Abstract
Background
Human landing catches (HLC) are an entomological collection technique in which humans are used as attractants to capture medically relevant host-seeking mosquitoes. The use of this method has been a topic of extensive debate for decades mainly due to ethical concerns. Many alternatives to HLC have been proposed; however, no quantitative review and meta-analysis comparing HLC to outdoor alternative trapping methods has been conducted.
Methods
A total of 58 comparisons across 12 countries were identified. We conducted a meta-analysis comparing the standardized mean difference of Anopheles captured by HLC and alternative traps. To explain heterogeneity, three moderators were chosen for analysis: trap type, location of study, and species captured. A meta-regression was fit to understand how the linear combination of moderators helped in explaining heterogeneity. The possibility of biased results due to publication bias was also explored.
Results
Random-effects meta-analysis showed no statistically significant difference in the mean difference of Anopheles collected. Moderator analysis was conducted to determine the effects of trap type, geographical location of study, and the species of Anopheles captured. On average, tent-based traps captured significantly more Anopheles than outdoor HLC (95% CI: [− .9065, − 0.0544]), alternative traps in Africa captured on average more mosquitoes than outdoor HLC (95% CI: [− 2.8750, − 0.0294]), and alternative traps overall captured significantly more Anopheles gambiae s.l. than outdoor HLC (95% CI: [− 4.4613, − 0.2473]) on average. Meta-regression showed that up to 55.77% of the total heterogeneity found can be explained by a linear combination of the three moderators and the interaction between trap type and species. Subset analysis on An. gambiae s.l. showed that light traps specifically captured on average more of this species than HLC (95% CI: [− 18.3751, − 1.0629]). Publication bias likely exists. With 59.65% of studies reporting p-values less than 0.025, we believe there is an over representation in the literature of results indicating that alternative traps are superior to outdoor HLC.
Conclusions
Currently, there is no consensus on a single “magic bullet” alternative to outdoor HLC. The diversity of many alternative trap comparisons restricts potential metrics for comparisons to outdoor HLC. Further standardization and specific question-driven trap evaluations that consider target vector species and the vector control landscape are needed to allow for robust meta-analyses with less heterogeneity and to develop data-driven decision-making tools for malaria vector surveillance and control.
Similar content being viewed by others
Background
The accurate understanding and quantification of the drivers of vector distribution and pathogen trans- mission could be critical for effective vector control. For mosquitoes, a number of environmental and human-associated factors shape pathogen transmission dynamics [1, 2]. These transmission parameters describe the relationship between entomological indicators (such as abundance, feeding behaviour, longevity) and epidemiological outcomes [3]. Thus, the first step in understanding the dynamics between mosquitoes and mosquito-borne diseases is the estimation of these parameters. Of these, entomological indicators that are a result of mosquito sampling/collection are the most pertinent. This is because collection methods are used to gather baseline information on mosquito abundance, diversity, distribution, biting frequency and behaviour, mosquito survival, and infection rates [1, 4]. Taken together, the synthesis of such key information is crucial for planning optimal mosquito intervention strategies.
The suitability of a mosquito collection method is species-specific and should be coupled with sampling methods that take advantage of specific behaviours. While there are about 4000 species of mosquito described today [5], only a few genera, such as Aedes and Anopheles, are efficient transmitters of human pathogens. Notably, Anopheles spp. transmit the parasites responsible for human malaria. Malaria is the most serious arthropod-vector borne disease causing morbidity and mortality in humans. To date, perhaps the greatest success recorded in the fight against malaria has been through the use of mosquito control interventions such as insecticide- treated nets (ITNs) and indoor residual spraying (IRS) that target specific biting and resting behaviours of Anopheles spp. adult females [6,7,8], behaviours that were identified through mosquito surveillance methods. Thus, mosquito surveillance methods that provide information about behaviours are essential for understanding parasite transmission dynamics and the impact of vector control tools. For example, one key metric in understanding malaria transmission dynamics is the entomological inoculation rate (EIR), this indicator takes into account Anopheles biting rate and the proportion of mosquitoes carrying infectious Plasmodium sporozoites. By combining this information, an estimate of the number of infectious bites an individual will get over a set period of time can be calculated and this value is often used as a key indicator of transmission. Data on vector bionomics gathered through Anopheles collection methods can also be used to develop and implement targeted vector control interventions that exploit specific Anopheles behaviours. In addition, the influence of socioenvironmental conditions on currently employed Anopheles surveillance methods should be studied [9, 10].
To date, several methods have been employed in the estimation of mosquito biting behaviours [10,11,12,13,14], however, human landing catches (HLC) have been suggested as the gold standard for malaria vector surveillance, being widely used and the most direct way to measure Anopheles vector biting on humans. HLC is a method of mosquito collection that uses humans and their natural production of carbon dioxide (CO2), heat, and odour as bait to capture host-seeking mosquitoes. HLC is an important collection method because it uses humans as an attractant to determine Anopheles abundance over a set period and human biting rate, the crucial metric that when combined with data on presence of infective human Plasmodium sporozoites gives the EIR. By using humans as baits, HLC facilitate the collection of human-biting mosquitoes capable of transmitting human malaria para- sites (Plasmodium spp.). By directly measuring biting hourly, HLC can also be used to determine peak human biting times and quantify differences in indoor/outdoor biting behaviours [15,16,17].
Despite the widespread use of HLC as an arguably unparalleled method in determining host seeking and biting behaviours relevant for understanding Anopheles vector exposure and human Plasmodium parasite transmission, the use of HLC has long been a topic of controversy. HLC requires collectors to stay awake during overnight collections, and although collectors may be provided with prophylaxis to protect them from malaria infection [18], they may be exposed to other vector- borne pathogens such as the causative agents of lymphatic filariasis, chikungunya, leishmaniasis, among other agents [16]. HLC also require expertise from both col- lectors and supervisors and are physically demanding, requiring collectors to work throughout the night [19]. The results obtained through HLC, and some other trap- ping tools, are also heavily influenced by the attractiveness of the human collector to the Anopheles species [20, 21]. Furthermore, HLC typically provides data on only mosquitoes that feed on human legs [16, 17] possibly ignoring populations that obtain a blood meal from other parts of the human body.
In Africa, as elsewhere, alternatives to HLC, many of which can measure human exposure and biting and therefore provide a proxy for EIR estimations have been proposed and evaluated under various conditions for the collection of Anopheles species. For example, varying designs of light traps including the Centers for Disease Control (CDC) light trap [22, 23] odour baited-traps [24, 25] electrocution traps [16, 26], decoy traps [27], tent traps [28, 29], barrier screens [30], and a combination of these traps [31] have all been explored as alternatives. Several of these alternative collection methods have been conducted in direct comparison with HLC with differing results [12, 20, 32,33,34,35,36]. For example, one study comparing methods showed CDC miniature light traps captured at least twice the number of Anopheles captured by HLC [37]. These comparative studies only use male volunteers, as the ethical concerns of using women and children are too great, even though they are the most vulnerable. Perhaps what is often overlooked, for the efficiency of a trap type, is the sensitivity and correlation between host-seeking/resting behaviour and malaria-pathogen transmission. In this respect, a trap would be appropriate if it collects representative populations (or species) of adult females (fed and unfed). Such trap data would provide essential information on Anopheles abundance, human biting rate (HBR), and EIR. In recent years several programmes have stopped HLC or had discussions about halting the use of HLC for various reasons including risk of exposure to other vector-borne diseases [16]. Therefore, a comparable and effective collection method is needed for malaria vector surveillance. Indoors, CDC light traps have been used as an alternative to indoor HLC where an individual sleeps under a bed net and acts as an attractant towards the light trap, with conversion factors being developed for EIR; however, there is no consensus on outdoor mosquito collection alternatives to HLC for use in malaria surveillance, despite a large number of individual field studies comparing alternative traps to outdoor HLC. To date, no systematic review or meta-analysis combining these results has been conducted.
Consequently, the aim of this literature review and meta-analysis is to compare mean differences in capture rates between alternative outdoor mosquito collection methods for malaria surveillance and outdoor HLC, to determine which tools could be used to replace outdoor HLC, and to examine variation in the literature and the effects of geography, general trap type, trap bias, and target species on collection results. Specifically, this study aimed to address whether publication bias, geographical location of the comparison study, species composition, and trap type (light trap, tent trap, electrocuting box trap), and categorical classification (biological, physical, chemical) had significant effects on the alternative trapping methods outdoors and their comparability to outdoor HLC.
Methods
Literature search, inclusion criteria, and study selection
Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) recommendations were followed for the literature search, creating the inclusion criteria, and data extraction [38]. Databases were searched independently during May 2020. Searching was done by a group of four researchers (SO, YW, SJ, VP) using (“human landing catches”) AND ((“human landing catches alternatives”) OR (“HLC”) OR (“vector surveillance”)) as keywords. Keywords were employed respectively and the search results were combined using advanced search tools. No language or date restrictions were set. The specific databases searched were left up to the discretion of the researcher with the only requirement being that each investigator searched three separate databases. A total of five databases were accessed (BASE, PubMed, Web of Science, Google Scholar, and Science.gov). After the removal of duplicates, there were 944 items left. For the next step, the paper titles and abstracts were screened by splitting into two groups (Group 1: JE, SZ, YW; Group 2: SO, SJ, VP, BM). Group 1 screened the first 472 papers and group 2 screened the last 472 sorted alphabetically by author’s last name. Each member of the group voted on the eligibility of each paper. For Group 2, tiebreakers were included as eligible. A paper was eligible for inclusion if it received a majority vote as per the inclusion criteria. The inclusion criteria were:
-
A paper must be an entomological malaria surveillance experiment
-
Outdoor HLC must have been performed
-
The study must have involved an alternative trapping method
-
The outcome of interest in the meta-analysis was the standardized mean difference between outdoor HLC and alternative traps. Therefore, the study must have recorded mean Anopheles captured per trap over a defined period or a similar metric/way of calculation.
-
At least one Anopheles mosquito must have been captured by both outdoor HLC. Similarly, at least one Anopheles mosquito must have been captured by the comparative alternative method.
Acceptance for publication was also taken as a criterion for inclusion. No grey literature or conference abstracts were included. Data from a total of 17 articles were extracted yielding a total of 58 comparisons of alternative traps to outdoor HLC. Figure 1 shows the PRISMA flow diagram. While all efforts were made to include eligible papers, some papers might have failed to be included due to limitations with search terms; papers with publication dates after the search period are failed to be included as well. Publications included for analysis were [11, 19, 24, 39,40,41,42,43,44,45,46,47,48,49,50,51,52].
Data extraction and preparation
Two researchers extracted the data (JE, SO). Any discrepancies were resolved by the lead author after revisiting the articles. The following variables were extracted from selected articles:
-
Author: author(s) of the included study
-
Year: included study publication year
-
Country: country the experiment was performed in
-
Coordinates: exact coordinates of experiment site if given in the included study. If testing was done at multiple sites or coordinates were not given, approximations were used
-
Trap name: name of trap being tested
-
Species name: name of species captured and identified during the experiment
-
Species: categorical variable of captured species into one of three groups. The species categories were:
-
‘Anopheles gambiae’ – species belonging to An. gambiae species complex
-
‘Anopheles funestus’—species belonging to An. funestus group
-
‘Anopheles spp.’—all other species not belonging to An. gambiae s.l. or An. funestus s.l.
-
-
Length: the number of days collections were conducted.
Three additional variables were created:
-
Category: categorical variable for the category classification of alternative trapping methods as defined in [20]. The type categories were:
-
‘Biological’
-
‘Chemical’
-
‘Physical’
-
‘Physical/Chemical’
-
-
Type: categorical variable for the classification of alternative trapping meth ods. The type categories were:
-
‘Tent’
-
‘Light’
-
‘Electrocuting’
-
‘Other—Mechanical’
-
‘Other—Passive’
-
-
Africa: categorical variable equal to 1 if the experiment was conducted in Africa or equal to 0 if the experiment was conducted outside of Africa. Africa was the only region with enough studies to be used as a moderator in analysis with enough statistical power.
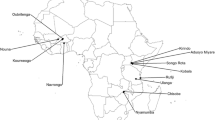
Publication dates of included studies ranged from 1995 to 2019. There were 28 different alternative traps included in the dataset. A total of thirty-one Anopheles species were represented in the meta-analysis. Four articles had experiments from South America, two from Asia, thirteen from Africa, and one from Oceania. This difference in experimental location was the reason behind creating a moderator for Africa, as opposed to a specific country or region. Across all included papers, there were a total of twelve unique countries (Fig. 2).
Study locations were distributed around the world, although most studies were conducted in the African continent. Heat map coloration indicates the number of studies in each location with darker colors indicating a higher number of studies. The complete list of studies are available on https://github.com/JordanEckert/malaria
If necessary, data were extracted from graphics using R version 3.6.3 [53] and the metaDigitise [54] package version 1.0.1. For articles that included multiple com- parisons to HLC, the individual comparisons were added. Treatment effect sizes and standard errors were calculated using esc [55] package version 0.5.1 and were recorded during data extraction.
Statistical analysis
Meta-analytic techniques were conducted using metafor [56] package version 2.4-0, meta [57] package version 4.11-0, and R. Some functions of the dmetar [58] package version 0.0.9 were also used in analysis which required installation from Github. The standardized mean difference (“Hedges’ g”) of mosquitoes captured in the two methods was used as the effect size. Effect sizes were calculated with the control being the outdoor HLC; negative effect sizes indicated that outdoor HLC captured fewer mosquitoes than the alternative method. Mosquitoes captured were chosen as the outcome variable due to capture numbers being universally available across HLC and all alternative trapping methods. A random effects framework was used for the modeling to account for heterogeneity. τ2, the variance of the distribution for the true effect size under such a framework was estimated using a restricted maximum likelihood (REML) approach. The model framework chosen assumes that the observed estimates of treatment effect can vary across studies because of real differences in the treatment effect in each study as well as sampling variability. The point estimate for an individual study then assumes that there is a second source of error that is hierarchical and that the observed effect sizes of a study deviate from their true value because of the sampling error. All random-effects models used the Hartung-Knapp adjustment for the variance of the pooled effects estimator. Moderator analysis was also performed with all moderators being considered random effects during their respective moderator analysis.
Outlier detection was done using the find.outliers() function in the dmetar package. The approach to classifying a study as an outlier was a brute force approach wherein an included study for which the upper bound of the 95% confidence interval was lower than the lower bound of the pooled effect confidence interval was considered an outlier, or similarly for when the lower bound of the 95% confidence interval was higher than the upper bound of the pooled effect confidence interval. The method described above is not comprehensive for finding outliers; it is possible that outliers existed that were not considered.
Multi-model inference was done using the multimodel.inference() function in R, wherein all possible combinations of the Type, Africa, and Species variables with their respective interactions were fitted in a meta-regression. Model selection was based on having the lowest corrected Akaike Information Criterion (AIC). Resampling methods were used to validate the robustness of the meta-regression. The standard in meta-analysis is to use permutation testing [59]. Using metafor’s built in permutest() function, one thousand iterations were run.
Results
Random-effect meta-analysis
Analysis performed on fifty-eight comparisons alternative traps to outdoor HLC yielded a Hedges’ g value of g = − 0.8544. I2 as a measure of heterogeneity was 98.3% which is quantified as substantial heterogeneity (Table 1). It is likely the meta-analysis results lack the ability to detect significant mean difference due to heterogeneity. High heterogeneity can be potentially caused by a single study with an anomalous effect size. Outlier analysis was performed to attempt to explain if the heterogeneity was caused by extreme points or other underlying factors. Of the original 58 comparisons, only 36 were synthesized after outlier removal. Substantial heterogeneity was still found after outlier removal, indicating that other sources might be contributing in tandem (Table 2). Using moderator analysis and meta-regression, an attempt was made to better explain the statistical heterogeneity present and quantify it.
Explaining heterogeneity
Meta-regression
Meta-regression was performed to see if the statistical heterogeneity could be explained using a linear combination of moderators instead of individual associations. The top regression model based on the AIC criterion included Type, Species, Africa, and the interaction of Type and Species (Fig. 3). Figure 3 shows the modelled average predictor importance plot. The fitted meta-regression model reported R2 = 55.77% which, in the context of meta-regression, implies that the linear combination of these four variables explains 55.77% of the heterogeneity present.
Moderator analysis
Three separate moderator analyses were performed wherein the random-effects meta-analysis framework was re-fit under observations being grouped into categories. Moderator analysis provides the framework to see between and within group heterogeneity. Some groups had to be combined into larger groups so that there was enough power to detect significant results. The first moderator analysis used the ‘Type’ variable to generate the groupings. There were four groups for this analysis—tent traps, light traps, a combined group of traps that were neither tent nor light but still used passive methods for collection, and another combined group of traps that were neither tent nor light but used mechanical methods for collection. The second moderator analysis used the ‘Africa’ variable to generate the groupings. There were only two groups for this analysis—studies performed in Africa and studies not performed in Africa. The third moderator analysis used the ‘Species’ variable to generate the groupings. There were three groups for this analysis—Anopheles gambiae complex, An. funestus group, and Anopheles spp. An additional moderator analysis looking at trap ‘Categories’ was also conducted, but due to the subjective nature of categorization those results are not included here (see Annex for trap category moderator findings).
Results showed that the tent group captured a significantly higher mean difference of Anopheles compared to outdoor HLC (95% CI: [− 0.9065, − 0.0544]). No other ‘type’ group had a significant mean difference between the alternative traps and outdoor HLC, indicating that there was no statistical difference between the mean capture numbers of these alternative trap groups and outdoor HLC. Significant results were found in the full comparisons of studies performed on the Africa subgroup ([− 2.8750, − 0.0294]) and the An. gambiae subgroup ([− 4.6475, − 0.2330]) in their separate, respective moderator analyses as well. None of the other 95% confidence intervals in these respective moderator groups found indicated that outdoor HLC collected on average more mosquitoes than their respective moderator groups. Results for each moderator analysis are found in Tables 3, 4, 5. Forest plots showing the effect sizes and confidence intervals within specific studies are found in Figs. 4, 5, 6. The assumption that there was not a common estimate of τ2 across subgroups was made for each analyses. For robustness, the results were computed under a change of assumption so that there was a common estimate of τ2 across subgroups. No changes to statistical significance were detected for any group between the two assumptions.
Analysis of subsets
Of the total 57 comparisons synthesized, 22 of the comparisons were from captures involving An. gambiae s.l. and 11 were An. funestus s.l., with the rest being other Anopheles spp. A separate moderator analysis using ‘Type’ was done independently for both An. gambiae (Table 6). s.l. and An. funestus s.l. (Table 7). For the An. gambiae s.l. subset, the light trap type had a statistically significant higher mean difference of An. gambiae s.l. caught when compared to outdoor HLC (95% CI: [− 18.3751, − 1.0629]).
Publication bias
An unfortunate weakness of any meta-analysis is the lack of ability to include all available data. The two most likely causes for not being able to include all available data are missing papers during searching, and the “file-drawer” problem i.e., many results that are not statistically significant are more likely to not become published [54, 56, 57]. The funnel plot of the data wherein each comparison’s standard error is plotted against the effect size is represented in Fig. 7. One would typically expect the data to follow the prescribed funnel shape if publication bias was not present. However, the figure shows a large grouping at the top of the funnel with some points landing well outside the funnel—a deviation from the typical shape. Further testing for publication bias was done using the Egger’s test of asymmetry (see Table 8 for results). The graphical representation of the data with the p-value for the t-test of the intercept was not significant (p∗ = 0.0508). This value, while not statistically significant, has a lot of practical significance- especially when coupled with the oddly shaped funnel plot. Given the closeness of the Egger’s test, funnel plot, and the trim-and-fill analysis (Table 9) the authors believe that it is reasonable to assume some moderate level of publication bias is present in the analysis. It is likely that certain entomological surveys that showed either no difference between the deployed trapping method and outdoor HLC, or even results where HLC performed better than the deployed method, were not published depending on the objective of the survey. Not including these results could be potentially biasing the results presented towards significance for alternative trapping methods in each of the mode.
Discussion
This meta-analysis aimed to compare the mean difference of Anopheles mosquitoes captured by outdoor human landing catches comparative to alternative trapping methods. The initial pooled results showed substantial heterogeneity in the literature and no clear evidence that one alternative trap is best to replace outdoor HLC. As a follow-up three different moderator analyses were used to explain heterogeneity. When examining trap type as a moderator, tent traps in particular collected an overall higher average number of Anopheles than HLC. Since the majority of studies comparing HLC and alternative traps were conducted in Africa, moderator analysis was conducted comparing studies in Africa with those conducted elsewhere in the world. It is possible that additional studies showing HLC collecting more mosquitoes than alternative traps in Africa may have been conducted, but not published. Similarly, papers might not have shown up in the search or been outside of the range of search dates or inclusion criteria. When examining species as a moderator, the results indicated that in general, alternative traps collected more An. gambiae s.l. than HLC. A meta-regression showed that over 55% of the heterogeneity can be explained by a linear combination of these three variables and the interaction of Type and Species variables. Subset analysis the An. gambiae s.l. shows that light traps capture on average more mosquitoes than HLC. Results for the An. funestus s.l. did not have enough statistical power to detect significant differences, if they truly exist.
Limitations
Many of the results presented in this meta-analysis showed no significant difference in the mean number of Anopheles captured by outdoor HLC and alternative methods. To determine if this was truly equivalency and not just a lack of evidence, statistical methods such as two one-sided test analyses should be conducted. If equivalency is determined, no conversion factor would be needed. A major limitation in this approach is that the trap comparisons are based on the total number of Anopheles collected/night, and accurately estimating human biting risk remains a challenge. Alternative methods may collect more Anopheles than HLC, but this does not mean that they more accurately estimate biting risk. Therefore, if alter- native approaches are used to replace HLC, correction factors may be necessary to estimate biting risk for the calculation of EIR. However, a precise conversion factor for metrics such as EIR may not be necessary. While EIR is a very valuable entomological indicator, these values are dynamic and absolute EIR numbers may not be necessary when a relative EIR could suffice to inform vector control decisions. Future work testing for equivalency will provide additional information, but the quality of collections not just the quantity of collections should be considered in future work. Additionally, to further understand the malaria vector landscape, a metric could be developed to show how HLC and alternative traps perform in the context of vector control interventions. Issues arise if the relationship between HLC and trapping methods is not a monotonic relationship—such as linear. The importance of the interaction of species and trap type in the meta-regression implies that the idea of a single “calibration” factor that can be broadly translated across ecological contexts is unlikely.
It has been reported that CDC light traps in a rice irrigation area had a bias towards younger An. arabiensis mosquitoes as indicated by parity dissections [60]. Such parity is not considered in this analysis, but could be incorporated in future work to get a full picture of the landscape. If an individual study had multiple comparisons to outdoor HLC, each individual comparison was added. It is possible that by adding these individual comparisons in such a manner, the results could have an over representation of certain locations where alternative traps do better. Future work should try to incorporate a more robust selection of spatial variables and their subsequent interactions to help calculate the true spatial effect on heterogeneity.
It is worth reiterating that while all efforts were made to include eligible papers, some papers might have failed to be included due to limitations with search terms, such as papers with publication dates after the search period. For example, [61] show that new double net traps and human odor baited CDC-LT caught more female An. arabiensis than traditional CDC-LT; however, this was published in 2020 and was not included due to publication after the search period. Future meta-analyses could include information on female and male trapping collection rates for both alternative traps and outdoor HLC.
Many comparative studies are recording data over the course of several months to even years. Temporal variables such as collection during the rainy and dry season are difficult to incorporate under the current reporting methods, and so were not used during this meta-analysis. Such variables could play a large role in further explaining heterogeneity. Changing the reporting standard to include data from collections in publications would allow for the interaction of temporal variables and alternative trapping methods to be observed.
No prior knowledge of the known differences between the An. gambiae subspecies was incorporated into analysis. Anopheles arabiensis readily feed on cattle [62]. By assuming that all feeding behaviours of An. gambiae subspecies are the same, it biases the results towards the alternative methods by collecting vectors that more do not as readily feed on humans, not to mention that humans have a specific close, medium, and long-range attraction to mosquitoes that is often difficult to replicate. Future research endeavours should look at including feeding behaviours of the An. gambiae subspecies, if there is enough readily available data. In that same vein, more studies on trap types that specifically capture An. funestus s.l. are necessary to statistically determine which trap type has the best potential for capture, if one exists. It is possible that additional studies showing HLC collecting more An. funestus s.l. than alternative traps may have been conducted, but not published.
In addition to ethical considerations, there are also biases (known but mostly uncharacterized) that may influence HLC’s data. Therefore, the use of HLC must be either critically examined and understood or alternatives to HLC that ablate these biases should be sought. For example, collector bias may impact the number and quality of collections from HLC as individuals may have differing degrees of mosquito attraction, although this may be addressed to some extent with appropriate study design. Additionally, HLC collectors who are recruited and trained are mostly men between the ages of 20–50, while the populations most vulnerable to malaria are women and children. Vector control interventions are often selected for implementation based on data from HLC collections; however, the possibility of differential attraction between men and women and children may not be adequately considered. Alternative methods that can be used to address this bias may provide a broader understanding of vector bionomics.
To date, despite claims that HLC may put collectors at risk for infection with vector-borne pathogens, there is only one study that examined the safety risks of HLC for collectors, and this study focused exclusively on the risk of malaria [18]. In this work, the authors showed that when HLC collectors were provided with malaria prophylaxis, malaria incidence was lower than in non HLC collectors. However, no published study has reported the risk of mosquito collectors being exposed to other arthropod vectors or vector-borne pathogens. Without these data, it is not possible to conclusively state whether HLC collectors are at increased health risk or not.
Recommendations
When determining whether HLC should be replaced with alternative trapping tools, National Malaria Control Programmes (NMCPs) should consider key data needs and select collection tools based on these priorities. A recent publication [63] was developed to help guide decision-makers on how to select appropriate mosquito collection tools for malaria programme needs. To determine equivalency between HLC and alternative traps, two one-sided test analyses should be conducted. If equivalency is determined, no conversion factor would be needed. A major limitation in this approach is that the trap comparisons are based on the total number of Anopheles collected/night, and accurately estimating human biting risk remains a challenge. Alternative methods may collect more Anopheles than HLC, but this does not mean that they more accurately estimate biting risk. Therefore, if alternative approaches are used to replace HLC, correction factors may be necessary to estimate biting risk for the calculation of EIR. However, a precise conversion factor for metrics such as EIR may not be necessary. While EIR is a very valuable entomological indicator, these values are dynamic and absolute EIR numbers may not be necessary when a relative EIR could suffice to inform vector control decisions. Future work testing for equivalency will provide additional information.
Additionally, to further understand the malaria vector landscape, a metric could be developed to show how HLC and alternative traps perform in the context of vector control interventions.
For this study, the number of Anopheles spp. collected per trap per night was used since this was the standard metric represented in the literature when comparing traps to HLC. Although this is the standard, it is not necessarily the best approach. Not all Anopheles spp. are malaria vectors, and without a question driven approach to identify which traps are best for certain species, it is not possible to determine which trap will be best in certain situations. A question driven and resource directed approach to trapping mosquitoes for malaria surveillance is necessary. An entomological surveillance planning tool (ESPT) helps guide decision-makers in deciding which trap is best for specific questions related to malaria vector control [63]. This tool may also be used to determine which trapping methods can or should be com- pared in future studies. It is important to note that one major advantage of HLC is that they provide the ability to understand the specific location and biting times of human-seeking mosquitoes. To date, alternative traps cannot reliably replicate this. If the question driven approach is asking when and where mosquitoes bite, there may not be a fully suitable alternative to HLC. There also needs to be a way to minimize heterogeneity. Moving forward, studies should be designed considering target species and trap types. For example, there may be one group or trap type that collects more total numbers of An. gambiae s.l., such as the light trap (Table 6), which does not perform as well for An. funestus s.l.
Meta-regression methods show that a linear combination of these variables can explain over 55% of the statistical heterogeneity present. Future work should go towards addressing the questions of trap comparisons to HLC should use standardized and modified methods to help address the remaining heterogeneity. Standardization of methods for future meta-analytic work should account for these variables. Future studies should address heterogeneity variables and publication bias by focusing on questions that address trap types and species outcome. Results should be published regardless of whether findings indicate alternative traps perform better than HLC. When deciding on a collection tool, multiple traps should be used to determine which traps are ideal for specific species. Future studies should report results whether they are capturing more or fewer mosquitoes than HLC along with a corresponding variance metric. Reporting these data will allow for a more robust meta-analysis; as such standardized reporting is necessary for robustness. Temporal analysis of when the mosquitoes were captured was purposely left out of this study because of the lack of standardization of reporting make it impossible to synthesize. Standardization techniques in this area could add another moderator to control for heterogeneity and account for the effects of seasonal effects.
Conclusions
The results of the meta-regression show that a large percentage of the heterogeneity present in the analysis comes from variations of traps, locations, and species collected. There is not a consensus among publications in the field over whether a specific trap can be used as a “magic bullet” alternative to HLC. Even so, the high between-study heterogeneity and publication bias cannot be ignored. Instead, research on alternative traps should be conducted by performing question-driven studies to address which traps are best for which species. If programmes want to examine Anopheles spp. diversity in an area, different trapping tools may be necessary than for programmes that are just interested in a specific vector, such as An. gambiae s.l., or have a specific bionomic question. Rather than aiming to determine which alternative trap can replace HLC, the goal should instead be to identify the optimal trapping tool for question-driven collections needed to inform decisions about appropriate malaria control interventions or for basic research. A baseline assessment of mosquito collection tools relative to HLC in specific locations could be conducted to determine the best tools in specific contexts in response to indicator-driven questions by using the ESPT tool and evaluating results at regular intervals to determine representativeness [63]. In addition, very few studies evaluating collection tools when compared to HLC describe the vector control context and landscape in which the study is being conducted. For example, conducting a study in a context where a vector control tool such as mass distribution of ITNs is used is likely to influence mosquito biting and resting behaviour and the resulting entomological indicators compiled by mosquito collection tools. Under this framework, future meta-analyses could better characterize the landscape of malaria vector behaviour by reducing between-study heterogeneity, allowing for recommendations for malaria vector control interventions that are tailored to local vector ecology.
Availability of data and materials
The datasets compiled, used, and/or analysed during the current study are available from the corresponding author on request. R scripts used for analysis are available in the malaria meta analysis repository, https://github.com/JordanEckert/malariametaanalysis.
Abbreviations
- HLCs:
-
Human landing catches
- ESPT:
-
Entomological surveillance planning tool
References
Sallum MAM, Conn JE, Bergo ES, Laporta GZ, Chaves LSM, Bickersmith SA, et al. Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malar J. 2019;18:117.
Reiner RC Jr, Perkins TA, Barker CM, Niu T, Chaves LF, Ellis AM, et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J R Soc Interface. 2013;10:20120921.
Smith DL, Perkins TA, Reiner RC, Barker CM, Niu TC, Chaves LF, et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg. 2014;108:185–97.
Molineaux L, Muir DA, Spencer HC, Wernsdorfer WH. The epidemiology of malaria and its measurement. In: Wernsdorfer WH, McGregod IA, editors. Malaria: principles and practice of malariology, vol. 2. Edinburgh: Churchill Livingstone; 1988. p. 999–1089.
Rueda LM. Global diversity of mosquitoes (Insecta: Diptera: Culicidae) in freshwater. Hydrobiologia. 2008;595:477–87.
Killeen GF, Kihonda J, Lyimo E, Oketch FR, Kotas ME, Mathenge E, et al. Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect Dis. 2006;6:161.
Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62.
Zittra C, Vitecek S, Obwaller AG, Rossiter H, Eigner B, Zechmeister T, et al. Landscape structure affects distribution of potential disease vectors (Diptera: Culicidae). Parasit Vectors. 2017;10:205.
Li Y, Su X, Zhou G, Zhang H, Puthiyakunnon S, Shuai S, et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors. 2016;9:446.
Sanou A, Moussa Guelbeogo W, Nelli L, Hyacinth Toe K, Zongo S, Ouedraogo P, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar J. 2019;18:386.
Mathenge EM, Misiani GO, Oulo DO, Irungu LW, Ndegwa PN, Smith TA, et al. Comparative performance of the Mbita trap, CDC light trap and the human landing catch in the sampling of Anopheles arabiensis, An. funestus and culicine species in a rice irrigation in western Kenya. Malar J. 2005;4:7.
Govella NJ, Maliti DF, Mlwale AT, Masallu JP, Mirzai N, Johnson PC, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J. 2016;15:465.
Cansado-Utrilla C, Jeffries CL, Kristan M, Brugman VA, Heard P, Camara G, et al. An assessment of adult mosquito collection techniques for studying species abundance and diversity in Maferinyah, Guinea. Parasit Vectors. 2020;13:150.
Service MW. A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res. 1977;67:343–82.
Achee NL, Youngblood L, Bangs MJ, Lavery JV, James S. Considerations for the use of human participants in vector biology research: a tool for investigators and regulators. Vector Borne Zoonotic Dis. 2015;15:89–102.
Mboera LE. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan Health Res Bull. 2005;7:117–24.
Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–8.
Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, et al. Comparison of two adult mosquito sampling methods with human landing catches in south-central Ethiopia. Malar J. 2017;16:30.
Lima JB, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenco-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? A review. Mem Inst Oswaldo Cruz. 2014;109:685–705.
Briët OJT, Huho BJ, Gimnig JE, Bayoh N, Seyoum A, Sikaala CH, et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: a pooled-analysis of 13 comparisons with human landing catches. Malar J. 2015;14:247.
Mbogo CNM, Glass GE, Forster D, Kabiru IEW, Githure JI, Ouma JH. Evaluation of light traps for sampling anopheline mosquitoes in Kilifi. Kenya J Am Mosq Control Assoc. 1993;9:260–3.
Costantini C, Sagnon NF, Sanogo E, Merzagora L, Coluzzi M. Relationship to human biting collections and influence of light and bednet in CDC light-trap catches of West African malaria vectors. Bull Entomol Res. 1998;88:503–11.
Dia I, Diallo D, Duchemin J-B, Ba Y, Konate L, Costantini C, et al. Comparisons of human-landing catches and odor-baited entry traps for sampling malaria vectors in Senegal. J Med Entomol. 2005;42:104–9.
Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, et al. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J. 2010;9:292.
Maliti DV, Govella NJ, Killeen GF, Mirzai N, Johnson PCD, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14:502.
Davidson JR, Baskin RN, Hasan H, Burton TA, Wardiman M, Rahma N, et al. Characterization of vector communities and biting behavior in South Sulawesi with host decoy traps and human landing catches. Parasit Vectors. 2020;13:329.
Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, et al. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157.
Govella NJ, Chaki PP, Mpangile JM, Killeen GF. Monitoring mosquitoes in urban Dar es Salaam: evaluation of resting boxes, window exit traps, CDC light traps, Ifakara tent traps and human landing catches. Parasit Vectors. 2011;4:40.
Pollard EJM, Russell TL, Burkot TR. Maximising mosquito collections from barrier screens: the impacts of physical design and operation parameters. Parasit Vectors. 2019;12:31.
Meza FC, Kreppel KS, Maliti DF, Mlwale AT, Mirzai N, Killeen GF, et al. Mosquito electrocuting traps for directly measuring biting rates and host-preferences of Anopheles arabiensis and Anopheles funestus outdoors. Malar J. 2019;18:83.
Gorsich EE, Beechler BR, van Bodegom PM, Govender D, Guarido MM, Venter M, et al. A comparative assessment of adult mosquito trapping methods to estimate spatial patterns of abundance and community composition in southern Africa. Parasit Vectors. 2019;12:462.
Marquetti MC, Navarro A, Bisset J, Garcia FA. Comparison of three catching methods for collecting anopheline mosquitoes. Mem Inst Oswaldo Cruz. 1992;87:457–8.
Overgaard HJ, Saebo S, Reddy MR, Reddy VP, Abaga S, Matias A, Slotman MA. Light traps fail to estimate reliable malaria mosquito biting rates on Bioko Island, Equatorial Guinea. Malar J. 2012;11:56.
Lines JD, Curtis CF, Wilkes TJ, Njunwa KJ. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84.
Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–42.
Davis JR, Hall T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo, Tanzania. Med Vet Entomol. 1995;9:249–55.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clin Research Edn). 2021;372: n160.
Abong’o B, Yu X, Donnelly MJ, Geier M, Gibson G, Gimnig J, et al. Host Decoy Trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasit Vectors. 2018;11:533.
Adde AG. Mosquito magnet® liberty plus trap baited with octenol confirmed best candidate for Anopheles surveillance and proved promising in predicting risk of malaria transmission in French Guiana. Malar J. 2014;13:384.
Batista EPA, Ngowo H, Opiyo M, Shubis GK, Meza FC, Siria DJ, et al. Field evaluation of the BG-Malaria trap for monitoring malaria vectors in rural Tanzanian villages. PLoS ONE. 2018;13: e0205358.
Chaki PP, Mlacha Y, Msellemu D, Muhili A, Malishee AD, Mtema ZJ, et al. An affordable, quality-assured community-based system for high-resolution entomological surveillance of vector mosquitoes that reflects human malaria infection risk patterns. Malar J. 2012;11:172.
Davidson JR, Wahid I, Sudirman R, Makuru V, Hasan H, Arfah AM, et al. Comparative field evaluation of kelambu traps, barrier screens and barrier screens with eaves for longitudinal surveillance of adult Anopheles mosquitoes in Sulawesi, Indonesia. Parasit Vectors. 2019;12:399.
Duo-quan W, Lin-hua T, Zhen-cheng G, Xiang Z, Man-ni Y, Wei-kang J. Comparative evaluation of light-trap catches, electric motor mosquito catches and human biting catches of Anopheles in the Three Gorges Reservoir. PLoS ONE. 2012;7: e28988.
Gama RA, da Silva IM, Geier M, Eiras AE. Development of the BG-Malaria trap as an alternative to human-landing catches for the capture of Anopheles darlingi. Mem Inst Oswaldo Cruz. 2013;108:763–71.
Hiwat H, Andriessen R, de Rijk M, Koenraadt CJM, Takken W. Carbon dioxide baited trap catches do not correlate with human landing collections of Anopheles aquasalis in Suriname. Mem Inst Oswaldo Cruz. 2011;106:360–4.
Krajacich BJ, Slade JR, Mulligan RF, LaBrecque B, About H, Grubaugh ND, et al. Sampling host-seeking anthropophilic mosquito vectors in West Africa: comparisons of an active human-baited tent-trap against gold standard methods. Am J Trop Med Hyg. 2015;92:415–21.
Kweka EJ, Mahande AM. Comparative evaluation of four mosquitoes sampling methods in rice irrigation schemes of lower Moshi, northern Tanzania. Malar J. 2009;8:149.
Kweka EJ, Mwang’onde BJ, Kimaro E, Msangi S, Massenga CP, Mahande AM. A resting box for outdoor sampling of adult Anopheles arabiensis in rice irrigation schemes of lower Moshi, northern Tanzania. Malar J. 2009;8:82.
Missawa NA, Maria Ribeiro AL, Moreira Lima Maciel GB, Zeilhofer P. Comparison of capture methods for the diagnosis of adult anopheline populations from State of Mato Grosso. Brazil Rev Soc Bras Med Trop. 2011;44:555–60.
Sikaala CH, Chinula D, Chanda J, Hamainza B, Mwenda M, Mukali I, et al. A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malar J. 2014;13:225.
Sikaala CH, Killeen GF, Chanda J, Chinula D, Miller JM, Russell TL, et al. Evaluation of alternative mosquito sampling methods for malaria vectors in Lowland South—East Zambia. Parasit Vectors. 2013;6:91.
Team R. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
Pick JL, Nakagawa S, Noble DWA. Reproducible, flexible and high-throughput data extraction from primary literature: the metaDigitise R package. Methods Ecol Evol. 2019;10:426–31.
Lüdecke D. esc: effect size computation for meta analysis. 0.5.1 edition2019.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package for the Guide ‘Doing Meta-Analysis in R’. 0.0.9000 edition2019.
Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82.
Githeko AK, Service MW, Mbogo CM, Atieli FA, Juma FO. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera, Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in Western Kenya. Bull Entomol Res. 1994;84:319–24.
Degefa T, Yewhalaw D, Zhou G, Atieli H, Githeko AK, Yan G. Evaluation of human-baited double net trap and human-odour-baited CDC light trap for outdoor host-seeking malaria vector surveillance in Kenya and Ethiopia. Malar J. 2020;19:174.
Mburu MM, Zembere K, Mzilahowa T, Terlouw AD, Malenga T, van den Berg H, et al. Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi. Malar J. 2021;20:353.
Entomological Surveillance Planning Tool (ESPT). http://www.shrinkingthemalariamap.org/tool/entomological-surveillance-planning-tool-espt.
Acknowledgements
The authors would like to thank Ash Abebe and Alan Wilson for their discussions about meta-analysis and would like to sincerely thank Seth Irish, John Gimnig, Jenny Carlson, Heather Ferguson, Nicodem Govella, Fredros Okumu, Krijn Paaijmans, Frances Hawkes, and Brian Foy for fruitful discussions on HLCs and alternative trapping tools and help with the classifications of traps.
Disclaimer
The findings and conclusions in this manuscript are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or the U.S. President’s Malaria Initiative.
Funding
The authors have no conflict of interests to declare. SZ was supported by funding from the U.S. President’s Malaria Initiative.
Author information
Authors and Affiliations
Contributions
JE, SO, BM, and SZ conceived the study and participated in its design and coordination. JE, SO, YW, SJ, VP, and SZ reviewed the literature. JE conducted all statistical analysis and figures. JE, SO, NL, SZ wrote the manuscript. YW created maps. JE, SO, BM, YW, SJ, VP, NL and SZ participated in data interpretation and revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Discussion on trap category
Initial results did not include moderator analysis results using the trap ‘Category’ variable. This is because classification for each trap is highly subjective; there was a grey area where traps would fall but under new categorization that would not be an issue. A more formal, rigorous definition within the academic community would allow trap category to be used in further analysis.
When examining trap category as a moderator there is still heterogeneity, and no trap type showed a significant difference in Anopheles collected per night com- pared to outdoor HLC. When examining sub trap types, there were 25 studies that used biological traps and 22 of those were human-baited alternative traps. Although there is high heterogeneity, there is evidence that human-baited alternative traps capture significantly more Anopheles on average per night than HLC (g = − 0.3940). Multimodel inference added ‘Category’ and the interaction between ‘Category’ and ‘Species’ to the regression model, but the R2 value decreased slightly. It is highly likely that there is a significant correlation between ‘Type’ and ‘Category’; this is one possible explanation as to why the results from adding the new variables do not improve the heterogeneity explained. When analysis was performed on the An. gambiae and An. funestus subsets, neither subset offered statistically significant results. However, many categories in both the subsets lack the necessary studies synthesized for statistical power.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eckert, J., Oladipupo, S., Wang, Y. et al. Which trap is best? Alternatives to outdoor human landing catches for malaria vector surveillance: a meta-analysis. Malar J 21, 378 (2022). https://doi.org/10.1186/s12936-022-04332-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04332-1