Abstract
Purpose
To investigate whether adherence to the Dutch Healthy Diet index 2015 (DHD15-index) is associated with change in glycemic control and cardio-metabolic markers over two-year follow-up in people with type 2 diabetes (T2D).
Methods
This prospective cohort study included 1202 individuals with T2D (mean age 68.7 ± 9.0 years; 62.5% male; mean HbA1c 53.8 ± 11.7 mmol/mol) from the Diabetes Care System cohort. Baseline dietary intake was assessed using a validated food frequency questionnaire, and adherence to the DHD15-index was estimated (range 0–130). HbA1c, fasting glucose, blood lipids (HDL and LDL cholesterol, cholesterol ratio), blood pressure, estimated glomerular filtration rate (eGFR), and BMI were measured at baseline, and after one- and two-year follow-up. Linear mixed model analyses were conducted to examine the associations between adherence to the DHD15-index and glycemic control and the cardio-metabolic outcomes, adjusting for energy intake, sociodemographic and lifestyle characteristics, and medication.
Results
Highest adherence (T3) to the DHD15-index was not associated with change in HbA1c, compared to lowest adherence (T1) [βT3vsT1: 0.62 mmol/mol (− 0.94; 2.19), Ptrend = 0.44]. There was a non-linear association with fasting glucose, where moderate adherence (T2) was associated with a decrease in fasting glucose [βT2vsT1: − 0.29 mmol/L (− 0.55; − 0.03), Ptrend = 0.30]. Higher adherence to the DHD15-index was associated with a decrease in BMI [β10point: − 0.41 kg/m2 (− 0.60; − 0.21), Ptrend < 0.001], but not with blood lipids, blood pressure or kidney function.
Conclusion
In this well-controlled population of people with T2D, adherence to the DHD15-index was associated with a decrease in BMI, but not with change in glycemic control or other cardio-metabolic parameters.
Similar content being viewed by others
Introduction
It is important to improve diabetes management to prevent comorbidities and complications among people with type 2 diabetes (T2D), such as cardiovascular disease, nephropathy, retinopathy and neuropathy [1]. Optimal glycemic control and cardio-metabolic health are therefore essential [2, 3], which can be achieved by lifestyle factors, including a healthy diet [1]. Studies have shown that adequate dietary management is associated with a reduction in hemoglobin A1c (HbA1c) levels of 11.0–22.0 mmol/mol [4].
Specifically, diets high in vegetables, fruits, legumes, nuts, poultry and vegetable oil, and low in solid fats, are associated with lower mortality rates in people with T2D [5], intake of low glycemic index fruits, nuts, whole grain products, and dairy is associated with improved glycemic control [6,7,8,9], and intake of low glycemic index fruits, nuts and wholegrain products with improved cardio-metabolic markers in people with T2D [6, 8, 10]. Systematic reviews of randomized controlled trials (RCTs) on dietary patterns, such as the Mediterranean diet score, have shown beneficial effects of the Mediterranean diet on glycemic control and cardio-metabolic markers in Western populations, reducing HbA1c by 1.1–6.6 mmol/mol [11,12,13,14], fasting glucose by 0.4–2.2 mmol/L [11, 13], low-density lipoprotein (LDL) cholesterol non-significantly by 0.08–0.19 mmol/L, increasing high-density lipoprotein (HDL) cholesterol by 0.04–0.09 mmol/L [11, 14, 15], and reducing systolic blood pressure (SBP) by 1.45 mm Hg, diastolic blood pressure (DBP) by 1.41 mm Hg, and body mass index (BMI) by 0.29 kg/m2 [11]. The Mediterranean diet however cannot directly be translated to the Dutch dietary behavior.
In 2015, the Dutch Health Council developed novel dietary guidelines largely consistent with the Mediterranean diet, focusing on food groups rather than nutrients, applicable to the Dutch general population and easy to understand and implement [16], aimed at facilitating adherence [17]. To evaluate adherence to the Dutch dietary guidelines, the Dutch Healthy Diet index of 2015 (DHD15-index) has been developed [18]. Higher adherence to the DHD15-index is associated with lower T2D incidence and lower all-cause mortality in the Netherlands [19, 20]. Dietary guidelines for people with T2D also mainly advocate a dietary pattern in line with the Dutch Healthy Diet guidelines and Mediterranean diet [21]. However, to date, no studies investigated the association between adherence to the DHD15-index and glycemic control or cardio-metabolic markers in people with T2D. Moreover, to our knowledge, no prior studies prospectively examined the association between dietary patterns and glycemic control or cardio-metabolic markers in people with T2D.
Therefore, the aim of this study was to investigate whether adherence to the DHD15-index was associated with change in glycemic control and cardio-metabolic markers over one- and two-year follow-up in people with T2D.
Methods
Study design and population
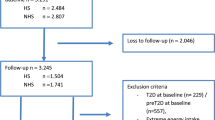
This study was embedded in the Diabetes Care System cohort (DCS), a prospective dynamic patient cohort including nearly all people with T2D with GPs located in the West-Friesland region of the Netherlands [22], consisting of ~ 15,000 persons in 2020. Participants visit the care center annually for monitoring visits during which routine measurements are performed. Between June 2017 and June 2018, 3592 individuals were invited to participate in a sub-study, of which 1549 participated (43%) (Fig. 1). Inclusion criteria were T2D diagnosis based on either having at least one classic T2D symptom (polyuria, polydipsia, polyphagia, unintended weight loss, pruritus) together with elevated blood glucose levels (fasting glucose ≥ 7.0 mmol/L, or random glucose ≥ 11.1 mmol/L), or, in case of no symptoms, having two or more elevated blood glucose levels on two separate occasions [22], and ability to provide informed consent. Routine measurements data were collected from the medical records during the annual visit, and for the present study additional data on dietary intake, lifestyle, behavior and health were obtained using questionnaires, and data on physical activity using accelerometers. The study was approved by the Ethical Review Committee of the VU University Medical Center in Amsterdam. All participants provided written informed consent.
Dietary assessment
Dietary intake was assessed at baseline using a self-reported 160-item food frequency questionnaire (FFQ): the FFQ-NL 1.0 [23]. This FFQ was specifically developed for Dutch observational studies, and validated against 24-h dietary recalls (on average 2.7 per person) and biomarkers in 24-h blood and urine. The FFQ was found to have a moderate validity in ranking individuals according to micronutrients, macronutrients and energy, and food groups, with median correlation coefficients of 0.30, 0.39 and 0.30, respectively [23]. The FFQ-NL 1.0 contains questions on frequency (times per month/week), portion size (standardized household measures) and preparation methods regarding 160 food items. The reference period for the FFQ was one year. Average daily intakes per food item (g/day) were derived and categorized into DHD15-index food groups.
Dutch healthy diet index
The DHD15-index comprises fifteen components, divided over five component types: adequacy, moderation, optimum, ratio and quality [18]. An overview of the calculation methods, cut-off and threshold values per component is provided in Online Resource 1, details are described elsewhere [18].
The adequacy components are vegetables, fruits, wholegrain products, legumes, nuts, fish and tea. In this component, a higher intake means a higher score (better diet quality), and intakes beyond the prescribed cut-off value are assigned 10 points (maximum score). The score is calculated by dividing the intake by the cut-off value and multiplying it by 10. Adequacy score = \(\frac{\mathrm{intake}}{\mathrm{cut}-\mathrm{off \: value}}*10\). The moderation components are red meat, processed meat, sugar-sweetened beverages, fruit juices, alcohol, and sodium, for which a lower intake means a higher score, and intakes below a prescribed threshold value are assigned 10 points. The score is calculated by taking the ratio between the intake minus the cut-off value and the threshold minus the cut-off value, multiplying it by 10 and subtracting it from 10. Moderation score = \(10- \frac{\mathrm{intake }-\mathrm{ cut}-\mathrm{off \: value}}{\mathrm{threshold \: value }-\mathrm{ cut}-\mathrm{off \: value}} *10\). For the optimum component, including dairy products, intakes between a specified optimal range are assigned the highest score. The score for intakes below the lower cut-off value of the optimal range is calculated by dividing the intake by the lower cut-off value and multiplying this ratio by 10. Optimum score lower intakes = \(\frac{\mathrm{intake}}{\mathrm{cut}-\mathrm{off \: value}} *10\). The score for intakes between the higher cut-off value of the optimal range and the threshold value is calculated by subtracting the cut-off value from the intake and dividing this by the difference between the threshold and cut-off value, multiplying it by 10 and subtracting it from 10. Optimum score higher intakes = \(10- \frac{\mathrm{intake }-\mathrm{ cut}-\mathrm{off \: value}}{\mathrm{threshold \: value}-\mathrm{cut}-\mathrm{off \: value}} *10\). In the ratio component, containing fats/oils and refined/wholegrain products, a higher healthy/unhealthy ratio is assigned a higher score, and ratios above the specified cut-off value are assigned 10 points. The score is calculated by subtracting the threshold value from the healthy/unhealthy ratio, and dividing this by the difference between the cut-off and threshold value. Ratio score = \(\frac{\mathrm{intake \: ratio }-\mathrm{ threshold \: value}}{\mathrm{cut}-\mathrm{off \: value}-\mathrm{ threshold \: value}}\). Since wholegrain products are included both as adequacy and ratio component, a score of 0 (minimum score) to 5 (maximum score) is assigned within each component, creating one total wholegrain products score. Lastly, in the quality component, comprising coffee, the recommended product within a particular type of product is assigned the highest score. In this case, no or filtered coffee consumption is assigned 10 points, and unfiltered coffee consumption 0 points. The FFQ used for the present study could not distinguish between unfiltered and filtered coffee and could not estimate sodium intake, these components were therefore excluded from the DHD15-index.
Each component received a score of 0–10 points. The final DHD15-index score is calculated by summing all components, generating scores ranging from 0 (lowest adherence) to 130 (highest adherence). For the analyses, the DHD15-index was used both as tertiles and continuously per 10 point increase.
Outcome assessment
The primary outcomes were HbA1c and fasting glucose levels, measured at baseline and after one and two years of follow-up. Fasting blood samples were drawn during the annual monitoring visits, after an overnight fast. HbA1c levels (percentage and mmol/mol) were assessed through the turbidimetric inhibition immunoassay for hemolyzed whole EDTA blood (Cobas c501, Roche Diagnostics, Mannheim, Germany). Fasting glucose levels (mmol/L) were assessed in fluorinated plasma with the UV-hexokinase test (Cobas c501, Roche Diagnostics) [22].
Secondary outcomes included HDL cholesterol, LDL cholesterol, cholesterol ratio, SBP, DBP, eGFR, and BMI, also measured at baseline and after one and two years of follow-up. Both HDL and LDL cholesterol levels were derived from fasting blood samples, HDL cholesterol levels were established enzymatically (Cobas c501, Roche Diagnostics), and LDL cholesterol levels were calculated according to the formula: \(LDL \,cholesterol=total \,cholesterol-HDL \,cholesterol-0.45*triglycerides\). The cholesterol ratio was calculated by dividing total cholesterol by HDL cholesterol. SBP and DBP were measured in duplicate per visit using an oscillometric device (Welch Allyn ProBP 3400, Skaneateles Falls, New York, USA), of which the averages were taken. The eGFR was measured using an overnight first morning urine sample, and calculated by means of the Modification of Diet in Renal Disease (MDRD) equation [24]. BMI was calculated by dividing body weight (kilograms) by height (meters) squared, measured barefoot and in light clothing.
Covariates
Sociodemographic characteristics were retrieved from self-reported general questionnaires. Age was determined based on date of birth (baseline and follow-up). Diabetes duration was calculated from medical records, by subtracting the diagnosis date from the annual visit date. Self-reported education was categorized into low (no completed education, primary education, secondary education (practical training)), middle (pre-vocational secondary education, vocational training, general secondary education or pre-university education), or high (professional university education, university). Employment status was categorized into employed (paid work, volunteer work) or unemployed/retired (unemployed, retired). Smoking status was categorized as current, former or never smoker. Physical activity was measured at baseline using accelerometers (ActiGraph GT3X, worn on the hip for 8 days) and reported as total hours of physical activity per week. Glucose-lowering medication was obtained from medication labels during the annual visits (baseline and follow-up), and categorized into no medication, one oral hypoglycemic agent (ATC code A10B), ≥ two oral hypoglycemic agents, or insulin (ATC code A10A)/combination therapy (oral hypoglycemic agents + insulin). Lipid-lowering and antihypertensive medication were also retrieved from medication labels during the visits and were dichotomous (no medication, medication).
Statistical analysis
Baseline characteristics were presented as mean ± SD, median (IQR), or proportions (n (%)), as appropriate based on unit and normality, for the total population and by tertiles of DHD15 adherence at baseline. Missing data in covariates (30.3% of participants had missing values for one or more covariates, ranging from 0.2% missing for smoking status and BMI, to 24.7% for physical activity) were imputed using multiple imputation, with five sets of imputed data based on predictive mean matching, and pooled according to Rubin’s rules [25].
Separate linear mixed models for repeated measures were used to examine the association between adherence to the DHD15-index at baseline and change in HbA1c (%), HbA1c (mmol/mol), fasting glucose, HDL cholesterol, LDL cholesterol, cholesterol ratio, SBP, DBP, eGFR and BMI over two years of follow-up. Unstandardized regression coefficients and 95% confidence intervals were presented per tertile (lowest tertile reference), representing the change in cardio-metabolic parameter over two years of follow-up from the moderate and highest tertile of adherence relative to the lowest tertile, and per 10 point increase in adherence to the index. Linearity across tertiles was assessed by adding the categorical DHD15-index variable as a linear term to the model. Non-linearity was explored by adding a quadratic DHD15-index term, which was not significant in any of the models.
For each outcome, four models were created (except for eGFR, where three models were created, and BMI, where two models were created). The first model was adjusted for age, sex and total energy intake. The second model was additionally adjusted for education, employment status, smoking, and physical activity. BMI was additionally added in a separate model (model 3) because of its potential role as mediator [26]. Finally, a fourth model was constructed where, depending on the outcome, glucose-lowering, lipid-lowering or antihypertensive medication was added to model 2, because these medications strongly affect glycemic control [27], blood lipids [28] and blood pressure [29], respectively. Confounders were added based on theory and previous literature. Age, sex, current smoking status and diabetes duration were checked for effect modification in model 2 by testing interaction. In case the interaction term was significant (p < 0.05), analyses were stratified. Each model included adherence to the DHD15-index at baseline and the confounding variables as fixed effects, whereas participant ID was treated as a random effect to account for potential within-participant correlations among the repeated measures of the cardio-metabolic parameter over follow-up. Likelihood ratio tests were conducted to compare model fit of the model including a random intercept on participant ID with the model additionally including a random slope. In case of significant improvement in model fit, the model including the random slope was used for analyses. To control the type I error rate, multiple testing was statistically accounted for using the post hoc Bonferroni correction [30].
We conducted a sensitivity analysis comparing baseline characteristics of the excluded participants with those of the included participants, to evaluate whether selection bias occurred. Moreover, complete case analyses including participants with complete data on all covariates and restricting to participants with both follow-up measurements, were conducted as sensitivity analyses, to assess the impact of data imputation and missing follow-up data. Additionally, we performed a sensitivity analysis excluding potential energy under-reporters (those with a reported energy intake to basal metabolic rate ratio below the lower confidence interval limit corresponding to their physical activity level, ranging from 0.68 to 1.24) according to the Goldberg method [31,32,33], to evaluate whether desire for social approval caused underreporting of discretionary foods, causing measurement error and differential misclassification, leading to underestimations. Lastly, sensitivity analyses excluding alcohol from the DHD15-index were conducted, to assess the impact of potential misclassification in the scoring of alcohol intake conforming to the DHD15-index, as this scoring is not entirely in line with previous studies observing a dose–response relationship between alcohol consumption and HDL cholesterol [34], and a J-shaped association between alcohol consumption and glycemic control [35] and eGFR decline [36, 37].
To evaluate associations, a significance level of p < 0.05 was used (p < 0.0045 after Bonferroni correction based on 11 comparisons). All statistical analyses were conducted using SPSS Statistics 26.
Results
For the current study, we excluded participants with invalid (n = 35) or missing (n = 244) data on dietary intake, or with extreme energy intake (top and bottom 0.5%) (n = 12). In addition, we excluded participants with missing HbA1c (n = 56) or fasting glucose (n = 89) data at baseline or both follow-up measurements (Fig. 1). The same procedure was applied for missing data in the secondary outcomes: HDL cholesterol (n = 56), LDL cholesterol (n = 57), cholesterol ratio (n = 56), SBP (n = 54), DBP (n = 54), estimated glomerular filtration rate (eGFR) (n = 55), and BMI (n = 56).
Baseline characteristics
Baseline characteristics in total and stratified by DHD15-index tertiles are presented in Table 1. The participants were on average 68.7 ± 9.0 years old, 62.5% were male. The mean score for adherence to the DHD15-index at baseline was 72.0 ± 14.9. Adherence was highest for red meat and legume guidelines (respectively 75.0% and 74.5% full adherence), and lowest for processed meat and grain guidelines (respectively 1.2% and 3.3% full adherence). None of the participants fully adhered to all guidelines. Highest adherers (T3; score 78.4–116.1) were more often female, higher educated, employed, non-smoking, more physically active, and had a lower BMI and total energy intake, compared to lowest adherers (T1; score 21.0–65.6). Participants had a mean HbA1c level of 53.8 ± 11.7 mmol/mol at baseline, with a mean change of 3.4 ± 11.1 after two years of follow-up, and a mean fasting glucose level of 8.6 ± 2.1 mmol/L at baseline, with a mean change of 0.7 ± 2.5, indicating that the participants are well-controlled (Table 2). There were no major differences in baseline values across the tertiles. Baseline characteristics of the excluded participants did not differ substantially from those of the included participants (Online Resource 2).
Primary outcomes
No random slopes were included in any of the primary models. In the model adjusted for age, sex, and total energy intake, highest adherence (T3) to the DHD15-index at baseline was not associated with change in HbA1c, compared to lowest adherence (T1) (model 1; T3vsT1: β = 0.37 mmol/mol [95% CI = − 1.16; 1.90]), as was the case for the fully adjusted model (model 2; T3vsT1: β = 0.62 mmol/mol [95% CI = − 0.94; 2.19]) (Table 3). No significant linear trend was found (Ptrend = 0.44). Additional adjustment for BMI amplified the association (model 3; T3vsT1: β = 1.10 mmol/mol [95% CI = − 0.47; 2.68]), whereas additional adjustment for glucose-lowering medication attenuated the association slightly (model 4; T3vsT1: β = 0.58 mmol/mol [95% CI = − 0.76; 1.92]). For comprehensibility, Online Resource 3 contains the HbA1c results in percentage units.
For changes in fasting glucose in all models, moderate adherence showed a significant decrease in fasting glucose, compared to lowest adherence (model 2; T2vsT1: β = − 0.29 mmol/L [95% CI = − 0.55; − 0.03]), and highest adherence pointed in the same direction (model 2; T3vsT1: β = − 0.14 mmol/L [95% CI = − 0.41; 0.13]), albeit not significant. No significant linear trend was observed (Ptrend = 0.30). Additional adjustment for BMI slightly attenuated the association (model 3; T3vsT1: β = − 0.09 mmol/L [95% CI = − 0.36; 0.18]), while additional adjustment for glucose-lowering medication did not alter the association (model 4; T3vsT1: β = − 0.14 mmol/L [95% CI = − 0.39; 0.11]).
The complete case analyses (n = 608) (Online Resource 4), the analyses excluding under-reporters (excluding n = 123) (Online Resource 5), and the analyses excluding alcohol from the DHD15-index (n = 1202) (Online Resource 6) all yielded similar results to the main analyses. Bonferroni correction for multiple testing did not lead to different conclusions.
Cardio-metabolic outcomes
A random slope on sex was included in the DBP analyses. Significant effect modification by sex was found for the HDL cholesterol analyses (p = 0.03). Stratification by sex showed that, although not significant, directionality of the association was different for men and women (Table 3). In men, highest adherence to the DHD15-index at baseline pointed toward a decrease in HDL cholesterol, compared to lowest adherence (model 2; T3vsT1: β = − 0.01 mmol/L [95% CI = − 0.07; 0.04], Ptrend = 0.58), while among women the association pointed toward an increase in HDL cholesterol (model 2; T3 vs T1: β = 0.08 mmol/L [95% CI = − 0.01; 0.17], Ptrend = 0.06). Higher adherence was not associated with changes in LDL cholesterol (model 2; T3vsT1: β = 0.04 mmol/L [95% CI = − 0.08; 0.15], Ptrend = 0.54), or the cholesterol ratio (model 2; T3 vs T1: β = − 0.06 [95% CI = − 0.22; 0.10], Ptrend = 0.44). In addition, no association was observed between higher adherence to the DHD15-index at baseline and SBP (model 2; T3vsT1: β = − 1.17 mm Hg [95% CI = − 3.60; 1.25], Ptrend = 0.34), or DBP (model 2; T3vsT1: β = − 0.65 mm Hg [95% CI = − 1.64; 0.34], Ptrend = 0.20). Higher adherence also was not associated with changes in eGFR (model 2; T3vsT1: β = 1.74 ml/min [95% CI = − 0.76; 4.25], Ptrend = 0.18). Finally, higher adherence to the DHD15-index at baseline was associated with a decrease in BMI (model 2; 10 point: β = − 0.41 kg/m2 [95% CI = − 0.60; − 0.21], Ptrend < 0.001).
The complete case analyses (Online Resource 4), the analyses excluding under-reporters (Online Resource 5), and the analyses excluding alcohol from the DHD15-index (Online Resource 6) all showed similar results to the main analyses. Application of the Bonferroni correction did not alter the results.
Discussion
This study aimed to investigate the association between adherence to the DHD15-index at baseline and change in glycemic control and cardio-metabolic markers over one and two years of follow-up in people with T2D being well-controlled (medically monitored) in the Diabetes Care System. Higher adherence to the DHD15-index was associated with a lower BMI, and with a higher HDL cholesterol only in women. There was no association between adherence and changes in HbA1c, FPG, LDL cholesterol, cholesterol ratio, SBP, DBP or eGFR.
Our results regarding glycemic control seem to contradict RCTs investigating the effect of adherence to the Mediterranean diet, which is comparable to the DHD15-index, on cardio-metabolic parameters in people with T2D. These studies showed that adherence to the Mediterranean diet reduced HbA1c by 1.1–6.6 mmol/mol [11,12,13,14], and fasting glucose by 0.4–2.2 mmol/L [11, 13], after follow-up periods ranging from four weeks to four years. Our study observed no association between highest adherence to the DHD15-index at baseline and change in HbA1c or fasting glucose, compared to lowest adherence. The observational design of our study may have limited us from finding an association, as opposed to these previous RCTs. RCTs implement interventions in controlled settings, leading to stronger effects in shorter time periods than observational studies conducted in real-life settings. Regarding previous observational studies, one cross-sectional study observed no association between adherence to the Mediterranean diet and odds of having a high HbA1c level (≥ 53 mmol/mol) [38], similar to our findings, while two cross-sectional studies observed a significant association between a high adherence to the Mediterranean diet and respectively 1.1 and 9.9 mmol/mol lower mean HbA1c levels, compared to a low adherence [39, 40]. An explanation for these inconsistent findings among previous cross-sectional studies could be that with a mean age of 69 years versus 62 and 58 years, respectively, and mean HbA1c levels of 52 mmol/mol versus 61 and 68 mmol/mol, participants in the former cross-sectional study were better controlled than those in the latter two studies and thereby showed weaker associations. The deviation in results between our study and the latter two cross-sectional studies may be explained by the study design. Within-person changes in longitudinal studies are more subtle than between-persons comparisons in cross-sectional designs. Furthermore, the discrepancies in food processing and preparation methods between the Netherlands and the countries where the previous studies were conducted (i.e., Spain, Italy, Greece, Israel, the United States, and Australia), and the differences between the Dutch dietary guidelines and the Mediterranean diet, may cause a distorted view in this comparison [41]. Finally, and most importantly, our study population generally had diabetes for a long time (average of 12.8 ± 5.9 years at baseline) and were well-controlled in the Diabetes Care System. Therefore, they may have been less likely to have variations in their glycemic control and had less room for improvement.
For the secondary outcomes, we only observed a significant association between higher adherence to the DHD15-index at baseline and a 0.41 kg/m2 decrease in BMI. This finding is in line with prior RCTs observing that adherence to the Mediterranean diet reduced BMI by 0.29 kg/m2 [11]. We expected to observe beneficial impacts of a higher adherence to the DHD15-index at baseline on cholesterol levels and blood pressure based on previous RCTs indicating that adherence to the Mediterranean diet non-significantly decreased LDL cholesterol by 0.08–0.19 mmol/L, and significantly increased HDL cholesterol by 0.04–0.09 mmol/L [11, 14, 15], and decreased SBP by 1.45 mm Hg and DBP by 1.41 mm Hg [11], in individuals with T2D. In our study, we observed no association between adherence to the DHD15-index at baseline and LDL cholesterol, SBP or DBP, although higher adherence was associated with higher HDL cholesterol, but only among women. A potential explanation for this deviation could, just as for our glycemic control results, lie within the observational longitudinal study design with only subtle changes in cardio-metabolic outcomes over time, that may require a longer time period to translate into significant improvement in cardiometabolic risk factors. Additionally, the observed association with HDL in women and close to significant association with HDL in men could be explained by the loss of statistical power after stratifying the study population. Moreover, we expected to observe a positive association between adherence to the DHD15-index and eGFR. One previous 15-year observational study concluded that a one point increase in Mediterranean diet score was associated with a 25% lower odds of rapid eGFR decline in participants without T2D after seven years of follow-up, but observed no association in participants with T2D [42]. Another two-year RCT found that adherence to the Mediterranean diet increased eGFR by 4.5% in participants without diabetes and by 6.7% in participants with diabetes, after two-year compliance [43]. These results are similar to ours: a non-significant 6.0 ml/min (8.1%) increase in eGFR in individuals with T2D in the highest tertile of adherence to the DHD15-index after two years of follow-up.
Strengths of the current study include the prospective two-year design with annual measurements, enabling the study to investigate temporality between exposure and outcome, the use of a population-based patient cohort, increasing the generalizability of the results to other populations with T2D, and the ability to adjust for many confounders. Moreover, contrary to previous studies, the present study was conducted two years after the implementation of the novel Dutch dietary guidelines in 2015. Participants therefore had the possibility to be aware of the guidelines and to adjust their diet accordingly. Nevertheless, some limitations should be considered. One limitation is that the cohort mainly consisted of Western European participants, and therefore the results cannot directly be generalized to other populations. Additionally, as in any observational study, our study could have suffered from (residual) confounding [44]. Furthermore, the FFQ was not specifically designed to measure DHD15-index food groups, which prevented (accurate) measurement of coffee and sodium, and led to the exclusion of these food groups from the DHD15-index, possibly affecting the results. However, although unfiltered coffee consumption, contrary to filtered coffee consumption, elevates total and LDL cholesterol levels [45], most coffee consumed in the Netherlands is filtered [46], indicating that there would be little variation among participants and therefore little impact on the results. Exclusion of sodium may have affected the SBP and DBP results, as excessive sodium intake is associated with increased blood pressure [47]. However, adding sodium intake to the FFQ probably would not have increased the validity of the results because FFQs are not accurate to estimate sodium intake, as added salt at the table or cooking cannot be accurately self-reported. Specifically, a previous study pooling data from five validation studies showed that the correlation coefficient for the correlation of sodium intake measured by an FFQ with true intake was only 0.16, and that the average underreporting of sodium measured by FFQs was 28–39%, leading to underestimations [48]. Another study on the previous DHD-index representing adherence to the Dutch Healthy Diet guidelines of 2006, showed a mean sodium component score of 2.4 when the score was estimated using 24-h urine samples, a score of 3.5 when using 24-h recalls, and a score of 4.8 when using FFQs, indicating substantial underestimations of sodium intake measured by 24-h recalls and FFQs within the DHD-index [49]. Finally, participants completed the FFQ only at baseline, while dietary intake may have changed over the two years of follow-up, which might lead to misclassification. Nevertheless, a prior study investigating the reproducibility of a validated FFQ in the European Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort concluded that with 60–70% of the participants being re-assigned to the same/adjacent food group intake quintile after 5.7 years of follow-up, food group intake is reasonably stable over time [50]. Since the time period of our study is shorter with only two years of follow-up, the misclassification is likely to be lower than in the EPIC-Heidelberg study. However, the EPIC-Heidelberg cohort recruited participants from the general population as opposed to from diabetes centers [51]. Dietary habits may differ between general populations and populations of people with T2D [52], and therefore caution should be taken when generalizing these results to our study population. Nonetheless, with the T2D duration among our participants ranging from 5.0 to 50.2 years at baseline, none of our participants were at the very beginning of medical treatment. Hence, it is likely that our participants had already adjusted and stabilized their diets to match the dietary prescriptions before they participated in our study, and that their diets did not alter greatly during the two years of follow-up.
In future research, larger-scale, prospective population-based studies with longer follow-up periods evaluating the association between adherence to dietary patterns and glycemic control in people with T2D are warranted. Evidence resulting from these studies may contribute to creating future dietary guidelines and improve dietary practices and disease management in individuals with T2D. Moreover, future Dutch dietary guidelines should additionally consider the effects of the guidelines on renal function, as chronic kidney disease is a serious public health burden associated with increased risks of kidney failure, cardiovascular disease, poor quality of life and mortality [53, 54], which a healthy diet may help prevent [55]. We conclude that there is no motive to advise individuals with T2D to deviate from the Dutch dietary guidelines.
In conclusion, in this population of people with T2D being well-controlled (medically monitored) in the Diabetes Care System, higher adherence to the DHD15-index at baseline was associated with a decrease in BMI over two years. Despite subtle changes in cardio-metabolic risk factors, this did not translate into associations with change in glycemic control or other cardio-metabolic risk factors.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
International Diabetes Federation (2019) IDF diabetes atlas, 9th Edition.
Kitsios K, Tsapas A, Karagianni P (2011) Glycemia and cardiovascular risk: challenging evidence based medicine. Hippokratia 15(3):199–204
Schwartz SS (2013) Optimizing glycemic control and minimizing the risk of hypoglycemia in patients with type 2 diabetes. Drugs Context 2013:212255. https://doi.org/10.7573/dic.212255
Canadian Diabetes Association (2013) Nutrition therapy. Can J Diabetes 37(Suppl 1):S45-55. https://doi.org/10.1016/j.jcjd.2013.01.019
Sluik D, Boeing H, Li K, Kaaks R, Johnsen NF, Tjonneland A, Arriola L, Barricarte A, Masala G, Grioni S, Tumino R, Ricceri F, Mattiello A, Spijkerman AM, van der AD, Sluijs I, Franks PW, Nilsson PM, Orho-Melander M, Fharm E, Rolandsson O, Riboli E, Romaguera D, Weiderpass E, Sanchez-Cantalejo E, Nothlings U (2014) Lifestyle factors and mortality risk in individuals with diabetes mellitus: are the associations different from those in individuals without diabetes? Diabetologia 57(1):63–72. https://doi.org/10.1007/s00125-013-3074-y
Jenkins DJ, Srichaikul K, Kendall CW, Sievenpiper JL, Abdulnour S, Mirrahimi A, Meneses C, Nishi S, He X, Lee S, So YT, Esfahani A, Mitchell S, Parker TL, Vidgen E, Josse RG, Leiter LA (2011) The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes. Diabetologia 54(2):271–279. https://doi.org/10.1007/s00125-010-1927-1
Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, Jayalath VH, Augustin LS, Chiavaroli L, Leiter LA, de Souza RJ, Jenkins DJ, Sievenpiper JL (2014) Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE 9(7):e103376. https://doi.org/10.1371/journal.pone.0103376
Fujii H, Iwase M, Ohkuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Joudai T, Hirakawa Y, Uchida K, Sasaki S, Nakamura U, Kitazono T (2013) Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J 12:159. https://doi.org/10.1186/1475-2891-12-159
Pasin G, Comerford KB (2015) Dairy foods and dairy proteins in the management of type 2 diabetes: a systematic review of the clinical evidence. Adv Nutr 6(3):245–259. https://doi.org/10.3945/an.114.007690
Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D (2015) Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr 102(6):1347–1356. https://doi.org/10.3945/ajcn.115.110965
Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, Yu X (2015) Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr 69(11):1200–1208. https://doi.org/10.1038/ejcn.2014.243
Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D (2015) A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 5(8):e008222. https://doi.org/10.1136/bmjopen-2015-008222
Esposito K, Maiorino MI, Ceriello A, Giugliano D (2010) Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 89(2):97–102. https://doi.org/10.1016/j.diabres.2010.04.019
Ajala O, English P, Pinkney J (2013) Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 97(3):505–516. https://doi.org/10.3945/ajcn.112.042457
Neuenschwander M, Hoffmann G, Schwingshackl L, Schlesinger S (2019) Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Eur J Epidemiol 34(9):837–852. https://doi.org/10.1007/s10654-019-00534-1
Health Council of the Netherlands (2015) Dutch dietary guidelines 2015. Health Council of The Netherlands, The Hague
Bechthold A, Boeing H, Tetens I, Schwingshackl L, Nothlings U (2018) Perspective: food-based dietary guidelines in Europe-scientific concepts, current status, and perspectives. Adv Nutr 9(5):544–560. https://doi.org/10.1093/advances/nmy033
Looman M, Feskens EJ, de Rijk M, Meijboom S, Biesbroek S, Temme EH, de Vries J, Geelen A (2017) Development and evaluation of the Dutch healthy diet index 2015. Public Health Nutr 20(13):2289–2299. https://doi.org/10.1017/S136898001700091X
den Braver NR, Rutters F, van der Spek A, Ibi D, Looman M, Geelen A, Elders P, van der Heijden AA, Brug J, Lakerveld J, Soedamah-Muthu SS, Beulens JWJ (2019) Adherence to a food group-based dietary guideline and incidence of prediabetes and type 2 diabetes. Eur J Nutr. https://doi.org/10.1007/s00394-019-02064-8
Biesbroek S, Verschuren WMM, Boer JMA, van de Kamp ME, van der Schouw YT, Geelen A, Looman M, Temme EHM (2017) Does a better adherence to dietary guidelines reduce mortality risk and environmental impact in the Dutch sub-cohort of the European Prospective Investigation into Cancer and Nutrition? Br J Nutr 118(1):69–80. https://doi.org/10.1017/s0007114517001878
Dutch Diabetes Federation (2020) NDF diabetes dietary guidelines 2020.
van der Heijden AA, Rauh SP, Dekker JM, Beulens JW, Elders P, t Hart LM, Rutters F, van Leeuwen N, Nijpels G, (2017) The Hoorn Diabetes Care System (DCS) cohort. A prospective cohort of persons with type 2 diabetes treated in primary care in the Netherlands. BMJ Open 7(5):e015599. https://doi.org/10.1136/bmjopen-2016-015599
Sluik D, Geelen A, de Vries JH, Eussen SJ, Brants HA, Meijboom S, van Dongen MC, Bueno-de-Mesquita HB, Wijckmans-Duysens NE, van ’t Veer P, Dagnelie PC, Ocke MC, Feskens EJ, (2016) A national FFQ for the Netherlands (the FFQ-NL 1.0): validation of a comprehensive FFQ for adults. Br J Nutr 116(5):913–923. https://doi.org/10.1017/s0007114516002749
Levey AS, Stevens LA (2010) Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55(4):622–627. https://doi.org/10.1053/j.ajkd.2010.02.337
Rubin DB (1996) Multiple imputation after 18+ years. J Am Stat Assoc 91(434):473–489. https://doi.org/10.1080/01621459.1996.10476908
Park YM, Zhang J, Steck SE, Fung TT, Hazlett LJ, Han K, Ko SH, Merchant AT (2017) Obesity mediates the association between Mediterranean diet consumption and insulin resistance and inflammation in US adults. J Nutr 147(4):563–571. https://doi.org/10.3945/jn.116.243543
Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E (2020) Comparative effectiveness of glucose-lowering drugs for Type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 173(4):278–286. https://doi.org/10.7326/M20-0864
Pahan K (2006) Lipid-lowering drugs. Cell Mol Life Sci 63(10):1165–1178. https://doi.org/10.1007/s00018-005-5406-7
Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, Province MA, Rao DC (2005) A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens 18(7):935–942. https://doi.org/10.1016/j.amjhyper.2005.01.011
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. BMJ 310(6973):170. https://doi.org/10.1136/bmj.310.6973.170
Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM (1991) Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 45(12):569–581
Black AE (2000) Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 24(9):1119–1130. https://doi.org/10.1038/sj.ijo.0801376
Food and Agriculture Organization of the United Nations (2001) Human Energy Requirements: Report of a Joint FAO/WHO/ONU Expert Consultation. Food and Agriculture Organization of the United Nations, Rome, Italy
Minzer S, Losno RA, Casas R (2020) The effect of alcohol on cardiovascular risk factors: is there new information? Nutrients. https://doi.org/10.3390/nu12040912
Pietraszek A, Gregersen S, Hermansen K (2010) Alcohol and type 2 diabetes. A review. Nutrition, metabolism, and cardiovascular diseases. NMCD 20(5):366–375. https://doi.org/10.1016/j.numecd.2010.05.001
Park M, Lee SM, Yoon HJ (2019) Association between alcohol intake and measures of incident CKD: an analysis of nationwide health screening data. PLoS ONE 14(9):e0222123. https://doi.org/10.1371/journal.pone.0222123
Sato KK, Hayashi T, Uehara S, Kinuhata S, Oue K, Endo G, Kambe H, Fukuda K (2014) Drinking pattern and risk of chronic kidney disease: the kansai healthcare study. Am J Nephrol 40(6):516–522. https://doi.org/10.1159/000370051
Diez-Espino J, Buil-Cosiales P, Serrano-Martinez M, Toledo E, Salas-Salvado J, Martinez-Gonzalez MA (2011) Adherence to the Mediterranean diet in patients with type 2 diabetes mellitus and HbA1c level. Ann Nutr Metab 58(1):74–78. https://doi.org/10.1159/000324718
Vitale M, Masulli M, Calabrese I, Rivellese AA, Bonora E, Signorini S, Perriello G, Squatrito S, Buzzetti R, Sartore G, Babini AC, Gregori G, Giordano C, Clemente G, Grioni S, Dolce P, Riccardi G, Vaccaro O, Group TIS (2018) Impact of a Mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: a real-life study. Nutrients. https://doi.org/10.3390/nu10081067
Esposito K, Maiorino MI, Di Palo C, Giugliano D (2009) Adherence to a Mediterranean diet and glycaemic control in Type 2 diabetes mellitus. Diabet Med 26(9):900–907. https://doi.org/10.1111/j.1464-5491.2009.02798.x
Hoffman R, Gerber M (2013) Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: a critical appraisal. Nutr Rev 71(9):573–584. https://doi.org/10.1111/nure.12040
Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS (2014) The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol 9(11):1868–1875. https://doi.org/10.2215/cjn.01080114
Tirosh A, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Rudich A, Kovsan J, Fiedler GM, Bluher M, Stumvoll M, Thiery J, Stampfer MJ, Shai I (2013) Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care 36(8):2225–2232. https://doi.org/10.2337/dc12-1846
Hajian Tilaki K (2012) Methodological issues of confounding in analytical epidemiologic studies. Caspian J Intern Med 3(3):488–495
Jee SH, He J, Appel LJ, Whelton PK, Suh I, Klag MJ (2001) Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 153(4):353–362. https://doi.org/10.1093/aje/153.4.353
Health Council of the Netherlands (2015) Dutch Dietary Guidelines 2015—background document coffee. Publication no. A15/14. Health Council of the Netherlands, The Hague
Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS (2015) Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 65(10):1042–1050. https://doi.org/10.1016/j.jacc.2014.12.039
Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, Moshfegh AJ, Kipnis V, Arab L, Prentice RL (2015) Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol 181(7):473–487. https://doi.org/10.1093/aje/kwu325
van Lee L, Feskens EJM, van Huysduynen EJCH, de Vries JHM, van’t Veer P, Geelen A, (2013) The Dutch healthy diet index as assessed by 24 h recalls and FFQ: associations with biomarkers from a cross-sectional study. J Nutr Sci. https://doi.org/10.1017/jns.2013.28
Nagel G, Zoller D, Ruf T, Rohrmann S, Linseisen J (2007) Long-term reproducibility of a food-frequency questionnaire and dietary changes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br J Nutr 98(1):194–200. https://doi.org/10.1017/S0007114507691636
Boeing H, Korfmann A, Bergmann MM (1999) Recruitment procedures of EPIC-Germany. Ann Nutr Metab 43(4):205–215. https://doi.org/10.1159/000012787
Ewers B, Trolle E, Jacobsen SS, Vististen D, Almdal TP, Vilsboll T, Bruun JM (2019) Dietary habits and adherence to dietary recommendations in patients with type 1 and type 2 diabetes compared with the general population in Denmark. Nutrition 61:49–55. https://doi.org/10.1016/j.nut.2018.10.021
Bello AK, Alrukhaimi M, Ashuntantang GE, Basnet S, Rotter RC, Douthat WG, Kazancioglu R, Kottgen A, Nangaku M, Powe NR, White SL, Wheeler DC, Moe O (2017) Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl 7(2):122–129. https://doi.org/10.1016/j.kisu.2017.07.007
van Westing AC, Kupers LK, Geleijnse JM (2020) Diet and kidney function: a literature review. Curr Hypertens Rep 22(2):14. https://doi.org/10.1007/s11906-020-1020-1
Ajjarapu AS, Hinkle SN, Li M, Francis EC, Zhang C (2019) Dietary patterns and renal health outcomes in the general population: a review focusing on prospective studies. Nutrients. https://doi.org/10.3390/nu11081877
Acknowledgements
We would like to thank the nurses at the Diabetes Care System for their recruitment support and the participants for their willingness to take part in this study.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
EB and NdB performed the analyses, interpreted the data and drafted the manuscript. NdB, JB and FR conceived the idea for the study and manuscript. AvdH, KBvdB, and NdB performed data collection and data cleaning. JB is the guarantor of this work. All authors read, provided major revisions and approved the final submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Diabetes Care System cohort was approved by the Medical Ethical Review Committee of VU University Medical Center, Amsterdam (Ethics Approval Number: 2017.027).
Consent to participate
All participants provided written informed consent.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bartels, E.C.M., den Braver, N.R., Borgonjen-van den Berg, K.J. et al. Adherence to the Dutch healthy diet index and change in glycemic control and cardiometabolic markers in people with type 2 diabetes. Eur J Nutr 61, 2761–2773 (2022). https://doi.org/10.1007/s00394-022-02847-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02847-6