Abstract
Background
The use of urban lowlands for agriculture contributes to the food security of city- dwellers, but promotes malaria transmission. The objective of the study was to characterize the entomological drivers of malaria transmission in two lowlands (N’Gattakro and Odiennekourani) in the city of Bouaké, Côte d’Ivoire.
Methods
The human landing catch technique was used to capture mosquitoes in houses located at the edge of two lowlands in Bouaké from February to December 2019. Cultivated surfaces were calculated monthly in both lowlands for each crop type (rice and market gardening) using images acquired by a drone. The different mosquito species were identified morphologically and by PCR analysis for the Anopheles gambiae complex. Anopheles infection by Plasmodium parasites was assessed by quantitative PCR. Mosquito diversity, biting behaviour and rhythmicity, and malaria transmission were determined in each lowland and compared.
Results
Anopheles gambiae sensu lato (s.l.) was predominant in N’Gattakro and Culex quinquefasciatus in Odiennekourani. Four Anopheles species were identified: An. gambiae s.l. and Anopheles funestus s.l. in both lowlands, Anopheles pharoensis in N’Gattakro, and Anopheles ziemanni in Odiennekourani. Within the An. gambiae complex, three species were caught: An. gambiae sensu stricto (s.s.), Anopheles coluzzii, and Anopheles arabiensis for the first time in Côte d’Ivoire (30.1%, 69.9% and 0% in N’Gattakro, and 45.1%, 52.6% and 2.4% in Odiennekourani, respectively). Anopheles gambiae s.l. species exhibited a significant exophagic behaviour in N’Gattakro (77.1% of outdoor bites versus 52.2% in Odiennekourani). In N’Gattakro, 12.6% of captures occurred before bedtime (09.00 pm) and after waking up (05.00 am), 15.1% in Odiennekourani. The mean human biting rate was higher in N’Gattakro than in Odiennekourani (61.6 versus 15.5 bites per person per night). Overall, Anopheles infection rate was 0.68%, with 0.539 and 0.029 infected bites per person per night in N’Gattakro and Odiennekourani, respectively.
Conclusion
The risk of malaria in urban agricultural lowland areas is uneven. The role of agricultural developments and irrigation patterns in the production of larval habitat should be explored. The exophagic behaviour of Anopheles vectors raises the question of the residual transmission that needs to be assessed to implement appropriate control strategies.
Similar content being viewed by others
Background
Malaria is the most prevalent and deadly vector-borne disease in the world. In 2020, the estimated number of malaria cases was 241 million worldwide of which 95% occurred in Africa populations [1]. In the same year, 627,000 malaria-related deaths were estimated with children under 5 facing the most significant burden among [1]. In Côte d’Ivoire, 7,571,801 malaria cases and 15,913 malaria deaths were reported in 2020 [1]. The overall incidence rate of malaria in Côte d’Ivoire was 230‰ in 2019 [2]. Malaria is endemic with 81% of population living in areas with an annual incidence ranging from 300 to over 500‰ [2]. The objectives of the National Strategic Plan for 2021–2025 are to reduce malaria mortality rates and incidence of malaria cases by at least 75% compared to 2015 [2].
Although malaria has long been considered a rural disease, it is now also present in cities [3]. Urban malaria is a very serious challenge because 50% of the world population is already living in cities and this percentage is expected to increase to 70% in 2050 [4]. In Côte d’Ivoire, 51.2% of the population currently lives in urban areas [5]. Moreover, population concentration in cities raises the issue of food supply, and more broadly of food security [6]. Urban agriculture has emerged as a promising opportunity to enhance food security [7]. It also contributes to the local economic development, poverty reduction and social inclusion of city dwellers as well to greening the city, urban waste recycling, and reducing vulnerability to climate change.
In sub-Saharan African cities, lowlands are privileged areas for market gardening and rice growing [8]. In Côte d’Ivoire, they have been used for agricultural purposes for decades [9]. However, they also constitute areas conducive to malaria vector development by offering permanent larval habitats [10,11,12].
The aim of this study was to assess malaria transmission in the neighbourhood of two lowlands in relation to their agricultural development practices in the city of Bouaké, Côte d’Ivoire.
Methods
Study site
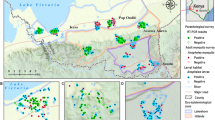
The study was conducted in the city of Bouaké that is located in the centre of Côte d’Ivoire, approximately 350 km for Abidjan, the capital city. According to the most recent census of 2014 [13], Bouaké has 536,719 inhabitants.
Annual malaria incidence in Bouaké was estimated at 400–499‰ in the southern health district and over 500‰ in the north-eastern and north-western health districts in 2018 [14]. In Bouaké, malaria prevention is mainly achieved through the distribution of long-lasting insecticidal nets (LLINs) to the entire population. The utilization rate of LLINs increased from 50% in 2016 to 63.2% in 2019 [15]. There are no indoor residual spraying (IRS) or larvicide campaigns, which are reserved for a few pilot areas in the country. The population normally receives health care advice on how to clean up their environment.
The climate is tropical humid with two seasons: a rainy season between April and October, and a dry season from November to March [16]. The monthly average temperatures range between 24 and 28 °C and the annual rainfall between 1000 and 1200 mm [17]. According to the national society of airports and meteorology (SODEXAM) estimates, the rainfall total was 1227 mm in 2019 (Fig. 1).
The city covers approximately 10,000 ha crossed by a large hydrographic network [9] (Fig. 2). This creates many lowlands that split the city and account for more than 1200 ha (i.e. ~ 12% of the total city area). These lowlands are intensively exploited for market gardening and rice cultivation.
For this study, two lowlands were selected. These two lowlands are quite similar in size and cropping patterns. The first one is in the N’Gattakro district, a structured district from the 1970s, located in the southwest part of the city, and covers 2.5 ha (Fig. 3). The farmers do not live close to the lowlands which are part of the Kan river basin. The second has a surface area of 2.8 ha and is located in the Odiennekourani district, an old district with a high building density in the surrounding area, located in the northwest part of the city (Fig. 4). This lowland is part of the Loka river basin and it is farmed by local residents. In these lowland, rice and market-garden crops (cabbage, okra, carrots, onions, lettuce, eggplants, corn) are grown. Unlike N’Gattakro, market gardening plots in Odiennekourani are watered manually. Rice plots are permanently irrigated in N’Gattakro by a watercourse, whereas the stream circulation can be interrupted in Odiennekourani. In addition, in Odiennekourani, the neighbourhood wastewater is discharged resulting in poor water quality.
Environmental data
The agricultural development of the two lowlands was monitored by monthly image acquisition with a drone (DJI Phantom 4 Pro V2) between January and December 2019. The drone flight parameters (flight height: 75 m, flight speed: 5.2 m/second, image capture every 7 s, spatial resolution: 2 cm/pixel, angle of view: 90° relative to the ground, percentage of overlap (front and side): 70%) were defined automatically and kept for each flight. The monthly images were assembled into a single georeferenced image using the OpenDroneMap platform (https://www.opendronemap.org/). Each lowland was divided into homogeneous spatial units, according to the agricultural use (rice cultivation, market gardening) by photo-interpretation with the free GIS software QGIS (version 3.16.4). This analysis gave 67 homogeneous spatial units in the Odiennekourani lowland and 85 in the N’Gattakro lowland. Each parcel was assigned an ID. A field survey was carried out monthly to assign each spatial unit a crop use (“rice” or “market gardening”) and to calculate the corresponding area.
Mosquito collection
Adult mosquitoes were caught by the human landing catching (HLC) method that is still considered the “gold standard” to measure Anopheles vector biting on humans [18], in inhabited areas located along the two lowlands from February to December 2019 (except in January, April and May due to human resource constraints). For comparison with a recent study in Bouaké [11], the entomological data were organized by season: dry season (February, March, November and December) and rainy season (June to October).
Mosquitoes were collected inside and outside two houses (Fig. 5) in each area (four person-nights per neighbourhood per month) from 07:00 pm to 07:00 am during one night per lowland and per month (for a total of 72 nights of HLC sampling). Houses were selected based on the agreement of their inhabitants and the same houses were used throughout the study. The mean distance between the house and the lowland was 26 m meters in N’Gattakro and 19 m in Odiennekourani. Mosquitoes were morphologically identified to species or complex level according to Mattingly [19] and Gilles & Coetzee [20]. Only Anopheles specimens were individually preserved in 1.5 ml Eppendorf tubes containing silica gel for further analysis.
Molecular analyses
DNA was extracted from the head and thorax following the method described by Yahouedo et al. [21]. Twin species of Anopheles gambiae sensu lato (s.l.) were identified by PCR according to Favia et al. [22] to distinguish Anopheles coluzzii and An. gambiae sensu stricto (s.s.) [23]. Anopheles arabiensis and An. gambiae s.l. were differentiated according to Scott et al. [24].
For the molecular identification of An. gambiae s.l. species, because of high densities in February, March, and September, only 56.4% and 92.6% of the Anopheles collected in N’Gattakro and in Odiennekourani, respectively, were analysed after random selection.
The presence of Plasmodium parasites was assessed by quantitative PCR (qPCR) based on Mangold et al. [25] in the subsample of An. gambiae complex specimens identified by molecular analysis and in all individuals of the other species (Anopheles funestus s.l., Anopheles ziemanni, Anopheles pharoensis).
Entomological parameters measured
The human biting rate (HBR) was derived from the number of host-seeking female mosquitoes landing on subjects. The HBR was calculated as the number of all female anopheles collected per person per night, which resulted in the number of bites per person per night (bpn) [26]; the infection rate (IR) as the proportion of Anopheles infected by Plasmodium parasites. The entomological inoculation rate (EIR) was calculated by multiplying the HBR by the IR, as the number of infected bites per person per night (ibpn) [27].
Statistical analysis
Statistical analyses were performed with the R software version 4.1 [28], and a 5% significant threshold. The Spearman test was used to assess the correlation between rainfall and cultivated areas, and between rainfall and mosquito abundance [29]. The Chi2 test was used to compare the mosquito species abundance according to the lowland, the season and the collection position (indoors and outdoors) [30]. The Fisher’s exact test was used to compare the number of infected Anopheles specimens between lowlands [30]. The Wilcoxon-Mann Whitney test was used to compare the human biting rate and the entomological inoculation rate between lowlands [30].
Results
Agricultural development of lowlands
The area devoted to rice fields was ~ 10,000 m2 in both lowlands. On average, market gardening represented 6600 m2 in N’Gattakro and 15,400 m2 in Odiennekourani. In N’Gattakro, the market gardening surface varied over time, and was largest between March and June, when rainfall started to increase. It remained almost constant in Odiennekourani (Fig. 6). Similarly, rice culture surface increased in N’Gattakro from August to January with a maximum in October at the peak of rainfall. Conversely, it did not show any major variability in Odiennekourani. These variations were not linked to the rainfall pattern (p > 0.1).
Mosquito characterization
In total, 7270 female mosquitoes were collected. They belonged to four genera (Aedes, Anopheles, Culex, and Mansonia) and to 18 species (Table 1). Species composition differed between the two lowlands: 16 species in N’Gattakro (2 species of Aedes, 3 species of Anopheles, 9 species of Culex, 2 species of Mansonia) and 13 species in Odiennekourani (2 species of Aedes, 3 species of Anopheles, 6 species of Culex, and 2 species of Mansonia).
In N'Gattakro, An. gambiae s.l. was the predominant species representing 54.08% of captures, whereas Culex quinquefasciatus accounted for 30.22% of captures. In Odiennekourani, Cx. quinquefasciatus was dominant (57.52%), followed by An. gambiae s.l. representing 16.97% of the captures. These differences in abundance between lowlands were significant (Chi2 = 966.7; p < 0.0001). Seasonal variations of abundance of An. gambiae s.l. followed a similar trend in both areas, with two peaks in March at rainfall onset, and in September just before the rainfall peak, but with different rates. Conversely, Cx. quinquefasciatus was mainly captured in June, July and November in N’Gattakro, and between February and July in Odiennekourani (Fig. 7). No correlation was found between rainfall and Cx. quinquefasciatus (p > 0.1) or An. gambiae s.l. (p > 0.1) abundances in both lowlands.
Anopheles composition
Anopheles gambiae s.l. was represented more than 96% of the captures regardless of the lowland and the season (Table 2). Anopheles funestus s.l. was captured at low frequency in both lowlands and seasons. Few specimens of An. pharoensis (n = 5) and An. ziemanni (n = 6) were collected only in N’Gattakro and in Odiennekourani, respectively, and only during the rainy season.
An. gambiae complex species
Among the 1740 An. gambiae s.l. analysed, 1129 (64.9%) were An. coluzzii, 599 (34.4%) were An. gambiae sensu stricto (s.s.), and 12 (0.7%) were An. arabiensis (Table 3). The distribution varied according to the lowland. An. coluzzii was significantly more frequent than An. gambiaes.s. in N’Gattakro compared to Odiennekourani in the rainy (Chi2 = 38.10; p < 0.0001) and also dry season (Chi2 = 4.75; p = 0.03). Anopheles arabiensis was found only at one site in Odiennekourani, mostly during the rainy season.
Anopheles gambiae complex biting behaviour
Species of the An. gambiae complex were more likely to be exophagic in N'Gattakro (77.1%, 951/1234) than in Odiennekourani (52.2%, 264/506). In N’Gattakro, significantly more An. coluzzii were collected outdoors than in Odiennekourani in the rainy (Chi2 = 13.4; p = 0.0002) and dry season (Chi2 = 45.4; p < 0.0001) (Table 4). It was the same for An. gambiae s.s. in the rainy (Chi2 = 19.28; p < 0.0001) and dry season (Chi2 = 24.3; p < 0.0001). The exophagy rate of An. arabiensis was 66.7%.
In N’Gattakro, the biting activity of An. coluzzii and An. gambiae s.s. peaked between 02:00 am and 03:00 am (Fig. 8). In Odiennekourani, the biting activity of An. gambiae s.s. followed the same pattern as in N'Gattakro, in contrast to An. coluzzii which exhibited a maximum activity between 03:00 and 04:00 am. Overall, 12.6% (in N’Gattakro) and 15.1% (in Odiennekourani) of An. gambiae s.l. were captured between 07:00 and 09:00 pm, and between 05:00 and 07:00 am.
Plasmodium parasite infection and transmission
Overall, the mean HBR of all Anopheles was 61.3 bpn in N’Gattakro and 15.4 bpn in Odiennekourani (Table 5). The difference was significant (w = 74; p = 0.0019). Among the 1766 Anopheles specimens analysed by qPCR, 12 were infected by Plasmodium parasites (n = 9 An. coluzzii and n = 3 An. gambiae s.s.); this gave an overall infection rate of 0.68%. Two Plasmodium species were identified: Plasmodium falciparum (n = 8 An. coluzzii; 0.71% and n = 3 An. gambiae s.s.; 0.5%) and Plasmodium malariae (n = 1 An. coluzzii; 0.09%).
Among the 12 infected mosquitoes, 11 were captured in N'Gattakro (n = 7 in the rainy season and n = 4 in the dry season) and 1 in Odiennekourani (rainy season) (Table 5). However, there is no relationship between the number of infected mosquitoes and the areas (OR = 4.57; IC95% = [0.66; 197.03]; p = 0.19). Eight infected specimens were captured outdoor and four indoor. Seven infected anopheles were captured before midnight in N’Gattakro, and the infected specimen in Odiennekourani was collected between 03:00 and 04:00 am.
The entomological inoculation rate was higher in N’Gattakro (0.539 ibpn) than in Odiennekourani (0.029 ibpn) (w = 69; p = 0.0059). It was 0 ibpn in the dry season in Odiennekourani.
Discussion
The study was designed to assess malaria transmission in the surroundings of two lowlands under agricultural development in the city of Bouaké. Some differences were observed in the entomological drivers of malaria transmission in the two studied lowlands despite the same cultivations.
Anopheles gambiae s.l. predominated in N’Gattakro and Cx. quinquefasciatus in Odiennekourani. The high abundance of Cx. quinquefasciatus in Odiennekourani could be associated with the permanent wastewater stream that favours its development, as observed in urban areas in Benin by Salako et al. [31]. The organic pollution of the watercourse that irrigates the rice plots and to the hand-watering of the market-gardening plots, could also explain the lower An. gambiae s.l. abundance in Odiennekourani than in N'Gattakro. Conversely, the higher water quality and its permanence for irrigation might explain its abundance in N’Gattakro. The An. gambiae s.l. preference for non-polluted water is generally accepted [32]. However, some studies showed that this species can be found also in polluted water [33, 34], although the pollution type and level have been rarely measured.
Overall, four Anopheles species were identified: An. gambiae s.l. and An. funestus s.l. are considered the main vectors of Plasmodium sp. in Côte d’Ivoire, while An. pharoensis and An. ziemanni appear as potential vectors. Anopheles coustani, which had been observed by Dossou-Yovo et al. [17], was not found. This could be due to the ongoing urbanization that might have altered the vector composition [35]. The dramatic increase in human densities in urban areas could lead to a shift in the feeding choices of Anopheles towards humans in many cities in sub-Saharan Africa. [36]. Unlike An. arabiensis which is highly flexible in its host preferences, An. coustani is a zoophilic species that may not have been able to adapt to the scarcity of animal hosts linked to the urban lifestyle. Furthermore, An. coustani may have failed to adapt to water pollution usually associated with urban development as opposed to An. gambiae s.l. [34].
Molecular analysis revealed the presence of An. gambiae s.s. and An. coluzzii, as previously reported in the region of Bouaké [11, 37]. Anopheles coluzzii was more abundant than An. gambiae s.s. in N’Gattakro. Anopheles coluzzii shows some adaptation to the urban environment and can colonize lowland areas where it might find more or less permanent larval habitats, unlike An. gambiae s.s. that prefers small and temporary sites [3, 38, 39]. Capturing Anopheles all over the district, not only along the lowlands, Adja et al. [11] identified more An. gambiae s.s. than An. coluzzii in N'Gattakro. The existence of more favourable larval habitats for this species within the district can be presumed.
Molecular analysis also resulted in the identification of An. arabiensis in Odiennekourani only. Its presence was recently reported for the first time in Côte d’Ivoire [40]. Anopheles arabiensis is generally associated with arid environments [41]. However, urbanization can provide favourable conditions for its development, as observed in Ghana, Nigeria [42]. The development of An. arabiensis in urban environments is consistent with its tolerance for polluted aquatic habitats as it was observed in different cities as Dakar [43] and Ouagadougou [44]. Anopheles arabiensis low abundance in Bouaké suggests that this species is currently in the process of being introduced in the country, possibly from Burkina Faso. Indeed, An. arabiensis is now prevalent in Bobo-Dioulasso (500 km from Bouaké) [45], and its transport via the intense commercial exchanges between these cities, cannot be excluded (e.g. with livestock carried by truck). Eritja et al. [46] demonstrated the importance of passive transportation in cars in Aedes albopictus colonization in Spain. This hypothesis needs to be tested by comparing the populations of Bouaké and Bobo-Dioulasso.
In N’Gattakro, An. gambiae complex species showed a trend towards exophagy that was not observed in Odiennekourani. From 2014 to 2015, Adja et al. [11] did not observe this trend in N’Gattakro. Conversely, in 2020, Assouho et al. [47] showed that all malaria vectors were frequently caught outdoors in urban and rural areas in different Côte d’Ivoire areas. Behavioural changes have been documented amongst Anopheles following the use of insecticide-treated nets (ITNs) and indoor residual spraying (IRS), including outdoor biting, time of biting and host choice preference [48,49,50]. As a limitation of the study, LLINs use among the human populations of both districts was not surveyed. The only hypothesis that can be formulated is that people in N’Gattakro are more likely to use LLINs than those in Odiennekourani, thus leading malaria vectors in N’Gattakro to be more exophagic.
Together with the presence of An. arabiensis (an exophilic and exophagic vector [51]) and the noticeable percentage of outdoor bites (70.9%) occurring when people are not supposed to be under bed nets in both lowlands, these results highlight the risk of residual transmission that could compromise malaria control. However, the risk of human population exposure to malaria vectors requires further investigation regarding the use of mosquito nets, the time of sleeping and of waking up [52].
People in N’Gattakro received 15-fold more anopheles bites than those in Odiennekourani, and they would annually suffer about 200 infected bites per human compared to about 10 in Odiennekourani. Such differences within a same area was already recorded [53]. These authors reported an average EIR around 300 infected bites per human and per year in Côte d’Ivoire, highlighting that the EIR in N’Gattakro is quite high for an urban area. Even if the association between the EIRs and the intensity of transmission still remains difficult to interpret, it can be considered that a greater risk of transmission occurred in N’Gattakro where the surface area of rice fields is similar to that of Odiennekourani but market gardening is less extensive.
Irrigated rice fields are known to produce important numbers of Anopheles, although a few of these are infected [54]. Shah et al. [55] recently showed that in complex mosaic cropping systems interspersed with natural vegetation, the risk of malaria increased in urban areas. These results prompt a further study of larval habitat production over time at the crop plot scale. The lowlands under study consist of crop mosaics that may have varying effects on Anopheles larvae production. Investigating them at a fine scale may allow to better understand larval production and design less productive crop assemblages, notably by introducing shade trees as suggested by Dongus et al. [56].
Conclusion
The results highlight the existence of uneven malaria transmission risk in the two surveyed lowlands, possibly associated with lowland management practices that should be thoroughly investigated, especially the management of parcels watering. They also indicate that living close to an agriculturally developed lowland may increase the risk of exposure to malaria vector bites. Given the importance of urban agriculture in Bouaké, Anopheles exophagic behaviour and the proportion of bites occurring when people are not supposed to be under mosquito nets, these results suggest a possible risk of residual transmission that could hamper the malaria control efforts through the use of long-lasting insecticidal nets. Several research topics could be explored to help the National Malaria Control Programme, including trials of additional control tools for LLINs, such as eave tubes, surveys to better understand population behaviour in terms of LLIN use, and also studies on developing lowland agriculture that would produce less Anopheles.
Availability of data and materials
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.
References
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
Azongnibo KRM, Guindo-Coulibaly N, Bonnet E, Kokro-Djahouri MNW, Assouho KF, Niamke MG, et al. Spatiotemporal analysis of malaria incidence in Côte d’Ivoire from 2015 to 2019. Trans R Soc Trop Med Hyg. 2022. https://doi.org/10.1093/trstmh/trac112.
De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med. 2012;2012: 819563.
United Nations. 2019 Department of economic and social affairs population division (2019). World Urbanization Prospects 2018: Highlights.
World Bank. 2022 Urban population Côte d’Ivoire. https://donnees.banquemondiale.org/indicateur/SP.URB.TOTL.IN.ZS?locations=CI
Poulsen MN, McNab PR, Clayton ML, Neff RA. A systematic review of urban agriculture and food security impacts in low-income countries. Food Policy. 2015;55:131–46.
Lovell ST. Multifunctional urban agriculture for sustainable land use planning in the United States. Sustainability. 2010;2:2499–522.
Albergel J, Lamachère JM, Lidon B, Mokadem A, Van Driel W. Mise en valeur agricole des bas-fonds au sahel. typologie, fonctionnement hydrologique, potentialités agricoles rapport final d’un projet CORAF-R3S. Ouagadougou: CIEH; 1993.
Konan JMKA. Compétition entre bâti et agriculture dans la conquête des bas-fonds de la ville de Bouaké: le savoir-faire ou les actions stratégiques des citadins-agriculteurs pour préserver les espaces agricoles. Vertigo. 2017. https://doi.org/10.4000/vertigo.18302.
Afrane YA, Klinkenberg E, Drechsel P, Owusu-Daaku K, Garms R, Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Trop. 2004;89:125–34.
Adja AM, Zoh DD, Sagna AB, Kpan DMS, Guindo-Coulibaly N, Yapi A, et al. Diversity of Anopheles gambiae s.l.. giles (Diptera: Culicidae) larval habitats in urban areas and malaria transmission in Bouaké Côte d’Ivoire. Vector Borne Zoonotic Dis. 2021;21(8):593–601.
Akono PN, Mbida JA, Tonga C, Belong P, Hondt OEN, Magne GT, et al. Impact of vegetable crop agriculture on anopheline aggressivity and malaria transmission in urban and less urbanized settings of the South region of Cameroon. Parasit Vectors. 2015;8:293.
INS. Recensement general de la population et de l’habitat. Région du Gbêkê: Répertoire des localités; 2014.
Ministère de la Santé et de l'Hygiène Publique. 2021 Plan stratégique national de lutte contre le paludisme 2021–2025. Côte d’Ivoire.
INS. Enquête par grappes a indicateurs multiples - Côte d’Ivoire 2016. Côte d’Ivoire: Ministère du plan et du développement; 2017.
Eldin M. Le climat. Paris (FR):ORSTOM (Mémoires ORSTOM); 1971. https://www.documentation.ird.fr/hor/fdi:16370.
Dossou-Yovo J, Doannio JM, Diarrassouba S, Chauvancy G. Impact d’aménagements de rizières sur la transmission du paludisme dans la ville de Bouaké. Côte d’Ivoire Bull Soc Pathol Exot. 1998;91:327–33.
Eckert J, Oladipupo S, Wang Y, Jiang S, Patil V, McKenzie BA, et al. Which trap is best? alternatives to outdoor human landing catches for malaria vector surveillance: a meta-analysis. Malar J. 2022;21:378.
Mattingly PF. The mosquitoes of ethiopian region. London: Sutcliffe; 1971.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region) South African institute for medical research. Johannesburg. 1987;55:141.
Yahouédo GA, Cornelie S, Djègbè I, Ahlonsou J, Aboubakar S, Soares C, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasit Vectors. 2016;9:385.
Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23.
Coetzee M, Kruger P, Hunt RH, Durrheim DN, Urbach J, Hansford CF. Malaria in South Africa: 110 years of learning to control the disease. S Afr Med J. 2013;103:770–8.
Scott JA, Broodon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–40.
Ryan SJ, Lippi CA, Boersch-Supan PH, Heydari N, Silva M, Adrian J, et al. Quantifying seasonal and diel variation in Anopheline and Culex human biting rates in Southern Ecuador. Malar J. 2017;16:479.
Birley MH, Charlewood JD. Sporozoite rate and malaria prevalence. Parasitol Today. 1987;3:231–2.
R Core Team. R: a language and environment for statistical computing. Vienna: R foundation for statistical computing; 2022.
Hollander M, Wolfe DA. Nonparametric statistical methods. New York: John Wiley & Sons; 1973.
Agresti A. Categorical data analysis. 2nd ed. New York: Wiley; 2002.
Salako AS, Ossè R, Padonou GG, Dagnon F, Aïkpon R, Kpanou C, et al. Population dynamics of Anopheles gambiae s.l. and Culex quinquefasciatus in rural and urban settings before an indoor residual. Vector Borne Zoonotic Dis. 2019;19:674–84.
Klinkenberg E, McCall P, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra. Ghana Malar J. 2008;7:151.
Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in western Kenya. J Med Entomol. 2001;38:282–8.
Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ, Unyimadu JP. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos. southwestern Nigeria. J Vector Borne Dis. 2007;44:241–4.
Gottdenker NL, Streicker DG, Faust CL, Carroll CR. Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth. 2014;11:619–32.
Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, et al. Climate and urbanization drive mosquito preference for humans. Curr Biol. 2020;21(30):3570-3579.e6.
Zoh DD, Yapi A, Adja MA, Guindo-Coulibaly N, Kpan DMS, Sagna AB, et al. Role of Anopheles gambiae s.s. and Anopheles coluzzii (Diptera: Culicidae) in human malaria transmission in rural areas of Bouaké in Côte d’Ivoire. J Med Entomol. 2020;57:1254–61.
Della Torre A, Tu Z, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem Mol Biol. 2005;35:755–69.
Simard F, Ayala D, Kamdem GC, Pombi M, Etoua J, Ose K, et al. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol. 2009;9:17.
Fournet F, Adja AM, Adou KA, Dahoui MMC, Coulibaly B, Assouha KF, et al. First detection of the malaria vector Anopheles arabiensis in Côte d’Ivoire: urbanization in question. Malar J. 2022;21:275.
Jones CM, Toé HK, Sanou A, Namountougou M, Hughes A, Diabaté A, et al. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso Burkina Faso. PLoS ONE. 2012;7:e45995.
Kristan M, Fleischmann H, Della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003;17:326–32.
Machault V, Gadiaga L, Vignolles C, Jarjaval F, Bouzid S, Sokhna C, et al. Highly focused anopheline breeding sites and malaria transmission in Dakar. Malar J. 2009;8:138.
Fournet F, Cussac M, Ouari A, Meyer P-E, Toé HK, Gouagna L-C, et al. Diversity in anopheline larval habitats and adult composition during the dry and wet seasons in Ouagadougou (Burkina Faso). Malar J. 2010;9:78.
Dabiré RK, Namountougou M, Sawadogo SP, Yaro LB, Toé HK, Ouari A, et al. Population dynamics of Anopheles gambiae s.l. in Bobo-Dioulasso city bionomics, infection rate and susceptibility to insecticides. Parasit Vectors. 2012;21:127.
Eritja R, Palmer JRB, Roiz D, Sanpera-Calbet I, Bartumeus F. Direct evidence of adult Aedes albopictus dispersal by car. Sci Rep. 2017;7:14399.
Assouho KF, Adja AM, Guindo-Coulibaly N, Tia E, Kouadio AMN, Zoh Dounin DDD, et al. Vectorial transmission of malaria in major districts of Côte d’Ivoire. J Med Entomol. 2020;57:908–14.
Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS ONE. 2014;9:e104967.
Wamae PM, Githeko AK, Otieno GO, Kabiru EW, Duombia SO. Early biting of the Anopheles gambiae s.s. and its challenges to vector control using insecticide treated nets in western Kenya highlands. Acta Trop. 2015;150:136–42.
Lefèvre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med Hyg. 2009;81:1023–9.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Soma DD, Zogo B, Taconet P, Somé A, Coulibaly S, Baba-Moussa L, et al. Quantifying and characterizing hourly human exposure to malaria vectors bites to address residual malaria transmission during dry and rainy seasons in rural Southwest Burkina Faso. BMC Public Health. 2021;21:251.
Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;23(8):19.
Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol. 2001;15:1–11.
Shah HA, Carrasco LR, Hamlet A, Murray KA. Exploring agricultural land-use and childhood malaria associations in sub-Saharan Africa. Sci Rep. 2022;12:4124.
Dongus S, Nyika D, Kannady K, Mtasiwa D, Mshinda H, Gosoniu L, et al. Urban agriculture and Anopheles habitats in dar es salaam. Tanzania Geospat Health. 2009;3:189–210.
Acknowledgements
We thank Patrick Akoliba, Kouame Brice Hervé Kouadio and the mosquito capture team for their technical assistance during mosquito collections and field analysis. We are also grateful to Bouaké authorities and to the Health Regional Direction of Gbêkê for their support with the project implementation.
Funding
This study is funded by the Agence Nationale de la Recherche (ANR) in France through the project COHESION (ANR 17 CE 22 0007).
Author information
Authors and Affiliations
Contributions
FF, KAA and AMA conceptualized and designed the study. MMCD, KLN and AK conducted the field work. MMCD, KLN, AK, BC and SC did the laboratory work. KAA was responsible with FF for the data management. MMCD, FF and NM analysed the data. MMCD, FF, NM and AMA wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Côte d’Ivoire National Life Sciences and Health Ethics Committee (agreement no. 156–18/MSHP/CNESVS-km of 14 November 2018). The participants who collected mosquitoes and their supervisors all gave their written informed consent. They received a vaccine against yellow fever as a prophylactic measure. Participants were treated free of charge for malaria according to the WHO recommendations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dahoui, M.M.C., Adou, K.A., Coulibaly, B. et al. Entomological drivers of uneven malaria transmission in urban lowland areas in Bouaké, Côte d’Ivoire. Malar J 22, 34 (2023). https://doi.org/10.1186/s12936-023-04457-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04457-x