Abstract
Background
Patients with concomitant type 2 diabetes mellitus (T2DM) and aortic regurgitation (AR) can present with right ventricular (RV) dysfunction. The current study aimed to evaluate the impact of AR on RV impairment and the importance of ventricular interdependence using cardiac magnetic resonance feature tracking (CMR‑FT) in patients with T2DM.
Methods
This study included 229 patients with T2DM (AR−), 88 patients with T2DM (AR+), and 122 healthy controls. The biventricular global radial strain (GRS), global circumferential strain (GCS), and global longitudinal peak strain (GLS) were calculated with CMR‑FT and compared among the healthy control, T2DM (AR−), and T2DM (AR+) groups. The RV regional strains at the basal, mid, and apical cavities between the T2DM (AR+) group and subgroups with different AR degrees were compared. Backward stepwise multivariate linear regression analyses were performed to determine the effects of AR and left ventricular (LV) strains on RV strains.
Results
The RV GLS, LV GRS, LV GCS, LV GLS, interventricular septal (IVS) GRS and IVS GCS were decreased gradually from the controls through the T2DM (AR−) group to the T2DM (AR+) group. The IVS GLS of the T2DM (AR−) and T2DM (AR+) groups was lower than that of the control group. AR was independently associated with LV GRS, LV GCS, LV GLS, RV GCS, and RV GLS. If AR and LV GLSs were included in the regression analyses, AR and LV GLS were independently associated with RV GLS.
Conclusion
AR can exacerbate RV dysfunction in patients with T2DM, which may be associated with the superimposed strain injury of the left ventricle and interventricular septum. The RV longitudinal and circumferential strains are important indicators of cardiac injury in T2DM and AR. The unfavorable LV-RV interdependence supports that while focusing on improving LV function, RV dysfunction should be monitored and treated in order to slow the progression of the disease and the onset of adverse outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Type 2 diabetes mellitus (T2DM) and its related cardiovascular diseases have extensive effects on the health and economies of communities [1]. Recently, the mortality rate of patients with T2DM has been decreasing, and the prevalence of diabetes has been increasing [2]. Therefore, the lifetime accumulation of T2DM complications and morbidities will increase worldwide [3]. Meanwhile, with the increased aging population, chronic aortic regurgitation (AR) can accompany T2DM. However, it is often overlooked due to its low frequency [4, 5]. Both T2DM and AR can lead to cardiac dysfunction and eventually heart failure [6, 7]. Previous studies commonly focused on left ventricular (LV) and right ventricular (RV) structural and functional impairments in patients with T2DM. These conditions can cause cardiovascular-related mortality [8, 9]. Notably, RV impairment can be a component of ventricular interdependence in diabetic cardiomyopathy [10]. Therefore, the synergistic effects of T2DM and AR on ventricular interdependence should be evaluated.
Echocardiography is a noninvasive technique frequently used in the examination of RV function, which is inhibited by the complex anatomical structure of the right ventricle, its reliance on operator skills, and limited spatial resolution [11, 12]. Cardiac magnetic resonance (CMR) imaging can quantitatively evaluate myocardial structure and function because of a high temporal and soft tissue resolution [13, 14]. In particular, CMR feature tracking (CMR-FT) can detect subclinical myocardial dysfunction. Hence, it can be an effective and feasible approach for assessing RV function and investigating the interactions between the right and left ventricles [15, 16]. In relation to this, the current study aimed to investigate the right ventricular structural and functional characteristics of patients with diabetes who present with AR and the mediating effects of LV dysfunction on RV strain using CMR-FT.
Methods
Study population
This study was approved by the Biomedical Research Ethics Committee of our hospital and was conducted in accordance with the Declaration of Helsinki.The need for informed consent was waived given the retrospective design of the study.
The study cohort comprised patients diagnosed with T2DM based on the current American Diabetes Association guidelines who underwent CMR examination at our institution between January 2015 and October 2023. The patients were divided into the healthy control, T2DM (AR−), and T2DM (AR+) groups. The exclusion criteria were as follows: patients with ischemic heart disease, rheumatic heart disease, congenital heart disease, primary cardiomyopathy, coexistence of other significant valvular disease, acute AR, and previous history of aortic valve surgery; those with incomplete clinical data; and those with CMR contraindications, poor image quality, and incomplete scans. Finally, 317 patients, including 229 with T2DM (AR−) and 88 with T2DM (AR+), were eligible for this study. Based on the echocardiographic findings, the T2DM (AR +) group was further classified into three subgroups, which were as follows: the mild AR (n = 41, 44.6%), moderate AR (n = 25, 28.4%), and severe AR (n = 22, 25%) groups. The healthy control group comprised patients who underwent cardiac MRI but did not present with a previous history of cardiac disease or symptoms and T2DM or impaired fasting glucose levels and inadequate cardiac MRI image quality (n = 122).
Data on the demographic characteristics, clinical history, cardiovascular risk factors, and laboratory test results of the patients were recorded in the Hospital Information System and Laboratory Information Management System.
MRI protocol
All patients were placed in the supine position, and they underwent imaging using a 3.0-T whole-body magnetic resonance scanner (TrioTim or MAGNETOM Skyra, Siemens Medical Solutions, Erlangen, Germany) equipped with 32-channel body-phased array coils and standard ECG trigger equipment. Balanced steady-state free precession cine images were obtained during breath-holding at the end of expiration with retrospective cardiac gating. Moreover, they comprised standard long-axis views and a short-axis stack that encompassed the entirety of the left and right ventricles. The following parameters were used for the two scanners: temporal time = 39.34/40 ms; echo time = 1.22/1.20 ms; slice thickness = 8.0 mm; field of view = 234 × 280/250 × 300 mm2; matrix size = 208 × 139/256 × 166 pixels; and flip angle = 40°/50°.
Image analysis
The CMR images were uploaded to an offline commercial software (Cvi42, v.5.11.2; Circle Cardiovascular Imaging, Calgary, Canada). Next, they were analyzed by two radiologists who were blinded to the clinical data of the participants. Both radiologists had > 3 years of experience in CMR imaging. The endo- and epicardial contours of both ventricles were automatically delineated at the end-diastolic and end-systolic phases on the short-axis cine images in the short three-dimensional module. Then, the two investigators manually adjusted the borders layer by layer, and the morphological and functional parameters, including LV and RV end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF), and myocardial masses (MM), were calculated automatically. The papillary muscle and trabeculae were excluded from MM but included in the ventricular volume analyses. The LV remodeling index and RV remodeling index were calculated as LVMM/LVEDV and RVMM/RVEDV, respectively.
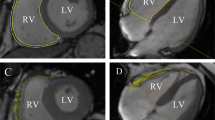
The short-, long-axis four- and two-chamber cine images were used to evaluate the myocardial strain of both ventricles using the tissue tracking module. The endocardium and epicardium of both ventricles were manually outlined at the end diastole after cautious exclusion of the papillary muscles and trabeculae. The biventricular and interventricular septal (IVS) global radial (GRS), circumferential (GCS) and longitudinal peak strains (GLS), RV regional peak strain (including the basal, middle, and apical cavities), and LV regional peak strain were generated automatically (Fig. 1). The 2, 3, 8, 9, and 14 LV segments represented the area of the interventricular septum, according to the 16-segment model of the AHA. The interventricular septum thickness (IVST) on each patient’s CMR was measured using the four-chamber long-axis view at the midway between the base and apex of the left ventricle at the end diastole (Additional file 1).
Examples of evaluations of left and right ventricular global strains using CMR-FT. A–C, the endo- and epicardial contours of the left and right ventricles are delineated on standard cardiac short-axis, two-chamber, and four-chamber planes in end diastole D–I, global longitudinal strains of the left (D–F) and right (G–I) ventricles among control, T2DM (AR−) group, and T2DM (AR+) group. The yellow and blue curves represent the endo- and epicardial contours of the right ventricle, and the red and green curves represent those of left ventricle. T2DM, type 2 diabetes mellitus; AR, aortic regurgitation; LV, left ventricular; RV, right ventricular
Reproducibility analysis
To assess the intraobserver variability of the RV global and regional strains, 50 participants selected randomly were evaluated by comparing two measurements conducted by LT.S. and taken 1 month apart. Interobserver variability was assessed by comparing the two measurements of all participants, which were conducted by LT.S. and K.S. double-blindly and independently.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences software (version 23.0, IBM, Armonk, New York, USA). Continuous variables with a normal distribution were presented as mean ± standard deviation. Meanwhile, variables with a non-normal distribution were expressed as median (interquartile range: 25–75%). One-way analysis of variance with Bonferroni post hoc correction was used to compare data with a normal distribution, and the Kruskal–Wallis test was used to compare data with a non-normal distribution among the healthy control, T2DM (AR−), and T2DM (AR+) groups. Categorical variables were expressed as frequencies (percentages) and analyzed using the chi-square test or the Fisher’s exact test based on the data distribution.
The associations between RV global strain, IVS global strain, RVEF, and LV geometric parameters and strains were assessed via Pearson’s or Spearman’s correlation coefficient analysis, as appropriate. Multivariate linear regression models adjusted for statistically significant parameters in the univariate analysis (p < 0.05) and traditional clinical risk factors (age, sex, body mass index, hyperlipidemia, fasting plasma glucose and hemoglobin A1c [HbA1c] levels, and diabetes duration) were used to determine the predictors of both ventricular global strains in the populations with T2DM and the independent effects of LV strains on RV strains. The intraobserver and interobserver variabilities of RV global and regional deformation were analyzed using the intraclass correlation coefficient. Two-tailed p values of < 0.05 were considered statistically significant.
Results
Clinical characteristics of the participants
The current study included 329 individuals. Among them, 229 presented with T2DM (AR−), 88 patients with T2DM (AR+), and 122 healthy controls. The three groups did not significantly differ in terms of sex, diastolic blood pressure, and lipid parameters (all p > 0.05). The resting heart rate and HbA1c) level of the T2DM (AR−) and T2DM (AR+) groups were higher than those of the control group (all p < 0.05). The T2DM (AR+) group was remarkably older and had a significantly higher systolic blood pressure than the T2DM (AR−) and healthy control groups (all p ≤ 0.001). Table 1 shows the demographic and clinical characteristics of the participants.
Characteristics of the biventricular volumetrics
Table 2 shows the comparisons of the left and right ventricular volumetric parameters. The LV MM and LV RI of the T2DM (AR−) and T2DM (AR+) groups were significantly higher than those of the healthy control group (all p < 0.05). The T2DM (AR+) group had a lower RV EF and LVEF than the T2DM (AR−) and control groups (45% ± 19.6% vs. 58.9% ± 13.4% vs. 61.2% ± 9.15%; 44.7% ± 16.6% vs. 58% ± 12.1% vs. 61.6% ± 6.5%, respectively; all p < 0.001). There was no significant difference in the RV ESD, RV EDV, and LV SV among the three groups (all p > 0.05). The T2DM (AR+) group had a higher IVST than the T2DM (AR−) and control groups (10.35[8.22, 12.5] vs. 9.4 [7.5–1.11] vs. 7.5[6.3, 8.5] mm).
Characteristics of the global biventricular and IVS strains
The RV GLS, LV GRS, LV GCS, LV GLS, IVS GRS and IVS GCS were decreased gradually from the controls through T2DM (AR−) group to the T2DM (AR+) group (all p < 0.001). The RV GRS and RV GCS did not significantly differ among the three groups (all p > 0.05). The IVS GLS of the T2DM (AR−) and T2DM (AR+) groups was lower than that of the control group (all p < 0.05) (Fig. 2).
Comparing LV-, IVS- and RV strains among controls, T2DM (AR−) group, and T2DM (AR+) group. T2DM, type 2 diabetes mellitus; AR, aortic regurgitation; LV, left ventricular; IVS, interventricular septum; RV, right ventricular; GRS, global radial strain; GCS, global circumferential strain; GLS, global longitudinal strain
Regional RV strain in patients in the T2DM group with different AR degrees
As shown in Table 3, patients with T2DM who did not present with AR and those with mild AR had decreased RV longitudinal strains in the mid and apical cavities compared with the healthy controls. However, the results did not significantly differ between the two groups. Patients with T2DM who presented with moderate AR had a lower RV mid and basal circumferential and basal longitudinal strains than healthy controls, patients with T2DM who did not present with AR, and those with T2DM with mild AR (all p < 0.05). Patients with T2DM who presented with severe AR had a significantly lower RV apical and basal longitudinal strains and RV apical, mid, and basal circumferential strains than healthy controls, patients with T2DM who did not present with AR, and those with mild AR (all p < 0.05). The RV mid longitudinal strain did not significantly differ among the T2DM subgroups.
Association between RV strains and LV and IVS function
In patients with T2DM (Additional file 2, Fig. 3), RVEF was significantly associated with AR (r = − 0.202), LVEF (r = 0.379), LVEDV (r = − 0.279), LVESV (r = − 0.377), LVGRS (r = 0.402), LVGCS (r = − 0.478), and LVGLS (r = − 0.293). RVGLS was significantly associated with AR (r = 0.215), LVEF (r = − 0.295), LVEDV (r = 0.256), LVESV (r = 0.263), LVGCS (r = 0.25), and LVGLS (r = 0.374). In addition, RVEF and RVGLS were associated with all IVS global strains. All RV radial and circumferential global strains were associated with LV and IVS global strains and AR.
Regression plots of the correlation between RV PS and LV PS. (a) RV GRS vs. LV GRS; (b) RV GCS vs. LV GCS; (c) RV GLS vs. LV GLS; (d) RVEF vs. IVS GRS; (e) RVEF vs. IVS GCS; (f) RVEF vs. IVS GLS. LV, left ventricular; RV, right ventricular; IVS, interventricular septum; GRS, global radial strain; GCS, global circumferential strain; GLS, global longitudinal strain
Associations between the biventricular strains and clinical variables in patients with T2DM
After adjusting for age, sex, body mass index, heart rate, triglyceride, cholesterol, high-density lipoprotein, low-density lipoprotein, FPG, and HbA1c levels, and diabetes duration, AR was found to be independently associated with LV GRS (β = − 0.385, p < 0.001, model R2 = 0.207), GCS (β = 0.430, p < 0.001, model R2 = 0.258), GLS (β = 0.348, p < 0.001, model R2 = 0.199), RV GCS (β = 0.149, p = 0.018, model R2 = 0.071), GLS (β = 0.220, p < 0.001, model R2 = 0.138), but not with RV GRS, in patients with T2DM (Table 4). When AR and LV GLS were included in the regression analyses, both AR and LV GLS (β = 0.146 and 0.214, p = 0.022 and < 0.001, model R2 = 0.174) were found to be independently associated with RV GLS (Table 4).
Intra- and interobserver variability in RV strain measurement
As depicted in Additional file 3, based on these results, the reproducibility of the global and regional RV strain measurement was excellent in both intraobserver and interobserver assessments (all intraclass correlation coefficients > 0.7).
Discussion
The current study used the CMR-FT technique to evaluate the effect of AR on global and regional RV myocardial strains in patients with T2DM and to explore the association between the RV and LV and IVS function. Results showed that the RV GLS, IVS GRS, IVS GCS, and LV global strains decreased significantly in patients with T2DM. Moreover, they were further deteriorated by AR. Deteriorating AR may aggravate the RV regional longitudinal and circumferential strains. The RV global strains were associated with that of the LV and IVS global strains in patients with T2DM. LV GLS impairment superimposed by coexisting AR was independently associated with RV GLS in patients with T2DM, which indicates an adverse interaction between ventricles.
RV dysfunction and pathophysiological mechanisms in T2DM
Several studies have revealed that the T2DM group had a significantly lower RV GLS than the control group. Further, RV dysfunction was an important component of diabetic cardiomyopathy [10, 17]. In the current study, the T2DM (AR−) group had a low RV global and regional longitudinal strain. However, their LVEF and RVEF were still within normal ranges. This phenomenon cold be explained by the synergistic effect of abnormally elevated glucose and lipid levels that leads to a decrease in the contractile function of the RV myocardium [18]. Recent evidence showed the role of myocardial lipotoxic injury from lipid oversupply in the development of diabetic heart disease [19]. Due to the predominance of longitudinal subendocardial myocytes, longitudinal shortening accounts for approximately 75% of RV contractions [20, 21]. Therefore, the RV longitudinal strain was a good indicator of RV dysfunction and good reproducibility in patients with diabetes combined with circumferential strain [10, 22, 23]. Further, another study found that the deterioration of RV performance was observed in the basal and apical segments of the RV free wall in asymptomatic patients with diabetes [19]. However, our study revealed a decreased RV longitudinal strain in the mid and apical segment, and we hypothesized that it is caused by different technical algorithms and endpoints [24,25,26].
AR aggravates RV dysfunction in T2DM
When AR occurs in patients with T2DM, few researches reported the additive damage to heart. Both T2DM and AR lead to myocardial dysfunction that do not often exhibit evident symptoms in the early stage but progresses to irreversible cardiac dysfunction in the absence of timely and adequate treatment [9, 27]. AR leads to increased LV filling pressure, with increased pulmonary venous and arterial pressure, thereby increasing RV afterload [28, 29]. The assessment of RV strain can be a useful adjunct to conventional clinical indices and LV function assessment in the timing of surgery in asymptomatic patients with severe AR [30, 31]. Our study found that the T2DM (AR+) group had a lower RV GLS, IVS GRS, and IVS GCS but a higher IVST than the T2DM (AR−) group, which indicates that coexisting AR further exacerbates RV and IVS dysfunction in T2DM.
In the RV, myocytes are arranged circumferentially in the superficial layer (approximately 25% of wall thickness) and longitudinally in the subendocardial layer (approximately 75% of wall thickness) [21]. Moreover, the orientation of RV apical myofibers is oblique at the subendocardium as compared to the basal region that preserves a more longitudinal orientation [32]. This could explain why only longitudinal strain damage in the apical and mid cavity occurred in the T2DM groups without AR and mild AR. The role of the RV apex may be maintaining a smooth and continuous blood flow rather than contributing to ejection because of a smooth curved path from the inflow to the outflow along the septum circumventing the RV apex [32, 33]. Another study revealed that the RV GCS plays an important role in maintaining normal RVEF when RV GLS was reduced [34]. In patients with T2DM who did not present with AR and those with mild AR, the longitudinal RV strain in the apex and mid segment decreased. However, the RVEF was maintained due to the compensation of the circumferential RV strain. As AR progressed to moderate or severe degree, these patients had a remarkable reduction in regional RV circumferential strain and the RVEF came to decreased. Thus, the regional RV strain should be evaluated to understand the progression of AR and provide timely interventions before the regional circumferential strain decreases in patients with T2DM.
Ventricular interdependence in patients with T2DM
Ventricular interdependence has been considered as an RV function associated with LV function via a common IVS and myocardial fibers and limited pericardial flexibility [35, 36]. The left ventricle is an important contributor to RV ejection, and LV contraction generates 20–40% of both RV SV and pulmonary flow [20]. Several animal and human studies have shown that changes in IVS function can be a sign of RV dysfunction. Therefore, RV dysfunction can primarily be a consequence of subclinical LV dysfunction [35, 37]. The current study found that the RV global strains were closely associated with LV and IVS global strains in all patients with T2DM. Further, the decreased RV GLS was associated with the superimposed impairment of LV GLS by the coexisting AR. The RV function is impaired under the influence of LV filling and contraction and IVS displacement and hypofunction caused by T2DM and AR [38]. It means the RV longitudinal and circumferential strain are indispensable parameters to reflect deterioration of LV systolic and diastolic function. Studies also found that patients with HF with concomitant ventricular interdependence experience worse cardiovascular outcomes [39]. Therefore, early intervention for LV and IVS dysfunction would be important for minimizing the detrimental impact on the right ventricle resulting from adverse ventricular interdependence.
Limitations
The current study had several limitations. First, although the sample size was adequate to support our conclusion, this was a single-center study. Therefore, a larger or multicenter study should be conducted in the future. Second, long-term follow-up data were unavailable. Therefore, further research must be performed to investigate the prognostic value of RV strain parameters in cardiovascular events. However, these studies could provide important information for preventing and improving RV dysfunction. Third, the current CMR data is insufficient to assess the severity of AR, which will be further improved in prospective studies. Finally, our study did not perform animal experiments, and future studies must assess the relevant pathological mechanisms of RV dysfunction.
Conclusions
AR can exacerbate RV dysfunction in patients with T2DM, which may be associated with the superimposed strain injury of the left ventricle and interventricular septum. Both RV longitudinal and circumferential strains are indispensable indicators of LV dysfunction in T2DM patients with AR.The unfavorable LV-RV interdependence supports that while focusing on improving LV function, RV dysfunction should be monitored and treated in order to slow the progression of the disease and the onset of adverse outcomes.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- AR:
-
Aortic regurgitation
- RV:
-
Right ventricular
- CMR-FT:
-
Cardiac magnetic resonance feature tracking
- GRS:
-
Global radial strain
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal peak strain
- LV:
-
Left ventricular
References
Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74.
Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838.
Qin P, Qin T, Liang L, Li X, Jiang B, Wang X, Ma J, Hu F, Zhang M, Hu D. The role of mitochondrial DNA copy number in cardiometabolic disease: a bidirectional two-sample mendelian randomization study. Cardiovasc Diabetol 2024, 23(1).
Galusko V, Thornton G, Jozsa C, Sekar B, Aktuerk D, Treibel TA, Petersen SE, Ionescu A, Ricci F, Khanji MY. Aortic regurgitation management: a systematic review of clinical practice guidelines and recommendations. Eur Heart J Qual Care Clin Outcomes. 2022;8:113–26.
Rawshani A, Sattar N, McGuire DK, Wallström O, Smith U, Borén J, Bergström G, Omerovic E, Rosengren A, Eliasson B, Bhatt DL. Left-sided degenerative valvular heart disease in type 1 and type 2 diabetes. Circulation. 2022;146:398–411.
Ajmone Marsan N, Delgado V, Shah DJ, Pellikka P, Bax JJ, Treibel T, Cavalcante JL. Valvular heart disease: shifting the focus to the myocardium. Eur Heart J. 2023;44:28–40.
Kanamori H, Naruse G, Yoshida A, Minatoguchi S, Watanabe T, Kawaguchi T, Tanaka T, Yamada Y, Takasugi H, Mikami A, Minatoguchi S. Morphological characteristics in diabetic cardiomyopathy associated with autophagy. J Cardiol. 2021;77:30–40.
Li XM, Yan WF, Jiang L, Shi K, Ren Y, Han PL, Peng LQ, Guo YK, Yang ZG. Impact of T2DM on right ventricular systolic dysfunction and interventricular interactions in patients with essential hypertension: evaluation using CMR tissue tracking. Cardiovasc Diabetol. 2022;21:238.
Shao G, Cao Y, Cui Y, Han X, Liu J, Li Y, Li N, Liu T, Yu J, Shi H. Early detection of left atrial and bi-ventricular myocardial strain abnormalities by MRI feature tracking in normotensive or hypertensive T2DM patients with preserved LV function. BMC Cardiovasc Disord. 2020;20:196.
Shi R, Yang ZG, Guo YK, Qian WL, Gao Y, Li XM, Jiang L, Xu HY, Li Y. The right ventricular dysfunction and ventricular interdependence in patients with DM: assessment using cardiac MR feature tracking. Cardiovasc Diabetol. 2023;22:93.
Fang H, Wang J, Shi R, Li Y, Li XM, Gao Y, Shen LT, Qian WL, Jiang L, Yang ZG. Biventricular dysfunction and ventricular interdependence in patients with pulmonary hypertension: a 3.0-T cardiac MRI feature tracking study. J Magn Reson Imaging. 2023.
Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther. 2018;8:101–17.
Fogel MA, Anwar S, Broberg C, Browne L, Chung T, Johnson T, Muthurangu V, Taylor M, Valsangiacomo-Buechel E, Wilhelm C. Society for cardiovascular magnetic resonance/European society of cardiovascular imaging/American society of echocardiography/society for pediatric radiology/north American society for cardiovascular imaging guidelines for the use of cardiovascular magnetic resonance in pediatric congenital and acquired heart disease: endorsed by the American heart association. J Cardiovasc Magn Reson. 2022;24:37.
Bawaskar P, Chaurasia A, Nawale J, Nalawade D, Shenoy C. Neoplastic arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2019;12:e009272.
Backhaus SJ, Metschies G, Billing M, Schmidt-Rimpler J, Kowallick JT, Gertz RJ, Lapinskas T, Pieske-Kraigher E, Pieske B, Lotz J, Bigalke B. Defining the optimal temporal and spatial resolution for cardiovascular magnetic resonance imaging feature tracking. J Cardiovasc Magn Reson. 2021;23:60.
Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, Sinagra G. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy: cardiac magnetic resonance feature-tracking study. Circ Cardiovasc Imaging. 2015;8:e003647.
Linssen PB, Veugen MG, Henry RM, van Der Kallen CJ, Kroon AA, Schram MT, Brunner-La Rocca HP, Stehouwer CD. Associations of (pre)diabetes with right ventricular and atrial structure and function: the Maastricht study. Cardiovasc Diabetol. 2020;19:88.
Wu T, Li X, Zhang D, Gong LG. Early impairment of right ventricular systolic function in patients with prediabetes and type 2 diabetes mellitus: an analysis of two-dimensional speckle tracking echocardiography. Echocardiography. 2023;40:831–40.
Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, Liu L, Ming Z, Tao X, Li Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol 2024, 23(1).
Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578–622.
Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, Forfia PR. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest. 2011;140:27–33.
Kowsari AA, Hosseinsabet A. Evaluation of the right ventricular function in prediabetes: a 2-D speckle tracking echocardiographic study. Ultrasound Med Biol. 2016;42:1321–9.
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48.
Lin K, Collins JD, Chowdhary V, Markl M, Carr JC. Heart deformation analysis for automated quantification of cardiac function and regional myocardial motion patterns: a proof of concept study in patients with cardiomyopathy and healthy subjects. Eur J Radiol. 2016;85:1811–7.
Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson. 2010;12:35.
Angel López-Candales NR, Gulyasy B, Edelman K, Bazaz R. Differential strain and velocity generation along the right ventricular free wall in pulmonary hypertension. Can J Cardiol. 2009;25:73–7.
Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7:352–62.
Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–60.
Leeuwenburgh BP, Helbing WA, Steendijk P, Schoof PH, Baan J. Effects of acute left ventricular unloading on right ventricular function in normal and chronic right ventricular pressure-overloaded lambs. J Thorac Cardiovasc Surg. 2003;125:481–90.
Sinning C. The additional use of strain measurements for timing of treatment in severe aortic regurgitation. Int J Cardiovasc Imaging. 2022;38:991–3.
Fernández-Golfín C, Hinojar-Baydes R, González-Gómez A, Monteagudo JM, Esteban A, Alonso-Salinas G, Fernández MA, García-Martín A, Santoro C, Pascual-Izco M, Jiménez-Nacher JJ. Prognostic implications of cardiac magnetic resonance feature tracking derived multidirectional strain in patients with chronic aortic regurgitation. Eur Radiol. 2021;31:5106–15.
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA, de Roos A. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–44.
Sengupta PP, Narula J. RV form and function: a piston pump, vortex impeller, or hydraulic ram? JACC Cardiovasc Imaging. 2013;6:636–9.
Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1463–82.
Todo S, Tanaka H, Yamauchi Y, Yokota S, Mochizuki Y, Shiraki H, Yamashita K, Shono A, Suzuki M, Sumimoto K, Tanaka Y. Association of left ventricular longitudinal myocardial function with subclinical right ventricular dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:212.
Colebank MJ, Taylor R, Hacker TA, Chesler NC. Biventricular interaction during acute left ventricular ischemia in mice: a combined in-vivo and in-silico approach. Ann Biomed Eng. 2023;51:2528–43.
Dibble CT, Lima JA, Bluemke DA, Chirinos JA, Chahal H, Bristow MR, Kronmal RA, Barr RG, Ferrari VA, Propert KJ, Kawut SM. Regional left ventricular systolic function and the right ventricle: the multi-ethnic study of atherosclerosis right ventricle study. Chest. 2011;140:310–6.
Driessen MM, Hui W, Bijnens BH, Dragulescu A, Mertens L, Meijboom FJ, Friedberg MK. Adverse ventricular-ventricular interactions in right ventricular pressure load: insights from pediatric pulmonary hypertension versus pulmonary stenosis. Physiol Rep. 2016;4:e12833.
Yoshida Y, Nakanishi K, Daimon M, Ishiwata J, Sawada N, Hirokawa M, Kaneko H, Nakao T, Mizuno Y, Morita H et al. Atrioventricular and ventricular functional interdependence in individuals without overt Cardiac Disease. J Am Heart Assoc. 2021, 10(23).
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National Natural Science Foundation of China (81771887, 81771897, 82371925), and the 1–3–5 project for disciplines of excellence of West China Hospital, Sichuan University (ZYGD23019).
Author information
Authors and Affiliations
Contributions
LTS. KS. and RS. YNJ. contributed to the study design, data analysis and manuscript writing. LTS. RS. and WFY. were responsible for collecting, sorting and analyzed the data. KS. and YL. participated in editing and review of the manuscript. YL. YKG. and ZGY. participated in the study design and revised the manuscript. All authors reviewed and approved the final version of submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of West China Hospital, Sichuan University (Chengdu, Sichuan, China), with a waiver of informed consent due to the retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, LT., Shi, K., Yang, ZG. et al. The right ventricular dysfunction and ventricular interdependence in patients with T2DM and aortic regurgitation: an assessment using CMR feature tracking. Cardiovasc Diabetol 23, 294 (2024). https://doi.org/10.1186/s12933-024-02372-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02372-2