Abstract
Background
Anopheles arabiensis, member species of the Anopheles gambiae complex, is the primary vector of malaria and is widely distributed in Ethiopia. Anopheles funestus, Anopheles pharoensis and Anopheles nili are secondary vectors occurring with limited distribution in the country. Indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) are pillars for the interventions against malaria control and elimination efforts in Ethiopia. However, the emergence and widespread of insecticide resistance in An. gambiae sensu lato (s.l.), might compromise the control efforts of the country. The aim of this study was to investigate composition of mosquito fauna and insecticide resistance status of An. gambiae s.l. in Itang special district ( woreda), Gambella, southwestern Ethiopia.
Methods
Adult mosquitoes were sampled from September 2020 to February 2021 using the CDC light trap and pyrethrum spray catch (PSC). CDC light traps were placed in three selected houses for two consecutive days per month to collect mosquitoes indoor and outdoor from 6:00 P.M. to 06:00 A.M. and PSC was used to collect indoor resting mosquitoes from ten selected houses once in a month from October 2020 to February 2021. Moreover, mosquito larvae were also collected from different breeding sites and reared to adults to assess susceptibility status of populations of An. gambiae s.l. in the study area. Susceptibility tests were conducted on two to three days old non blood fed female An. gambiae s.l. using insecticide impregnated papers with deltamethrin (0.05%), alpha-cypermethrin (0.05%), propoxur (0.1%), pirimiphos-methyl (0.25%) and bendiocarb (0.1%) following World Health Organization (WHO) standard susceptibility test procedure. Molecular diagnostics were done for the identification of member species of An. gambiae s.l. and detection of knockdown resistance (kdr) allele using species specific polymerase chain reaction (PCR) and allele specific PCR.
Results
In total, 468 adult mosquitoes were collected from different houses. Culex mosquitoes were the most dominant (80.4%) followed by Anopheles mosquitoes. Three species of Anopheles (Anopheles coustani, An. pharoensis, and An. gambiae s.l.) were identified, of which An. coustani was the dominant (8.1%) species. Higher number of mosquitoes (231) were collected outdoor by CDC light traps. Out of 468 adult mosquitoes, 294 were blood fed, 46 were half-gravid and gravid whereas the remaining 128 were unfed. WHO bioassay tests revealed that the populations of An. gambiae s.l. in the study area are resistant against alpha-cypermethrin and deltamethrin, but susceptible to bendiocarb, pirimiphos-methyl and propoxur. Of the total 86 An. gambiae s.l. specimens assayed, 79 (92%) successfully amplified and identified as An. arabiensis. West African kdr (L1014F) mutation was detected with high kdr allele frequency ranging from 67 to 88%.
Conclusion
The detection of target site mutation, kdr L1014F allele, coupled with the phenotypic resistance against alpha-cypermethrin and deltamethrin call for continuous resistance monitoring.
Similar content being viewed by others
Background
In Africa, malaria is vector-borne disease caused by four Plasmodium species, namely Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, and Plasmodium vivax. The parasite is transmitted to human through the bite of infective female Anopheles mosquito [1]. There are over 445 recognized species of Anopheles mosquito of which around 70 species are potential malaria vectors [2]. The major vectors are Anopheles gambiae and Anopheles funestus species complexes, but there are also a number of primary and secondary vectors that contribute to the malaria transmission [2].
Approximately 60% of the Ethiopian populations live in malaria risk areas [3]. The disease primarily occurs up to the 2000 m elevation, but can also occasionally affect areas over 2000 m elevation in response to the spatial and temporal changes [4, 5]. Malaria transmission is unstable and seasonal which produces little immunity in the community; hence malaria epidemics are common and lead to high mortality and morbidity [3].
In Ethiopia, there are 47 documented Anopheles mosquito species [6], of which only four species are malaria vectors. Anopheles arabiensis, member species of the An. gambiae complex, is the primary vector of malaria widely distributed in the country [7, 8]. Anopheles funestus, Anopheles pharoensis and Anopheles nili are secondary vectors occurring with varying population densities, limited distribution and vector competence [3]. Very recently, an invasive Anopheles species, Anopheles stephensi, has been documented in the country [9], which might complicate the malaria elimination effort of the country [10].
Chemical based vector control intervention is the pillar strategy to combat malaria. Indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) are instrumental for the significant reduction of malaria morbidity and mortality [11]. However, the emergence and widespread of insecticide resistance in the major malaria vectors might compromise effectiveness of chemical based (IRS and LLINs) interventions against malaria control and elimination efforts [12,13,14,15,16,17].
Insecticide resistance has become a serious challenge for the control and elimination of malaria due to the fact that malaria vectors are resistant to the four commonly used insecticide classes (pyrethroids, organochlorines, carbamates and organophosphates) [11, 17]. In Ethiopia, DDT resistance by An. gambiae s.l. was reported in the 1990s; since then widespread DDT resistance was documented throughout the country [12, 13, 18,19,20,21]. Moreover, resistance against other classes of insecticides, such as carbamates (bendiocarb), organophosphates (malathion) and pyrethroids (permethrin, deltamethrin) has been reported from various regions of the country [21, 22].
In the last decade, the number of malaria cases has declined due to a high coverage of IRS and scaling-up of LLINs [11, 17]. The national malaria control and elimination programme of Ethiopia has developed a malaria elimination road map to eliminate malaria from the country by 2030 [23]. However, this plan might be compromised as the magnitude of resistance against several insecticides is increasing in the An. gambiae s.l. populations [11, 21, 22].
Insecticide resistance largely caused by two major mechanisms. The first one is due to target-site insensitivity as a result of mutations in the target site of the insecticide that changes binding. The second mechanism is metabolic based resistance, where the insecticide is either degraded, sequestered or transported/excreted out of the cell before binding to the target site [24, 25].
Target-site and metabolic based resistance mechanisms operating in malaria vectors in several malaria endemic African countries have been documented. Knockdown resistance (kdr) is target site mutations in the voltage-gated sodium channel gene of mosquito nerve membranes is associated with DDT and pyrethroids resistance. In Anopheles, this involves the substitution of leucine (TTA) to phenylalanine (TTT) (kdr L1014F) or to serine (TCA) (kdrL1014S) [26, 27]. In addition, substitution of asparagine to tyrosine (N1575Y) is linked with resistance in An. gambiae sensu stricto (s.s.) [28], but not in An. arabiensis [21]. There is also an acetylcholinesterase gene (ace-1R) mutation, substitution of glycine (GGC) to a serine (AGC) which confers resistance to organophosphates and carbamates [29]. In Ethiopia, target site resistance mechanism, kdr L1014F (West Africa kdr), in populations of An. arabiensis documented in several populations across the country [12, 13, 19, 21, 22]. Metabolic based resistance in Anopheles mosquitoes has been reported from several countries in Africa [30,31,32,33]. Moreover, modifications in the cuticle either through cuticle thickening and/or altering of the cuticle composition of arthropods, which can slow down the penetration of chemical compounds [34,35,36] has also been reported in Anopheles populations [37].
Gambella is one of the malarious areas of Ethiopia with high malarial endemicity. Itang special district is known for its a stable form of malaria transmission [38]. Despite the current effort of the country, malaria incidence rate in Itang did not decline unlike many other malarious areas of Ethiopia [39]. Therefore, this study aimed to investigate the composition mosquito fauna and insecticide resistance status of An. gambiae s.l. in Itang, and detect target site mutations associated with DDT and pyrethroid resistance.
Methods
Study area
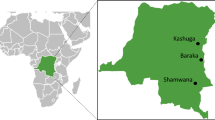
Gambella is administrative regional state located in south western Ethiopia 777 km away from Addis Ababa (Fig. 1). Most of the region is flat, hot, and humid, with an altitude range of 300–2300 m above sea level and sloping westward. The annual average temperature of the region is 21.1–35.9 °C, with an average annual rainfall of 600 mm .The region has a total area of 25,802 km2 and administratively divided into three Zones (Nuwer, Agnua, Mezeng) and one special district, Itang [40]. According to the 2017 Ethiopian population projection, Gambella is sparsely populated with the total population approximately 436,000 [41].
The study was conducted from September 2020-Feburary 2021 in Itang special district, which is situated 42 km to the west of the regional capital of Gambella. Itang has 23 kebeles, with estimated total population of 45,772 and 9154 households [41]. The district’s average annual temperature and rainfall are 29 °C and 1000 mm, respectively. The climatic conditions of the district are favourable for the existence of a stable form of malaria throughout the year [38].
Mosquito rearing
Potential mosquito breeding habitats (Baro river fringes, paddle or farming field ditches, sewerage, and stagnant water pools) were first inspected for the presence of mosquito larvae and positive habitats were sampled with a 350 ml capacity mosquito scoop. Dipping was done following World Health Organization (WHO) guidelines and standard operating procedures for entomological surveillances [42]. Anopheline mosquito larvae were sampled from various breeding sites, and kept in a room at constant 80% ± 10% RH and 27 ± 2 °C. The larvae were fed baking powder. The pupae were sorted and transferred to beakers and placed inside a cage adults to emerge. Adults were provided 10% sugar in the cage and, 2–3 days old female mosquitoes were used for insecticide susceptibility tests. The anopheline larvae collected from different breeding sites were not used for species composition analysis.
Adult mosquito collection
CDC Light trap catches (LT)
Adult Anopheles were collected each month from September 2020 to February 2021 using CDC light traps from selected houses in study area. CDC light traps were placed in three selected houses indoor and outdoor from 6:00 P.M. to 06:00 A.M. to collect female Anopheles for two consecutive days per month from September 2020 to February2021. Due to scarcity of CDC light traps only three houses were selected for adult collections.
Pyrethrum spray catch (PSC)
In addition to CDC light traps, indoor resting mosquitoes were collected using PSC from ten selected houses from 6:00 A.M. to 9:00 A.M. once in a month from October 2020 to February 2021. Before spray the whole house was covered by white sheet and closed every opening such as windows, door, and others. After fifteen minute of spraying knockdown mosquitoes were collected and recorded and separately placed in Eppendorf tube. Anopheles were morphologically identified to the species using taxonomic keys [43, 44].
Insecticide susceptibility test
Two to three days old non blood fed adult females morphologically identified as An. gambiae s.l. (from now on referred to as An. gambiae) were exposed to insecticide impregnated papers with discriminating doses of deltamethrin (0.05%), alpha-cypermethrin (0.05%) propoxur (0.1%) pirimiphos-methyl (0.25%), bendiocarb (0.1%) and control papers impregnated with oil. Insecticides were selected based on their current operational significance in the national malaria control programme. Pirimiphos-methyl, propoxur and bendiocarb are currently used for IRS in Ethiopia and pyrethroid is incorporated in LLINs. The insecticide impregnated and control papers were obtained from the WHO collaboration Centre, Vector Control Research Unit, School of Biological Sciences, Penang, Malaysia. The insecticides were first tested on susceptible strain An. arabiensis collected from Jimma University Tropical and Infectious Disease Research Center insectary to assure the quality of the impregnated papers. Then, the susceptibility test was carried out on non-blood fed female mosquitoes collected and reared from the study area. Batches of 25 mosquitoes in four replicates were exposed in test kit tubes with insecticide-impregnated papers and a control in two replicates, each with equal number of mosquitoes, exposed to papers impregnated with silicone oil was run in parallel for all bioassays for 1 h. Knockdown were recorded at 10, 15, 20, 30, 40, 50, and 60 min [45]. After 1 h, mosquitoes were transferred into holding tubes and provided with 10% sucrose solution with soaked cotton pads. Mortality was recorded 24 h post exposure. Mosquitoes, both dead and alive, were individually preserved in Eppendorf tubes over silica-gel for further molecular assays. The same numbers of mosquitoes were exposed to insecticide free papers as controls.
DNA extraction of An. gambiae
Genomic DNA of individual Anopheles mosquito was extracted from 75 survived and 11 dead mosquitoes (sampled from mosquitoes exposed to alpha-cypermethrin and deltamethrin) using DNAzol reagent (MRCgene, USA) with minor modification of the protocol [46]. Due to limitations of reagents and consumables fewer samples of mosquitoes were used for the molecular assays.
Molecular identification of An. gambiae s.l.
Morphologically identified An. gambiae female mosquitoes (alpha-cypermethrin and deltamethrin survived and dead mosquitoes) were selected for the molecular identification of members of An. gambiae species complex using species specific polymerase chain reaction (PCR) at Molecular Biology Laboratory, Tropical and Infectious Diseases Research Centre (TIDRC), Sekoru, Jimma University. Anopheles arabiensis susceptible colony strain was used as a positive control. The PCR assay was done adapting the established protocol [47]. In brief, PCR reaction was prepared for 20 µl final volume of 10 µl master mix, 0.5 µl of each primer (Universal primer (5′-GTGTGCCCCTTCCTCGATGT-3′), An. arabiensis (5′-AAGTGTCCT TCTCCATCCTA-3′), An. amharicus (5′-CAGACCAAGATGGTTAGTAT-3′)), 7.5 µl nuclease free water and 1 µl template DNA. The PCR program was set for an initial step at 94 °C for 10 min and then, for 30 cycles at 94 °C, 50 and 72 °C for 30 s each, respectively and a final extension step at 72 °C for 5 min. Finally, the band size of PCR products for each species was visualized on a 2% agarose gel.
Detection of L1014F (kdr allele)
Allele specific PCR assay was conducted on the same specimens used for the identification of member species of An. gambiae s.l. The presence of West Africa kdr (L1014F) mutation was detected adapting the established protocols [26, 27]. In brief, the PCR was done with 25 µl final volume including 2.5 µl PCR buffer, 0.5 µl dNTP mixture, 0.75 µl MgCl, 0.8 µl Agd 1 (5′-ATAGATTCCCCGACCATG-3′) primer, 0.8 µl Agd 2(5′-AGACAAGGATGATGAACC-3′) primer, 1.5 µl Agd3(5′-AATTTGCATTACTTACGACA-3′) primer, 1.5 µl Agd4 (5′-CTGTAGTGATAGGAAATTTA-3′) primer, 0.15 µl Taq DNA polymerase and 1 µl DNA template to detect kdr L1014F allele. The following PCR program was set 94°C for 3 min, and 94 °C for 1 min, 52 °C for 30 s, 72 °C for 30 s for 40 cycles and a final extension step at 72 °C for 10 min. Finally, the band size of the PCR products for kdr allele was visualized on a 2% agarose gel to determine the genotype to homozygous resistant, heterozygous resistant and susceptible or wild type kdr allele. Due to reagents and scarcity of consumables more samples were not analysed. Moreover, East Africa kdr (L1014S) allele was not screened.
Data analysis
Susceptibility status data was analysed based on the WHO 2016 classification criteria. As per the criteria 24 h mortality rate 98% and above considered fully susceptible; between 90 and 98%, possible resistance or suspected resistance; and mortality below 90% classified as resistant. When the control mortality was between 5 and 20%, the average observed mortality was corrected using Abbott’s formula [48]. When the control mortality was above 20%, the test result was discarded and the test was repeated.
Results
Mosquito densities and species composition
In total, 468 mosquitoes were collected using CDC light trap and PSC collection methods from different houses (Table 1). The majority of mosquitoes were Culex spp. (80.4%) followed by Anopheles mosquitoes. Three species of Anopheles mosquitoes, such as Anopheles coustani, An. pharoensis, and An. gambiae s.l. were identified, of which An. coustani was dominant among others (8.1%; n = 38) (Table 1). As shown in Table 1 the higher number of mosquito was collected outdoor by CDC light traps-night.
Abdominal status of different mosquito species
The abdominal conditions of all collected mosquito samples were classified into unfed, freshly fed, half gravid, and gravid. Out of 468 adult mosquitoes collected, 294 were fed while 46 were half-gravid and gravid (Table 2).
Insecticide resistance status of An. gambiae s.l. against different insecticides
As per WHO criterion, the local mosquito populations of An. gambiae s.l. were resistant to two groups of pyrethroid insecticides (deltamethrin and alpha-cypermethrin). Mortality rates of An. gambiae s.l. against deltamethrin and alpha-cypermethrin was 58% and 42%, respectively. However, the populations of An. gambiae s.l. were completely susceptible to pirimiphos-methyl, propoxur and bendiocarb, where 100% mortality rate was recorded for the three insecticides (Table 3).
Molecular identification of An. gambiae s.l. and detection of L1014F kdr allele
Out of the total 86 An. gambiae s.l. samples assayed using species specific PCR, 79 (92%) of the specimens were successfully amplified and genotyped, and all were identified as An. arabiensis. The allele specific PCR revealed the presence of the knock-down resistance (kdr) L1014F allele with 92.7% (n = 51) homozygous and 7.3% (n = 4) heterozygous kdr resistance allele respectively with the kdr allele frequency ranging from 67 to 88% (Table 4).
Discussion
Anopheles coustani, An. pharoensis and An. gambiae s.l. were the three species identified from Itang, southwestern Ethiopia. Of all Anopheles mosquitoes, An. coustani was the most prominent species followed by An. pharoensis and An. gambiae s.l. In the study site, the abundance of mosquito species collected by CDC light trap was higher outdoor than indoor. Unlike many other localities in Ethiopia [49,50,51], the abundance of An. coustani dominated An. gambiae s.l. A study from Lare, Gambella documented higher density of An. gambiae than An. coustani [50], contrasting the result of the current study. This difference might be due to sampling period difference which leads to variation or shifts in density for various species of mosquitoes. Taye and his coauthors sampled mosquitoes from May to September 2017 whereas in our study sampling time was between September and February. Moreover, the difference might also be due to the type of breeding habitat. The breeding site where the Anopheles mosquitoes sampled was shore to the Baro River and due to this reason the breeding sites were covered by plants and shaded the water which might be favorable for An. coustani [52]. Very recently, higher density of An. coustani than An. gambiae s.l. populations were reported from non-irrigated swampy and river edges in Arjo Didessa, south west Ethiopia [53]. Unlike An. coustani, An. gambiae s.l. typically breeds in small, clear, sunlit temporary water pools [54] where vegetative cover is low [52, 55]. In Ethiopia the role of An. coustani in malaria transmission not clearly documented. Very recently, An. coustani, was found being infected with Plasmodium species [56] showing evidence of possible malaria transmission by An. coustani in the country. Therefore, the high abundance of An. coustani in Itang might call attention for further studies [57, 58].
The molecular identification of member species of An. gambiae complex confirmed that An. arabiensis is the only member species found in the study area. This finding is similar to previously reported studies from central, south west and southern parts of Ethiopia [10, 17, 19, 20]. Anopheles arabiensis populations from the study area were found highly resistant to deltamethrin like other populations of An. arabiensis from different localities of Ethiopia [10, 19, 20] Sudan [59] and Uganda [60]. The present study also showed that An. arabiensis populations were resistant to alpha-cypermethrin. Similarly, studies from South-West Ethiopia [61] and Congo [62] reported very high resistance to pyrethroids, but susceptible to bendiocarb, propoxur and pirimiphos-methyl. In contrast, populations of An. arabinesis form Malawi were found susceptible to alpha-cypermethrin [63].
The population of An. arabiensis collected from Itang found fully susceptible to bendiocarb and propoxur. This report is in agreement with other studies from different regions in Ethiopia [11, 19], Sudan [59], Yemen [64], Burkina Faso and Chad [65]. However, unlike the current finding, resistance against bendiocarb has been developed by An. arabiensis populations in some other parts of Ethiopia [17, 20]. The current result is similar to previously reported studies from different parts of Ethiopia [11, 66, 67] and Sudan [59] where propoxur fully killed An. gambiae s.l. However, in some other parts of Ethiopia resistance against propoxur has been observed [19, 20]. Similar to propoxur and bendiocarb the An. arabiensis populations from the study area were found susceptible to pirimiphos-methyl. This is similar with studies from different parts of Ethiopia [11, 19], Uganda [68] and Congo [62].
In this study, the population of An. arabiensis were screened for West African kdr allele (L1014F). A very high frequency of the West African kdr allele (L1014F), was observed with higher kdr allele indicating that target site resistance mechanism might contribute for the observed high level of pyrethroid (deltamethrin and alpha-cypermethrin) resistance in the population. This is similar with populations of An. arabiensis in some other areas of Ethiopia [11, 17, 19, 20]. The mutation, L1014F, is widespread in West and West Central Africa [69, 70] also becoming common in East African countries including Sudan [71]; Tanzania [72, 73] and Kenya [74, 75]. The L1014F kdr mutation in the voltage gated sodium channel is a target point mutation against pyrethroids and DDT [25, 76] which might occurred as a result of long and extensive use of DDT and pyrethroids for ITNs.
LLINs and IRS are the two most widely implemented malaria vector control interventions, and have resulted in a significant reduction of malaria-related mortality and morbidity in Ethiopia [9, 39, 77]. Universal access and coverage of LLINs by all household members regardless of age or gender [78] is not yet achieved in Ethiopia [78,79,80].
Conclusion and recommendations
Overall, this study demonstrated that An. coustani was the predominant Anopheles species recorded among adult Anopheles mosquito collected during the study period in the study area. The high abundance of the species in the study area might call attention to further study the species for its role in malaria transmission. It also revealed that An. arabiensis, a member of An. gambiae complex, has developed high level of resistance against deltamethrin and alpha-cypermethrin. Moreover, target site mutation L1014F kdr allele with high frequency was detected in the populations.
For the success of malaria elimination from Ethiopia by 2030 [3], it is important to control the malaria vectors and focus on successful treatment. Therefore, understanding the type and density of malaria vector species in specific localities, their feeding behavior and interaction with humans is crucial for effective malaria vector control strategies. Moreover, evidence based insecticide susceptibility status of the malaria vector populations and understanding mechanisms of resistance operating in the populations is key for effective insecticide resistance management.
Availability of data and materials
Data used for this study are included in the manuscript.
Abbreviations
- CCEs:
-
Carboxylcholinesterases
- CDC:
-
Center for Diseases Control and Prevention
- CSA:
-
Central Statistics Authority
- DDT:
-
Dichlorodiphenyltrichloroethane
- DNA:
-
Deoxyribonucleic acid
- FMoH:
-
Federal Minister of Health
- GSTs:
-
Glutathione S-transferases
- ITNs:
-
Insecticide-treated nets
- kdr :
-
Knock down resistance
- LLINs:
-
Long-lasting insecticidal nets
- MSF:
-
Medicins sans Frontières
- OCs:
-
Organochlorines
- Ops:
-
Organophosphates
- P450s:
-
Cytochrome-P450 monooxygenase
- PSC:
-
Pyrethrum Spray Catches
- PY:
-
Pyrethroids
- SPSS:
-
Statistical package for the social sciences
- VGSC:
-
Voltage gated sodium channel
- WHO:
-
World Health Organization
References
Cox FE. History of the discovery of the malaria. Parasit Vectors. 2010;3:5.
Sinka ME, Banks MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphab T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69.
Guidelines FMoHNMalaria, Fourth edition. 2017;1–108. https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/eth_national_malaria_guidline_4th_edition.pdf.
Lyon B, Dinku T, Raman A, Thomson MC. Temperature suitability for malaria climbing the Ethiopian Highlands. Environ Res Lett. 2017;12:064015.
Haile M, Lemma H, Weldu Y. Population movement as a risk factor for malaria infection in high-altitude villages of Tahtay-Maychew District, Tigray, Northern Ethiopia: a case-control study. Am J Trop Med Hyg. 2017;97:726–32.
Gaffigan TV, Wilkerson RC, Pecor JE, Stoffer JA, Anderson T. Systematic Catalog of Culicidae. Walter Reed Biosystematics Unit, Walter Reed Army Institute of Research, Silver Spring. 2018. http://www.mosquitocatalog.org. Accessed 22 Mar 2021.
Lulu M, Hadis M, Makonnen Y, Asfaw T. Inversion polymorphisms on Anopheles arabiensis chromosomes from several regions of Ethiopia. Insect Sci its Appl. 1999;19:207–10.
Abose T, Ye-ebiyo Y, Olana D, Alamirew D, Beyene Y, Regassa L, et al. Re-orientation and definition of the role of malaria vector control in Ethiopia. WHO/MAL/981085. Geneva, World Health Organization; 1998.
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6.
Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J. 2021;20:263.
WHO. World Malaria Report 2020 [Internet]. Geneva. Organization WH. 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020.
Yewhalaw D, Van Bortel W, Denis L, Coosemans M, Duchateau L, Speybroeck N. First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am J Trop Med Hyg. 2010;83:122–5.
Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, van Bortel W, Denis L, et al. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS ONE. 2011;6:e16066.
Nkya TE, Akhouayri I, Poupardin R, Batengana B, Mosha F, Magesa S, et al. Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: a case study in Tanzania. Malar J. 2014;13:28.
Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS ONE. 2014;9:e110058.
Chanda E, Hemingway J, Kleinschmidt I, Rehman AM, Ramdeen V, Phiri FN, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS One. 2011;6:e24336.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010;3:40.
Yewhalaw D, Asale A, Tushune K, Getachew Y, Duchateau L, Speybroeck N. Bio-efficacy of selected long-lasting insecticidal nets against pyrethroid resistant Anopheles arabiensis from South-Western Ethiopia. Parasit Vectors. 2012;5:159.
Alemayehu E, Asale A, Eba K, Getahun K, Tushune K, Bryon A, et al. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasit Vectors. 2017;10:407.
Messenger LA, Shililu J, Irish SR, Anshebo GY, Tesfaye AG, Ye-Ebiyo Y, et al. Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): a nationwide study for insecticide resistance monitoring. Malar J. 2017;16:469.
FMoH. Malaria Operational Plan FY. 2016. President’s Malaria Initiative Ethiopia. 2016;1–76.
Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59.
Feyereisen R, Dermauw W, Van Leeuwen T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol. 2015;121:61–77.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA. 2012;109:6614–9.
Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7.
Vulule JM, Beach RF, Atieli FK, Mcallister JC, Brogdon WG, Roberts JM, et al. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Med Vet Entomol. 1999;13:239–44.
Matowo J, Kulkarni MA, Mosha FW, Oxborough RM, Kitau JA, Tenu F, et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malar J. 2010;9:193.
Edi CV, Djogbénou L, Jenkins AM, Regna K, Muskavitch MAT, Poupardin R, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10:e1004236.
Ibrahim SS, Riveron JM, Stott R, Irving H, Wondji CS. The cytochrome P450 CYP6P4 is responsible for the high pyrethroid resistance in knockdown resistance-free Anopheles arabiensis. Insect Biochem Mol Biol. 2016;68:23–32.
Balabanidou V, Grigoraki L, Vontas J. Insect cuticle: a critical determinant of insecticide resistance. Curr Opin Insect Sci. 2018;27:68–74.
Balabanidou V, Kampouraki A, Maclean M, Blomquist GJ, Tittiger C, Juárez MP, et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc Natl Acad Sci USA. 2016;113:9268–73.
Balabanidou V, Kefi M, Aivaliotis M, Koidou V, Girotti JR, Mijailovsky SJ, et al. Mosquitoes cloak their legs to resist insecticides. Proc R Soc B Biol Sci. 2019;286:20191091.
Simma EA, Dermauw W, Balabanidou V, Snoeck S, Bryon A, Clark RM, et al. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag Sci. 2019;75:1808–18.
FMoH. National Malaria Control Team, Ethiopian Public Health Institute, World Health Organization, Addis Ababa University and the INFORM Project. An epidemiological profile of malaria in Ethiopia. A report prepared for the Federal Ministry of Health, Ethiopia, the roll Back malaria partnership and the department for international development, UK; 2014.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103.
Demeke D, Legesse M, Bati J. Trend of tuberculosis and treatment outcomes in Gambella region with special emphasize on Gambella Regional Hospital, Western Ethiopia. Mycobact Dis. 2013;3:2.
CSA. Population Projection of Ethiopia for All Regions, Wereda Level from 2014–2017; Central Statistical Agency. 2018.
WHO. Entomological field techniques for malaria control. Part I. Learners guide. Geneva, World Health Organization; 1992.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. S Afr Inst Med Res. 1987;55:1–43.
Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2nd Edn. Geneva, World Health Organization; 2016.
Asghar U, Malik MF, Anwar F, Javed A, Raza A. DNA extraction from insects by using different techniques: a review. Adv Entomol. 2015;3:132–8.
Fanello C, Santolamazza F, Della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–4.
Abbott WS. A method of computing the effectiveness of insecticide. J Econom Entomol. 1925;18:265–7.
Adugna T, Getu E, Yewhalaw D. Species diversity and distribution of Anopheles mosquitoes in Bure district, Northwestern Ethiopia. Heliyon. 2020;6:e05063.
Taye B, Seid M, Gindaba A. Entomological study on species composition, behavior, longevity and probability of surviving sporogony of Anopheles mosquitoes in Lare District, Ethiopia. J Parasitol Vector Biol. 2017;9:137–45.
Getachew D, Gebre-Michael T, Balkew M, Tekie H. Species composition, blood meal hosts and Plasmodium infection rates of Anopheles mosquitoes in Ghibe River Basin, southwestern Ethiopia. Parasit Vectors. 2019;12:257.
Kiszewski AE, Teffera Z, Wondafrash M, Ravesi M, Pollack RJ. Ecological succession and its impact on malaria vectors and their predators in borrow pits in western Ethiopia. J Vector Ecol. 2014;39:414–23.
Hawaria D, Demissew A, Kibret S, Lee MC, Yewhalaw D, Yan G. Effects of environmental modification on the diversity and positivity of anopheline mosquito aquatic habitats at Arjo-Dedessa irrigation development site, Southwest Ethiopia. Infect Dis Poverty. 2020;9:9.
Kenea O, Balkew M, Gebre-Michael T. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the rift valley, Central Ethiopia. J Vector Borne Dis. 2011;48:85–92.
Aklilu E, Kindu M, Gebresilassie A, Yared S, Tekie H, Balkew M. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in Metema District, Northwestern Ethiopia. J Arthropod Borne Dis. 2020;14:153–61.
Bamou R, Rono M, Degefa T, Midega J, Mbogo C, Ingosi P, et al. Entomological and anthropological factors contributing to persistent malaria transmission in Kenya, Ethiopia, and Cameroon. J Infect Dis. 2021;223:155–70.
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:114.
Stephen A, Nicholas K, Busula AO, Webale MK, Omukunda E. Detection of Plasmodium sporozoites in Anopheles coustani s.l.: a hindrance to malaria control strategies in highlands of western Kenya. BioRxiv. 2021.
Abdalla H, Wilding CS, Nardini L, Pignatelli P, Koekemoer LL, Ranson H, et al. Insecticide resistance in Anopheles arabiensis in Sudan: temporal trends and underlying mechanisms. Parasit Vectors. 2014;7:213.
Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16.
Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West Ethiopia. Parasit Vectors. 2013;6:44.
Loonen JACM, Dery DB, Musaka BZ, Bandibabone JB, Bousema T, van Lenthe M, et al. Identification of main malaria vectors and their insecticide resistance profile in internally displaced and indigenous communities in Eastern Democratic Republic of the Congo (DRC). Malar J. 2020;19:425.
PMI. Malawi Entomological Monitoring 2017 Final Report: Anopheles species abundance, sporozoite infections and insecticide resistance. 2017;1–30.
Al-Koleeby Z, El Aboudi A, Assada M, Al-Hadi M, Abdalr Ahman M, Awash A, et al. The current insecticide resistance in main malaria vector Anopheles arabiensis in Yemen. J Trop Med. 2020;2020:5625019.
Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbé C, Yangalbé-Kalnoné E, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J. 2009;8:299.
Deressa W, Loha E, Balkew M, Hailu A, Gari T, Kenea O, et al. Combining long-lasting insecticidal nets and indoor residual spraying for malaria prevention in Ethiopia: study protocol for a cluster randomized controlled trial. Trials. 2016;17:20.
Abate A, Getu E, Wale M, Hadis M, Mekonen W. Impact of bendiocarb 80% WP indoor residual spraying on insecticide resistance status of Anopheles arabiensis. Ethiop J Sci Technol. 2020;13:215–28.
Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, Eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013;27:276–83.
Koffi AA, Ahoua Alou LP, Adja MA, Chandre F, Pennetier C. Insecticide resistance status of Anopheles gambiae s.s population from M’Bé: a WHOPES-labelled experimental hut station, 10 years after the political crisis in Côte d’Ivoire. Malar J. 2013;12:151.
Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A, et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7:74.
Nardini L, Christian RN, Coetzer N, Ranson H, Coetzee M, Koekemoer LL. Detoxification enzymes associated with insecticide resistance in laboratory strains of Anopheles arabiensis of different geographic origin. Parasit Vectors. 2012;5:113.
Kulkarni MA, Rowland M, Alifrangis M, Mosha FW, Matowo J, Malima R, et al. Occurrence of the leucine-to-phenylalanine knockdown resistance (kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method. Malar J. 2006;5:56.
Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, et al. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health. 2014;19:331–41.
Owuor KO, Machani MG, Mukabana WR, Munga SO, Yan G, Ochomo E, et al. Insecticide resistance status of indoor and outdoor resting malaria vectors in a highland and lowland site in Western Kenya. PLoS ONE. 2021;16:e0240771.
Ochomo E, Subramaniam K, Kemei B, Rippon E, Bayoh NM, Kamau L, et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasit Vectors. 2015;8:616.
Dabiré KR, Diabaté A, Namountougou M, Toé KH, Ouari A, Kengne P, et al. Distribution of pyrethroid and DDT resistance and the L1014F kdr mutation in Anopheles gambiae s.l. from Burkina Faso (West Africa). Trans R Soc Trop Med Hyg. 2009;103:1113–20.
Otten M, Aregawi M, Were W, Karema C, Medin A, Bekele W, et al. Initial evidence of reduction of malaria cases and deaths in Rwanda and Ethiopia due to rapid scale-up of malaria prevention and treatment. Malar J. 2009;8:14.
WHO. Achieving and maintaining universal coverage with long-lasting insecticidal nets for malaria control. Geneva, World Health Organization. 2017. http://www.who.int/malaria/publications/atoz/who_recommendation_coverage_llin/en/.
Nigatu W, Woyessa A, Tafesse H, Sisay A, Getachew A, Fentie G, et al. A survey for long-lasting insecticidal net coverage and use in Ethiopia. Ethiop J Public Health Nutr. 2020;3:34–42. Special issue.
Seyoum D, Speybroeck N, Duchateau L, Brandt P, Rosas-Aguirre A. Long-lasting insecticide net ownership, access and use in southwest Ethiopia: a community-based cross-sectional study. Int J Environ Res Public Health. 2017;14:1312.
Acknowledgements
The authors would like to thank Department of Biology, Tropical and Infectious Disease Research Center (TIDRC) and technical staff members of TIDRC, Jimma University, Ethiopia.
Funding
This work was supported by Tropical and Infectious Disease Research Center (TIDRC), Jimma University, Ethiopia.
Author information
Authors and Affiliations
Contributions
TC, DY and EAS conceived and designed the study. TC performed the field and laboratory experiments and analysed data and drafted the manuscript. GN drafted the manuscript. DA developed the map of the study site. DY and EA analysed and interpreted and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval letter (Ref. No. RPG/ 056/2020) was obtained from research and ethical review board of College of Natural Sciences, Jimma University, Ethiopia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chanyalew, T., Natea, G., Amenu, D. et al. Composition of mosquito fauna and insecticide resistance status of Anopheles gambiae sensu lato in Itang special district, Gambella, Southwestern Ethiopia. Malar J 21, 125 (2022). https://doi.org/10.1186/s12936-022-04150-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04150-5