Abstract
Background and aim
Metabolic syndrome is one of the major public-health challenges, affecting one-quarter of the world population. Fatty acid quality indices are novel determinants of this disease and their interactions with genetic factors may have an impact on metabolic syndrome risk. Therefore, we aimed to investigate the interaction between genetic risk score (GRS) and fatty acid quality indices with metabolic syndrome (MetS) among overweight and obese women.
Methods
In the present cross-sectional study, 279 overweight and obese women (18–48 years old) were included. Several anthropometric measurements such as weight, height, body mass index (BMI), waist circumference (WC), and body fat percent (BF%) were measured. Also, systolic and diastolic blood pressure (SBP and DBP) were measured. Biochemical determination was performed for fasting blood glucose (FBS), triglyceride (TG), and high-density lipoprotein (HDL). MetS was determined according to National Cholesterol Education Program (NCEP ATP III) criteria. Dietary intake was evaluated by a validated and reliable 147-item semi-quantitative food frequency questionnaire. Cholesterol-saturated fat index (CSI) and the ratio of omega-6/omega-3 (ω-6/ω-3) essential fatty acids were considered as fat quality indices. The salting-out method was used to extract the total DNA. The unweighted GRS was calculated using the risk alleles of the three single nucleotide polymorphisms. The total average GRS value was 2 and the sum of the risk alleles of the 3 polymorphisms was 6.
Result
The results of our analysis showed that after controlling for age, energy intake, BMI, and physical activity, there was a positive interaction between T2 of GRS and T2 of N6/N3 ratio on WC (β = 7.95, 95%CI = 0.83,15.08, P = 0.029), T3 of GRS and T2 of N6/N3 ratio on DBP (β = 5.93, 95%CI= -0.76,12.63, P = 0.083), and FBS (β = 6.47, 95%CI = 0.59,13.53, P = 0.073), T3 of GRS and T3 of N6/N3 ratio on TG (β = 54.42, 95%CI = 1.76,107.08, P = 0.043), and T3 of GRS and T3 of CSI on BF% (β = 3.55, 95%CI= -0.35,7.45, P = 0.075). Also T2 of GRS in the interaction with T3 of CSI leads to an decrease − 8.35 mg/dl in HDL level after adjustment in (β= -8.35, 95%CI= -17.34,0.62, P = 0.068).
Conclusion
It seems the interaction of GRS and fatty acid quality indices is positively associated with several components of metabolic syndrome such as WC, TG and BF%. Our findings are of importance to public health, considering the high consumption of foods that are high on fatty acids. Conflicting evidence of many previous studies regarding the effect of fat intake and obesity and cardiovascular diseases could be because of the gene-diet interactions and genetic heterogeneity across various ethnic groups. Hence, the synergism effect of genetic and dietay intakes should be considered in future studies.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a cluster of components including obesity, hypertriglyceridemia, high-density lipoprotein cholesterol (HDL), hypertension, and high fasting blood glucose [1], which is associated with an increased risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [2]. Given that 20–25% of adults worldwide have MetS, it is regarded as one of the major contributors to serious global health challenges over the current century [3, 4]. Furthermore, the prevalence of this syndrome in Asian countries varies, ranging from 10 to 20% [5] with an estimated 8–35% of the Iranian population affected [6,7,8,9].
As a multifactorial disease, environmental conditions such as dietary intake and genetic variations are involved in its pathogenesis [10,11,12,13,14,15]. Apart from a positive connection between total fat intake and risk of MetS, as reported in a Japanese-Brazilian population, it is crucial to mention that dietary fat quality is also of utmost importance [16]. During the preceding decade, Connor et al. proposed the Cholesterol-Saturated Fat Index (CSI), a novel index of dietary fat quality [17]. Alongside CSI, Simopoulos indicated the importance of the omega-6/omega-3 essential fatty acids (EFA) ratio [18]. The CSI is a dietary self-monitoring tool that reflects the cholesterol and saturated fat content of food and helps patients to improve their cholesterol-lowering eating plan, by its influence on self-management and better food selection. As a matter of fact, lower CSI represents reduced saturated fatty acid (SFA) and cholesterol [19]. Studies have reported that following dietary pattern high in SFA might be attributed to weight gain and elevated risk of metabolic disturbances [20, 21]. Moreover, polyunsaturated fatty acids such as linoleic acid have shown a tendency to attenuate the risk of MetS due to their relation with insulin resistance [16]. In this regard, a balanced ratio of omega-6/omega-3 EFA plays a central role in the prevention and management of chronic diseases [18]. Of note is that, genetic predisposition has been recognized as a significant risk factor for MetS [22] and genetic risk score (GRS), calculated through the summing of risk alleles for each single nucleotide polymorphisms (SNP) [23], was developed to determine the association between MetS and genetic factors. Here, large-scale genome-wide association studies (GWAS) identified obesity-related SNPs for three novel genes of Melanocortin-4 Receptor (MC4R), Caveolin (CAV), and Cryptochrome (CRY) [24,25,26]. Following this identification, the “gene-environment interaction” hypothesis was suggested [27]. In accordance with this hypothesis, individuals with adherence to a western dietary pattern that is high in saturated fat and low in linoleic acid, indicated an increased risk for MetS, considering genetic predisposition [28]. However, to the author’s knowledge, no literature has been generated on the interaction between BMI-GRS, based on aforementioned genetic variants, and dietary fat quality indices on MetS thus far, and most of them evaluated single SNPs interactions [29]. Therefore, this study aimed to investigate the interaction between BMI-GRS including MC4R (rs17782313), CAV-1 (rs3807992), and Cry-1 (rs2287161) with dietary fat quality indices according to CSI and omega-6/omega-3 EFA ratio on MetS in overweight and obese women.
Method and materials
Study population
In the present cross-sectional study, among all health centers of Tehran University of Medical Sciences, 20 health centers were selected randomly in 2018. Through multi-stages simple random sampling, 279 overweight and obese women who were referred to one of those health centers were entered. Participants (overweight and obese) with BMI of 25 to 40 kg/m2 [30] and ages range of 18 to 48 years were included. All subjects signed the written informed consent at begin of the study and the Tehran University of Medical Sciences (TUMS) approved them. Exclusion criteria were as follows: patients with malignancies; liver, kidney, or cardiovascular diseases; all types of diabetes; thyroid disease; any other acute and chronic diseases, menopause or pregnant women, lactation, weight loss supplementation, antihypertensive or lowering glucose and lipid medications, dieting during the last year, and smoking. The present study was approved by The Ethics Committee of the TUMS (assigned number: IR.TUMS.VCR.REC.1398.636). Regarding the following formula, sample size of 279 was estimated to sufficiently evaluate the outcomes (both primary and secondary) and achieve r = 0.25 [31], β = 0.95, and a type I error α = 0.05. Formula: n= (([Z1−α+Z1−β) ×<![CDATA[ \surd ]]>1-r2]/r) 2+2 [32].
Anthropometric and blood pressure assessment
Several anthropometric measurements were measured by bioelectrical impedance analyzer BIA, including weight, body mass index (BMI), and body fat percent (BF%); following the manufacturer’s protocol (InBody 770 scanner from InBody Co. (Seoul, Korea)) [33]. Subjects were required to remove extra clothing and metal objects such as rings, earrings, watches, sweaters, coats, and shoes.
Moreover, the height was measured using a non-stretch tape measure in a standing up position with 0.5 cm precision. Waist circumference (WC) was measured using the most prominent portion and the narrowest portion respectively with 0.5 cm precision.
Blood pressure was measured using an appropriate cuff according to arm size. It was measured for two times after 5 min of rest. Finally, the average of two measurements was recorded.
Physical activity assessment
Physical activity (PA) was assessed based on the validated and reliable self-report instrument called the short-form of the International Physical Activity Questionnaire (IPAQ). The IPAQ assesses the duration and frequency of typical daily activities throughout a week in the preceding year. It quantifies the participants’ weekly physical activity levels in metabolic equivalent hours (MET-h/week) [34].
Biochemical and hormonal determination
Venous blood was collected between 8:00 to 10:00 a.m. after fasting overnight. Serum samples were centrifuged, stored at − 80 °C, and analyzed by using a single assay technique. Fasting blood glucose (FBS), and triglyceride (TG) were measured by using glucose oxidase-phenol 4-aminoantipyrine peroxidase (GOD-PAP) and glycerol-3-phosphate oxidase–phenol 4-aminoantipyrine peroxidase (GPOPAP) enzymatic endpoint, respectively. We measured high-density lipoprotein (HDL) cholesterol using by direct enzymatic clearance assay. Randox Laboratories (Hitachi 902) kit was used for all measurements.
All samples were assessed by standard methods at the Nutrition and Biochemistry Laboratory of the School of Nutritional and Dietetics at TUMS.
Assessment of metabolic syndrome (MetS)
MetS was determined according to National Cholesterol Education Program (NCEP ATP III) criteria [35]. Presence of 3 or more of the following criteria was considered as MetS: (1) abdominal obesity [≥ 102 cm for men and WC ≥ 88 cm for women]; (2) hypertriglyceridemia [≥ 150 mg/dL]; (3) reduced HDL [< 40 mg/dL for men and < 50 mg/dL for women]; (4) raised FBS [FBS > 100 mg/dL]; and (5) raised blood pressure [systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mm Hg].
Dietary intake assessment
Dietary intake was evaluated by a validated and reliable 147-item semi-quantitative food frequency questionnaire (FFQ) [36]. Participants recorded their usual diet consumption frequency through a day, week, or month in the last year in the presence of a dietitian. Dietary intake was analyzed for energy intake, macronutrients, and micronutrients utilizing the NUTRITIONIST 4 (First Data Bank, San Bruno, CA) food analyzer [37].
Dietary fat quality indices
FFQ was evaluated to determine those food items to be included. Cholesterol-saturated fat index (CSI) and the ratio of omega-6/omega-3 (ω-6/ω-3) essential fatty acids were considered as fat quality indices. CSI indicates the concentrations of cholesterol and saturated fat in foods. By dividing cholesterol by saturated fat content of food items that were derived from FFQ, CSI was presented [19]. A low CSI represents low cholesterol and/or saturated fat content, therefore a diet with lower CSI has hypocholesterolemic and low atherogenic potential. Also, the ratio of ω-6 to ω-3 was calculated according to dividing ω-6 to ω-3 contents of food items which had been evaluated by FFQ [17, 18].
FFQ was evaluated to determine those food items to be included. Cholesterol-saturated fat index (CSI) and the ratio of omega-6/omega-3 (ω-6/ω-3) essential fatty acids were considered as fat quality indices. CSI indicates the concentrations of cholesterol and saturated fat in foods. By dividing cholesterol by saturated fat content of food items that were derived from FFQ, CSI was presented [19]. A low CSI represents low cholesterol and/or saturated fat content, therefore a diet with lower CSI has hypocholesterolemic and low atherogenic potential. Also, the ratio of ω-6 to ω-3 was calculated according to dividing ω-6 to ω-3 contents of food items which had been evaluated by FFQ [17, 18].
Genotyping and GRS
The salting-out method was used to extract the total DNA [38]. 1% agarose gel was used to assess the DNA integrity and a nanodrop 8000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used to assess DNA concentration. SNP genotyping was carried out using the TaqMan Open Array (Life Technologies Corporation, Carlsbad, CA, USA) [39].
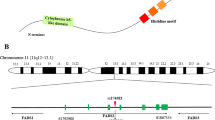
The CAV-1 (rs3807992) forward primer is 3′AGTATTGACCTGATTTGCCATG 5′ and the reverse primer is 5′ GTCTTCTGGAAAAAGCACATGA 3′. The fragments containing three genotypes were distinguished: GG, AA, and GA. The Cry1 (rs2287161) forward primer is 5′-GGAACAGTGATTGGCTCTATCT − 3′ and the reverse primer is 5′-GGTCCTCGGTCTCAAGAAG-3′. Then, the fragments containing three genotypes were distinguished: CC, GG, and GC. The MC4R gene primer was selected based on a previous study [40]. The MC4R (rs17782313) forward primer is 5- AAGTTCTACCTACCATGTTCTTGG-3 and the reverse primer is 5-TTCCCCCTGAAGCTTTTCTTGTCATTTTGAT-3. Then, fragments containing three genotypes were distinguished: CC, TT, and CT. We created the GRS by combining three single nucleotide polymorphisms [CAV-1 (rs3807992), Cry-1 (rs2287161), and MC4R (rs17782313)] that had previously been linked to obesity-related traits in GWAS and other studies [26, 41, 42]. The risk alleles for higher BMI were assigned to each SNP by recoding them into 0, 1, or 2. The unweighted GRS was calculated using the risk alleles of the three SNPs. Higher scores indicate greater genetic susceptibility to higher BMI on the GRS scale, which ranges from 0 to 6 [43].
Statistical analyses
The normal distribution of data was assessed by the Kolmogorov-Smirnov test. General characteristics of participants were presented as mean ± standard deviation, minimum and maximum. Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were conducted to compare anthropometric indices, blood pressure, FBS, and lipid profile among participants. A generalized linear model (GLM) was used in crude and adjusted models to evaluate the associations of MetS components (dependent variable) and GRS (independent variable). Adjustments were performed for age, energy intake, PA, and BMI. All statistical analysis was performed using SPSS version 23.0 (SPSS, Chicago, IL, USA). A P-value lower than 0.05 was considered statistically significant and a P-value lower than 0.1 was considered marginally significant.
Result
Study population characteristics
A total of 279 overweight and obese women were evaluated in this study. The mean height, weight, BMI and WC of participants were 161.28 cm, 80.75 kg, and 31.03 kg/m2 and 99.22 cm respectively. Also, the mean of metabolic factors including FBS, TG, and HDL of participants were 87.26 mg/dl, 120.80 mg/dl, and 46.45 mg/dl, respectively.
Mean and standard deviation (SD) of general characteristics according to tertiles of CSI and N6/N3
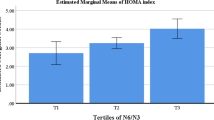
The general characteristics of study participants among tertiles of the CSI and N6/N3 ratio were presented in Table 1. According to this table, p-values for all variables were reported in the crude and adjusted model after controlling for potentially confounding variables (age, energy intake, physical activity, and BMI). In the crude model, a significant mean difference was observed among tertiles of the CSI in terms of age (P = 0.003), and TG (P = 0.010), while none of the variables were significant among tertiles of the N6/N3. After adjustment with potential cofounders, the mean difference of age (P = 0.021) and TG (P = 0.020) remained significant and the PA (P = 0.048) of participants among tertiles of the CSI became significant, while no significant difference was observed in any of the variables among tertiles of the N6/N3 ratio (P > 0.05). BMI was considered as collinear for anthropometrics variables.
Mean and SD of general characteristics according to tertiles of GRS
The baseline characteristics of study participants, categorized according to the GRS, were presented in Table 2; Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10. As shown in this table, in the crude model, a significant mean difference was observed among tertiles of the GRS in terms of height (P = 0.010) and marginally significant for BMI (P = 0.051). After controlling for potentially confounding variables (age, energy intake, PA, and BMI), the mean difference of height (P = 0.020) remained significant among tertiles of the GRS.
Mean and SD of dietary intake according to tertiles of CSI and N6/N3
Dietary intakes of participants across tertiles of CSI and N6/N3 ratio were presented in (Additional file 1: Table S1). After adjustment with the energy intake, there were significant mean differences of legumes (P = 0.049), vegetables(P = 0.001), high fat dairy(P = 0.001), carbohydrate(P = 0.001), total fat(P = 0.001), monounsaturated fatty acid (MUFA) (P = 0.001), SFA(P = 0.001), vitamin C(P = 0.001), folate(P = 0.001), vitamin B12(P = 0.001), total fiber (P = 0.001), linolenic acid (P = 0.005), potassium (P = 0.002), niacin (P = 0.002), thiamin (P = 0.012), and vitamin B6 (P = 0.016) across tertiles of CSI, also a significant mean difference was observed among tertiles of the N6/N3 in terms of MUFA (P = 0.034), polyunsaturated fatty acid (PUFA) (P = 0.029), linoleic acid (P = 0.030), and sodium (P = 0.046).
The interaction between GRS with CSI and N6/N3 ratio on MetS risk factors
The interaction between tertiles of the GRS with tertiles of the CSI and N6/N3 ratio on MetS risk factors were presented in Table 3. In the crude model, a marginally positive interaction was observed between T3 of GRS and T3 of CSI on FBS (β = 7.21, 95%CI= -0.02,14.46, P = 0.051), and also a marginally negative interaction was observed between T2 of GRS with T3 of on HDL (β= -8.74, 95%CI= -17.68,0.19, P = 0.055), also the interaction between T2 of GRS and T2 of N6/N3 ratio on WC (β = 8.45, 95%CI = 1.33, 15.57, P = 0.020), and T3 of GRS and T3 of N6/N3 ratio on TG (β = 62.00, 95%CI = 7.52, 116.48, P = 0.026) were positive. After controlling for potential confounders including age, energy intake, PA and BMI in model 1, there was a positive interaction between T2 of GRS and T2 of N6/N3 ratio on WC (β = 7.95, 95%CI = 0.83,15.08, P = 0.029), T3 of GRS and T2 of N6/N3 ratio on DBP (β = 5.93, 95%CI= -0.76,12.63, P = 0.083), and FBS (β = 6.47, 95%CI = 0.59,13.53, P = 0.073), T3 of GRS and T3 of N6/N3 ratio on TG (β = 54.42, 95%CI = 1.76,107.08, P = 0.043), and T3 of GRS and T3 of CSI on BF% (β = 3.55, 95%CI= -0.35,7.45, P = 0.075). Also, T2 of GRS in the interaction with T3 of CSI leads to an decrease − 8.35 mg/dl in HDL level after adjustment in model 1 (β= -8.35, 95%CI= -17.34,0.62, P = 0.068).
Discussion
In the current cross-sectional study, we investigated the interaction between GRS and fatty acid quality indices with MetS among 279 overweight and obese women. Accoring to findings, after controlling for potential confounders, we observed that the interaction of GRS and N6/N3 has a positive significant association with WC, DBP, FBS and TG. Also, the interaction of GRS and CSI had a positive significant association with BF%. Moreover, the interaction of GRS and CSI had a negative significant association with HDL.
The etiology of MetS is complicated, however documents have indicated that dietary patterns, physical activity level and genetic polymorphism take a part in its pathogenesis [10, 44]. Moreover, Asians are more likely prone to develop MetS than non-Asians, Europeans and Americans [45]. Individual SNPs have been studied as a useful genetic tool to predict the tendency to MetS or obesity in different age groups [46]. GRS as an non-modifiable factor have been recognized as an associated factor for obesity, MetS, and type 2 diabetes in previous studies [25, 47, 48]. On the other hand, dietary patterns which contain too much fried foods, soda and meat can increase the risk of MetS [12, 49]; however, other dietary ingrediants such as fruits and vegetables can have a protective role against MetS and other chronic diseases [10].
Findings on FBS and anthropometric indices
The findings on interaction between GRS and dietary patterns especially different dietary quality indices with MetS incidence is rare and unclear. In a prospective nested case-control study among 1196 diabetic and 1337 nondiabetic men, the highest risk of type 2 diabtes in relationship with a western dietary pattern was belonged to the highest GRS tertile [50]. Our findings revealed that the interaction of the highest tertile of GRS with the N6/N3 positively was associated with FBS level. A study which conducted among both men and women found that increasing ratio of saturated fat to carbohydrate related to higher HOMA levels in minor allele carriers (PLIN11482G > A) [51].
According to our findings, the interaction of GRS and N6/N3 and the interaction of GRS and CSI were positively associated with WC and BF%, respectively. This finding suggest that dietary fatty acid amouts and composition may potentially influence on genetic susceptibility of being obese [52, 53]. Findings from 18 cohorts of European ancestry found that GRS and diet may increase risk of obesity [54]. Morover, a cross-sectional study among 476 Iranian participants assessed the interaction of a high fat and sugar intake with a SNP of vascular endothelial growth factor (rs10738760), and revealed a increased risk of MetS [55]. A study among Ghanaian population revealed an interaction between 4-SNP GRS and fat intake on WC which are associated with higher amounts of mortality [56]. Studies in 354 Spanish children and adolscents, 1754 French individulas and 2163 American participants have shown a significant interaction of FTO SNP rs9939609 with MUFAs and SFAs on BMI [57,58,59]. Previous studies among 28,449 individuals in Malmo [60] and 2163 individulas in United States of America have indicated significant intractions of the FTO SNP rs9939609 and fat intake on BMI [57], however, a meta-analysis on 177,330 participants failed to identify this intraction [52]. High SFA intake presented significantly a higher BMI in the GG carriers than in A carriers [61]. On the other hand, low PUFA intake revealed an inverse association with risk of BMI of more than 30 kg/m2 in the presence of ADAM17i33708A polymorphism among 936 men amd women [62]. Overally, conflicting evidence in many previous studies regarding the effect of fat intake and obesity-related parameters could be because of the gene-diet interactions and genetic heterogeneity across various ethnic groups [63, 64]. Hence, the synergism effect of genetic and dietay intakes should be considered in future studies. According to findings of a parallel controlled-feeding trial, the mechanisms which can explain the increase in anthropometric indices by adhereing a high SFA diet including as increase in the expression of inflammatory genes in adipose tissue, and decrease in the expression of genes involved in fatty acid β-oxidation and synthesis of TG [65].
Findings on lipid profile indices
A study in 1680 South Asians has demonstrated a significant interaction of fat intake with the risk allele ‘T’ of the TCF7L2 SNP rs 12,255,372 on HDL [66]. In a population-based study findings showed that the women who carries the A allele of APOA1 gene (G-A polymorphism) had higher HDL in response of high PUFA intake [67]. While, in our study the interaction of GRS and CSI showed a negative significant association with HDL. Accoring to our findings, after controlling for potential confounders, we observed that the interaction of GRS and N6/N3 has a positive significant association with TG. In a population-based study, the interaction of PUFA intake and PPARAL162V revealed a lower TG level with higher PUFA intake in the V carries [68]. The mechanism which is related to the alteration of lipid profile maybe because of altering the lipoprotein lipase activity in adipose tissue and muscles and decreasing energy expenditure [69, 70].
An eleven year longitudinal study revealed that the western dietary pattern increased MetS risk among GRS tertiles in Whites participants with age range of 45–64 years [28]. However, in the mentioned study, high-fat dairy pattern showed a protective effect against MetS especially in the lowest GRS tertile [28]. There are several studies which have shown a protective role of high-fat dairy products on MetS and type 2 diabetes [11, 71], but deleterious effect of western dietary pattern is related to red and processed meat, fried foods and sweets [28]. Also, Hardy et al. found that FAD1 and FAD2 genes were linked to rs174548 which is a SNP in the GRS [28]. FAD1 and FAD2 genes involves in long chain polyunsaturated fatty acid synthesis and are linked with CVD and other health outcomes [72]. Different fatty acids indicate various effect on metabolic outcomes, for instance conjugated linoleic acid has been observed to decrease insulin resistance and inflammation, while arachidonic acid has indicated pro-inflammatory condition and has increased atherosclerotic damage [72]. High ratio of arachidonic acid to linoleic acid among individuals who carrying FAD may be detrimental due to more susceptibility to inflammatory conditions [73]. Overally, various findings of different studies could be due to the different age, gender, population, continent, or specific criteria to define MetS and other related outcomes. Moreover, analyzing the genetic associations with main outcomes in Iranian population may not be ideal, because of differences between risk allele frequency of the Iranian population and the other poulations [74, 75]. These inconsistencie maybe due to the variation in the genetic architecture between different ancestries [76].
To the best of authors knowledge, this is the first study that investigated the interaction between GRS and fatty acid quality indices with MetS among overweight and obese women. Also, our study population was highly homogeneous, because it conducted only among Iranian subjects. However, findings cannot be applied to reveal cause and effect regarding the cross-sectional type of the study. Also, using FFQ to assess dietary intake is one of limitations due to its recall bias. Moreover, dietary intakes can vary by socioeconomic status and culture; although we adjusted findings to several confounders, remain effect of these factors may impact on results. Thus, it is suggested to replicate the study in other large populations.
Conclusion
As theses days, MetS, obesity and other non-communicable diseases occur in a wide range, it is fundamental to develop health prevention programs which help to detection, early diagnosis and treatment of MetS. It seems the interaction of GRS and fatty acid quality indices is positively associated with several components of metabolic syndrome such as WC, TG and BF%. However, more studies with larger sample size are needed to confirm these findings.
Data availability
The datasets analysed during the current study are not publicly available due ethical issues but are available from the corresponding author on reasonable request.
Abbreviations
- CAV:
-
Caveolin
- CRY:
-
Cryptochrome
- CVD:
-
Cardiovascular disease
- CSI:
-
Cholesterol-Saturated Fat Index
- EFA:
-
Essential fatty acids
- GRS:
-
Genetic risk score
- GWAS:
-
Genome-wide association studies
- HDL:
-
High-density lipoprotein
- MC4R:
-
Melanocortin-4 Receptor
- PA:
-
Physical Activity
- SFA:
-
Saturated fatty acid
- SNP:
-
Single nucleotide polymorphisms
- T2DM:
-
Type 2 diabetes mellitus
References
Alberti KGER, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645.
Pérez-Martínez PMD, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307–26.
Alberti GZ, Shaw P. J. The IDF Consensus Worldwide Definition of the metabolic syndrome. Brussels, Belgium: International Diabetes Federation; 2006.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
Nestel PLR, Low LP, et al. Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007;16(2):362–7.
Iravani SSB, Sedaghat S, et al. The association of elevated serum alanine aminotransferase with metabolic syndrome in a military population in Southern Iran. Iran Cardiovasc Res J. 2010;4(2):74–80.
Sarrafzadegan NKR, Baghaei A, et al. Metabolic syndrome: an emerging public health problem in Iranian women: Isfahan Healthy Heart Program. Int J Cardiol. 2008;131(1):90–6.
Azizi FSP, Etemadi A, et al. Prevalence of metabolic syndrome in an urban population: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2003;61(1):29–37.
Delavari AFM, Alikhani S, et al. First Nationwide Study of the prevalence of the metabolic syndrome and optimal cutoff points of Waist circumference in the Middle East. Diabetes Care. 2009;32(6):1092–97.
de Oliveira EPMK, Vaz de Arruda Silveira L, Burini RC. Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J. 2012;11:13–20.
Lutsey PLSL, Stevens J. Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation. 2008;117(6):754–61.
Damiao RCT, Cardoso MA, Gimeno SG, Ferreira SR, JapaneseBrazilian Diabetes Study Group. Dietary intakes associated with metabolic syndrome in a cohort of Japanese ancestry. Br J Nutr. 2006;96(3):532–8.
Mohamadi A, Shiraseb F, Mirzababaei A, Hosseininasab D, Rasaei N, Clark CC et al. Circulating inflammatory markers may mediate the relationship between healthy plant-based diet and metabolic phenotype obesity in women: A cross-sectional study. International Journal of Clinical Practice. 2022;2022.
Rasaei N, Ghaffarian-Ensaf R, Shiraseb F, Fallah M, Gholami F, Clark CC, et al. The association between healthy beverage index and quality of life among overweight and obese women: a cross-sectional study. BMC Public Health. 2023;23(1):176.
Rasaei N, Gholami F, Samadi M, Shiraseb F, Khadem A, Yekaninejad MS, et al. The interaction between MALAT1 and TUG1 with dietary fatty acid quality indices on visceral adiposity index and body adiposity index. Sci Rep. 2024;14(1):12.
Freire RDCM, Gimeno SG, Ferreira SR, Japanese-Brazilian Diabetes Study Group. Dietary fat is associated with metabolic syndrome in Japanese brazilians. Diabetes Care. 2005;28(7):1779–85.
Connor SL, Gustafson JR, Artaud-Wild SM, Flavell DP, Classick-Kohn CJ, Hatcher LF, et al. The cholesterol/saturated-fat index: an indication of the hypercholesterolaemic and atherogenic potential of food. Lancet (London England). 1986;1(8492):1229–32.
AP S. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother Elsevier Masson. 2002;56:365–79.
Mitchell DTKM, Brewer BK, Novascone MA. Development and validation of the cholesterol-saturated fat index (CSI) scorecard: a dietary self-monitoring tool. J Am Diet Assoc. 1996;96:132–6.
van Dam RMWW, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–24.
Feskens EJVS, Rasanen L, Tuomilehto J, Stengard J, Pekkanen J, Nissinen A, Kromhout D. Dietary factors determining diabetes and impaired glucose tolerance: a 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care. 1995;18:1104–12.
Cornier MADD, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822.
Belsky DWMT, Sugden K, Williams B, Houts R, McCarthy J, et al. Development and evaluation of a genetic risk score for obesity. Biodemography Social Biology. 2013;59(1):85–100.
Vera BDH, Gómez-Abellán P, Hernández-Martínez AM, Esteban A, Scheer FA, et al. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Reportsv. 2018;8(1):1–11.
Zhao XXB, Shen Y, Wu L, Hou D, Cheng H, et al. An obesity genetic risk score is associated with metabolic syndrome in Chinese children. Gene. 2014;535(2):299–302.
Abaj FKF, Rafiee M, Alvandi E, Yekaninejad MS, Mirzaei K. Interactions between Caveolin-1 (rs3807992) polymorphism and major dietary patterns on cardio-metabolic risk factors among obese and overweight women. BMC Endocr Disorders. 2021;21(1):1–14.
Garver WSNS, Gonzales-Pacheco DM, Castillo JJ, Jelinek D, Heidenreich RA, et al. The genetics of childhood obesity and interaction with dietary macronutrients. Genes Nutr. 2013;8(3):271–87.
Hardy DSRS, Garvin JT, Gebrekristos HT, Mersha TB. Ancestry specific associations of a genetic risk score, dietary patterns and metabolic syndrome: a longitudinal ARIC study. BMC Med Genomics. 2021;1(1):118.
Abaj FMK. Caveolin-1 genetic polymorphism interacts with PUFA to modulate metabolic syndrome risk. Br J Nutr. 2021;21:1–8.
Organization WH. Obesity: preventing and managing the global epidemic. 2000.
Appelhans BMWM, Schneider KL, Ma Y, Oleski JL, Merriam PA, Waring ME, Olendzki BC, Mann DM, Ockene IS, Pagoto SL. Depression severity, diet quality, and physical activity in women with obesity and depression. J Acad Nutr Diet. 2012;112(5):693–8.
Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences (3rd edition). Mahwah, NJ: Lawrence Earlbaum Associates; 2003.
Fernandez-Lazaro CI, Martínez-González MÁ, Aguilera-Buenosvinos I, Gea A, Ruiz-Canela M, Romanos-Nanclares A, et al. Dietary antioxidant vitamins and minerals and breast cancer risk: prospective results from the SUN cohort. Antioxidants. 2021;10(3):340.
Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80.
Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007;77(2):251–7.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–62.
Ghaffarpour M, Houshiar-Rad A. H. K. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 1999;7(213):42–58.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215.
Myakishev MV, Khripin Y, Hu S, Hamer DH. High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11(1):163–9.
Zlatohlavek L, Vrablik M, Motykova E, Ceska R, Vasickova L, Dlouha D, et al. FTO and MC4R gene variants determine BMI changes in children after intensive lifestyle intervention. Clin Biochem. 2013;46(4–5):313–6.
Yu K, Li L, Zhang L, Guo L, Wang C. Association between MC4R rs17782313 genotype and obesity: a meta-analysis. Gene. 2020;733:144372.
Tangestani H, Emamat H, Yekaninejad MS, Keshavarz SA, Mirzaei K. Variants in Circadian Rhythm Gene Cry1 interacts with healthy Dietary Pattern for serum leptin levels: a cross-sectional study. Clin Nutr Res. 2021;10(1):48–58.
Miranda AM, Steluti J, Norde MM, Fisberg RM, Marchioni DM. The association between genetic risk score and blood pressure is modified by coffee consumption: gene-diet interaction analysis in a population-based study. Clin Nutr. 2019;38(4):1721–8.
Mazidi M, Pennathur S, Afshinnia F. Link of dietary patterns with metabolic syndrome: analysis of the National Health and Nutrition Examination Survey. Nutr Diabetes. 2017;7(3):e255.
Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48.
Janssens AC, Aulchenko YS, Elefante S, Borsboom GJ, Steyerberg EW, van Duijn CM. Predictive testing for complex diseases using multiple genes: fact or fiction? Genetics in medicine: official. J Am Coll Med Genet. 2006;8(7):395–400.
Belsky DW, Moffitt TE, Sugden K, Williams B, Houts R, McCarthy J, et al. Development and evaluation of a genetic risk score for obesity. Biodemography Social Biology. 2013;59(1):85–100.
Ntalla I, Kanoni S, Zeng L, Giannakopoulou O, Danesh J, Watkins H, et al. Genetic risk score for Coronary Disease identifies predispositions to Cardiovascular and Noncardiovascular diseases. J Am Coll Cardiol. 2019;73(23):2932–42.
Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2009;32(4):688–94.
Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr. 2009;89(5):1453–8.
Smith CE, Arnett DK, Corella D, Tsai MY, Lai CQ, Parnell LD, et al. Perilipin polymorphism interacts with saturated fat and carbohydrates to modulate insulin resistance. Nutr Metab Cardiovasc Dis. 2012;22(5):449–55.
Qi Q, Kilpeläinen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23(25):6961–72.
Rasaei N, Fallah M, Nemati M, Gholami F, Ghaffarian-Ensaf R, Mirzaei K. Investigation the interaction of dietary fat quality indices and the MC4R gene in metabolically healthy and unhealthy overweight and obese women. Sci Rep. 2023;13(1):12183.
Nettleton JA, Follis JL, Ngwa JS, Smith CE, Ahmad S, Tanaka T, et al. Gene × dietary pattern interactions in obesity: analysis of up to 68 317 adults of European ancestry. Hum Mol Genet. 2015;24(16):4728–38.
Ghazizadeh H, Esmaily H, Sharifan P, Parizadeh SMR, Ferns GA, Rastgar-Moghadam A, et al. Interaction between a genetic variant in vascular endothelial growth factor with dietary intakes in association with the main factors of metabolic syndrome. Gene Rep. 2020;21:100813.
Alsulami S, Nyakotey DA, Dudek K, Bawah AM, Lovegrove JA, Annan RA et al. Interaction between metabolic genetic risk score and dietary fatty acid intake on central obesity in a Ghanaian Population. Nutrients. 2020;12(7).
Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr. 2011;141(12):2219–25.
Phillips CM, Kesse-Guyot E, McManus R, Hercberg S, Lairon D, Planells R, et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J Nutr. 2012;142(5):824–31.
Moleres A, Ochoa MC, Rendo-Urteaga T, Martínez-González MA, Azcona San Julián MC, Martínez JA, et al. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr. 2012;107(4):533–8.
Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90(5):1418–25.
Fernández-Real JM, Corella D, Goumidi L, Mercader JM, Valdés S, Rojo Martínez G, et al. Thyroid hormone receptor alpha gene variants increase the risk of developing obesity and show gene-diet interactions. Int J Obes (Lond). 2013;37(11):1499–505.
Junyent M, Parnell LD, Lai CQ, Arnett DK, Tsai MY, Kabagambe EK, et al. ADAM17_i33708A > G polymorphism interacts with dietary n-6 polyunsaturated fatty acids to modulate obesity risk in the Genetics of lipid lowering drugs and Diet Network study. Nutr Metab Cardiovasc Dis. 2010;20(10):698–705.
Heianza Y, Qi L. Gene-Diet Interaction and Precision Nutrition in obesity. Int J Mol Sci. 2017;18(4).
Gholami F, Rasaei N, Samadi M, Yekaninejad MS, Keshavarz SA, Javdan G, et al. The relationship of genetic risk score with cardiometabolic risk factors: a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):459.
van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, et al. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90(6):1656–64.
Bodhini D, Gaal S, Shatwan I, Ramya K, Ellahi B, Surendran S, et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE. 2017;12(11):e0188382.
Ordovas JM, Corella D, Cupples LA, Demissie S, Kelleher A, Coltell O, et al. Polyunsaturated fatty acids modulate the effects of the APOA1 G-A polymorphism on HDL-cholesterol concentrations in a sex-specific manner: the Framingham Study. Am J Clin Nutr. 2002;75(1):38–46.
Tai ES, Corella D, Demissie S, Cupples LA, Coltell O, Schaefer EJ, et al. Polyunsaturated fatty acids interact with the PPARA-L162V polymorphism to affect plasma triglyceride and apolipoprotein C-III concentrations in the Framingham Heart Study. J Nutr. 2005;135(3):397–403.
Piers LS, Walker KZ, Stoney RM, Soares MJ, O’Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream). Int J Obes Relat Metab Disord. 2002;26(6):814–21.
Jones PJ, Jew S, AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism. 2008;57(9):1198–203.
Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, et al. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29(7):1579–84.
Koletzko B, Reischl E, Tanjung C, Gonzalez-Casanova I, Ramakrishnan U, Meldrum S, et al. FADS1 and FADS2 polymorphisms modulate fatty acid metabolism and Dietary Impact on Health. Annu Rev Nutr. 2019;39:21–44.
Martinelli N, Girelli D, Malerba G, Guarini P, Illig T, Trabetti E, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88(4):941–9.
Adeyemo AA, Tekola-Ayele F, Doumatey AP, Bentley AR, Chen G, Huang H, et al. Evaluation of Genome Wide Association Study Associated Type 2 diabetes susceptibility loci in sub Saharan africans. Front Genet. 2015;6:335.
Chen J, Sun M, Adeyemo A, Pirie F, Carstensen T, Pomilla C, et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia. 2019;62(7):1204–11.
Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–33.
Acknowledgements
The authors thank the laboratory of Nutrition Sciences and Dietetics in Tehran University of Medical Sciences (TUMS). We are grateful to all of the participants for their contribution to this research. This study was approved by the Research ethics committee of the Tehran University of Medical Sciences (TUMS), Tehran, Iran with ethics number IR.TUMS.MEDICINE.REC.1399.636. All participants signed a written informed consent that was approved by this committee prior to enrollment in the study.
Funding
This study was supported by Tehran University of Medical Sciences (grant ID: 99-3212-51715).
Author information
Authors and Affiliations
Contributions
NR and KM designed the search; NR, ED and KM conducted the sampling; FG performed statistical analysis; NR, ED, AK, FG, MS and KM wrote the paper, KM primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of the Tehran University of Medical Sciences.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rasaei, N., Daneshzad, E., Khadem, A. et al. Investigation of the interaction between genetic risk score (GRS) and fatty acid quality indices on metabolic syndrome among overweight and obese women. BMC Med Genomics 17, 113 (2024). https://doi.org/10.1186/s12920-024-01838-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-024-01838-2