Abstract
Stress is deeply rooted in the modern society due to limited resources and large competition to achieve the desired goal. Women are more frequently exposed to several stressors during their reproductive age that trigger generation of reactive oxygen species (ROS). Accumulation of ROS in the body causes oxidative stress (OS) and adversely affects ovarian functions. The increased OS triggers various cell death pathways in the ovary. Beside apoptosis and autophagy, OS trigger necroptosis in granulosa cell as well as in follicular oocyte. The OS could activate receptor interacting protein kinase-1(RIPK1), receptor interacting protein kinase-3 (RIPK3) and mixed lineage kinase domain-like protein (MLKL) to trigger necroptosis in mammalian ovary. The granulosa cell necroptosis may deprive follicular oocyte from nutrients, growth factors and survival factors. Under these conditions, oocyte becomes more susceptible towards OS-mediated necroptosis in the follicular oocytes. Induction of necroptosis in encircling granulosa cell and oocyte may lead to follicular atresia. Indeed, follicular atresia is one of the major events responsible for the elimination of majority of germ cells from cohort of ovary. Thus, the inhibition of necroptosis could prevent precautious germ cell depletion from ovary that may cause reproductive senescence and early menopause in several mammalian species including human.
Similar content being viewed by others
Background
Stress has affected physical, social and psychological status of a person in the modern society [1, 2]. Although both genders are exposed to various kinds of stressors, females are more frequently exposed to one or other type of stressors during their reproductive life [3,4,5]. Several factors such as lifestyle, pressure and demands may generate psychological stress [2]. The psychological stress triggers the release of cortisol and generation of reactive oxygen species (ROS) in the body. Further, accumulation of ROS in the ovary results in oxidative stress (OS) [1, 6]. Studies suggest that high level of cortisol as well as OS induce granulosa cell death [2, 6, 7]. The granulosa cell death deprives follicular oocytes from nutrients, growth factor, survival factors and reduces estradiol biosynthesis [6]. The reduced level of estradiol-17β affects folliculogenesis and deteriorates oocyte quality by inducing various cell death pathways in somatic cells as well as in follicular oocyte [6,7,8]. Studies suggest that estradiol-17β could act as an antioxidant [9, 10] and protect OS-mediated apoptosis in pig [11] and ovine follicles [10, 12]. Although ovary is a dynamic organ and has its own antioxidant enzymes to scavenge ROS during final stages of folliculogenesis, depletion of antioxidants system could result in the accumulation of ROS and thereby OS in the ovary [13].
ROS affects oocyte physiology by modulating meiotic cell cycle resumption/arrest and cell death depending upon its level [6, 7, 14,15,16,17,18,19,20,21]. For instance, a moderate level of ROS triggers oocyte meiotic resumption from diplotene as well as M-II arrest [19, 22], while supplementation of antioxidants inhibits spontaneous resumption under in vitro culture condition [16, 17, 23]. Further, high level of ROS generates OS and induces meiotic cell cycle arrest and thereby apoptosis in rat oocytes cultured in vitro [6,7,8, 24,25,26,27]. The extremely high level of ROS induces necrosis in oocytes of several mammalian species including mouse [28], rat [29], ewe [30] and human [31].
Necrosis is morphologically characterized by organelle swelling, increase of cell volume and rupture of cell membrane [32]. Studies suggest that regulated form of necrosis so called necroptosis shows morphological features similar to necrosis [33]. A few studies indicate the occurrence of OS-mediated necroptosis in cow [34] and human ovary [35]. The OS-mediated necroptosis in granulosa cells and oocyte remains ill understood. This review article updates the information on stress-mediated necroptosis and proposes a possible molecular mechanism underlying OS-mediated necroptosis in mammalian ovary.
Stress and necroptosis in granulosa cells
Increase of ROS in the follicular fluid under physiological range is beneficial for follicular oocyte. For instance, a moderate increase of ROS is associated with spontaneous meiotic resumption, fertilization rate and reproductive outcome in rat [16] and human [16, 17, 23]. However, sustained high level of ROS generates OS and increased OS trigger granulosa cell death in rat [6, 7, 13]. The possible source for the increased level of ROS in the follicular fluid seems to be macrophages and the extracellular ROS together with TNF-α produced by macrophages, may trigger necroptosis of encircling granulosa cells [34]. The granulosa cell death subsequently starves oocyte and results in more vulnerable to cell death. The elevated intracellular ROS would trigger apoptosis, necrosis or necroptosis in response to the extent of insult and different stress conditions. In addition, ROS is cell permeable and it can easily enter in granulosa cells from follicular fluid. Thus, it is not possible to distinguish the necroptosis triggered by extracellular or intracellular ROS within the follicular microenvironment.
The prolonged starvation causes generation of ROS and induces necroptosis in human granulosa cells [35]. The increased level of ROS has been reported to inhibit cleavage of caspase and result in necroptosis in human ovary [33, 35]. The high level of ROS increases receptor interacting protein kinase 1 (RIPK1) and receptor interacting protein kinase 3 (RIPK3) in human granulosa cells [35]. Dehydroepiandrosterone (DHEA) reduces ROS production and RIPK expression in starvation-mediated necroptosis in human granulosa cells [35]. DHEA has been reported to improve oocyte quality probably by its antioxidant property [36]. The OS increases the expression of few enzymes that induce necroptosis in human granulosa cells [37]. Read-through acetylcholinesterase (AChE-R) is a stress form of acetylcholinesterase (AChE) and its expression increases in response to OS [38, 39]. The acetylcholine (ACh) is cleaved by AChE-R and triggers granulosa cell necroptosis in human [37]. The phosphorylated mixed lineage kinase domain-like protein (p-MLKL) has been reported during necroptosis in mouse and human ovary [40]. This is supported by the observation that the p-MLKL triggers granulosa cell necroptosis. The use of necrosulfonamide reduces necroptosis in human granulosa cells cultured in vitro [37, 41].
The increased number of macrophages has been reported during luteolysis in bovine corpus luteum [34, 42] and produce tumor necrosis factor alpha (TNF-α) as well as interferon-γ (IFNG) [34, 43]. The high level of cytokines triggers expression of RIPK1 and RIPK3 in luteal cell of cow ovary [34], human T-cells [44] and mouse dendritic cells [45]. The RIPK1 and RIPK3 act as stress sensors that promote necroptosis in cow ovary [34, 46]. On the other hand, necrostatin-1 treatment inhibits TNF-α and IFNG-mediated increase of RIPK-1 as well as RIPK-3 expression in granulosa cells of cow ovary [34]. These studies suggest that the increase of ROS generate OS in the follicular fluid of ovary. The OS triggers necroptosis in encircling granulosa cells through RIPK as well as MLKL-signaling pathways. The OS-mediated granulosa cell necroptosis could increase susceptibility of follicular oocyte in several mammalian species including human.
Environmental pollutants including cadmium (Cd) have been reported to trigger necroptosis in chinese hamster ovary (CHO) cells [47]. The Cd exposure increases intracellular calcium [(Ca2+)i] and generation of ROS and thereby calpain activity [47, 48]. Increased calpain activity reduces mitochondrial membrane potential (MMP), while increased level of ROS inhibits NF-κB activity [47]. The reduced level of MMP and NF-κB activities lead to Cd-induced necrotic cell death possibly through necroptosis. This possibility is further supported by the observations that the specific inhibitor of necroptosis, necrostatin-1, attenuates Cd-induced necroptosis by restoring the NF-κB activity in CHO cells [47].
Stress and necroptosis in oocyte
The endogenous burst of internal calcium stores result in extremely high level of [(Ca2+)i] that induce necrotic cell death through the generation of ROS in oocyte [22]. The follicular oocyte secretes GDF-9 that helps in the cumulus cell expansion and granulosa cell proliferation [49,50,51]. The possible involvement of necroptosis in oocyte comes from the observations that the necrostatin-1 treatment increases GDF-9 and mitotic arrest deficient 2 (Mad2) expressions required for meiotic competency of mouse oocyte [49]. Further, necrostatin-1 also increases Bcl-2 expression that ensures oocyte survival in mouse [49]. In addition, necrostatin-1 prevents OS-mediated deterioration of oocyte quality in mouse by modulating RIPK1 activity [49].
Immune suppression causes necrotic cell death in follicular oocyte and depletes ovarian reserve in mouse [52]. The death of oocyte under compromised immune system could be a programmed necrosis due to the involvement of a cytokine such as TNF-α [53, 54]. The TNF-α binds to it’s receptor and triggers necroptosis through RIPK1-mediated pathway [33, 55]. The increased TNF-α attenuates the development of ovary [56], decreases number of mature follicles in ovary and impairs oocyte meiotic maturation in mouse [52]. This is further supported by the observation that inhibition of TNF-α protein synthesis prevents spontaneous meiotic maturation in mouse oocyte cultured in vitro [52]. Thus, ROS-mediated TNF-α signaling may induce necroptosis in follicular oocytes in the ovary as described in granulosa cells.
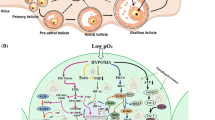
Oocyte is one of the major sources for TNF-α [57, 58] and its receptors are reported on oocyte as well as granulosa cells of rat ovary [58]. TNF-α binds to its receptor (TNFR) and triggers trimerization of cytoplasmic domain of TNFR1 containing TNFR1-associated death domain protein (TRADD). The TRADD binds to RIPK1 as well as TNFR associated factor 2 (TRAF2) forming complex-I [59, 60]. Cylindromatosis (CYLD) deubiquitinates RIPK1 [60, 61] and helps in the dissociation of complex-I from plasma membrane. The complex-I get associated with Fas-associating protein with death domain (FADD) and caspase-8 forming complex-II in the cytoplasm [62, 63]. Caspase-8 cleaves RIPK1 and RIPK3 resulting in apoptotic signals [55, 60, 64,65,66]. On the other hand, inhibition of caspase-8 results in RIPK1 and RIPK3 association and their autophosphorylation induces necroptosis [67, 68]. Activated RIPK3 then phosphorylates MLKL that finally leads to rupture of plasma membrane [40, 60, 69,70,71,72] (Fig. 1). The plasma membrane rupture is one of the important morphological features of necroptosis. The disruption of transfer of nutrients and signal molecules from dying granulosa cells to the oocyte is sufficient to trigger necroptosis. Further, nutrient deprivation may generate ROS in the oocyte and calcium burst from internal stores may trigger TNF-α signaling to induce oocyte necroptosis. The cross-talk between granulosa cells and oocyte decide the fate of each other, hence it is not yet clear which pathway is responsible for oocyte necroptosis.
Pathological conditions, environmental pollutants, starvation and lifestyle changes generate stress in the body. Stress increases extracellular ROS production from macrophages, OS and cytokines level in the granulosa cell of mammalian ovary. Stress as well as enhanced OS generates TNF-α that binds to its receptor present on the granulosa cell membrane and induces conformational changes that results a binding of TRADD with death domain of a receptor. TRADD recruits RIPK1 as well as TRAF2 forming complex-I. CYLD deubiquitinates RIPK1 allowing complex-I to dissociate from membrane. Complex-I moves into cytoplasm and associates with FADD as well as caspase-8 forming complex-II. Complex-II is responsible for the induction of apoptosis. Inhibition of caspase-8 allows the formation of necrosome and association of RIPK1 with RIPK3 that induce autophosphorylation of RIPK1 as well as RIPK3. RIPK1-RIPK3 complex phosphorylates MLKL that triggers damage of cell membrane resulting in necroptosis. Granulosa cell death deprives oocyte from survival factor, nutrients and cyclic nucleotides that lead to generation of ROS and thereby OS. The OS as well as increased level of intracellular calcium triggers oocyte necroptosis following a similar pathway as described for granulosa cell necroptosis
Beneficial impact of necroptosis in pathological ovary
Growing body of evidences suggest the beneficial role of necroptosis in controlling tumor growth in pathological ovary [73]. The majority of patients (60–85%) respond to primary therapy initially but later disease recurrence has been observed [74, 75]. This could be due to the evasion of apoptosis [76]. Studies suggest that the inhibitors of apoptosis protein (IAP) antagonism triggers necroptosis in apoptosis-resistant ovarian cancer cell [73]. It has been observed that necroptosis selectively occurs in apoptosis resistant cells [73]. The oncolytic vaccinia virus has been used to induce programmed necrosis in ovarian cancer cell [77]. The inhibition of RIPK1 and its substrate MLKL attenuate ovarian cancer cell death [77]. This is supported by the observations that the inhibition of RIPK1 and MLKL protect from vaccinia-mediated necroptosis in ovarian cancer cell [78]. These studies suggest that the induction of necroptosis in pathological ovary having ovarian cancer could be beneficial to control the cancer cell proliferation and may be used in the therapeutic design of ovarian cancer management [73].
Conclusions
Stress is frequently observed at each level of society and affecting day to day as well as social life of a person. Stress not only increases the cortisol production but also induce generation of ROS. High level of ROS causes OS that negatively affects physiology of mammalian ovary. An increased OS induces granulosa cell and oocyte necroptosis by operating RIPK as well as MLKL-mediated signaling pathways. Although necroptosis could be beneficial to prevent tumor growth in pathological ovary, its involvement in granulosa cell and oocyte may cause precautious depletion of germ cells from the cohort of ovary. Thus, prevention of necroptosis in normal and healthy ovary may prevent reproductive senescence as well as early menopause in several mammalian species including human.
Abbreviations
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AChE-R:
-
Read-through Acetylcholinesterase
- Cd:
-
Cadmium
- CHO:
-
Chinese hamster ovary
- DHEA:
-
Dehydroepiandrosterone
- FADD:
-
Fas associating protein with death domain
- GDF9:
-
Growth differentiation factor 9
- IAP:
-
inhibitors of apoptosis protein
- IFNG:
-
interferon γ
- Mad2:
-
Mitotic arrest deficient 2
- MLKL:
-
Mixed lineage kinase domain like protein
- MMP:
-
Mitochondrial membrane potential
- OS:
-
Oxidative stress
- RIPK1:
-
Receptor interacting protein kinase-1
- RIPK3:
-
Receptor interacting protein kinase-3
- ROS:
-
Reactive oxygen species
- TNFR:
-
Tumor necrosis factor receptor
- TNF-α:
-
Tumor necrosis factor alpha
References
Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66.
Prasad S, Tiwari M, Tripathi A, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on female reproductive outcome. J Biomed Sci. 2016;23:36.
Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21(16):6292–7.
Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64(4):303–8.
Xu M, Sun J, Wang Q, Zhang Q, Wei C, Lai D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS One. 2018;13(3):e0194894.
Tripathi A, Shrivastav TG, Chaube SK. An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int J Appl Basic Med Res. 2013;3(1):27–36.
Tiwari M, Chaube SK. Spontaneous exit from diplotene arrest associates with the increase of calcium and reactive oxygen species in rat oocytes cultured in vitro. React Oxygen Species. 2017;4(12):418–33.
Tiwari M, Prasad S, Tripathi A, Pandey AN, Ali I, Singh AK, Shrivastav TG, Chaube SK. Apoptosis in mammalian oocytes: a review. Apoptosis. 2015;20(8):1019–25.
Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol. 1993;45(6):509–11.
Sugino N. Reactive oxygen species in ovarian physiology. Reprod Med Biol. 2005;4(1):31–44.
Murdoch WJ. Inhibition by oestradiol of oxidative stress-induced apoptosis in pig ovarian tissues. J Reprod Fertil. 1998;114(1):127–30.
Lund SA, Murdoch J, Van Kirk EA, Murdoch WJ. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: relevance to luteal function. Biol Reprod. 1999;61(2):388–92.
Tiwari M, Prasad S, Tripathi A, Pandey AN, Singh AK, Shrivastav TG, Chaube SK. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React Oxygen Species. 2016;1(2):110–6.
Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem. 2010;111(3):521–8.
Tripathi A, Shrivastav TG, Chaube SK. Aqueous extract of Azadirachta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J Assist Reproduction Genet. 2012;29(1):15–23.
Pandey AN, Chaube SK. A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Biores Open Access. 2014;3(4):183–91.
Pandey AN, Chaube SK. Reduction of nitric oxide level leads to spontaneous resumption of meiosis in diplotene-arrested rat oocytes cultured in vitro. Exp Biol Med (Maywood). 2015;240(1):15–25.
Chaube SK, Prasad S, Tiwari M, Gupta A. Rat: an interesting model to study oocyte meiosis in mammals. Res Rev J Zool Sci. 2016;4(3):25–7.
Premkumar KV, Chaube SK. Nitric oxide signals postovulatory aging-induced abortive spontaneous egg activation in the rat. Redox Rep. 2015;20(4):184–92.
Tiwari M, Chaube SK. Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. J Obstet Gynaecol Res. 2016;42(5):536–46.
Yadav PK, Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN, Chaube SK. Germ cell depletion from mammalian ovary: possible involvement of apoptosis and autophagy. J Biomed Sci. 2018;25(1):36.
Premkumar KV, Chaube SK. RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim. 2014;50(7):640–7.
Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am J Physiol Endocrinol Metab. 1999;276(4 Pt 1):e684–8.
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10(4):863–74.
Tripathi A, Premkumar KV, Pandey AN, Khatun S, Mishra SK, Shrivastav TG, Chaube SK. Melatonin protect against clomiphene citrate-induced generation of free radicals and egg apoptosis in rat. Eur J Pharmacol. 2011;667(1–3):419–24.
Tripathi A, Chaube SK. High level of cytosolic free calcium signals apoptosis through the mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis. 2012;17(5):439–48.
Tripathi A, Chaube SK. Roscovitine induces metaphase-II arrest and apoptosis through FasL-mediated pathway in rat eggs cultured in vitro. In Vitro Cell Dev Biol Anim. 2015;51(2):174–82.
Paleos GA, Powers RD. The effect of calcium on the first meiotic division of the mammalian oocyte. J Exp Zool. 1981;217(3):409–16.
Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43(3):287–94.
Alonso-Pozos I, Rosales-Torres AM, Avalos-Rodrıguez A, Vergara-Onofre M, Rosado-Garcia A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology. 2003;60(6):1071–81.
Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13(6):451–8.
Zhou Z, Han V, Han J. New components of the necroptotic pathway. Protein Cell. 2012;3(11):811–7.
Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82(3):249–58.
Hojo T, Siemieniuch MJ, Lukasik K, Pioprowska-Tomala KK, Jonczyk AW, Okuda K, Skarzynski DJ. Programmed necrosis-a new mechanism of steroidogenic luteal cell death and elimination during luteolysis in cows. Sci Rep. 2016;6:38211.
Tsui KH, Wang PH, Lin LT, Li CJ. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction. 2017;154(2):101–10.
Pellissier MA, Trap C, Malewiak MI, Morfin R. Antioxidant effects of dehydroepiandrosterone and 7alpha-hydroxy-dehydroepiandrosterone in the rat colon, intestine and liver. Steroids. 2004;69(2):137–44.
Blohberger J, Kunz L, Einwang D, Berg U, Berg D, Ojeda SR, Dissen GA, Frohlich T, Arnold GJ, Soreq H, et al. Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis. 2015;6(3):e1685.
Hartl R, Gleinich A, Zimmermann M. Dramatic increase in readthrough acetylcholinesterase in a cellular model of oxidative stress. J Neurochem. 2011;116(6):1088–96.
Zimmermann M. Neuronal AChE splice variants and their non-hydrolytic functions: redefining a target of AChE inhibitors? Br J Pharmacol. 2013;170(5):953–67.
He S, Huang S, Shen Z. Biomarkers for the detection of necroptosis. Cell Mol Life Sci. 2016;73(11–12):2177–81.
Du Y, Bagnjuk K, Lawson MS, Xu J, Mayerhofer A. Acetylcholine and necroptosis are players in follicular development in primates. Sci Rep. 2018;8(1):6166.
Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fertil. 1999;115(1):87–96.
FriedmanA WS, Levy N, Meidan R. Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol Reprod. 2000;63(6):1905–12.
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–11.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–95.
Declercq W, VandenBerghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–32.
Hsu TS, Yang PM, Tsai JS, Lin LY. Attenuation of cadmium-induced necrotic cell death by necrostatin-1: potential necrostatin-1 acting sites. Toxicol Appl Pharmacol. 2009;235(2):153–62.
Yang PM, Chen HC, Tsai JS, Lin LY. Cadmium induces Ca2+-dependent necrotic cell death through calpain-triggered mitochondrial depolarization and reactive oxygen species-mediated inhibition of nuclear factor-KB activity. Chem Res Toxicol. 2007;20:406–15.
Jo JW, Lee JR, Jee B, Suh CS, Kim SH. Exposing mouse oocytes to necrostatin 1 during in vitro maturation improves maturation, survival after vitrification, mitochondrial preservation, and developmental competence. Reprod Sci. 2015;22(5):615–25.
Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146(6):2798–806.
Hirao Y. Oocyte growth in vitro: potential model for studies of oocyte–granulosa cell interactions. Reprod Med Biol. 2011;11(1):1–9.
Elena S, Victor D, Tatyana V, Nataliya G, Roman Y. Changes in expression of TNF alpha and its receptors mRNAs in oocytes and granulosa cells in mice with experimental immune ovarian failure. J Health Sci. 2014;4(8):81–90.
Osnes LT, Nakken B, Bodolay E, Szodoray P. Assessment of intracellular cytokines and regulatory cells in patients with autoimmune diseases and primary immunodeficiencies - novel tool for diagnostics and patient follow-up. Autoimmun Rev. 2013;12(10):967–71.
Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol. 2013;93(1):51–61.
Li D, Meng L, Xu T, Su Y, Liu X, Zhang Z, Wang X. RIPK1-RIPK3-MLKL-dependent necrosis promotes the aging of mouse male reproductive system. elife. 2017;6:e27692.
Bagavant H, Adams S, Terranova P, Chang A, Kraemer FW, Lou Y, Kasai K, Luo AM, Tung KS. Autoimmune ovarian inflammation triggered by proinflammatory (Th1) T cells is compatible with normal ovarian function in mice. Biol Reprod. 1999;61(3):635–42.
Terranova PF. Potential roles of tumor necrosis factor-alpha in follicular development, ovulation, and the life span of the corpus luteum. Domest Anim Endocrinol. 1997;14(1):1–15.
Marcinkiewicz JL, Balchak SK, Morrison LJ. The involvement of tumor necrosis factor-alpha (TNF) as an intraovarian regulator of oocyte apoptosis in the neonatal rat. Front Biosci. 2002;7:d1997–2005.
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90.
LuJV CHC, Walsh CM. Necroptotic signaling in adaptive and innate immunity. Semin Cell Dev Biol. 2014;35:33–9.
Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8(10):e76841.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–23.
Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703.
Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–26.
Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, Lollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, et al. Caspase-8 prevents sustained activation of NF-kappa B in monocytes undergoing macrophagic differentiation. Blood. 2007;109(4):1442–50.
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–67.
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277(11):9505–11.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildform M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23.
Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65.
Cai Q, Gan J, Luo R, Qu Y, Li S, Wan C, Mu D. The role of necroptosis in status epilepticus-induced brain injury in juvenile rats. Epilepsy Behav. 2017;75:134–42.
Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111(43):15438–43.
Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–21.
McCabe KE, Bacos K, Lu D, Delaney JR, Axelrod J, Potter MD, Vamos M, Wong V, Cosford ND, et al. Triggering necroptosis in cisplatin and IAP antagonist-resistant ovarian carcinoma. Cell Death Dis. 2014;5:e1496.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126.
Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park). 2013;27(4):288–94.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Whilding LM, Archibald KM, Kulbe H, Balkwill FR, Oberg D, McNeish IA. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol Ther. 2013;21(11):2074–86.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIPK3 kinase. Cell. 2012;148(1–2):213–27.
Acknowledgements
Not applicable.
Funding
This article is supported by Department of Science and Technology, Ministry of Science and Technology, Government of India (Grant No. EMR/2014/000702).
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
GRC searched the literature and wrote the initial draft of manuscript. SKC suggested the structure. PKY, AKY, MT, AG, AS, ANP and AKP revised and finished the final version of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chaudhary, G.R., Yadav, P.K., Yadav, A.K. et al. Necroptosis in stressed ovary. J Biomed Sci 26, 11 (2019). https://doi.org/10.1186/s12929-019-0504-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-019-0504-2