Abstract
Background
Recent studies have shown that dietary intakes and gene variants have a critical role in the obesity related comorbidities. This study aimed to evaluate the effects of the interactions between Fatty acid desaturase 2 (FADS2) gene rs174583 polymorphism and two dietary indices on cardiometabolic risk factors.
Methods
This cross-sectional study was carried out on 347 obese adults aged 20-50 years old in Tabriz, Iran. Healthy eating index (HEI) and Diet quality index-international (DQI-I) were evaluated by a validated semi-quantitative 147-item Food frequency questionnaire (FFQ). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to determine FADS2 gene variants. Multivariate analysis of covariance (MANCOVA) was used to identify gene-diet interactions on metabolic parameters.
Results
Waist circumference (WC) and serum triglyceride (TG) levels were significantly higher among carriers of TT genotype of FADS2 gene (P < 0.05). In addition, the interactions between FADS2 gene rs174583 polymorphism and DQI-I had significant effects on weight (P interaction = 0.01), fat mass (P interaction = 0.04), fat free mass (P interaction = 0.03), and Body mass index (BMI) (P interaction = 0.02); the highest level of these parameters belonged to TT carriers. Similarly, the interactions between FADS2 gene variants and HEI had significant effects on insulin (P interaction < 0.001), Homeostasis model assessment of insulin resistance (HOMA-IR) (P interaction < 0.001), Quantitative insulin check index (QUICKI) (P interaction = 0.001), and alpha Melanocyte stimulating hormone (α-MSH) (P interaction = 0.03).
Conclusion
In this study, for the first time, we reported the effects of gene-diet interactions on metabolic traits. Compliance with dietary indices (DQI-I and HEI) ameliorated the adverse effects of gene variants on metabolic risk factors, especially in heterogeneous genotypes. Further prospective cohort studies are needed to confirm these results.
Similar content being viewed by others
Background
The prevalence of obesity has been doubled since 1980 and now accounts for one-third of the world’s population [1, 2]. This ascending trend in the prevalence of obesity across the globe is true in Iranian population too. Likewise, the prevalence of obesity was estimated to be 21.7% in Iran, which demands urgent actions [3]. Obesity is a multifactorial disorder which contributes to increased risk of chronic diseases such as type two diabetes mellitus (T2DM), cancers, and cardiovascular disease (CVD); it has also negative effects on life expectancy, quality of life, and health care costs [1, 4,5,6]. Obesity is characterized by excessive body fat accumulation due to increased energy intake and decreased physical activity [7,8,9,10]. Although it is internationally accepted to use Body mass index (BMI) to classify the obesity status, it is not an accurate index for body fat distribution and body composition [1, 11].
Despite significant progress to overcome the current health problem and the need to transit from basic nutritional science to clinically relevant dietary recommendations, nutrigenomics and nutrigenetics seem to be challenging to fill the gap in clinical nutrition. This addresses the substantial between-individual variability in response to dietary interventions in a molecular and metabolic level and considers the gene-diet interactions [12].
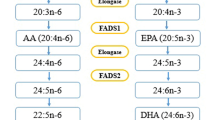
The development of genome studies have generated opportunities to design a personalized nutrition based on genetic makeup in population [13]. Genome wide association studies (GWAS) have paved the way for identification of Single nucleotide polymorphisms (SNPs) as drivers of cardiometabolic diseases. Therefore, SNPs have been known as a proper choice to identify phenotypic differences and address inter-individual variability [14]. In this regard, it is of great importance to identify SNP in the fatty acid desaturase (FADS) genes and determine their interaction with environmental factors such as diet in relation to risk of metabolic diseases. FADS1 and FADS2 appear side-by-side on chromosome 11 and therefore a degree of overlap may exist and it has been shown that SNP of rs174547 in FADS1 and rs174583 in FADS2 are in high linkage disequilibrium [15]. However, it has been shown that FADS2 gene rs174583 polymorphism is involved in fatty acids metabolism [16]. FADS2 is an endoplasmic reticulum membrane-bound protein located on chromosome 11 (11q12–13.1) from 61,792,980 to 61,867,354 in forward strand, which is comprised of 12 exons and 11 introns and rs174583 is located in 61,842,278 kb [17,18,19,20]. FADS2 has the desaturation domain on the C-terminal with histidine rich region and a cytochrome b5 domain in N-terminal position (Fig. 1) [19]. It also encodes delta 6 desaturase (D6D) as a rate limiting enzyme in the synthesis of Long chain poly unsaturated fatty acids (LC-PUFAs) from essential fatty acids [17, 21, 22]. Since w-3 and w-6 pathways share the same enzymes for desaturation and elongation, D6D is involved in the desaturation of dietary obtained linoleic acid (LA) and alpha linolenic acid (α-ALA) to gamma linoleic acid (γ-ALA) and stearidonic acid, respectively [19]. Arachidonic acid (AA) and eicosapentaenoic acid (EPA), produced in the LA and ALA pathways respectively, are affected by elongase and D6D; this facilitates the production of docosahexaenoic acid (DHA) in n-3 pathway subsequently [19, 21]. Although initial steps of LC-PUFA biosynthesis are performed in endoplasmic reticulum, DHA production is conducted in the peroxisome where β-oxidation of LC-PUFAs takes place (Fig. 2) [23, 24]. Although knowledge about the effect of FADS2 gene polymorphism and their potential role in the development of metabolic diseases is limited. However, several studies have pointed to the association of FADS polymorphism with AA which is known as a precursor of low-grade inflammation and it will facilitate the condition accompanied with lipid metabolism disorders including obesity, CVDs, T2DM and non-alcoholic fatty liver disease [25,26,27]. PUFAs act as biological ligands to functional pathways and modulate the function of membrane lipid rafts, so fatty acid composition may alter metabolic function [26]. Also, PUFAs are involved in immunometabolic responses and the pathogenesis of chronic diseases associated with lipid metabolism disorders [28]. In addition, some studies have illustrated the effects of FADS2 gene polymorphism in glucose hemostasis too [25]. While, Mazoochian et al. showed no significant differences about the distribution of rs174583 genotypes between diabetic patients and control groups [29].
Previous studies have suggested that dietary intakes may interact with FADS2 gene rs174583 polymorphism to modify cardiometabolic factors in obese individuals [17, 29, 30]. So far, several dietary approaches have been developed. However, since the nutrients are not used in isolation as an individual dietary component, multidimensional method is preferred to single nutrient assessment to provide a comprehensive diet quality assessment [31]. Healthy eating index (HEI) and Diet quality index-international (DQI-I) may provide a broader and more realistic image of dietary intake by exerting cumulative and interactive effects of nutrients [32, 33] and help to clarify the gene-diet interactions. Therefore, our primary outcome was to evaluate metabolic factors among different FADS2 genotypes. Accordingly, for the first time, we evaluated the effects of the interactions between FADS2 gene rs174583 polymorphism and two dietary indices (HEI and DQI-I) on cardiometabolic risk factors among obese individuals as a secondary outcome.
Methods
Study population
This cross-sectional study included 347 obese individuals. The samples were obtained from two separate projects including 188 and 159 obese participants in Tabriz, Iran. The inclusion criteria were as follows: age range of 20-50 years, BMI range of 30-40 kg/m2, with no history of alcohol or drug abuse, and signing a written informed consent. We also excluded the subjects with a history or presence of chronic diseases (such as T2DM, cardiovascular diseases, cancers, kidney diseases, and infections), individuals reporting any possible changes in their usual diet, pregnant or lactating women, and those using drugs affecting weight such as corticosteroids. The sample size calculation, using G-power software was based on the r of 0.25, α = 0.05 and β = 0.20, and power 80%, the minimum sample size was 290 and considering 15% drop-out, the final sample size was 347 individuals. The statistical power of 80% was used to avoid false negative associations and to determine a cost-effective sample size. Therefore, we decided to subgroup the individuals into tertiles for maintaining the power of 80% and also for assigning the comparable number of participants to each group accordingly.
The study protocol was fully explained to all participants and a written informed consent was obtained from them. The protocols of both projects had already been approved by the ethics committee of Tabriz University of Medical Science, Tabriz (IR.TBZMED.REC.1399.062. and IR.TBZMED.REC.1398.460).
Anthropometric measurements
First, the demographic data of participants was collected by interviewing. Additional data on socioeconomic status (SES), including educational status, occupation, marital status, and family size was obtained and the subjects were classified into three tertiles (low, middle, and high) according to the total scores. The International Physical Activity Questionnaire (IPAQ) was used to investigate the physical activity status in subjects [34]. Weight assessment was performed by calibration of the Seca scale (Seca, Germany) in standing unassisted position with light clothing and no shoes to the nearest 0.1 kg. Height was measured by standing on heels against the corner where the wall and floor meet considering head, shoulders, and buttocks touching the wall by a stadiometer near to 0.5 cm. BMI was calculated according the Quetelet’s formula [4]. Waist circumference (WC) was measured with a non-stretchable tape around the middle and above the umbilicus without compressing the skin to the nearest 0.1 cm over light clothing. Hip circumference (HC) was measured according to the widest part of buttocks. In addition, bioelectrical impedance analysis (BIA) was used to estimate body composition. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in a relaxing position with mercury sphygmomanometer.
Measurements of biochemical parameters
Fasting blood samples (10 ml) were obtained after a 12-hour overnight fasting. Serum and plasma isolations were done using centrifugation at 4500 rpm at 4 °C for 10 min immediately. Then, aliquots were frozen at − 80 °C until use. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and serum glucose concentrations were measured using a commercial kit (Pars Azmoon, Tehran, Iran). Serum low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald’s equation using TC, TG, and HDL-C concentrations [35]. In addition, enzyme-linked immunosorbent assay (ELISA) kits were used to detect insulin levels. Homeostasis Model Assessment-Insulin Resistance Index (HOMA-IR) and Quantitative Insulin Sensitivity Check Index (QUICKI) were calculated according to the specified formula [36, 37]. Moreover, plasma Agouti-Related Peptide (Ag-RP) and α-Melanocyte-Stimulating Hormone (α-MSH) were assayed using ELISA kits (Bioassay Technology Laboratory, China).
Appetite assessment
Appetite was measured by Visual Analogue Scale (VAS) (100-mm line) to record the feelings about hunger, satiety, fullness, and desire to eat salty, sweet, or fatty foods. This was done by making a mark on a 100-mm line with ‘I’m not hungry at all’ and ‘I have not been so hungry’ at tow ends. The distance from the left end up to marked one was considered as appetite score [38].
Dietary assessment
A trained dietitian estimated the usual dietary intakes of participants using a validated semi-quantitative Food Frequency Questionnaire (FFQ) with 147 food items [39, 40]. The questions were explained to all subjects, and they were asked to report the frequency (daily, weekly, monthly, or yearly) and amount of each food item. Afterward, all these responses were changed to gram using household measurements [41]. Accordingly, nutrient and energy intakes were calculated using Food Composition Table (FCT).
DQI-I was designed to capture and represent both food and nutrient intake together. The DQI-I, as a healthy diet index, is based on four factors, including variety, adequacy, moderation, and balance to resolve all nutritional concerns. Each item has its own score as follows: the range of dietary variety score is 0-15 for overall dietary diversity and 0-5 for a protein source (fish, meat, poultry, dairy, eggs, and beans); adequacy scores range between 0 and 40 according to the amount of protein, vegetable, fiber, fruit, grain, calcium, iron, and vitamin C intake to ensure consumption of a healthy diet; and moderation scores such as saturated fat, cholesterol, total fat, sodium, and junk foods get 30 and 10 points, allocated to the overall balance based on fatty acid and macronutrient ratio. Finally, the total DQI-I score constitutes the sum of these categories and higher DQI-I level indicates better diet quality [42].
HEI-2015 was developed to demonstrate the compliance of dietary intakes with Dietary Guidelines for Americans (DGA). HEI constitutes of 13 components (four moderation and nine adequacy), which are scored separately; total score of HEI is derived from the sum of each component. Three adequacy components (e.g., dairy, whole grains, and fatty acids) score between 0 and 10, where a higher score indicates a higher intake. Other six adequacy components, including total fruits (canned fruit, fruit, and fruit juice), whole fruits (fruits except fruit juice), plant proteins, total vegetables, greens, seafood, beans, and total protein score 5 for the highest intake and 0 for the poorest intake. Also, higher intake of four moderation components such as refined grains, added sugars, sodium, and saturated fats reflect lower intakes [43].
DNA extraction and genetic sequencing
Single nucleotide polymorphism (SNP) was chosen from studies claimed to be associated with obesity [29] and according to minor allele frequency from previous studies [44]. Chloroform technique was used to extract genomic DNA from whole blood. In this technique the cell lysis buffer was used to remove proteins binding to DNA and membrane of red blood cell. Likewise, chloroform was used to remove the remaining proteins. Accordingly, three phases were formed in the micro tubes that the upper phase contained DNA.
The concentration of extracted DNA was checked using Nano Drop ND-1000 spectrophotometer. Likewise, all available DNAs were expected to be genotyped for rs174583. Then, polymerase chain reaction-restricted length polymorphism (PCR–RFLP) method was carried out to determine genotypes of SNP rs174583 located in the 61,842,278 position of chromosome 11 in the intron of FADS2 (Fig. 3). The forward and reverse primers were 5′ AGGAAGCAGACCACAGAGTC 3′ and 5′ TCCTTCGTCTGGTGTCTCAG 3′, respectively. PCR reactions were performed in a final volume of 10 μl containing 2 μl of extracted DNA, 5 μl of Master Mix (Ampliqon; Denmark), 2 μl of distilled water, and 1 μl of primers. The PCR cycles in a DNA thermocycler (BIO RAD T100 Thermal Cycler) were optimized to 10 min of denaturation at 95 °C; amplification consisted of 35 cycles at 94 °C; annealing at 60 °C for 20 s, 50 s of extension at 74 °C, and final extension for 10 min at 74 °C. In addition, TauI (cat. Num ER1652, USA), as a restriction enzyme, was used to digest amplified DNA. The enzymatic digestion with a final volume of 20 μl containing the PCR product, TauI enzyme and buffer was performed at 56 °C. Three possible genotypes of FADS2 rs174583 were detected as follows: homozygous mutated TT (572 bp), homozygous wild-type CC (380 and 192 bp), and heterozygous mutated CT (572, 380, and 192 bp). Finally, all the digested PCR products were visualized by green stained gel of electrophoresis on 1.5% agarose gel in a Gel Doc-system (U.V.P Company, Cambridge, UK).
Statistical analysis
Data were analyzed using SPSS software (SPSS Inc., USA, version 25). Normality of variables was checked according to mean, standard deviation (SD), skewness, and kurtosis. Normal and non-normal data were expressed as mean ± SD and median (min, max), respectively. Meanwhile, categorical values were reported as frequency (%) using χ2 test. Chi-square and independent sample t-test tests were used to compare qualitative and quantitative variables, respectively. Multivariate analysis of covariance (MANCOVA) was used to investigate the effect of interactions between FADS2 rs174583 polymorphism and diet quality indices on cardiometabolic risk factors. P-values less than 0.05 were considered as statistically significant.
Results
This cross-sectional study was conducted among 347 obese individuals (58.2% males vs. 41.8% females). The demographic, clinical, and biochemical characteristics of participants are presented in Table 1. The mean (SD) age, weight, and BMI were 40.78 ± 9.23 years, 92.11 ± 14.44 kg, and 32.62 ± 4.80 kg/m2, respectively. As can be seen, the majority of participants (47.9%) had low physical activity with middle SES (52.90%). Genotype distribution was as follows: CC (37.8%), CT (51.9%), and TT (10.3%).
The comparison of anthropometric and biochemical parameters across genotypes of FADS2 is presented in Table 2. As the table shows, there were significant differences in WHR (P = 0.04), WC (P = 0.04), and TG (P = 0.03) levels between FADS2 genotypes. Moreover, adults with adverse lipid profile were more likely to have TT carriers. However, there was no significant differences between genotypes except for TG value. Also, TT carriers had higher levels of anthropometric values, including weight, BMI, WC, and fat mass. Also, dietary vitamin are compared between FADS2 genotype and are provided in supplementary table 1. In addition, Table 3 presents analysis for the association between biochemical values and FADS2 rs174583 genotypes. There was no significant association between dietary biochemical values and FADS2 rs174583 genotypes in either crude or multivariate-adjusted model except for HDL value. Furthermore, there were significant interactions between FADS2 gene rs174583 polymorphism and DQI-I in terms of weight (P = 0.01), FM (P = 0.04), FFM (P = 0.03), BMI (P = 0.02), and HC (P = 0.005) (Fig. 4) even after adjusting for potential confounders such as age and sex. We witnessed higher levels of weight, BMI, FM, FFM, and HC in subjects with lower adherence to DQI-I diet in TT genotype. In contrast, by increasing adherence to DQI-I, we observed the downward trend for these parameters in this genotype compared to the first tertile of DQI-I, indicating that DQI-I may modify the adverse effects of having TT genotype. Moreover, insulin (P < 0.001), QUICKI (P = 0.001), HOMA-IR (P < 0.001), and α-MSH (P = 0.03) had a significant interaction with FADS2 genotypes and HEI in obese adults (Fig. 5). With respect to gene-diet interactions, the highest level of insulin and HOMA-IR were seen in TT carriers even in those with high compliance to HEI. This shows that TT carriers may experience high levels of insulin and HOMA-IR irrespective of having high-quality diet. All other gene-diet interactions (gen-DQI-I and gene HEI) in term of biochemical values are provided in supplementary Tables 2 and 3.
Discussion
To the best of our knowledge, this is the first study to investigate the effects of the interactions between FADS2 gene rs174583 polymorphism and two dietary indices (DQI-I and HEI) on cardiometabolic risk factors, including serum lipids, markers of glucose hemostasis, blood pressure, AgRP, and α-MSH in obese individuals. According to our results, it seems that while obese individuals with TT homozygote genotype are more prone to have higher levels of weight, BMI, WC, FM, TC, LDL, TG, and insulin, they have lower HDL levels. Similarly, Mazoochian et al. evaluated the effect of FADS2 gene rs174583 polymorphism on biochemical parameters among T2D patients in Iran and reported the highest levels of TC and TG among homozygotes for T alleles, and the difference was significant in term of TG. Also, in line with our findings, they illustrated that the lowest level of HDL belonged to TT carriers [29]. Moreover, a study in South Korea showed a significant difference in terms of TG and glucose across FADS2 genotypes, but other metabolic parameters including WC, HDL, SBP, and DBP did not differ significantly. Also, they reported an association between FADS2 genotypes and cardiometabolic components [45]. Our results were also in agreement with another study conducted among Japanese males, reporting higher levels of TG in homozygotes for T allele of FADS2 gene rs174583 polymorphism [46].
In our study, FADS2 gene-diet interactions affected some anthropometric and glycemic markers with no significant interaction in terms of lipid profile. It is expected that high adherence to DQI-I and HEI with higher intake of EPA and DHA may contribute to reduced level of TG through increased β-oxidation and clearance of TG-rich lipoproteins; while gene variants have affected the level of serum lipids in response to dietary intervention [47]. Interestingly, Abu-Mweis et al. illustrated more beneficial effects of DHA combined with high-oleic canola oil supplementation on TG levels in comparison to canola oil supplemented group with heterozygous CT genotypes; however, the difference was not statistically significant [47]. Although the underling mechanism for the effect of FADS2 gene variants on HDL concentration remains unclear, it is suggested that its gene variants may impair desaturase function contributing to low level of n-6 and n-3 production. PUFAs have been introduced as a ligand for peroxisome proliferator activating receptor-α and are involved in the regulation of lipoprotein lipase and apo-lipoprotein A-I, A-II, and C-III, modulating HDL metabolism [15, 48, 49]; these discrepancies may be attributed to the study population and dietary patterns and habits. Furthermore, Warensjo et al. indicated that D6D and triacylglycerol in adipose tissue were correlated with BMI and insulin resistance [50]. Indeed, desaturase enzymes are incorporated in the synthesis of LC-PUFAs, which are involved in cell membrane affecting permeability of cells. This may affect insulin signaling and receptor binding affinities. In other words, the products of D6D have been proposed as a ligand for transcription factors, such as sterol regulatory element binding protein 1 (SREBP-1) and peroxisome proliferators activated receptors, and they may interact with genes involved in lipogenesis and fatty acid oxidation subsequently [15, 48, 49].
Despite the higher levels of weight, BMI, and FM in homozygote TT genotypes, moderate adherence to DQI-I improved the level of these values and attenuated the adverse effects of risk allele. Similarly, the highest levels of glycemic markers, including insulin, HOMA-IR, QUICKI, and α-MSH were reported among TT carriers. Nevertheless, high compliance with HEI could not modify the adverse effects of having TT genotype on insulin and HOMA-IR. On the other hand, high adherence to HEI exerted its favorable effect on heterogeneous genotype in terms of insulin and HOMA-IR. In contrast, higher adherence to HEI had ameliorative effects on QUICKI and α-MSH even in TT carriers after adjusting for potential confounders. Evidence shows that HOMA-IR is related to fatty acid composition (LA and DGLA). Kim et al. [51] found an interactive effect of rs174575 SNP of FADS2 and the proportion of DGLA or AA on HOMA-IR levels. In addition, previous studies indicated the relationship between serum phospholipid fatty acid composition or dietary fat with insulin resistance, reporting higher levels of palmitic acid in people with insulin resistance, which was also confirmed in another study by Warensjo et al. [50]. Positive relationships have also been reported between obesity and insulin resistance and palmitoleic acid and DGLA in serum phospholipids [50, 51].
It is worth noting that despite ever-increasing knowledge in this field, there is no study to evaluate the effect of interactions between FADS2 gene polymorphism and dietary indices on cardiometabolic risk factors. The present study focused on the modifiable effects of dietary indices (DQI-I and HEI) on obesity outcomes in different FADS2 genotypes with different susceptibility to risk factors among a relatively acceptable sample size, which provides an effective and dynamic nutritional approach to design tailored nutrition recommendations. However, the cross-sectional design of the study limits understanding the causal relationships. Therefore, further longitudinal studies are necessary to confirm our findings.
Conclusion
This study, for the first time, evaluated the effects of the interactions between FADS2 gene rs174583 polymorphism and two dietary indices (DQI-I and HEI) on cardiometabolic risk factors. According to the results, compliance with these dietary indices ameliorated the adverse effects of gene variants on metabolic risk factors, especially in heterogeneous genotypes. Further prospective cohort studies are needed to confirm these results.
Availability of data and materials
The author have some restrictions from the Ethics Committee of Tabriz University of Medical Sciences for sharing data of the current study. All the data are available with reasonable request from the corresponding author.
Abbreviations
- T2DM:
-
Type two diabetes mellitus
- BMI:
-
Body mass index
- GWAS:
-
Genome wide association studies
- SNP:
-
Single nucleotide polymorphisms
- FADS2:
-
Fatty acid desaturase-2
- D6D:
-
Delta 6 desaturase
- LC-PUFAs:
-
Long chain poly unsaturated fatty acids
- LA:
-
Linoleic acid
- α-ALA:
-
Alpha linolenic acid
- DGLA:
-
Dihomo gamma-linoleic acid
- AA:
-
Arachidonic acid
- EPA:
-
Ecosapantanoic acid
- DQI-I:
-
Diet quality index-International
- HEI:
-
Healthy eating index
- SES:
-
Socioeconomic status
- IPAQ:
-
International Physical Activity Questionnaire
- HC:
-
Hip circumference
- BIA:
-
Bioelectrical impedance analysis
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance index
- QUICKI:
-
Quantitative insulin sensitivity check index
- Ag-RP:
-
Agouti-related peptide
- α-MSH:
-
α-melanocyte-stimulating hormone
- VAS:
-
Visual analogue scale
- FFQ:
-
Food frequency questionnaire
- FCT:
-
Food composition table
- PCR-RFLP:
-
Polymerase chain reaction-restricted length polymorphism
- SREBP-1:
-
Sterol regulatory element binding protein 1
References
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metab Clin Exp. 2019;92:6–10.
Tang D, Bu T, Feng Q, Liu Y, Dong X. Differences in overweight and obesity between the north and south of China. Am J Health Behav. 2020;44(6):780–93.
Rahmani A, Sayehmiri K, Asadollahi K, Sarokhani D, Islami F, Sarokhani M. Investigation of the prevalence of obesity in Iran: a systematic review and meta-analysis study. Acta Medica Iranica. 2015;53(10):596–607.
Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17.
Schroeder K, Kubik MY, Sirard JR, Lee J, Fulkerson JA. Sleep is inversely associated with sedentary time among youth with obesity. Am J Health Behav. 2020;44(6):756–64.
Valerio A, Nisoli E, Rossi AP, Pellegrini M, Todesco T, El Ghoch M. Obesity and higher risk for severe complications of Covid-19: what to do when the two pandemics meet. J Popul Ther Clin Pharmacol. 2020;27(SP1):e31–e6.
Crovesy L, Rosado EL. Interaction between genes involved in energy intake regulation and diet in obesity. Nutrition (Burbank, Los Angeles County, Calif). 2019;67-68:110547–52.
Ulaganathan V, Kandiah M, Shariff ZM. A case–control study on the association of abdominal obesity and hypercholesterolemia with the risk of colorectal cancer. J Carcinogenesis. 2018;17:4.
Nasiri A. Parental care challenges in childhood obesity management: a qualitative study. Evid Based Care. 2022;11(4):7–15.
Aghajani R, Nemati N, Hojjati Zidashti Z, Bagherpour T. Effect of aerobic program in the morning and afternoon on Obestatin and the body composition of overweight and obese women. J Chem Health Risks. 2020;10(2):117–25.
Kamolthip R, Fung XC, Lin C-Y, Latner JD, O'Brien KS. Relationships among physical activity, health-related quality of life, and weight stigma in children in Hong Kong. Am J Health Behav. 2021;45(5):828–42.
Gasmi A, Noor S, Piscopo S, Menzel A. Lifestyle genetics-based reports in the treatment of obesity. Arch Razi Institute. 2021;76(4):707.
de Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolicsSyndrome. Nutrients. 2017;9(8):1–28.
Abdullah MM, Vazquez-Vidal I, Baer DJ, House JD, Jones PJ, Desmarchelier C. Common genetic variations involved in the inter-individual variability of circulating cholesterol concentrations in response to diets: a narrative review of recent evidence. Nutrients. 2021;13(2):695.
Huang MC, Chang WT, Chang HY, Chung HF, Chen FP, Huang YF, et al. FADS gene polymorphisms, fatty acid desaturase activities, and HDL-C in type 2 diabetes. Int J Environ Res Public Health. 2017;14(6):572–83.
Atanasovska B, Kumar V, Fu J, Wijmenga C, Hofker MH. GWAS as a driver of gene discovery in cardiometabolic diseases. Trends Endocrinol Metab. 2015;26(12):722–32.
Gol S, Pena RN, Rothschild MF, Tor M, Estany J. A polymorphism in the fatty acid desaturase-2 gene is associated with the arachidonic acid metabolism in pigs. Sci Rep. 2018;8(1):14336–45.
He Z, Zhang R, Jiang F, Zhang H, Zhao A, Xu B, et al. FADS1-FADS2 genetic polymorphisms are associated with fatty acid metabolism through changes in DNA methylation and gene expression. Clin Epigenetics. 2018;10(1):113–26.
Bláhová Z, Harvey TN, Pšenička M, Mráz J. Assessment of fatty acid desaturase (Fads2) structure-function properties in fish in the context of environmental adaptations and as a target for genetic engineering. Biomolecules. 2020;10(2):1–13.
Naghibi D, Mohammadzadeh S, Azami-Aghdash S. Barriers to evidence-based practice in health system: a systematic review. Evid Based Care. 2021;11(2):74–82.
Brown KM, Sharma S, Baker E, Hawkins W, Merwe MVD, Puppa MJ. Delta-6-desaturase (FADS2) inhibition and omega-3 fatty acids in skeletal muscle protein turnover. Biochem Biophys Rep. 2019;18:100622–9.
Guerra ZC, Moore JR, Londoño T, Castro Y. Associations of acculturation and gender with obesity and physical activity among Latinos. Am J Health Behav. 2022;46(3):324–36.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52–67.
Glaser C, Heinrich J, Koletzko B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metab Clin Exp. 2010;59(7):993–1000.
Shetty SS, Devi H, Sharmila K, Rai S. Association of FADS2 rs174575 gene polymorphism and insulin resistance in type 2 diabetes mellitus. Afr Health Sci. 2020;20(4):1770–6.
Kytikova OY, Novgorodtseva TP, Antonyuk M, Gvozdenko T. Associations of delta fatty acid desaturase gene polymorphisms with lipid metabolism disorders. Russian Open Med J. 2021;10(4):403.
Shetty SS, Kumari NS. Fatty acid desaturase 2 (FADS 2) rs174575 (C/G) polymorphism, circulating lipid levels and susceptibility to type-2 diabetes mellitus. Sci Rep. 2021;11(1):1–8.
Margină D, Ungurianu A, Purdel C, Nițulescu GM, Tsoukalas D, Sarandi E, et al. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem Toxicol. 2020;143:111558.
Mazoochian L, Sadeghi H, Pourfarzam M. The effect of FADS2 gene rs174583 polymorphism on desaturase activities, fatty acid profile, insulin resistance, biochemical indices, and incidence of type 2 diabetes. J Res Med Sci. 2018;23:47–54.
Pavlos S, Nikiforou C. Medicinal plants against obesity: a met-analysis of literature. J Complement Med Res. 2022;12(4):244.
Jontony N, Hill EB, Taylor CA, Boucher LC, O'Brien V, Weiss R, et al. Diet quality, carotenoid status, and body composition in NCAA division I athletes. Am J Health Behav. 2020;44(4):432–43.
Anvigh B, Abedi V, Heydari S, Karamati D, Babajafari S, Najafi A, et al. Healthy eating index-2015 and bone mineral density among adult Iranian women. Arch Osteoporos. 2020;15(1):1–11.
Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the healthy eating index–2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150(2):312–21.
Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(sup2):114–20.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–62.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. Am J Epidemiol. 2010;20(2):150–8.
Ghaffarpour M, Rad AH, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods, vol. 7. Tehran: Nashre Olume Keshavarzy; 1999. p. 213.
Kim S, Haines PS, Siega-Riz AM, Popkin BM. The diet quality index-international (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133(11):3476–84.
Smith SMK, Pannucci TRE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602.
Malerba G, Schaeffe L, Xumerle L, Klopp N, Trabetti E, Biscuola M, et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43(4):289–99.
Park S, Kim DS, S. K. Carrying minor allele of FADS1 and haplotype of FADS1 and FADS2 increased the risk of metabolic syndrome and moderate but not low fat diets lowered the risk in two Korean cohorts. Eur J Nutr. 2019;58(2):831–42.
Sone Y, Kido T, Tomomi A, Sonoda M, Ichi I, Kodama S, et al. Genetic variants of the fatty acid desaturase gene cluster are associated with plasma LDL cholesterol levels in Japanese males. J Nutr Sci Vitaminol. 2013;59(4):325–35.
AbuMweis S, Panchal S, Jones P. Triacylglycerol-lowering effect of docosahexaenoic acid is not influenced by single-nucleotide polymorphisms involved in lipid metabolism in humans. Lipids. 2018;53(9):897–908.
Nakayama K, Bayasgalan T, Tazoe F, Yanagisawa Y, Gotoh T, Yamanaka K, et al. A single nucleotide polymorphism in the FADS1/FADS2 gene is associated with plasma lipid profiles in two genetically similar Asian ethnic groups with distinctive differences in lifestyle. Hum Genet. 2010;127(6):685–90.
Chen Y, Estampador AC, Keller M, Poveda A, Dalla-Riva J, Johansson I, et al. The combined effects of FADS gene variation and dietary fats in obesity-related traits in a population from the far north of Sweden: the GLACIER study. Int J Obes. 2019;43(4):808–20.
Warensjö E, Rosell M, Hellenius ML, Vessb B, De Faire U, Risérus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8(1):37–44.
Kim OY, Lim HH, Yang LI, Chae JS, Lee JH. Fatty acid desaturase (FADS) gene polymorphisms and insulin resistance in association with serum phospholipid polyunsaturated fatty acid composition in healthy Korean men: cross-sectional study. Nutr Metab. 2011;8(1):24–35.
Acknowledgements
We thank all the study participants. We also thank Research Undersecretary of Tabriz University of Medical Sciences for their financial support (Grant number: 64111).
Funding
This study was financially supported by a grant from Tabriz University of Medical Sciences, Iran (Identifiers: IR.TBZMED.REC.1399.062. and IR.TBZMED.REC.1398.460).
Author information
Authors and Affiliations
Contributions
MM contributed to data collection and manuscript writing. She also performed the statistical analysis and data interpretation. MAF and SK conceptualized and designed the study. Moreover, MAF and LSB revised the manuscript and approved the final manuscript as submitted. HK was involved in lab works and genetic assays. EF was involved in statistical analysis, manuscript writing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Each participant was completely informed about the study protocol and provided a written informed consent form before taking part in the study. The study protocols of both projects were approved by the ethics committee of Tabriz University of Medical Science, Iran (IR.TBZMED.REC.1399.062. and IR.TBZMED.REC.1398.460).
Human and Animal Ethics
This study was performed according to the guidelines of declaration for Helsinki. All of the study participants signed a written informed consent prior participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Comparison of dietary vitamins between FADS2 gene polymorphism. Supplementary Table 2. Representing the interactions between FADS2 gene polymorphism and DQI-I in term of metabolic factors. Supplementary Table 3. Representing the interactions between FADS2 gene polymorphism and HEI in term of metabolic factors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmoudinezhad, M., Khosravaniardakani, S., Saljoughi Badelou, L. et al. The integrative panel of fatty acid desaturase-2 (FADS2) rs174583 gene polymorphism and dietary indices (DQI-I and HEI) affects cardiovascular risk factors among obese individuals. BMC Endocr Disord 23, 41 (2023). https://doi.org/10.1186/s12902-023-01289-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01289-3