence of DKD using data obtained from the NHANES.
Materials & methods
Study population
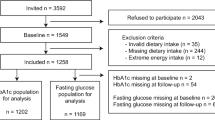
The NHANES was undertaken by the Centers for Disease Control and Prevention with the aim of gathering comprehensive data on the nutritional and health status of both children and adults residing in the United States. The NHANES study used a sampling method that adhered to the principles of multi-stage, complicated, and probabilistic sampling to get a nationally representative sample [21]. The present study used the whole dataset including four cycles of the NHANES spanning the years 2013 to 2020. This research included individuals who were of adult age and diagnosed with diabetes. Diabetes was operationally defined as the presence of a documented medical history of diabetes and/or a HbA1c level equal to or over 6.5%. Subjects who had missing data about meal times, calorie intake, renal function, and urine albumin-to-creatinine ratio (UACR) were eliminated from the study. In addition, those who were following an extremely low-calorie diet were also eliminated from the study. Ultimately, the research included 2194 individuals. The detailed criteria of study population selection were seen in Fig. 1.
Eating parameters
During each cycle, two 24-hour dietary recalls were administered to gather data pertaining to eating patterns, including meal timing and composition, and energy intake. The first dietary memory interviews were conducted by skilled dietary interviewers, followed by a subsequent dietary recall gathered by telephone after a time interval ranging from 3 to 10 days. The parameters obtained included the initial and final meal times, as well as the calorie consumption. The eating duration was determined by measuring the time gap between the first and final meals, with the first meal being considered to have taken place after 5 AM. A five-step methodology was employed to measure the consumption of food and beverages throughout a 24-hour period. The term “meal” was operationally defined as a dietary consumption event that resulted in an energy intake above 1 kcal within a certain time frame. The eating midpoint was operationally defined as the midpoint between the eating window [20]. This was mathematically represented by the formula (eating duration/2) + first meal time [20]. The average of each metric was calculated based on the data obtained from the two 24-hour dietary recalls.
Clinical variables
The demographic information obtained from the NHANES data included age, race, gender, family income, alcohol intake, smoking status, and body mass index (BMI).
Laboratory variables
The detailed measurement method of renal function, UACR and HbA1c were given in the references [22, 23]. The Chronic Kidney Disease Epidemiology Collaboration algorithm was used to calculate estimated glomerular filtration rate (eGFR) [24].
Diagnosis of DKD
The diagnosis of DKD was established by assessing the loss in renal filtration function or the presence of higher levels of urine albumin. The parameters used to indicate a decline in renal filtration function and an increase in urine albumin levels were an eGFR < 60 ml/min/1.73 m2 and a UACR ≥ 30 mg/g [25].
Statistical analysis
The data from the NHANES dataset were processed via R (version 4.2.2). Significant findings were defined as having a two-sided P value < 0.05. The individuals were categorized into four groupings according on the quartiles of their eating midpoint. Continuous variables that follow a normal distribution were presented using the mean ± standard deviation, while continuous variables that do not follow a normal distribution were presented using the median and the interquartile range (25th and 75th percentiles). The representation of categorical data was done via frequencies, expressed as percentages. The study examined the variations in normally distributed, nonnormally distributed, and categorical data among the four subgroups using the one-way analysis of variance (ANOVA), Kruskal‒Wallis, and chi-square tests, respectively. UACR values did not follow a normal distribution, and therefore were transformed using natural logarithms. Two multivariate linear regression analyses were conducted to examine the mean differences in eGFR and ln(UACR) among the four subgroups, using the first quartile (Q1) as the reference. Additionally, a binomial logistic regression analysis was performed to evaluate the odds ratios (ORs) for DKD among the four subgroups. Since age, sex, ethnicity, household income, BMI, smoking and drinking status, energy intake, HbA1c and so on were all clinical variables which might affect the incidence of DKD, they were adjusted as covariables multivariate linear regression analyses and binomial logistic regression analysis. Furthermore, the whole population was categorized into two groups based on the duration of eating window: those who had meals for less than 12 h and those who consumed meals for 12 h or more. Afterwards, a comparison was made between the two categories in terms of the disparities in eGFR, UACR, and DKD incidence. Finally, the ORs for DKD were examined in participants with an eating window of less than 12 h, as opposed to those with an eating window of 12 h or more.
Results
Clinical characteristics of the study participants
The clinical features of the whole population and the four subgroups, categorized by the quartiles of eating midpoint, are shown in Table 1. The research included 2194 diabetic individuals, with an average age of 60.83 ± 13.15 years. Among these, 46.4% had DKD. Significant variations were observed among the four subgroups in terms of age, male proportion, race, BMI, smoking status, UACR, DKD incidence, first meal time, last meal time, and eating duration (P = 0.001, < 0.001, < 0.001, < 0.001, 0.037, 0.023, 0.037, < 0.001, < 0.001 and < 0.001, respectively). However, no significant differences were found in HbA1c, household income, drinking status, eGFR, and energy intake (all P > 0.05).
Multivariate regression analysis of eGFR and UACR in the eating midpoint quartiles
As shown in Table 2, in unadjusted and adjusted models the mean differences (B) in eGFR of the participants of other eating midpoint quartiles versus Q1 were not significant (all P > 0.05). The non-linear association between eating midpoint and eGFR was seen in Supplementary Figure S1.
Table 3 also showed that only the adjusted mean difference (B) in ln(UACR) of the participants in the second quartile (Q2) of eating midpoint versus Q1 was significant (P = 0.003), and the corresponding (B) were 0.29 mg/g (95% CI, 0.10 to 0.49). After gradually adjusting for possible clinical variables, the adjusted mean difference (B) in ln(UACR) of Q2 versus Q1 remained significant (P = 0.003). In the fully adjusted model, the corresponding (B) were 0.31 mg/g (95% CI, 0.11 to 0.52). The non-linear association between eating midpoint and ln(UACR) was seen in Supplementary Figure S2.
Multivariate analysis of factors influencing DKD according to the eating midpoint quartiles
According to the findings shown in Table 4, individuals in the second and fourth quartiles (Q4) had higher ORs for DKD compared to those in the first one (Q1). The OR for Q2 was 1.31 (95% CI, 1.03 to 1.67), while the OR for Q4 was 1.33 (95% CI, 1.05 to 1.70) (P = 0.028 and 0.019, respectively). After controlling for additional clinical factors, the ORs for DKD remained significant in the individuals within the Q2 and Q4 (P = 0.016 and 0.027, respectively). The OR for Q2 was 1.42 (95% CI, 1.07 to 1.90), while the OR for Q4 was 1.39 (95% CI, 1.04 to 1.85).
Association between eating duration and incidence of DKD
In Supplementary Table S3, no significant differences were seen between individuals with an eating duration of less than 12 h and those with an eating window of 12 h or more in terms of eGFR, UACR, and the incidence of DKD (all P > 0.05). Besides, when comparing individuals with an eating duration of less than 12 h to those with an eating duration of 12 h or more, the ORs for DKD were not significant, regardless of whether or not adjustment was used (all P > 0.05) (Supplementary Table S4).
Discussion
The results of the current research indicate that a delayed eating midpoint may independently lead to a higher likelihood of developing DKD. Furthermore, the present investigation could not identify a distinct correlation between the duration of eating window and DKD. Moreover, it can be suggested that restricting meals to earlier timepoints throughout the daytime might potentially have positive effects on renal outcomes in individuals with diabetes.
Previous studies have proven the role of CR in improving diabetic renal outcomes. CR has the capacity to play a positive role in enhancing autophagy, diminishing inflammation and oxidative stress, and enhancing insulin sensitivity [26]. In rats with diabetes and obese individuals with type 2 diabetes, CR intervention can significantly reduce kidney damage [27, 28]. The aforementioned studies highlight the significance of CR in the context of diabetes and DKD. Nevertheless, the findings of this research demonstrated a significant correlation between the eating midpoint, irrespective of caloric intake, and the prevalence of DKD. Therefore, it may be necessary to direct focus not alone towards daily energy consumption, but also towards the eating midpoint.
DKD is a disease involving multiple factors, including abnormal glucose and lipid metabolism, inflammation, oxidative stress, abnormity in hemodynamics and so on [29]. In the present study, patients with a later eating midpoint tended to have a higher BMI. A later eating midpoint was associated with a later first meal time and last meal time. Clinical studies have confirmed the adverse effects of late meals [14, 15], and similar results were also observed in animal studies. Delaying the first meal of the day significantly increased body weight, fat deposition, and even affected the circadian rhythm of lipid metabolism associated genes in animals [30, 31]. In mice, late-night eating also led to weight gain, increased inflammation levels, and alternations in peripheral clock rhythms [32,33,34]. Therefore, eating late may influence the renal prognosis of patients with diabetes via multiple pathways, and adjusting the timing of meals may exert multiple benefits. A randomized crossover investigation conducted on males who were at risk of developing diabetes revealed a noteworthy reduction in the average blood glucose levels among participants who underwent the eTRE intervention, as opposed to those who received the delayed time-restricted eating (dTRE) intervention [35]. In addition, a group of healthy male participants underwent a 2-week eTRE intervention, which resulted in a significant decrease in systemic insulin sensitivity even ad libitum intake was permitted [36].
The benefits of eating early in the day may involve a variety of hormones and metabolic enzymes. Fibroblast growth factor-21 (FGF-21) and adiponectin are important regulators of energy metabolism, and the secretion of FGF-21 and adiponectin mostly occurs throughout 8 AM to 4 PM [37]. This temporal pattern of secretion is associated with the promotion of fat oxidation and glycolysis, while concurrently preventing fat formation [37]. Hence, it may be inferred that consumption of food during this period enhances the efficiency of nutrient digestion and metabolism in humans [38, 39]. Furthermore, it was observed that consuming the identical meal in the morning resulted in a greater glucose tolerance as compared to its consumption in the evening [40]. The phenomenon of nocturnal feeding is associated with a diminished responsiveness of the body to insulin, perhaps leading to oscillations in glucose levels [41]. In addition, due to the strong diurnal pattern of liver hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase expression and cholesterol synthesis [42], eating earlier in the day may play a role in regulating lipid metabolism. Thus, having an early eating midpoint may be beneficial in the kidney by improving glycolipid metabolism.
Alireza and his colleagues conducted two trials of dietary interventions in treating experimental acute kidney injury (AKI), and found that restricting feeding for 5 h had renoprotective effects to rats with AKI by inhibiting the apoptosis of kidney cells, improving antioxidant status, and inhibiting renal fibrosis [43, 44]. Likewise, receiving short-term early time-restricted feeding could also exert the reno-protective effects of anti-oxidation and inhibition of renal fibrosis to rats by regulating mitochondrial dynamics [45]. Additionally, early time-restricted feeding could regulate the local immunity in the kidney of mice with hypertension [46]. In patients with CKD, eTRE intervention significantly improved the renal function, which might be attributed to alternations in gut microbiota [47]. These results provided several explanations in mechanisms for the results of this study.
Eating late in the day may contribute to circadian rhythm disruption as described previously. The circadian clock genes are not only present in the central nervous system, but they are also widely expressed in the kidney [6]. Research has shown the existence of a diurnal rhythm in both glomerular filtration function and urine protein excretion [48, 49]. Similarly, this study revealed that an earlier eating midpoint was possibly associated with less urinary protein excretion. Furthermore, the aberrant expression of clock genes may also expedite renal damage by several mechanisms, including elevated blood pressure, exacerbated inflammation, heightened oxidative stress, and intensified hypoxia [50]. Hence, eating early in the day has significant potential in enhancing renal outcomes in individuals with diabetes.
The challenge of dietary modification is that it possibly increases hunger of the subjects, making it difficult to sustain dietary modification. It is noteworthy that those who were subjected to the eTRE intervention exhibited a significant reduction in their appetite [51, 52]. Therefore, eating earlier in the day may be acceptable to patients with diabetes.
This study observed that the association between a long duration of eating window and the incidence of DKD trended towards statistical significance. Similarly, Nayara et al. also found that in elderly, the adults with a longer eating window had a non-significant lower prevalence of obesity and abdominal obesity, which was opposite to the results of non-elderly [20]. The underlying mechanism is that a short duration of eating window may be partly attributable to skipping breakfast, while ample evidence has confirmed the adverse effects of skipping breakfast in several metabolic morbidities [53,54,55,56]. In Korean middle-aged and older adults, skipping breakfast was significantly associated with increased risk of CKD [57].
The current research has provided novel insights by demonstrating that implementing time-restricted meals throughout the early part of the day may provide favorable kidney outcomes in diabetic individuals. Nevertheless, it is important to acknowledge certain limitations inherent in this research. The causal link between the two variables cannot be completely determined owing to the inherent limitations of the cross-sectional research design. Nor can it be ruled out that the relationship is in the opposite direction, specifically that subjects with DKD or other diabetic complications may alter their eating habits. Furthermore, the diagnostic criteria used in this investigation to identify DKD may introduce the possibility of confounding with other forms of CKD. Thirdly, in accordance with the eating midpoint algorithm, an earlier eating midpoint might perhaps indicate an earlier and shorter eating window. However, it is essential to get more accurate indications to further validate this assertion.
Conclusions
The findings of this research provide evidence to support the notion that a more advanced eating midpoint is correlated with a reduced likelihood of developing DKD. Clinically, eating early in the day may have the potential to provide positive effects on renal outcomes among patients with diabetes. Further longitudinal and interventional investigations are required to substantiate the findings of this research.
Data availability
No datasets were generated or analysed during the current study.
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
Cheng HT, Xu X, Lim PS, Hung KY. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care. 2021;44(1):89–97.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80.
Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, Guerrero-Hue M, García-Caballero C, Vázquez-Carballo C, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci. 2020;21:3798.
Petersen MC, Gallop MR, Flores Ramos S, Zarrinpar A, Broussard JL, Chondronikola M, et al. Complex physiology and clinical implications of time-restricted eating. Physiol Rev. 2022;102(4):1991–2034.
Benjamin JI, Pollock DM. Current perspective on circadian function of the kidney. Am J Physiol Ren Physiol. 2023 Dec 22.
Kolbe I, Brehm N, Oster H. Interplay of central and peripheral circadian clocks in energy metabolism regulation. J Neuroendocrinol. 2019;31:e12659.
Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, et al. Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607–608:1073–84.
Mohebbi I, Shateri K, Seyedmohammadzad M. The relationship between working schedule patterns and the markers of the metabolic syndrome: comparison of shift workers with day workers. Int J Occup Med Environ Health. 2012;25(4):383–91.
Puttonen S, Härmä M, Hublin C. Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36(2):96–108.
Oza MJ, Laddha AP, Gaikwad AB, Mulay SR, Kulkarni YA. Role of dietary modifications in the management of type 2 diabetic complications. Pharmacol Res. 2021;168:105602.
Afsar B, Afsar RE, Copur S, Sag AA, Ortiz A, Kanbay M. The effect of energy restriction on development and progression of chronic kidney disease: review of the current evidence. Br J Nutr. 2021;125:1201–14.
Makarem N, Sears DD, St-Onge MP, Zuraikat FM, Gallo LC, Talavera GA, et al. Habitual nightly fasting duration, eating timing, and eating frequency are associated with cardiometabolic risk in women. Nutrients. 2020;12:3043.
Wirth MD, Zhao L, Turner-McGrievy GM, Ortaglia A. Associations between fasting duration, timing of first and last meal, and cardiometabolic endpoints in the National Health and Nutrition Examination Survey. Nutrients. 2021;13:2686.
Palomar-Cros A, Andreeva VA, Fezeu LK, Julia C, Bellicha A, Kesse-Guyot E, et al. Dietary circadian rhythms and cardiovascular disease risk in the prospective NutriNet-Santé cohort. Nat Commun. 2023;14(1):7899.
Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–15.
Cho Y, Hong N, Kim KW, Cho SJ, Lee M, Lee YH, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. 2019;8:1645.
Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database Syst Rev Implement Rep. 2018;16:507–47.
Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–e12213.
Bernardes da Cunha N, Teixeira GP, Madalena Rinaldi AE, Azeredo CM, Crispim CA. Late meal intake is associated with abdominal obesity and metabolic disorders related to metabolic syndrome: a chrononutrition approach using data from NHANES 2015–2018. Clin Nutr. 2023;42:1798–805.
Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat 2. 2012;155:1–39.
Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fbrosis in patients with type 2 diabetes. Metabolism. 2021;121:154752.
Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, non-obese and lean NAFLD in the United States, 1999–2016. J Intern Med. 2020;288:139–51.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–9.
Ruggenenti P, Abbate M, Ruggiero B, Rota S, Trillini M, Aparicio C, et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes. 2017;66:75–86.
Dong D, Cai GY, Ning YC, Wang JC, Lv Y, Hong Q, et al. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget. 2017;8:16109–21.
Ruggenenti P, Cortinovis M, Trillini M, Parvanova A, Abbate M, Satriano C, et al. Long-term kidney and systemic effects of calorie restriction in overweight or obese type 2 diabetic patients (C.Re.S.O. 2 randomized controlled trial). Diabetes Res Clin Pract. 2022;185:109804.
Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in diabetic kidney disease. Nephron. 2019;143(1):12–6.
Shimizu H, Hanzawa F, Kim D, Sun S, Laurent T, Umeki M, et al. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE. 2018;13:e0206669.
Kim D, Hanzawa F, Sun S, Laurent T, Ikeda S, Umeki M, et al. Delayed meal timing, a breakfast skipping model, increased hepatic lipid accumulation and adipose tissue weight by disintegrating circadian oscillation in rats fed a high-cholesterol diet. Front Nutr. 2021;8:681436.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obes (Silver Spring). 2009;17:2100–2.
Kuroda H, Tahara Y, Saito K, Ohnishi N, Kubo Y, Seo Y, et al. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci Rep. 2012;2:711.
Ni Y, Wu L, Jiang J, Yang T, Wang Z, Ma L, et al. Late-night eating-induced physiological dysregulation and circadian misalignment are accompanied by microbial dysbiosis. Mol Nutr Food Res. 2019;63:e1900867.
Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obes (Silver Spring). 2019;27:724–32.
Jones R, Pabla P, Mallinson J, Nixon A, Taylor T, Bennett A, et al. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr. 2020;112:1015–28.
Charlot A, Hutt F, Sabatier E, Zoll J. Beneficial effects of early time-restricted feeding on metabolic diseases: importance of aligning food habits with the circadian clock. Nutrients. 2021;13(5):1405.
Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–41.
Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32:S13–8.
Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–34.
Qian J, Scheer FAJL. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–93.
Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005.
Raji-Amirhasani A, Khaksari M, Soltani Z, Saberi S, Iranpour M, Darvishzadeh, et al. Beneficial effects of time and energy restriction diets on the development of experimental acute kidney injury in rat: Bax/Bcl-2 and histopathological evaluation. BMC Nephrol. 2023;24(1):59.
Raji-Amirhasani A, Khaksari M, Shahrokhi N, Soltani Z, Nazari-Robati M, Darvishzadeh Mahani F, et al. Comparison of the effects of different dietary regimens on susceptibility to experimental acute kidney injury: the roles of SIRT1 and TGF-β1. Nutrition. 2022;96:111588.
Rojas-Morales P, León-Contreras JC, Granados-Pineda J, Hernández-Pando R, Gonzaga G, Sánchez-Lozada LG, et al. Protection against renal ischemia and reperfusion injury by short-term time-restricted feeding involves the mitochondrial unfolded protein response. Free Radic Biol Med. 2020;154:75–83.
Sims BM, Goodlett BL, Allbee ML, Pickup EJ, Chiasson VL, Arenaz CM, et al. Time restricted feeding decreases renal innate immune cells and blood pressure in hypertensive mice. J Hypertens. 2022;40(10):1960–8.
Lao BN, Luo JH, Xu XY, Fu LZ, Tang F, Ouyang WW, et al. Time-restricted feeding’s effect on overweight and obese patients with chronic kidney disease stages 3–4: a prospective non-randomized control pilot study. Front Endocrinol (Lausanne). 2023;14:1096093.
Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond). 1989;77:105–11.
Koopman MG, Krediet RT, Koomen GC, Strackee J, Arisz L. Circadian rhythm of proteinuria: consequences of the use of urinary protein:creatinine ratios. Nephrol Dial Transpl. 1989;4:9–14.
Olaoye OA, Masten SH, Mohandas R, Gumz ML. Circadian clock genes in diabetic kidney disease (DKD). Curr Diab Rep. 2019;19(7):42.
Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obes (Silver Spring). 2019;27:1244–54.
Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11:2854.
Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O’Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013;16:2073-82.
Lee TS, Kim JS, Hwang YJ, Park YC. Habit of eating breakfast is associated with a lower risk of hypertension. J Lifestyle Med. 2016;6(2):64–7.
Ballon A, Neuenschwander M, Schlesinger S. Breakfast skipping is associated with increased risk of type 2 diabetes among adults: a systematic review and meta-analysis of prospective cohort studies. J Nutr. 2019;149:106–13.
Uzhova I, Fuster V, Fernández-Ortiz A, Ordovás JM, Sanz J, Fernández-Friera L, et al. The importance of breakfast in atherosclerosis disease: insights from the PESA study. J Am Coll Cardiol. 2017;70:1833–42.
Gahm C, Park S. The association between skipping breakfast and chronic kidney disease. Int Urol Nephrol. 2023;55:3209–15.
Acknowledgements
The authors of this study thank the participants of the NHANES and the NHANES staff.
Funding
The authors declare no conflict or competing interest. The study was supported by the Medical Research Project of Health Commission of Nantong (MB2020012, QNZ2022019, MS2022018, MB2021012, JCZ21099, MS2023032) and the Science and Technology Support Program of Nantong (HS2020005, HS2022004, MS22021008, JC2021118).
Author information
Authors and Affiliations
Contributions
CL and XC participated in the design of the study, analysis of the data, and drafting of the manuscript. XW, LZ and FX conceived of the study, participated in its design and revised the manuscript. WL, LW, and HH participated in extracting, merging and cleaning data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The institutional review board approved the NHANES protocol of the Centers for Disease Control and Prevention (CDC), and each participant provided written informed consent.
The authors of this study thank the participants of the NHANES and the NHANES staff.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Cf., Cang, Xm., Liu, Ws. et al. A late eating midpoint is associated with increased risk of diabetic kidney disease: a cross-sectional study based on NHANES 2013–2020. Nutr J 23, 39 (2024). https://doi.org/10.1186/s12937-024-00939-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00939-z