Abstract
Cardiovascular diseases (CVDs) are the main cause of death among patients with type 2 diabetes mellitus (T2DM), particularly in low- and middle-income countries. To effectively prevent the development of CVDs in T2DM, considerable effort has been made to explore novel preventive approaches, individualized glycemic control and cardiovascular risk management (strict blood pressure and lipid control), together with recently developed glucose-lowering agents and lipid-lowering drugs. This review mainly addresses the important issues affecting the choice of antidiabetic agents and lipid, blood pressure and antiplatelet treatments considering the cardiovascular status of the patient. Finally, we also discuss the changes in therapy principles underlying CVDs in T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a persistent state of hyperglycemia and glucose intolerance that occurs when the body cannot respond fully to insulin, followed by an increase in insulin production and a subsequent insulin deficiency. Once mainly limited to older adults in the twentieth century, T2DM is now the largest global health crisis of the twenty-first century, and although its prevalence and incidence have shown the fastest increase in adults, T2DM is increasingly occurring in children and adolescents [1]. Premature mortality from diabetes increased by 5% during the period 2000–2016 [2]. Between 2000 and 2019, trends in deaths due to diabetes exhibited a 3% increase in age-standardized rates [3]. Of note, T2DM accounts for approximately 90% of all diabetes cases, and cardiovascular (CV) events in people with T2DM are a major cause of the increased risk of early death and have become a rising threat to human health worldwide.
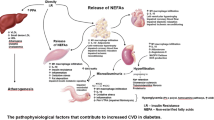
The major CV diseases (CVDs) associated with T2DM include ischemic heart disease, heart failure (HF), stroke, coronary artery disease (CAD), and peripheral artery disease, and these complications can result in death for at least 50% of patients with T2DM [4]. Therefore, CVDs are of great concern in the disease progression and prognosis of T2DM. T2DM is characterized by insulin resistance and hyperglycemia, which is usually, but not always, accompanied by abnormal lipid metabolism. Insulin resistance generally occurs early in the progression of T2DM and CVD [5]. Importantly, insulin resistance is associated with a higher relative risk of CV events [6]. Elevated blood glucose is strongly associated with the risk of both macrovascular and microvascular complications in patients with T2DM [7]. Furthermore, excess accumulation of lipids may result in cardiac insulin resistance, fibrosis and diastolic dysfunction [8]. Moreover, hypoglycemia, which has generally been recognized as an adverse effect of glucose reduction, has also been reported as a risk factor for CVD among patients with T2DM [9]. Overall, CVD associated with diabetes is a major cause of death and disability among patients with T2DM.
To date, limited progress has been made in the prevention of T2DM, and no country or health care system is immune to the threat of T2DM. Although the incidence and mortality rate of CVD have decreased in high-income countries, these countries account for only approximately 10% of the world population, and CVD incidence and mortality trends in T2DM patients in middle- and low-income countries remains unclear [10]. In addition, considering the clinical burden of CVD complications in T2DM patients, attention to the joint management of T2DM and CVD has been increasing. In this review, we focus more thoroughly on the current state of the epidemiology of CVD in T2DM and on preventive measures and then explore measures to reduce the threat from CVD in T2DM patients.
Current trends in the epidemiology of cardiovascular disease in T2DM
While globally the incidence of CVD among patients with T2DM is 2–3 times higher than among those without T2DM, data suggest a decreasing trend worldwide in the overall prevalence of CVD attributed to T2DM. The currently available data are shown in Table 1. Before 2016, the prevalence of all CVDs in T2DM ranged from 14.3 to 46.9%[11], while a meta-analysis for the years 2007–2017 indicated a prevalence of 32%[4]. The weighted CVD prevalence in 2019 was reported to be 34.8% across 13 countries, although there was a wide range between countries from 18.0% in Saudi Arabia to 56.5% in Israel. [12]. There was a general trend of reduced incidence of coronary artery disease, myocardial infarction, stroke and HF, and peripheral artery disease [12]. The study by Artime et.al demonstrated that the prevalence of CVD among patients with T2DM in Spain was ranged from 7 to 41%, and in-hospital mortality rates due to CVD were between 6 and 11%[13].
The reduction in the incidence of CVD in T2DM is consistent with new findings from Ethiopia [14], Sweden [15], and South Korea [16]. However, in the study from South Korea the risk of HF increased over the years 2006 to 2015 [16], and this finding was also observed among patients with a T2DM duration over 10 years and among those with a history of hypertension [17]. The occurrence of HF has been found to be related to diabetes duration, with a 17% increase in HF risk strongly associated with each 5-year increase in diabetes duration [18].
The decreasing incidence of CVD among T2DM patients has been reported in high-income countries, such as Sweden, the USA, Canada, and the United Kingdom, with a 3–5% yearly decline in the rates of CVD since the early 1990s [19]. However, a large gap remains in the incidence of CV morbidity and mortality between people with T2DM and those without the disease. Unfortunately, there is a paucity of data on CVD incidence among people with T2DM from middle- and low-income countries and in global investigations.
Consistent with the declining trend of CVD incidence, the incidence of death from CVD in T2DM steadily dropped in 2019 [3]. There was approximately a 20% reduction in the mortality rates in Sweden from 1998 through 2014 [15]. Additionally, a general trend toward a reduction in T2DM mortality was reported in countries from the European Union between 1990 and 2019 [20]. Likewise, reductions in the death rate and the proportion of deaths from vascular causes decreased from 1988–94 to 2010–15 in the USA [21]. A similar trend was also observed in Hong Kong [22] and South Korea [16]. However, in contrast to the findings of observational studies, a systematic review of diabetes CV outcome clinical trials reported no improvement in CV mortality in T2DM; the possible cause underlying the difference between randomized controlled trials (RCTs) and real world data may be the lack of standardized definitions of CVD outcomes (including HF) [23]. The overall estimated CVD mortality rates among patients with diabetes are higher in low- and middle-income countries than in high-income countries [24]. Thus, the global CVD-related mortality among people with diabetes in low-income countries may decrease in time, in-line with high-income countries.
Despite the trend in the reduction in the overall CVD mortality, the incidence and mortality rate of T2DM continue to increase annually, and along with population growth and aging, the total number of CVD- and T2DM-related deaths have increased worldwide [3]. The highest number of deaths due to diabetes in adults in 2019 occurred in the Western Pacific region, followed by Southeast Asia, and the highest proportion of deaths due to diabetes among patients under the age of 60 in 2019 was in Africa [24]. CVD and diabetes were among the top ten causes of death globally in 2019 and are a considerable threat to human health.
A transition in the major cause of death in T2DM from CVD to non-CVD was observed in the ALTITUDE trial [25]. Non-vascular, non-cancer causes of death among American adults diagnosed with diabetes accounted for approximately 46.5% of all deaths during 2010–2015, and the proportion of cancer-related deaths did not change from 1988 to 2015 [21]. A change in the leading cause of death from vascular causes to cancer among people with diabetes was observed in England from 2001 to 2018 [26]. In addition, a decrease in mortality due to cancer and CVD in older people with T2DM in Australia was observed, masking the rising excess risk among patients of younger ages [27]. There is a suggestion that younger-onset T2DM may be associated with an increase in CVD-related mortality but a slight decrease in cancer-related mortality [28]. In summary, notwithstanding some reductions in CVD mortality in T2DM, there is a paucity of data about the leading cause of death among T2DM patients, especially at younger ages.
Prevention of cardiovascular disease in T2DM
A focus on efforts to prevent CVD events in high-risk populations might reduce mortality and decrease the economic burden of heart attack and stroke. CVD can be prevented or delayed by controlling blood glucose, blood pressure and cholesterol and with lifestyle changes such as stopping smoking, eating healthily, and increasing physical activity.
Primary prevention involves preventing or delaying new-onset CVD in patients with T2DM. Early detection of CVD-related risk factors in community-based high-risk populations and early diagnosis of T2DM in subjects without CVD are needed. Secondary prevention involves treatment of risk factors in diabetic patients with established CVD. Comprehensive medical intervention for secondary prevention among diabetic patients with clinical CVD was approved by the American Heart Association (AHA) in 1999 [29]; a position statement by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) in 2012 outlined the need for individualized glycemic targets and glucose-lowering therapies in combination with comprehensive CV risk reduction, indicating that patient-centered care must be a consideration in decision-making for secondary prevention among T2DM patients with established CVD [30]. Beginning in 2018, the glucocentric strategy for the management of CVD in T2DM transitioned to patient-centered care [31], and individual medical history, lifestyle behaviors, and CV and metabolic risk factors were included as considerations. Precision medical therapeutic approaches for patients with T2DM with CVD will be gradually achieved [32].
CVD risk assessment aims to provide appropriate regimens for the prevention and treatment of CVD in T2DM. The American College of Cardiology (ACC) and AHA have developed a risk stratification tool for primary prevention in patients with T2DM; the Risk Estimator Plus tool can be used to calculate ten-year atherosclerosis CVD risk and provide individualized advice for patients aged 40–79 years. Risk stratification for secondary prevention is under clinical testing [33]. Alternatively, the European Society of Cardiology (ESC) recommends that CVD risk stratification be divided into moderate-, high-, and very high-risk levels for patients with either pre-DM or established DM based on comorbidities and the duration of the disease, rather than into primary or secondary prevention groups. These three levels of risk stratification support individualized diagnostic and therapeutic approaches to patient care in diabetes [34].
The ACC/AHA and ESC methods differ somewhat in the criteria they use for risk stratification. The Risk Estimator Plus does not account for the comorbidities and duration of diabetes, especially for adolescents due to the increase in obesity incidence, but it does consider age ≥ 40 years, sex, blood pressure, blood lipids, history of diabetes, smoking and drug use. Risk stratification for secondary prevention accounts for age ≥ 75 years, DM, hypertension, peripheral artery disease, previous stroke, previous coronary artery bypass graft, history of HF, active smoking, and renal dysfunction. The ESC method based on three levels of risk stratification recognizes the complexity of developing CVD by considering comorbidities and the duration of diabetes, and also accounts for obesity, renal impairment, left ventricular hypertrophy, and retinopathy rather than a history of CV-related disease. A comparison of the benefits of the risk estimator and that of risk stratification would be useful to obtain a consensus to facilitate the choice of individualized care in patients with T2DM and CVD.
Individualized glycemic management in patients with T2DM and CVD
A meta-analysis of 102 prospective studies showed that fasting blood glucose concentrations ≥ 7 mmol/L conferred an elevated risk of coronary heart disease [35]. Additionally, impaired glucose tolerance and elevated HbA1c (> 5.7%) levels have been associated with an increased risk of CVD [36]. An increased blood glucose concentration in the general population or in patients with atherosclerotic CVD confers an increased risk of all-cause mortality and CVD [37]. Individualized glycemic management may lead to improved prevention of CVD in T2DM. In this section, we focus on current aspects of individualized management of T2DM.
Glycemic targets
With respect to glycated hemoglobin (HbA1c) targets, the AHA, ADA, ESC and ESC/EASD guidelines propose a target of < 7%, and gives further recommendations for less stringent targets in older or more frail adults. A target of ≤ 6.5% for those with an early diagnosis, who are not frail, and who do not have established atherosclerotic CVD. This is consistent with current ESC, ADA and AHA guidelines on DM and CVD [38,39,40,41](Table 2).
Achieving a glycemic target of an HbA1c level < 7% for patients with T2DM reduces the incidence of the development of microvascular complications and CVD. A more stringent HbA1c goal (< 6.5%) is defined for selected children and adolescents, those with a short duration of DM, lesser degrees of β-cell dysfunction and no evidence of CVD. An HbA1c target of < 6% may be optimal during pregnancy without significant hypoglycemia. Furthermore, a less stringent HbA1c goal (< 8.0% or even slightly higher) may be appropriate for patients with limited life expectancy, a history of advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing T2DM [38]. The association of HF mortality with HbA1c goals in T2DM has a “U” shape, where a modest HbA1c level of 7.1–8.0% is associated with the lowest mortality among patients with HF and T2DM [42], whereas an HbA1c level < 6.0% or > 8%, and even > 10%, may increase the risk of incident HF or HF-related hospitalization [43].
Antidiabetic treatment choice
The novel glucose-lowering agents, sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists, have been shown to lower atherosclerotic CVD and HF risks independently of baseline HbA1c[44]; this has led to newer treatment approach. A reasonable regimen of glycemic control is associated with both glycemic targets and strategies of glucose-lowering therapy, and the medical provider must prioritize the patient’s CV state based on preference and the prescription history.
Metformin
The UK Prospective Diabetes Study (UKPDS 34) found that metformin therapy reduced all-cause mortality by 36% and heart attack incidence by 39% among overweight patients with newly diagnosed T2DM [45]. Moreover, a continued benefit of reductions in microvascular risk, myocardial infarction and death over 10 years after metformin therapy in that trial were reported [46]. Metformin administration may be the primary preventive measure against the risk of CVD among patients with newly diagnosed T2DM without established CVD. However, there is insufficient evidence for the use of metformin for secondary prevention in patients with T2DM and established CVD [47]. Only one RCT that included 68 patients with CAD showed a cardioprotective role of metformin [48], and in another trial, 36 patients with HF with reduced ejection fraction (HFrEF) showed increased myocardial efficiency after metformin treatment [49]. Nonetheless, the additional benefits of metformin may include prevention of the occurrence of new T2DM and cancer [50, 51], weight gain and hypoglycemia, and prolonged gestation in preterm preeclampsia [52]. Thus, metformin administration is suitable for the treatment of patients with T2DM without established CVD as well as those with CVD.
Sodium-glucose cotransporter 2 inhibitors
A meta-analysis of three studies showed that sodium-glucose cotransporter 2 (SGLT2) inhibitors reduced major adverse CV events by 11% in patients with T2DM and atherosclerotic CVD and reduced the risk of CV death or hospitalization for HF by 23% and the risk of renal disease progression by 45% among patients with T2DM [53]. SGLT2 inhibitors also reduced the risk of cardiac arrhythmias and atrial fibrillation/atrial flutter progression [54, 55].
Currently, the approved SGLT2 inhibitors are empagliflozin [56], canagliflozin [57], dapagliflozin [58], ertugliflozin [59], tofogliflozin [60], luseogliflozin [61] and ipragliflozin [61] (Table 3). The available studies focused on CVD are mainly limited to the United States and Europe; however, more recent real-world data have also shown a reduced risk of CV events across a wide range of patient characteristics from 6 countries in the Asia Pacific region, the Middle East, and North American regions [61]. In HF, empagliflozin administration was associated with improved clinical outcomes for the HFrEF phenotype [62], and the overall risk of HF events between HFrEF and HFpEF was reduced by canagliflozin [63], dapagliflozin [64], and ertugliflozin [65].
Overall, data suggest that SGLT2 inhibitors have moderate benefits in CVD and robust benefits in HF and renal disease and should be considered for primary and secondary prevention of CVD and renal outcomes in patients with T2DM, regardless of existing atherosclerotic CVD or a history of HF [53]. A recent meta-analysis by Giugliano et al. provided an update of all large CV outcome trials (CVOTs) with SGLT-2 inhibitors, with findings indicating that treatment with SGLT-2 inhibitors in patients with T2DM resulted in a sustained to moderate reduction of the composite CV death or hospitalization for HF, robust reduction of HF, moderate reduction of CV mortality, total mortality and major adverse cardiac events (MACE) [66]. Furthermore, a comprehensive systematic review and network meta-analysis of RCTs provided direct evidence for the absolute benefits and harms of SGLT-2 inhibitors in reducing CV outcomes in patients with type 2 diabetes [67]. Importantly, this comprehensive systematic review found that SGLT-2 inhibitors reduced all cause and CV mortality, myocardial infarction, admission for heart failure and kidney failure, without a reduction in non-fatal stroke.
In addition to the role of individual SGLT2 inhibitors, inhibitors of both SGLT1 and SGLT2 (dual SGLT1/2 inhibitors) have the ability to reduce intestinal glucose absorption after meals since SGLT1 in the small intestine [68]. Sotagliflozin, the first dual SGLT1/2 inhibitor, approved for T1DM and T2DM in the EU and T2DM in the US, has exhibited CV benefits in patients with T2DM and chronic kidney disease [69]; a similar result was shown in patients with T2DM and HFrEF or HFpEF [70]. The adverse events of sotagliflozin include diarrhea, genital mycotic infections, volume depletion, and diabetic ketoacidosis. Dual inhibition of SGLT1/2 with licogliflozin treatment showed CV benefits in patients with T2DM and HF, and licogliflozin has a greater effect than empagliflozin on glucose lowering, weight loss, and systolic blood pressure reduction [71]. The CV benefits of sotagliflozin seem to be equal to those of SGLT2 inhibitors. Sotagliflozin and licogliflozin may be recommended as first-line therapies among patients with T2DM, regardless of the presence of atherosclerotic CVD or a history of HF.
Other trials of SGLT1/2 inhibitors, including LX4211 [72], LX-2761, and YG-1699, for treating CVD in patients with T2DM are underway.
Glucagon-like peptide-1 receptor agonists
Five CVOTs, namely, LEADER3, SUSTAIN-6, REWIND, HARMONY, and AMPLITUDE-O [73] have consistently shown the safety and efficacy of GLP-1 agonists in patients with T2DM and have provided evidence of a secondary preventive effect of GLP-1 agonists in T2DM patients with CVD and kidney disease. Nevertheless, the ELIXA and EXSCEL trials did not show any benefit on CVD or kidney outcomes (Table 4). Currently, a notable decrease in CVD outcomes has been observed with several GLP-1 agonists; furthermore, GLP-1 agonists promote a reduction in CV risk factors, and dulaglutide showed strong evidence of primary prevention.
A systematic review and meta-analysis of RCTs revealed that GLP-1 receptor agonists reduced MACE by 14% and also reduced all-cause mortality by 12%, hospital admission for HF by 11%, and the composite kidney outcome by 21%, with no increase in risk of severe hypoglycemia, retinopathy, or pancreatic adverse effects [73]. Another meta-analysis showed that GLP-1 receptor agonists are efficacious for treating obesity and T2DM in children [74]. GLP-1 receptor agonists also safely reduce total MACEs by 12%, hospital admission for HF by 9%, and composite kidney outcomes (reduction in urinary albumin excretion) by 17% in adult T2DM patients [75]. Unexpectedly, a beneficial effect on HF has been observed with GLP-1 agonists. Furthermore, the largest and most current systematic review showed that GLP-1 agonists reduced all cause and CV mortality, myocardial infarction, non-fatal stroke, kidney failure and admission for HF in subjects with T2DM [67]. Overall, these important data provide novel insights into the benefits of GLP-1 receptor agonists in CVD patients; however, the benefits they may confer to primary prevention patients are unclear.
In summary, GLP-1 agonists show a beneficial effect on CVD, HF, and kidney outcomes and a reduction in weight gain, blood pressure, and levels of HbA1c and LDL-C. Therefore, GLP-1 agonists are recommended in the context of secondary prevention in patients with T2DM and CVD.
Finally, because the dual GIP/GLP-1 agonists will soon be introduced into clinical practice, it is worth addressing their potential. A phase 3 trial of tirzepatide showed that the mean HbA1c level decreased from baseline by 1.87–2.07%, more participants met their HbA1c targets, and weight loss ranged from 7.0 to 9.5 kg [76]. The efficacy of tirzepatide treatment in patients with T2DM was shown to be superior to that of treatment with 1.5 mg dulaglutide [77] and semaglutide [78]. Unfortunately, there are still no data on CVOTs with tirzepatide. However, tirzepatide treatment showed a good safety profile in T2DM patients with a high risk of or established CVD, an improved effect on HbA1c in comparison with insulin glargine, and no increases in MACE events compared to insulin glargine [79]. Available data show an important effect of tirzepatide in terms of HbA1c reduction, weight loss, blood pressure and lipid profiles, with this effect persisting for up to 2 years without increased CVD risk [80]. Tirzepatide displays primary preventive effects in T2DM with or without CVD risk and may be a well-positioned glucose-lowering agent in future therapeutic regimens.
Clinical trials of dual GIP/GLP-1 agonists, such as combined GIP and GLP-1 infusion [81] and NNC0090-2746 [82], are ongoing.
Other antidiabetic agents
Dipeptidylpeptidase-4 (DPP4) inhibitors have been reported to be neutral with regard to CV outcomes [83]. However, the use of saxagliptin is associated with an increase in hospitalization for HF [84], which should be carefully considered.
Regarding sulfonylureas, these older agents are inexpensive and widely available and are used as glucose-lowering agents for T2DM patients with CVD. The UKPDS 33 has confirmed the reduced microvascular risk of sulfonylureas [85]. In addition, the TOSCA.IT trial confirmed the CV safety of sulfonylureas [86]. Insulin is a widely extended therapeutic option. A meta-analysis showed that insulin treatment does not increase the risk of CV mortality and myocardial infarction [87]. A RCT showed that insulin glargine had a neutral effect on CV outcomes [88], and insulin is widely used to treat T2DM with CVD.
Finally, pioglitazone reduced key secondary macrovascular outcomes in people with T2DM without CV events whose glucose levels were controlled with metformin monotherapy [86]. Although the PROactive trial failed to show an effect of pioglitazone on the primary composite outcome, this drug resulted in a decrease in the secondary outcome (i.e., the classical 3 component MACE) [89]. Pioglitazone was also associated with a reduced risk of diabetes, ischemic stroke, and myocardial infarction in patients without diabetes [90]. An adverse effect of pioglitazone is an increased risk of developing HF, thus pioglitazone should not be used to treat subjects with HF. Therefore, pioglitazone may be used as an add-on treatment to metformin for people with T2DM, as these drugs are widely available and affordable.
Current recommendations on the use of antidiabetic drugs with regard to CVD
Different scientific societies recommend the use of antidiabetic therapies in CVD, including the AHA [39], the ADA [91], the ESC [41] and the ESC/EASD [40]; Chinese scientific bodies, however, do not [92]. The overall medication regimen is roughly similar, with a key focus on metformin, GLP-1 agonists and SGLT2 inhibitors in guidelines for treating CVD in T2DM patients (Table 5). The ADA and the EASD issued an update of their joint 2018 recommendations on management of hyperglycemia [93]. The major updates included: 1) SGLT2 inhibitors are recommended in patients with T2DM and HF, particularly those with HFrEF, to reduce hHF, MACE, and CV death and to prevent the progression of CKD, HF, MACE, and CV death in patients with T2DM with CKD; 2) to reduce risk of MACE, GLP-1 receptor agonists can also be considered in patients with type 2 diabetes without established CVD with indicators of high risk, specifically, patients aged 55 years or older with coronary, carotid, or lower extremity artery stenosis > 50%, and left ventricular hypertrophy. 3) for patients with type 2 diabetes and established atherosclerotic CV disease (such as those with prior myocardial infarction, ischemic stroke, unstable angina with ECG changes, myocardial ischemia on imaging or stress test, or revascularization of coronary, carotid, or peripheral arteries) where MACE is the gravest threat, the level of evidence for MACE benefit is greatest for GLP-1 receptor agonists.
The use of glucose-lowering agents should depend on CV status and T2DM complications. SGLT2 inhibitors (empagliflozin, canagliflozin, and dapagliflozin) are associated with a lower risk of HF hospitalization in patients with DM, and are recommended [94]. More importantly, the largest available systematic review found that SGLT2 inhibitors and GLP-1 receptor agonists lowered all-cause mortality, CV mortality, non-fatal myocardial infarction, and kidney failure. However, SGLT-2 inhibitors reduced admission to hospital for HF more than GLP-1 receptor agonists, and GLP-1 receptor agonists reduced non-fatal stroke more than SGLT-2 inhibitors (which appeared to have no effect) [67].
One RCT has shown the efficacy of metformin in patients with T2DM and HF [48]. Accordingly, metformin should be considered for treating patients with T2DM and HF (eGFR > 30 mL/min) [94]. Pioglitazone and saxagliptin are contraindicated in patients with HF or at risk of HF. Although initial data from RCTs of GLP-1 agonists supported a neutral effect on the risk of HF, a recent meta-analysis showed benefits of GLP-1 agonists in HF and diabetes [67]. However, current guidelines recommend SGLT2 inhibitors as the most suitable treatment in patients with HF.
Hypertension
The presence of hypertension in patients with T2DM significantly increases the risk of CVD development, and a study revealed that evaluated blood pressure down to < 120 mm Hg and < 70 mm Hg could decrease mortality, macrovascular, and microvascular events regardless of baseline systolic blood pressure [39]. Thus, lowering blood pressure reduces CVD events and microvascular complications and has a favorable effect on CVD outcomes in patients with and without T2DM.
Blood pressure treatment target
A target of 140/90 mmHg may be reasonable among patients with T2DM and stable CAD or patients with higher blood pressure targets; lower blood pressure targets of < 130/80 mmHg can be recommended for patients with a higher risk of stroke and microvascular complications, according to the AHA [95]. A target of < 130/80 mmHg may be appropriate for patients with diabetes and hypertension at higher CV risk (existing atherosclerotic cardiovascular disease (ASCVD) or 10-year ASCVD risk ≥ 15%), whereas a blood pressure target of < 140/90 mmHg is recommended for individuals with diabetes and hypertension at lower risk for CVD (10-year ASCVD risk < 15%). The ADA advises that a reading higher than 120/80 mmHg in patients with T2DM indicates the need for lifestyle intervention. In pregnant patients with diabetes and preexisting hypertension, a blood pressure target of 110–135/85 mmHg is suggested in the interest of reducing the risk for accelerated maternal hypertension [91]. ESC guidelines recommend that the initial target of < 140/90 mm Hg with goal of 120–130/ < 80 mm Hg is suitable for patients aged 18–69; the target of 130–139/ < 80 mm Hg, with < 130/ < 80 mm Hg being acceptable if tolerated is suitable for adults ≥ 70 years of age [41], although the blood pressure target for T2DM patients with CVD is unclear. Overall, individualized blood pressure targets are recommended based on CVD status and individual condition (Table 6).
Treatment choice
The ADA and ESC guidelines consistently recommend that initial treatment should involve the drug classes demonstrated to reduce CVD events in patients with T2DM and hypertension, and dual therapy, ACE inhibitors or ARBs in conjunction with dihydropyridine calcium channel blockers or thiazide diuretics are recommended as the first-line treatment[41, 94]. For patients with a urine albumin-to-creatinine ratio ≥ 30 mg/g, initial treatment should include an ACE inhibitor or ARB. For patients with pre-DM, ACE inhibitors or ARBs rather than diuretics or β-blockers are recommended due to the increased risk of new-onset T2DM from diuretics and β-blockers [96]. Nebivolol does not reduce insulin sensitivity and may be used as an antihypertensive treatment for T2DM patients [97].
In addition, a double-blind clinical trial has shown that the non-steroidal potent MRA blocker finerenone is efficacious and safe to reduce HF cardiac biomarkers, HF, and albuminuria in T2DM. Different trials also demonstrated a significant reduction in the primary outcome (CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for HF) in those subjects with T2DM and chronic kidney disease receiving finerenone [98,99,100]. Indeed, finerenone significantly improved cardiorenal outcomes in patients with T2DM and kidney disease irrespective of HbA1c levels or insulin use [101]. Therefore, finerenone should be considered for treatment of patients with T2DM, and this agent is likely to be included as an important treatment option in future updates of current guidelines.
Lipid management
The presence of diabetic dyslipidemia in patients with T2DM is, at least in part, a cause of CVD. Moreover, diabetic dyslipidemia, including elevated triglycerides, low-density lipoprotein cholesterol (LDL-C) and low high-density lipoprotein cholesterol (HDL-C), is associated with increased CV events, especially in high-risk populations [102]. Accounting for metabolic dyslipidemia in CVD risk stratification is necessary for patients with T2DM [103].
Lipid targets
The concept of “lower is better” regarding LDL levels has been supported for reducing the risk of CVD in patients with T2DM and CVD [104]. The ADA guidelines recommend that lifestyle interventions be initiated for individuals with abnormal triglyceride levels (> 150 mg/dL) and/or HDL-C levels (< 40 mg/dL for men, 50 mg/dL for women), and an LDL-C target reduction of ≥ 50% or more from baseline is reasonable for patients with diabetes and 10-year ASCVD risk of 20% or higher [91]. The ESC/EASD guidelines recommend an LDL-C target of < 55 mg/dL for those with T2DM and at very high CV risk [94]. The AHA guidelines recommend an LDL-C target reduction of ≥ 50% from baseline for those with diabetes and clinical CVD [39]. The ESC established goals for LDL-C based on level of ASCVD risk [41]. With respect to lipid targets, the AHA/ADA and the ESC/EASD guideline are shown in Table 7.
Treatment choice
Meta-analysis has shown that statins are the most effective therapy for reducing CV mortality, followed by PCSK9 inhibitors and statins in combination with ezetimibe. PCSK9 inhibitors effectively reduce MACEs [105], and statin therapy and add-on treatment with PCSK9 inhibitors or ezetimibe exhibit significant benefit in CVD outcomes [106]. Fibrate therapy reduces major CVD events [107], and icosapent ethyl exerts a risk reduction in CVD outcomes beyond lipid-lowering effects [108]. Overall, these lipid-lowering drugs have CV benefits for secondary prevention.
Depending on the baseline LDL-C levels, the initiation of statin therapy remains the first-line treatment in patients (aged < 50 years) with a T2DM duration < 10 years and without CVD risk factors or with an LDL-C of > 100 mg/dL. In patients at very high CVD risk, if the LDL-C target is not reached, despite treatment with the maximum tolerated statin dose, combination therapy with ezetimibe or a PCSK9 inhibitor is recommended [41, 94]. In high- and very high-risk patients with triglycerides of 200–500 mg/dL, statins combined with a fibrate or icosapent ethyl may be considered for both macro- and microvascular benefits in patients with T2DM [109]. Statin therapy is contraindicated in patients with pregnancy.
Antiplatelet therapy
T2DM is associated with increased blood thrombogenicity among patients with non-ST elevation acute coronary syndrome [110]. Platelet P2Y12 expression is increased fourfold in patients with T2DM, and platelet activation and hypercoagulation in T2DM induce a prothrombotic state and result in an increased risk for CVD events [111].
Aspirin
The use of aspirin decreases the risk of T2DM among healthy men but not among women [112]. Low-dose aspirin (81 or 100 mg) is neutral for CVD outcomes and increases the risk for gastrointestinal bleeding when used for primary prevention among patients with T2DM [113]. Contemporary meta-analyses show that the use of aspirin for the primary prevention of CV events needs to be reconsidered [114, 115] due to inconsistent CVD benefits and an increased bleeding risk.
In secondary prevention trials, meta-analyses showed that medium-dose aspirin (75–325 mg/day) reduces vascular events over 2 years [116], an aspirin dose of 75–150 mg daily is a protective regimen for patients with occlusive vascular events [117], and aspirin therapy for the secondary prevention of CVD has long been established [118]. Nonetheless, the longest duration of the included trials was just 4 years, and the aspirin effect for longer term use this requires caution, particularly in the post-acute setting [119]. Meta-analyses showed that early aspirin discontinuation in patients with acute coronary syndrome or percutaneous coronary intervention (PCI) prevented bleeding events with a neutral effect on CVD outcomes [120], and the duration of aspirin use (3 months) in patients undergoing complex PCI reduced the risk of bleeding without increasing the risk of ischemic events [121]. In acute settings, reducing the duration of aspirin use (< 3 months) for secondary prevention of CVD may be proposed.
P2Y 12 inhibitors
Compared with aspirin, P2Y12 inhibitors reduce the risk of myocardial infarction and stroke in secondary prevention [122]; thus, P2Y12 inhibitors may be a useful option for secondary prevention. P2Y12 inhibitors include prasugrel, ticagrelor and clopidogrel. Long-term administration of clopidogrel reduces the risk of myocardial infarction or vascular death, and the overall efficacy is superior to that of aspirin [123]. The benefits of prasugrel and ticagrelor over clopidogrel are even greater, and a reduction in CVD mortality was observed only with ticagrelor [124]. Ticagrelor exerts similar or greater inhibition of platelet reactivity than prasugrel in diabetic patients with CAD [125]. For patients after PCI, discontinuation of aspirin within 1 to 3 months with continued P2Y12 inhibitor monotherapy has a neutral effect on MACE outcomes with a reduction in bleeding [121].
Rivaroxaban
Rivaroxaban has been shown to reduce the incidence of CVD and an increased risk of bleeding among patients with chronic coronary syndrome [126]. Compared to warfarin, rivaroxaban reduces stroke, myocardial infarction and MACEs with a lower risk of bleeding among patients with atrial fibrillation and diabetes [127].
Treatment choice
Depending on risk stratification and the risk of bleeding, ESC guidelines recommend that aspirin be considered for primary prevention among patients with a risk of CVD or a diagnosis of CVD and low risk of bleeding [41, 94].
Intensive secondary prevention is indicated for patients with T2DM and CVD. Aspirin therapy (75–162 mg/day) is recommended as a secondary preventive strategy for patients with T2DM and atherosclerotic CVD. For diabetic patients with an aspirin allergy, clopidogrel (75 mg/day) should be used [128]. Long-term dual antiplatelet therapy is approved for patients with additional high-risk markers.
The addition of clopidogrel to aspirin for people with CVD risk or established CVD is associated with a reduction in myocardial infarction and ischemic stroke, however it also leads to an increase in bleeding [129]. This regimen may increase CVD death among patients with DM and microalbuminuria (≥ 30 μg/mL) [130]. The CV benefit of clopidogrel plus aspirin is reduced in T2DM due to high platelet reactivity, and increasing the dose of clopidogrel and aspirin may enhance antiplatelet effects [131, 132]. The benefit of an intensive antiplatelet regimen in these patients is still unclear. The National Institute for Health and Care Excellence (NICE) recommends prasugrel plus aspirin for people with ST elevation myocardial infarction after PCI. Prasugrel or ticagrelor plus aspirin is recommended for people with non-ST elevation myocardial infarction after PCI. Clopidogrel and oral anticoagulants other than prasugrel or ticagrelor for up to one year are recommended for people with acute coronary syndrome and atrial fibrillation after PCI [133]. The recommended duration of these regimens is > 30 months [134], while meta-analyses have indicated inconsistent results on the duration of dual antiplatelet therapy after PCI with drug-eluting stents [135, 136], and discontinuation of aspirin within 1 to 3 months with continued P2Y12 inhibitor monotherapy is recommended [137].
For patients with DM and atrial fibrillation or peripheral artery disease, ESC guidelines recommend rivaroxaban therapy [94]. Rivaroxaban plus aspirin is the preferred long-term antithrombotic regimen for patients with chronic coronary syndrome and high-risk factors [126].
Conclusion
The current main targets for the control of glycemia, lipids and blood pressure levels in patients according to the most commonly used guidelines should be included as an individualized strategy to prevent CVD in T2DM (Tables 2, 6 and 7). Although the incidence and mortality rate of T2DM-related CVD have decreased, the prevalence and mortality rate of CVDs in patients with T2DM continues to rise, and most T2DM-related CVDs may be prevented by lifestyle modification and the use of adjunctive drugs. The notion of T2DM-related CVD care has transitioned from comprehensive medical intervention to precision diabetes therapy. For T2DM patients with established CVD, the GLP-1 agonists, SGLT2 inhibitors, and blood-pressure and lipid-lowering drugs provide an improved precision treatment approach.
Availability of data and materials
Not applicable.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- CVD:
-
Cardiovascular disease
- HF:
-
Heart failure
- CAD:
-
Coronary artery disease
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- HbA1c:
-
Hemoglobin A1c
- SGLT2:
-
Sodium-glucose cotransporter 2
- GLP-1:
-
Glucagon-like peptide-1 receptor
- DPP4:
-
Dipeptidylpeptidase-4
- RCT:
-
Randomized controlled trial
- AHA:
-
American Heart Association
- ADA:
-
American Diabetes Association
- ESC:
-
European Society of Cardiology
- EASD:
-
European Association for the Study of Diabetes
- GFR:
-
Glomerular filtration rate
- pre-DM:
-
Prediabetes
- ACE:
-
Angiotensin-converting enzyme
- ARB:
-
Angiotensin II receptor blockers
- TGs:
-
Triglycerides
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- PCSK9:
-
Proprotein convertase subtilisin/kexin 9
- PCI:
-
Percutaneous coronary intervention
- MACE:
-
Major adverse cardiac event
- NICE:
-
National Institute for Health and Care Excellence
References
Diabetes. World Health Organization 2021, https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 10 Nov 2021.
World health statistics 2020: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
World health statistics 2021: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization 2021. Licence: CC BY-NC-SA 3.0 IGO.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83–83.
James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021. https://doi.org/10.1038/s41580-021-00390-6.
Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, Collins D. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care. 2003;26(5):1513–7.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ (Clinical research ed). 2000;321(7258):405–12.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8.
Stephanie A, Amiel PA, et al. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7(5):385–96.
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16.
Federation ID. Diabetes and cardiovascular disease. Brussels: International Diabetes Federation; 2016.
Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154.
Artime E, Romera I, Díaz-Cerezo S, Delgado E. Epidemiology and economic burden of cardiovascular disease in patients with type 2 diabetes mellitus in spain: a systematic review. Diabetes Ther. 2021;12(6):1631–59.
Tesfaye A, Josef H, Wube TB, Girma Z, Negasa B, Muche T, Zewude B. Magnitude of, and factors associated with cardiovascular disease among type two diabetes mellitus patients. DMSO. 2020;13:4123–9.
Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–18.
Park JH, Ha KH, Kim BY, Lee JH, Kim DJ. Trends in cardiovascular complications and mortality among patients with diabetes in South Korea. Diabetes Metab J. 2021;45(1):120–4.
Oo MM, et al. Observational study investigating the prevalence of asymptomatic stage B heart failure in patients with type 2 diabetes who are not known to have coronary artery disease. BMJ Open. 2021;11(1): e039869.
Echouffo-Tcheugui JB, Zhang S, Florido R, Hamo C, Pankow JS, Michos ED, Goldberg RB, Nambi V, Gerstenblith G, Post WS, et al. Duration of diabetes and incident heart failure: the ARIC (Atherosclerosis Risk In Communities) study. JACC Heart failure. 2021;9(8):594–603.
Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838.
Goodall R, Alazawi A, Hughes W, Bravis V, Salciccioli JD, Marshall DC, Crowley C, Shalhoub J. Trends in type 2 diabetes mellitus disease burden in European Union countries between 1990 and 2019. Sci Rep. 2021;11(1):15356–15356.
Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet (London, England). 2018;391(10138):2430–40.
Luk AOY, Hui EMT, Sin MC, Yeung CY, Chow WS, Ho AYY, Hung HF, Kan E, Ng CM, So WY, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: the hong kong diabetes database. Diabetes Care. 2017;40(7):928–35.
Vetrone LM, Zaccardi F, Webb DR, Seidu S, Gholap NN, Pitocco D, Davies MJ, Khunti K. Cardiovascular and mortality events in type 2 diabetes cardiovascular outcomes trials: a systematic review with trend analysis. Acta Diabetol. 2019;56(3):331–9.
Luo L-J, Wang D-D, Wang J, Yang F, Tang J-H. Diverse roles of miR-335 in development and progression of cancers. Tumor Biol. 2016. https://doi.org/10.1007/s13277-016-5385-3.
Jhund PS, McMurray JJ, Chaturvedi N, Brunel P, Desai AS, Finn PV, Haffner SM, Solomon SD, Weinrauch LA, Claggett BL, et al. Mortality following a cardiovascular or renal event in patients with type 2 diabetes in the ALTITUDE trial. Eur Heart J. 2015;36(36):2463–9.
Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, Gregg EW. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–73.
Sacre JW, Harding JL, Shaw JE, Magliano DJ. Declining mortality in older people with type 2 diabetes masks rising excess risks at younger ages: a population-based study of all-cause and cause-specific mortality over 13 years. Int J Epidemiol. 2021. https://doi.org/10.1093/ije/dyaa270.
Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus — implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16(6):321–31.
Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–96.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Perreault L, Skyler JS, Rosenstock J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(6):364–77.
Fegers-Wustrow I, Gianos E, Halle M, Yang E. Comparison of American and European Guidelines for primary prevention of cardiovascular disease: JACC guideline comparison. J Am Coll Cardiol. 2022;79(13):1304–13.
Grant PJ, Cosentino F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: new features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019;40(39):3215–7.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England). 2010;375(9733):2215–22.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355: i5953.
Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297–m2297.
Committee ADAPP: 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45(Supplement 1):S83-S96.
Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, Palo KED, Golden SH, Sperling LS. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement From the American Heart Association. Circulation. 2022;145(9):e722–59.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
Elder DH, Singh JS, Levin D, Donnelly LA, Choy AM, George J, Struthers AD, Doney AS, Lang CC. Mean HbA1c and mortality in diabetic individuals with heart failure: a population cohort study. Eur J Heart Fail. 2016;18(1):94–102.
Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, et al. Type 2 diabetes mellitus and heart failure: a Scientific Statement From the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–324.
Ipp E, Genter P, Childress K. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2017;376(9):890–1.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–65.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Hong J, Zhang Y, Lai S, Lv A, Su Q, Dong Y, Zhou Z, Tang W, Zhao J, Cui L, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304–11.
Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, Baig F, Hussain MS, Bhalraam U, Khan F, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. 2019;40(41):3409–17.
Larsen AH, Jessen N, Nørrelund H, Tolbod LP, Harms HJ, Feddersen S, Nielsen F, Brøsen K, Hansson NH, Frøkiaer J, et al. A randomised, double-blind, placebo-controlled trial of metformin on myocardial efficiency in insulin-resistant chronic heart failure patients without diabetes. Eur J Heart Fail. 2020;22(9):1628–37.
Warrilow A, Somerset S, Pumpa K, Fleet R. Metformin use in prediabetes: is earlier intervention better? Acta Diabetol. 2020;57(11):1359–66.
Martinez JA, Chalasani P, Thomson CA, Roe D, Altbach M, Galons JP, Stopeck A, Thompson PA, Villa-Guillen DE, Chow HH. Phase II study of metformin for reduction of obesity-associated breast cancer risk: a randomized controlled trial protocol. BMC Cancer. 2016;16:500.
Cluver CA, Hiscock R, Decloedt EH, Hall DR, Schell S, Mol BW, Brownfoot F, Kaitu’u-Lino TJ, Walker SP, Tong S. Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial. BMJ (Clinical research ed). 2021;374: n2103.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet (London, England). 2019;393(10166):31–9.
Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020;19(1):130.
Li H-L, Lip GYH, Feng Q, Fei Y, Tse Y-K, Wu M-Z, Ren Q-W, Tse H-F, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20(1):100–100.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347–57.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, Torimoto K, Umayahara Y, Kaneto H, Osonoi T, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol. 2020;19(1):110–110.
Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–39.
Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42(6):700–10.
Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. 2019;139(22):2591–3.
Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23(7):1217–25.
Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142(23):2205–15.
Giugliano D, Longo M, Scappaticcio L, Bellastella G, Maiorino MI, Esposito K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol. 2021;20(1):236.
Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, Tunnicliffe D, Ruospo M, Natale P, Saglimbene V, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573.
Powell DR, Zambrowicz B, Morrow L, Beysen C, Hompesch M, Turner S, Hellerstein M, Banks P, Strumph P, Lapuerta P. Sotagliflozin decreases postprandial glucose and insulin concentrations by delaying intestinal glucose absorption. J Clin Endocrinol Metab. 2020;105(4):e1235-1249.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2020;384(2):129–39.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384(2):117–28.
de Boer RA, Núñez J, Kozlovski P, Wang Y, Proot P, Keefe D. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br J Clin Pharmacol. 2020;86(7):1346–56.
Rosenstock J, Cefalu WT, Lapuerta P, Zambrowicz B, Ogbaa I, Banks P, Sands A. Greater dose-ranging effects on A1C levels than on glucosuria with LX4211, a dual inhibitor of SGLT1 and SGLT2, in patients with type 2 diabetes on metformin monotherapy. Diabetes Care. 2015;38(3):431–8.
Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–62.
Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: systematic review and meta-analysis. Obes Rev. 2021;22(6): e13177.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85.
Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (London, England). 2021;398(10295):143–55.
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet (London, England). 2018;392(10160):2180–93.
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15.
Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet (London, England). 2021;398(10313):1811–24.
Slomski A. Tirzepatide tested for type 2 diabetes with high cardiovascular risk. JAMA. 2021;326(24):2464–2464.
Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62(4):665–75.
Frias JP, Bastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RH, DiMarchi RD. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):343-352.e342.
Mannucci E, Mosenzon O, Avogaro A. Analyses of results from cardiovascular safety trials with dpp-4 inhibitors: cardiovascular outcomes, predefined safety outcomes, and pooled analysis and meta-analysis. Diabetes Care. 2016;39(Suppl 2):S196-204.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352(9131):837–853.
Vaccaro O, Masulli M, Nicolucci A, Bonora E, Del PS, Maggioni AP, Rivellese AA, Squatrito S, Giorda CB, Sesti G, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5(11):887–97.
Rados DV, Falcetta MRR, Pinto LC, Leitão CB, Gross JL. All-cause mortality and cardiovascular safety of basal insulin treatment in patients with type 2 diabetes mellitus: a systematic review with meta-analysis and trial sequential analysis. Diabetes Res Clin Pract. 2021;173: 108688.
Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A. The prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 2004;27(7):1647–53.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31.
Committee ADAPP: 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45(Supplement 1):S144-S174.
Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, Zou D, Guo L, Ji Q, Chen L, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6): e3158.
Buse JB, Wexler DJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, Kosiborod M, Leiter LA, Lipska KJ, Newman JD, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the american heart association. Circulation. 2020;141(19):e779–806.
Nazarzadeh M, Bidel Z, Canoy D, Copland E, Wamil M, Majert J, Smith Byrne K, Sundström J, Teo K, Davis BR, et al. Blood pressure lowering and risk of new-onset type 2 diabetes: an individual participant data meta-analysis. Lancet (London, England). 2021;398(10313):1803–10.
Ayers K, Byrne LM, DeMatteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension. 2012;59(4):893–8.
Redon J. New insights of cardiovascular and renal protection in diabetic chronic kidney disease with finerenone. Cardiovasc Res. 2022;118(5):e36–7.
Agarwal R, Filippatos G, Pitt B. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–84.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63.
Rossing P, Burgess E. Finerenone in patients with chronic kidney disease and type 2 diabetes according to baseline HbA1c and insulin use: an analysis from the FIDELIO-DKD study. Diabetes Care. 2022;45(4):888–97.
Ye X, Kong W, Zafar MI, Chen LL. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol. 2019;18(1):48.
Kaze AD, Santhanam P, Musani SK, Ahima R, Echouffo-Tcheugui JB. Metabolic dyslipidemia and cardiovascular outcomes in type 2 diabetes mellitus: findings from the look AHEAD study. J Am Heart Assoc. 2021;10(7): e016947.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England). 2010;376(9753):1670–81.
Khan SU, Talluri S, Riaz H, Rahman H, Nasir F, Bin Riaz I, Sattur S, Ahmed H, Kaluski E, Krasuski R. A Bayesian network meta-analysis of PCSK9 inhibitors, statins and ezetimibe with or without statins for cardiovascular outcomes. Eur J Prev Cardiol. 2018;25(8):844–53.
Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Räber L, Mach F, Windecker S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39(14):1172–80.
Zhu L, Hayen A, Bell KJL. Legacy effect of fibrate add-on therapy in diabetic patients with dyslipidemia: a secondary analysis of the ACCORDION study. Cardiovasc Diabetol. 2020;19(1):28.
Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19): e013543.
Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, Magni P, März W, Masana L, Mello ESA, et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European Atherosclerosis Society Task Force. Atherosclerosis. 2021;325:99–109.
Viswanathan GN, Marshall SM, Schechter CB, Balasubramaniam K, Badimon JJ, Zaman AG. Thrombus and antiplatelet therapy in type 2 diabetes mellitus. A prospective study after non-ST elevation acute coronary syndrome and a randomised, blinded, placebo-controlled study in stable angina. Thromb Haemost. 2012;108(5):937–45.
Pretorius L, Thomson GJA, Adams RCM, Nell TA, Laubscher WA, Pretorius E. Platelet activity and hypercoagulation in type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):141.
Hayashino Y, Hennekens CH, Kurth T. Aspirin use and risk of type 2 diabetes in apparently healthy men. Am J Med. 2009;122(4):374–9.
Saito Y, Okada S, Ogawa H, Soejima H, Sakuma M, Nakayama M, Doi N, Jinnouchi H, Waki M, Masuda I, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation. 2017;135(7):659–70.
Mahmoud AN, Gad MM, Elgendy AY, Elgendy IY, Bavry AA. Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur Heart J. 2019;40(7):607–17.
Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277–87.
Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81–106.
Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324(7329):71–86.
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet (London, England). 2009;373(9678):1849–60.
Jacobsen AP, Raber I, McCarthy CP, Blumenthal RS, Bhatt DL, Cusack RW, Serruys PWJC, Wijns W, McEvoy JW. Lifelong aspirin for all in the secondary prevention of chronic coronary syndrome. Circulation. 2020;142(16):1579–90.
Guedeney P, Mesnier J, Sorrentino S, Abcha F, Zeitouni M, Lattuca B, Silvain J, De Rosa S, Indolfi C, Collet J-P, et al. Early aspirin discontinuation following acute coronary syndrome or percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(3):680.
O’Donoghue ML, Murphy SA, Sabatine MS. The Safety and efficacy of aspirin discontinuation on a background of a P2Y(12) inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation. 2020;142(6):538–45.
Chiarito M, Sanz-Sánchez J, Cannata F, Cao D, Sturla M, Panico C, Godino C, Regazzoli D, Reimers B, De Caterina R, et al. Monotherapy with a P2Y(12) inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet (London, England). 2020;395(10235):1487–95.
Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. (CAPRIE) CAPRIE Steering Committee. Lancet. 1996;348(9038):1329–39.
Navarese EP, Khan SU, Kołodziejczak M, Kubica J, Buccheri S, Cannon CP, Gurbel PA, De Servi S, Budaj A, Bartorelli A, et al. Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation. 2020;142(2):150–60.
Franchi F, Rollini F, Aggarwal N, Hu J, Kureti M, Durairaj A, Duarte VE, Cho JR, Been L, Zenni MM, et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 Study. Circulation. 2016;134(11):780–92.
Zhu H, Xu X, Fang X, Ying F, Song L, Gao B, Tong G, Zhou L, Chen T, Huang J. Efficacy and safety of long-term antithrombotic strategies in patients with chronic coronary syndrome: a network meta-analysis of randomized controlled trials. J Am Heart Assoc. 2021;10(6): e019184.
Hua Y, Sun JY, Su Y, Qu Q, Wang HY, Sun W, Kong XQ. The safety and efficacy of rivaroxaban compared with warfarin in patients with atrial fibrillation and diabetes: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2021;21(1):51–61.
Disease C, Management R. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-s123.
Squizzato A, Bellesini M, Takeda A, Middeldorp S, Donadini MP. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;12(12):Cd005158.
Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, Mak KH, Fox KA, Montalescot G, Weber MA, Haffner SM, et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). Am J Cardiol. 2009;103(10):1359–63.
Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007;115(6):708–16.
Bethel MA, Harrison P, Sourij H, Sun Y, Tucker L, Kennedy I, White S, Hill L, Oulhaj A, Coleman RL, et al. Randomized controlled trial comparing impact on platelet reactivity of twice-daily with once-daily aspirin in people with Type 2 diabetes. Diabet Med. 2016;33(2):224–30.
Corbett SJ, Ftouh S, Lewis S, Lovibond K. Acute coronary syndromes: summary of updated NICE guidance. BMJ (Clin Res Ed). 2021;372: m4760.
Garratt KN, Weaver WD, Jenkins RG, Pow TK, Mauri L, Kereiakes DJ, Winters KJ, Christen T, Allocco DJ, Lee DP. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after TAXUS Liberté paclitaxel-eluting coronary stent placement. Circulation. 2015;131(1):62–73.
Yin SHL, Xu P, Wang B, Lu Y, Wu Q-Y, Zhou M-L, Wu J-R, Cai J-J, Sun X, Yuan H. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ. 2019;365:l2222.
Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, Khan MS, Mani P, Kapadia SR, Michos ED, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. 2020;142(15):1425–36.
Wang Q, Yang K, Bundhun PK. Discontinuing aspirin after short term use versus continuous use with a P2Y12 inhibitor for the treatment of patients with type 2 diabetes mellitus following percutaneous coronary intervention: a meta-analysis. Diabetes Ther. 2020;11(10):2299–311.
Acknowledgements
None to report.
Funding
The authors disclose receipt of the following forms of financial support for the research, authorship, and publication of this article: this work was supported by grants from the Lanzhou Chengguan District Science and Technology Plan Project (2021-9-19), the Hospital Fund of the First Hospital of Lanzhou University (ldyyyn2020-93), the Construction Program of Gansu Provincial Clinical Medical Research Center for Endocrine Diseases (20JR10FA667), the Gansu Provincial Natural Science Foundation (20JR10RA681), and the Special Funds of Science and Technology Development of the Chinese Central Government to Guide Local in 2020 (1004TCYA032).
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature search and interpretation of the available evidence. C-XM drafted the manuscript, and X-NM, C-HG, Y-DL, DM and S-BF critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave consent for the publication of the article.
Competing interests
I declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, CX., Ma, XN., Guan, CH. et al. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol 21, 74 (2022). https://doi.org/10.1186/s12933-022-01516-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01516-6