Abstract
Background
Caveolin-1 (CAV-1) is a cholesterol-dependent essential component located in caveolae. Several studies have been CAV-1 related to cardio-metabolic parameters in animal models, however, there are few studies in humans. Importantly, there is no study has investigated the interaction between CAV-1 rs3807992 gene and dietary patterns (DPs) on cardio-metabolic risk factors.
Methods
The current cross-sectional study was conducted on 404 overweight and obese women. Dietary intake was obtained from FFQ with 147 items. The CAV-1 genotype was measured by the PCR-RFLP method. The anthropometric measurements, serum lipid profile, and inflammatory markers were measured by standard protocols.
Results
There was a significant interaction between CAV-1 rs3807992 and healthy DP on high-density cholesterol (HDL) (P-interaction = 0.03), TC/HDL (P-interaction = 0.03) and high sensitivity C-reactive protein (hs-CRP) (P-interaction = 0.04); in A-allele carriers, higher following a healthy DP was related to a higher level of HDL and lower TC/HDL and hs-CRP. As well as, the significant interactions were observed between CAV-1 rs3807992 and unhealthy DP in relation to triglyceride (TG) (P-interaction = 0.001), aspartate aminotransferase (AST) (P-interaction = 0.01) and monocyte chemoattractant protein-1(MCP-1) (P-interaction = 0.01); A-allele carriers were more following the unhealthy DP had lower levels of TG, AST and MCP-1.
Conclusions
Our study revealed a significant gene-diet interaction between rs3807992 SNPs and DPs in relation to cardio-metabolic risk factors; A-allele carriers might be more sensitive to dietary composition compared to GG homozygotes. Following a healthy DP in A-allele-carriers may be improved their genetic association with cardio-metabolic risk factors.
Similar content being viewed by others
Background
The mortality rate of cardiovascular diseases (CVDs) rise to 23.6 million by 2030 [1]. Obesity-induced dyslipidemia, high blood pressure (BP) and inflammation have a key role in the pathogenesis of CVDs [2, 3]. Modifiable factors such as dietary intake and physical activity and non-modifiable including age, gender, and genes associated with the etiology of CVDs [4]. In several previous epidemiologic studies, the various nutrients separately have been considered without other components of dietary intakes [5, 6]. A complete analysis of dietary intakes can be provided precious insights into both nutrient intakes and dietary patterns (DPs) [7, 8]. Interestingly, DPs were associated with CVD risk in several studies [9,10,11]. Additionally, genome-wide association studies (GWAS) have discovered several usual variants correlated with CVD and obesity [12]. Caveolin-1 (CAV-1) is the most principal structural protein of caveolae, which is encoded by the genes CAV-1 [13]. Caveolae are found in most numerous cells but are especially abundant in adipocytes [14]. The physiological functions of caveolae are not fully illuminated. However, CAV-1 and caveolae are known to interact with a variety of physiologic and biologics pathways, including insulin sensitivity, lipid regulation especially cellular cholesterol and glucose homeostasis, maybe most importantly, cell signaling and receptors [15]. Some experimental studies have reported that CAV-1 plays a substantial role in the development of dyslipidemia, hypertension, and atherosclerosis [16,17,18]. Thus, has been reported CAV-1 single-nucleotide polymorphism (SNP) and the risk for various types of diseases and disorders [13, 19]. In some human studies, the two variant rs3807989 and rs926198 of CAV-1 genotypes may contribute to metabolic disorders and chronic diseases such as dyslipidemia, insulin resistance, metabolic syndrome, diabetes and CVDs [18, 20, 21]. Importantly, the association of CAV-1 rs3807992 polymorphism with CVDs has not been reported up to now, present study is the first report to investigate this association.
On the other hand, animal studies have shown that various nutrients might modify the genetic susceptibility of CAV-1 to CVD or obesity risk [22, 23]. Particularly, CAV1 knockout mice have shown severe lipodystrophy and stay lean even after being fed high-fat diets [12]. In this regard, Cohen et al. [13] have found that rats fed with high-fat diet have higher CAV-1 expression. The previous animal studies on rabbits have revealed that, expression of the CAV-1 gene increase in response to high-cholesterol diet [24]. In another animal study, caveolin-1 has enhanced resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model [25]. In this regard, we have previously found insights about CAV-1 and insulin signaling in modifying dyslipidemia and fat composition in overweight and obese women [26]. Given the lack of human studies and contradictory results of animal and experimental studies, we conducted this study in order to determine the association between CAV-1 rs3807992 and CVD risk factors and investigated whether dietary intake modulates this association.
Methods
Study population
The present study is a descriptive cross-sectional study carried out in Tehran, Iran. The recruitment of subjects was realized from the health center using a multi-stage cluster random sampling method. The inclusion criteria were women with age ≥ 18 years, health status, obesity and overweight (body mass index (BMI) ≥ 25 kg/m2). The participants were not pregnant, not the history of diseases including type 2 diabetes (T2D), CVDs, polycystic ovary syndrome (PCO), stroke, non-alcoholic fatty liver disease (NAFLD), Inflammatory disease include allergy, asthma, autoimmune diseases, coeliac disease and inflammatory bowel disease, hypertension, cancer, and thyroid, and also have not used weight loss program and supplements during our study. Additionally, women taking medications such as lipid-lowering (e.g. Atorvastatin, Cholestyramine, etc.), antihypertensive (Captopril, etc.) and blood glucose controlling (Metformin, etc.) and also their total calorie intake was not in range between 800 and 4200 were excluded. A self-administered questionnaire was provided from all participants for their health status and the exclusion criteria of the study. Finally, total 404 eligible women were included into the present study. All protocols of this study were conducted in accordance with Helsinki Declaration and approved by the Ethical Committee of the Tehran University of Medical Sciences (TUMS) (NO: 97–03–161-41,017). All of the participants completed a written informed consent form before taking part in the study.
Dietary assessments
Dietary intakes were assessed by expert dietitians using a validated 147-items semi-quantitative food frequency questionnaire (FFQ) (Table 1) [27]. Subjects were reported the frequency of each food item consumed on a daily, weekly, monthly, or yearly during the past year. Then, was converted to grams per day using household measures. Total energy and dietary nutrients were assessed by the Iranian Food Composition Table (FCT) and N4 software. Finally, 17 food groups were extracted for an examination of DPs of dietary intake.
General and anthropometric assessments
General data such as age, educational level, marital status, family history of obesity was collected via standard questionnaires. Body weight and height were measured using a digital scale (Seca, Germany) and tape measure with a precision of 100 g and 0.1 cm, respectively, when the subjects were minimally clothed and not wearing shoes in a standing position. Waist circumference (WC, cm) was measured at the narrowest part of the abdomen to the nearest 0.1 cm. BMI was calculated as weight (kilograms) divided by the height (meters) squared. Physical activity was assessed by the validated International Physical Activity Questionnaire [28].
Laboratory tests
All samples were collected after overnight fasting (10–12 h) at the Nutrition and Biochemistry laboratory of the School of Nutritional Sciences and Dietetics, TUMS. indices of serum lipids and lipoproteins (total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C) and TG), liver enzymes (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), hs-CRP, MCP-1, interleukin 1 beta (IL-1β) and transforming growth factor beta1(TGF-β1) were measured via standard protocols. Plasminogen activator inhibitor-1 (PAI-1) (Human PAI-1*96 T ELISA kit Crystal Company) was measured in triplicate.
DNA analysis
Genomic DNA was extracted from the whole blood sample by a Mini Columns kit (Type G; Genall; Exgene) based on the manufacturer’s guidebook. The CAV-1 rs3807992 SNP (major allele: G; minor allele: A) was genotyped by PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism) technique as follows. The PCR amplification of the genomic DNA fragment for rs3807992 was performed by the forward primer 3′ AGTATTGACCTGATTTGCCATG 5′ and reverse primer 5′ GTCTTCTGGAAAAAGCACATGA 3′. The final volume of PCR product was contained 20 μl including 1 μl extracted DNA, 1 μl forward primers, 1 μl reverse primers, 7 μl distilled water, and 10 μl Taq DNA Polymerase Master Mix (Amplicon; Germany). PCR cycles were designed with initial denaturation step at 94 °C for 3 min (40 cycles), annealing at 53 °C for 30 s and 30 s of extension at 72 °C, eventually a final extension at 72 °C for 3 min. Amplified DNA (10 μl) was digested using 0.5 μl of Hin1II (NlaIII) restriction enzyme (Fermentase, Germany) at 37 °C overnight. Finally, electrophoresis of the PCR products was performed on 3.5% agarose gel. Three DNA fragments appeared with different lengths: homozygous AA (1 band: 213 bp), heterozygous GA (3 bands: 118 & 95 & 213 bp and homozygous GG (2 bands: 118 & 95 bp). Importantly, 10% of the samples were directly sequenced for confirmation of the PCR-RFLP results. The sequencing process performed using the ABI PRISM 3730 automated sequencer (Applied Biosystems, Foster City, Calif, USA).
Statistical analyses
The normality distribution was checked by the Kolmogorov- Smirnov test. Non-normal data including TG and AST (p < 0.05) were normalized by logarithmic transformation. Continuous variables were expressed as mean ± SD. Pearson’s chi-square test was used for the Hardy-Weinberg Equilibrium (HEW). Genotype groups were considered as a dominant inherent model. DPs were examined using principal factor analysis (PCA) and factors were extracted by varimax rotation to ensure unrelated conditions and improve interpretability. Eventually, factors were obtained by considering an eigenvalue of more than 1.5 and a scree plot. The factor scores were evaluated as the sum of each factor loading ≥|0.3| and the reference daily intake of each food correlated to the DPs. These factors containing: vegetables, low-fat dairy, starchy vegetables, fruits, legumes, fish, nuts& olive, red meat, poultry, spices, sweet snack & drinks, processed foods, high-fat dairy and Tea& coffee. Thus, the high score was associated with more adaptation to the extracted pattern dietary. Subjects were categorized into tertiles (T1-T3) by scores of 17 factors for further analyses. Differences of continuous variables in genotype groups and DPs were compared by the independent-sample t-test and the One-Way ANOVA test in the crude model, respectively. ANCOVA analysis was performed for adjustment model (adjusting for age, physical activity and energy intake). A generalized linear model (GLM) was exerted to analyze the interactions between CAV-1 polymorphism (rs3807992) and DPs concerning metabolic risk factors of cardiovascular disease. Data were analyzed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 25) and P-value < 0.05 was considered as significant.
Results
Study population characteristics
The present study was carried out on 404 women with a mean (SD) age of 36.67 ± 9.1 years. The majority of women were married (70.8%) and had a university education (54%) and a history of obesity (71.2%). The genotypic frequency among participants was as follow: GG (50%), AG (23.3%) and AA (26.7%). Further, the minor frequency allele (A) was 38% in this study. The genotype distributions had a deviation from in Hardy-Weinberg equilibrium (P < 0.05).
Difference in means of cardio metabolic variables between CAV-1 rs3807992 genotypes
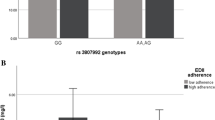
The comparison of variables including age, BMI and biochemical parameters according to two genotypes groups (GG and AG + AA) is given in Table 2. Results show that A-allele carriers have significantly higher and lower BMI and age, respectively, compared to GG homozygotes (P = 0.02 and P = 0.05, respectively). Additionally, there was a significant association between CAV-1 polymorphism (rs3807992) and serum LDL-C, HDL in both unadjusted (crude) and adjusted models (adjusted for age, physical activity and energy intake) (P < 0.0001 and P = 0.006, P = 0.003 and P = 0.001, respectively), TC/HDL in unadjusted model (P = 0.01) and TC in the adjusted model (P = 0.04). In particular, serum LDL-C, HDL-C, and TC concentration were significantly higher in GG homozygous compared to A-allele carriers. Although, the TC/HDL ratio was significantly lower in those. Moreover, there was no significant association between this polymorphism and other biochemical parameters including TG, inflammatory markers, and liver enzyme in both crude and adjusted models (P > 0.05) (Table 2).
Difference in means of cardio metabolic variables between major DPs
The factor-loading scores for each DP are represented in Table 3. Two major dietary patterns were identified; the healthy dietary pattern was loaded extremely on raw vegetables, fruits, starchy vegetables, legumes, low-fat dairy, nuts and olives, red meat, poultry, fish, and spices, whereas the unhealthy dietary pattern comprised sweet snack& drinks, refined grain, salty snack, processed foods, animal oil, high-fat dairy, coffee &tea, and with low for legumes.
The results displayed that women in the highest tertile (T3) of the unhealthy pattern were significantly younger than those in T1 and T2 (p = 0.002). Women in T1 of the healthy pattern had significantly higher serum TC concentration than those in T2 and T3 of the same pattern in the unadjusted model (p = 0.01). Although, this significance was less in the adjusted model (for age, energy intake, physical activity and BMI) (P = 0.05) (Table 4).
Interaction between major DPs and CAV-1 rs3807992 genotypes on cardio-metabolic risk factors
The interaction effects between CAV-1 variants at rs3807992 and two DPs (healthy and unhealthy) on cardio-metabolic markers including TC, HDL, LDL, TC, TC/HDL, LDL/HDL, TG, ALT, AST, hs-CRP, MCP-1, PAI-1 and IL-1β were shown in Tables 5 and 6. Furthermore, the significant interactions were reported via bar chart in Figs. 1 and 2.
There is a gen-diet interaction for healthy pattern and CAV-1 polymorphism (rs3807992) on HDL (β: 7.74, 95%CI: − 0.76 to 14.72, P:0.03), TC/HDL (β: -1.01, 95%CI: − 1.97 to 0.05, P:0.03), and hs-CRP (β: -0.34, 95%CI: − 0.68 to − 0.007, P:0.04), in adjusted model (adjusting for age, energy intake, physical activity and DBP) (Table 5). Thus, the A-allele carriers who were placed in the last tertile of the healthy DP had a higher HDL-C level and lower TC/HDL and hs-CRP compared to GG homozygotes (Fig. 1). No significant interactions were found between CAV-1 rs3807992 variants and healthy dietary DP intake for other metabolic-related traits (p > 0.05).
Furthermore, there is significant interactions between unhealthy DP and rs3807992 on TG (β: -64.63, 95%CI: 103.68 to 25.58, P:0.001), AST (β: -0.18, 95%CI: − 0.33 to 0.04, P:0.01) and MCP-1 (β: 82.27, 95%CI: 17.42 to 147.12, P:0.03) in adjustment model (adjusting for age, energy intake, physical activity and DBP) (Table 6). In particular, A-allele carriers were characterized by lower serum TG and AST when had the highest following an unhealthy DP compared to GG homozygote. (Fig. 2). Also, risk-allele carriers who consumed higher unhealthy DP had higher inflammatory marker include MCP-1 concentration. No significant interactions were found between CAV-1 rs3807992 variants and unhealthy dietary DP intake for other metabolic-related traits (p > 0.05).
Discussion
The present study provides information on the interaction between dietary patterns and genetic polymorphism of CAV-1 (rs3807992) in association with cardio-metabolic traits. Our findings represent that following a healthy DP was associated with lower serum TC level. Besides, in our study younger women have the highest unhealthy DP score. In particular, following an unhealthy DP was associated with higher BP. Additionally, findings showed that A-allele carriers of the rs3807992 polymorphism had higher BMI and lower TC, HDL-C, and LDL-C compared with GG homozygous. In the current study, we observed A-allele carriers of rs3807992 had a higher serum HDL-C, lower TC/HDL and hs-CRP concentration when placed in the last tertile of healthy DP compared to GG homozygotes. Another novel significant interaction was found between rs3807992 polymorphism and unhealthy DP on TG, AST, and MCP-1. In particular, A-allele carriers with the highest following an unhealthy DP had lower HDL, AST, and MCP-1 concentrations compared to the GG genotype. It is now well accepted, that the prevalence of obesity and CVD is due to changes in DPs [29, 30]. In line with our study, several studies have shown the protective effects of healthy DPs as an independent factor on decrease of CVD risk, which has not been reported precise mechanisms yet. However, a possible mechanism has suggested that high content of phytosterols, pectin and beta-glucagon in healthy patterns can decrease intestinal absorption of cholesterol [31, 32]. Moreover, in line with our study, several studies have shown that subjects who follow the unhealthy DP (comprise of foods with high-sodium and low potassium and magnesium and also caffeine) have statistically significant higher DBP [33, 34]. It can be due to raises renin activity disruption and systemic vascular resistance [35,36,37].
Although the underlying mechanism of the CAV-1 gene on cardio-metabolic risk factors is not completely known, some studies have shown that CAV-1 regulated the intracellular transport of fatty acids and cholesterol through direct binding to them [38,39,40]. Given this mentioned above, CAV-1 plays an important role in lipid and lipoprotein metabolism such as HDL-C, TC, VLDL and TG [17, 41,42,43]..
Moreover, our study revealed that risk-allele carriers who follow a healthy DP have higher HDL and lower hs-CRP and MCP-1 levels. It has now been suggested that diet and plasma-derived nutrients may modulate metabolic biomarkers through interacting with caveolae-associated cellular signalling [44]. In this regard, we revealed higher polyunsaturated fatty acid consumption might attenuate the CAV-1 rs3807992 associations with metabolic syndrome (MetS), and risk-allele appeared to have a higher risk of MetS, associated with higher saturated fatty acid consumption [45]. In regarding to the interaction between CAV-1 variant and diet on inflammatory markers, some studies have suggested that CAV-1 binds to endothelial nitric oxide synthase (eNOS) and HDL receptor in the caveolae and inhibits their activity, but the exact mechanism is not clear yet. Thus, the anti-inflammatory diet can be displaced the CAV-1 from caveolae to the cytoplasm which led to a decrease in the CAV-1 level, as a result, disappears the inhibitory effects on HDL and eNOS receptors [31]. As respects, Oberleithner et al. have claimed that serum sodium and potassium can regulate the binding of eNOS to the caveolae membrane and its activity [32]. Hence, the favourable effects of the healthy DP may be attributed to components such as vegetables and fruits, which more influence on balance potassium and sodium. Besides, this study revealed that A-allele carriers who had more follow unhealthy DP have lower levels of serum TG and LDL. These findings are advocated by Philippe et al., in which CAV-1(+/+) mice fed to high cholesterol has lower TC and TG levels compared to CAV-1(−/−) [33]. According to our finding, probably the lower AST and TG concentration in A-allele carriers following an unhealthy DP be dependent on the caffeine intake. According to probable mechanism, the low AST and TG concentration in A-allele carriers following an unhealthy DP be dependent on the caffeine intake. Regarding, there is particular region of a candidate gene for caffeine intake near to the CAV-1 gene [59], we have suggested a possible interaction between caffeine and CAV-1, and have hoped that will prove completely in further research. Besides, reduced NO production due to increased CAV-1 expression has been thought to result in prolonged exposure to high glucose, which may play a potential role in inflammatory pathways and development of inflammation [34]. Hence, it is not surprising that higher following an unhealthy DP can increase MCP-1 levels by altering the expression of CAV-1 and other genes. However, because of limitations in the financial source, we could not perform western blot analysis to find out whether rs-3,807,992 SNP alters the expression of CAV-1.
Limitation and strength
Limitations of the present study including the cross-sectional design, so any causality cannot be argued; the use of FFQ for dietary assessing, which may have resulted in memory bias; small sample size, which may have led to weak statistical to determine significant results and we could not eliminate all confounder factors, which can be affected on our results. Furthermore, our participants were from the Iranian country which may not be generalized due to racial and regional differences (52). Finally, we did not include a normal-weight participant due to financial constraints and instead focused on overweight and obese women as high-risk categories for metabolic traits [61]. However, as this is the first study of its kind, we propose that future studies focus on women of normal weight. Despite the limitations mentioned above, this is the first effort to study the interaction between CAV-1 rs3807992 polymorphism and DPs on cardio-metabolic risk factors. Recognition of these gene-diet interactions could be determining in prescribe personalized nutritional recommendations for the improvement and management of CVD risk. Finally, these results can be used in combination with a patient’s genetic history to provide more applicable and tailored nutritional advice for preventing or attenuating cardiovascular disease in overweight and obese women.
Conclusions
In conclusion, our results were in agreement with the differential-susceptibility model, which is defined as the risk alleles that could be modified with environmental factors by positive or negative effects. In particular, A-allele carriers might be more sensitive to dietary composition compared to GG homozygotes. Our study revealed a significant gene-diet interaction between rs3807992 SNPs and DPs in relation to cardio-metabolic risk factors; following a healthy DP in A-allele-carriers may be improved their genetic association with HDL, TC/HDL, and hs-CRP, as well as following an unhealthy DPs may be modified on genetic susceptibility to TG, AST, and MCP-1. We believe that our research will consider as a framework for future studies on CAV-1 gene and diet interaction. Large prospective studies are needed to confirm the present results.
Availability of data and materials
The data are not publicly available due to containing private information of participants. Data are however available from the authors upon reasonable request and with permission of Khadijeh Mirzaei.
Abbreviations
- AST:
-
Aspartate aminotransferase
- ApoA-I:
-
Apolipoprotein A-I
- BIA:
-
Bioelectrical impedance analysis
- BMI:
-
Body mass index
- bp:
-
Base pair
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- DNA:
-
Deoxyribonucleic acid
- DP:
-
Dietary pattern
- eNOS:
-
Endothelial nitric oxide synthase
- FFQ:
-
Food frequency questionnaire
- GLM:
-
General linear model
- GWAS:
-
Genome-wide association studies
- HDL:
-
High-density lipoprotein cholesterol
- IR:
-
Insulin receptor
- hs-CRP:
-
High sensitivity C-reactive protein
- IPAQ:
-
International physical activity questionnaire
- LDL:
-
Low-density lipoprotein
- MA:
-
Minor allele
- PCR:
-
Polymerase chain reaction
- RFLP:
-
Restriction fragment length polymorphism
- SNP:
-
Single nucleotide polymorphism
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- VAT:
-
Visceral adipose tissue
- WC:
-
Waist circumference
- WHO:
-
World health organization
References
World Health Organization. Global status report on noncommunicable diseases. Geneva: WHO; 2014. Available from [http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1]
McMillan DC, Sattar N, McArdle CS. ABC of obesity. obesity and cancer. BMJ. 2006;333(7578):1109–11.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282(16):1523–9. https://doi.org/10.1001/jama.282.16.1523.
Cannon CP. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin Cornerstone. 2007;8(3):11–28. https://doi.org/10.1016/S1098-3597(07)80025-1.
Bowen KJ, Sullivan VK, Kris-Etherton PM, Petersen KS. Nutrition and cardiovascular disease-an update. Curr Atheroscler Rep. 2018;20(2):8. https://doi.org/10.1007/s11883-018-0704-3.
Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and Cardiovascular Health. Int J Mol Sci. 2018;19(12):3988. https://doi.org/10.3390/ijms19123988.
Jacobs DR Jr, Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 2007;65(10):439–50. https://doi.org/10.1111/j.1753-4887.2007.tb00269.x.
Jew S, Antoine J-M, Bourlioux P, Milner J, Tapsell LC, Yang Y, et al. Nutrient essentiality revisited. J Funct Foods. 2015;14:203–9.
Aljefree N, Ahmed F. Association between dietary pattern and risk of cardiovascular disease among adults in the Middle East and North Africa region: a systematic review. Food Nutr Res. 2015;59(1):27486. https://doi.org/10.3402/fnr.v59.27486.
Asadi Z, Shafiee M, Sadabadi F, Heidari-Bakavoli A, Moohebati M, Khorrami MS, et al. Association of dietary patterns and risk of cardiovascular disease events in the MASHAD cohort study. J Hum Nutr Diet. 2019;32(6):789–801. https://doi.org/10.1111/jhn.12669.
Najafi M, Mozaffari H, Yahya J, Mokhtari P, Teymouri M, Faghih S. The associations between dietary patterns and cardiovascular risk factors among adults: a cross-sectional study. Clin Nutr ESPEN. 2020;40:300–8. https://doi.org/10.1016/j.clnesp.2020.09.001.
Burton PR, Clayton DG, Cardon LR, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661-78. https://doi.org/10.1038/nature05911.
Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res. 2006;70(1):42–9. https://doi.org/10.1016/j.cardiores.2005.11.029.
Thorn H, Stenkula KG, Karlsson M, Ortegren U, Nystrom FH, Gustavsson J, et al. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Mol Biol Cell. 2003;14(10):3967–76. https://doi.org/10.1091/mbc.e03-01-0050.
Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15(3):92–6. https://doi.org/10.1016/j.tcm.2005.04.001.
Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177(2):998–1003. https://doi.org/10.2353/ajpath.2010.091287.
Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol Cell Physiol. 2008;295(1):C242–8. https://doi.org/10.1152/ajpcell.00185.2008.
Pojoga LH, Underwood PC, Goodarzi MO, Williams JS, Adler GK, Jeunemaitre X, et al. Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab. 2011;96(8):E1288–92. https://doi.org/10.1210/jc.2010-2738.
Cohen AW, Hnasko R, Schubert W, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341-79. https://doi.org/10.1152/physrev.00046.2003.
Chen S, Wang X, Wang J, Zhao Y, Wang D, Tan C, et al. Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis. 2016;246:148–56. https://doi.org/10.1016/j.atherosclerosis.2016.01.008.
Cohen AW: Role of caveolin and caveolae in insulin signaling and diabetes. 2003, Role of caveolin and caveolae in insulin signaling and diabetes.
Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta. 2011;412(17–18):1493–514.
Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Western-style fast food intake and cardiometabolic risk in an eastern country. Circulation. 2012;126(2):182–8. https://doi.org/10.1161/CIRCULATIONAHA.111.084004.
Wei-Wen Lin Y-CL, Chang TY, Tsai SH, Ho HC, Chen YT, Yang VC. Caveolin-1 expression is associated with plaque formation in hypercholesterolemic rabbits. https://doi.org/10.1369/jhc.5A6869.2006.
Yang H-l, W-q C, Cao X, Worschech A, Du L-F, Fang W-Y, et al. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009;7(1):22. https://doi.org/10.1186/1479-5876-7-22.
Abaj F, Saeedy SAG, Mirzaei K. Are caveolin-1 minor alleles more likely to be risk alleles in insulin resistance mechanisms in metabolic diseases? BMC Res Notes. 2021;14(1):185. https://doi.org/10.1186/s13104-021-05597-6.
Mirmiran P, Hosseini-Esfahani F, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2009;13:654–62.
Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003;35(7):1196–202. https://doi.org/10.1249/01.MSS.0000074446.02192.14.
Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004;7(1a):123–46. https://doi.org/10.1079/phn2003585.
Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;369(10):954–64. https://doi.org/10.1056/NEJMra1203528.
Matveev S, Uittenbogaard A, van Der Westhuyzen D, Smart EJ. Caveolin-1 negatively regulates SR-BI mediated selective uptake of high-density lipoprotein-derived cholesteryl ester. Eur J Biochem. 2001;268(21):5609–16. https://doi.org/10.1046/j.1432-1033.2001.02496.x.
Oberleithner H, Kusche-Vihrog K, Schillers H. Endothelial cells as vascular salt sensors. Kidney Int. 2010;77(6):490–4. https://doi.org/10.1038/ki.2009.490.
Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(1):98–105. https://doi.org/10.1161/01.ATV.0000101182.89118.E5.
Haddad D, Al Madhoun A, Nizam R, Al-Mulla F. Role of Caveolin-1 in diabetes and its complications. Oxidative Med Cell Longev. 2020;2020:9761539.
Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–78. https://doi.org/10.1056/NEJMra064486.
Drenjančević-Perić I, Jelaković B, Lombard JH, Kunert MP, Kibel A, Gros M. High-salt diet and hypertension: focus on the renin-angiotensin system. Kidney Blood Press Res. 2011;34(1):1–11. https://doi.org/10.1159/000320387.
Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther. 1991;256(3):850–60.
Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100(6):1152–7. https://doi.org/10.1017/S0007114508992576.
Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 1999;255(1):34–9. https://doi.org/10.1006/bbrc.1998.0123.
Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92(22):10339–43. https://doi.org/10.1073/pnas.92.22.10339.
Fernandez-Rojo MA, Gongora M, Fitzsimmons RL, Martel N, Martin SD, Nixon SJ, et al. Caveolin-1 is necessary for hepatic oxidative lipid metabolism: evidence for crosstalk between caveolin-1 and bile acid signaling. Cell Rep. 2013;4(2):238–47. https://doi.org/10.1016/j.celrep.2013.06.017.
Frank PG, Cheung MW-C, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Phys Heart Circ Phys. 2006;291(2):H677–86. https://doi.org/10.1152/ajpheart.01092.2005.
Hahn-Obercyger M, Graeve L, Madar Z. A high-cholesterol diet increases the association between caveolae and insulin receptors in rat liver. J Lipid Res. 2009;50(1):98–107. https://doi.org/10.1194/jlr.M800441-JLR200.
Majkova Z, Toborek M, Hennig B. The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. J Cell Mol Med. 2010;14(10):2359–70. https://doi.org/10.1111/j.1582-4934.2010.01064.x.
Abaj F, Mirzaei K. Caveolin-1 genetic polymorphism interact with polyunsaturated fatty acids to modulate metabolic syndrome risk. Br J Nutr. 2021:1-23. https://doi.org/10.1017/S0007114521002221. Epub ahead of print. PMID: 34155967.
Acknowledgements
We would like to thank all participants who gave their time to carry out this project.
Funding
This study was supported by a grant from Tehran University of Medical Sciences (97–03–161-41017).
Author information
Authors and Affiliations
Contributions
FA: Conceptualization; Methodology; Investigation; Formal analysis; Writing Original draft. FK: Validation. FA and EA carried out the experiment. MSY: Formal analysis; Software. MR: Editing. KhM: Supervision; Validation; Project administration. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. The protocol of the study was approved by the ethics committee of TUMS (Ethics number: 97–03–161-41017). All participants completed a written informed consent.
Consent for publication
‘Not applicable’.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abaj, F., Koohdani, F., Rafiee, M. et al. Interactions between Caveolin-1 (rs3807992) polymorphism and major dietary patterns on cardio-metabolic risk factors among obese and overweight women. BMC Endocr Disord 21, 138 (2021). https://doi.org/10.1186/s12902-021-00800-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-021-00800-y