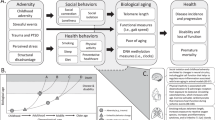
Abstract
Background
Psychological and trauma-related factors are associated with many diseases and mortality. However, a comprehensive assessment of the association between psycho-trauma exposures and aging acceleration is currently lacking.
Methods
Using data from 332,359 UK Biobank participants, we calculated biological aging acceleration, indexed by the presence of leukocyte telomere length (LTL) deviation (i.e., the difference between genetically determined and observed LTL > 0). The acceleration of facial aging (i.e., looking older than the chronological age) was assessed using a self-report question. Then, we estimated the associations of each psycho-trauma factor with biological and facial aging acceleration, using logistic regression models adjusted for multiple important covariates. Furthermore, restricted to 99,180 participants with complete psychological and trauma-related data, we identified clusters of individuals with distinct psycho-trauma patterns using the latent class analysis method and assessed their associations with aging acceleration using similar models.
Results
We observed most of the studied psycho-trauma factors were associated with biological and facial aging acceleration. Compared to the “Absence of trauma and psychopathology” cluster, the “adverse childhood experiences (ACEs) with psychopathology” cluster showed strong associations with those aging measurements (odds ratio [OR] = 1.13 [1.05 − 1.23] for biological and 1.52 [1.18 − 1.95] for facial aging acceleration), while no such association was observed for the “ACEs without psychopathology” cluster (1.04 [0.99 − 1.09] and 1.02 [0.84 − 1.24].
Conclusions
Our study demonstrated significant associations of psycho-trauma factors with both biological and facial aging acceleration. The differential aging consequences observed among ACEs exposed individuals with and without psychopathology prompt interventions aimed to improve individuals’ psychological resilience to prevent aging acceleration.
Similar content being viewed by others
Background
It has been estimated that approximately 970 million people worldwide were living with psychiatric disorders in 2019 [1]. Notably, individuals with psychiatric disorders (e.g., depression and substance use) were reported to have a significant excess mortality risk [2] and a reduction of 10–20 years in life expectancy [3], compared to the general population. Likewise, people suffering from current psychological symptoms (e.g., depressive and anxiety symptoms) were also associated with an elevated risk of major diseases, such as cancer [4] and cardiovascular diseases [5]. Furthermore, given that one third (31.4%) of adults reported having experienced at least one lifetime traumatic experience [6], while close to half (47%) of individuals experienced at least one adverse childhood event (ACE) according to reports from England [7], the accumulated negative effects of traumatic experience on physiological, behavioral, and psychological functions could be substantial at the population level [8, 9].
Telomeres are conserved complex DNA structures at the ends of chromosomes that ensure their stability and prevent damage [10]. In most human cells, the telomere shortens with cell division, making it a biomarker of human aging [11]. Leukocyte telomere length (LTL) is a practicable measure of TL and has a close correlation with TL across different tissues within individuals [12]. Therefore, LTL could serve as a biomarker for biological aging, and shorter LTL has been significantly associated with increased morbidity and mortality [13]. However, telomere attrition is highly associated with age, gender, and genetic background, rendering it difficult to measure changes of LTL (i.e., aging acceleration) virtually induced by environmental factors, such as those aforementioned psychological and trauma-related factors (e.g., depression [14], anxiety [15], and ACEs [16]). This could be an explanation of inconsistent results observed for associations between adulthood traumatic experiences and LTL [17].
In addition, besides the biological measures of aging acceleration, facial aging acceleration (i.e., if the person looks older than his/her chronological age) is also considered as a valid indicator of accelerated aging. In a cohort of 1826 twins, the twin individual who looked older seemed to have a higher risk of mortality during a 7-year follow-up, after adjustment for chronological age and sex, than his/her twin partner [18]. However, with limited evidence for some specific psychological symptoms (e.g., depression [19]) or severe trauma (e.g., financial difficulty [20]), a comprehensive assessment on the effects of psychological and trauma-related factors on facial aging acceleration is currently lacking.
Taking advantage of enriched data on phenotypes and genotypes in the UK Biobank, we aimed to examine the association of a broad set of psychological and trauma-related factors with aging acceleration indexed by both biological and facial aging acceleration. Importantly, our study features the application of data-driven approaches to identify psycho-trauma exposure patterns, as illustrations of various existing stimulus–response constellations among the study population. Also, the conceptualization of the aging acceleration index, which was calculated based on an algorithm involving genetic determinants of LTL, sex, and chronological age, might facilitate the accurate measurement of possible changes of LTL due to an environmental exposure.
Methods
Data source
The UK Biobank prospectively recruited 502,507 participants aged 40 to 69 across the UK between 2006 and 2010 [21]. All participants filled in questionnaires providing information on socio-demographic, economic, and lifestyle factors as well as self-reported information about their mental and physical health at baseline. Physical examinations and biological sample collection were also performed at the initial health center visit. UK Biobank data were periodically linked with several national registries to track health-related outcomes with the consent of the participants [22]. Inpatient hospital data were obtained through linked data from Hospital Episode Statistics in England, the Scottish Morbidity Record, and the Patient Episode Database in Wales that has covered all UK Biobank participants since 1997 [23]. Primary care data, covering approximately 45% of the entire UK Biobank cohort [24], were derived from multiple data providers, including the Phoenix Partnership and Egton Medical Information Systems.
In the UK Biobank, genotyping data of 488,377 participants were released, based on DNA extracted from blood samples collected at baseline and measured using two similar genotyping arrays with 95% of marker content [21]. Based on the extracted DNA from 474,074 UK Biobank participants, LTL was determined as the ratio of telomere repeat copy number (T) relative to that of a single copy gene (S) using an established qPCR multiplex method [25].
Study design
As this study focused on both biological and facial aging acceleration, indexed by the presence of non-genetically determined portion of shortened LTL (i.e., LTL deviation > 0) and self-reported facial aging acceleration (see ascertainment of those indexes in the “Indexes of aging acceleration” section), respectively, we restricted our analyses to European ancestry participants with eligible genotyping data and the relevant questionnaire data (n = 334,765, Fig. 1A). To assess the biological aging acceleration (i.e., the primary outcome of the study), participants with non-European ancestry were excluded for calculating polygenic risk score (PRS) for LTL, which served as a proxy of genetically determined LTL and was calculated based on publicly available genome-wide association study (GWAS) data derived from European ancestry samples. Furthermore, we excluded 2406 individuals who had outliers of LTL deviation (i.e., values surpassed three standard deviations from the mean), leaving 332,359 participants in the analytic dataset for assessing the association of each psychological or trauma-related factor (i.e., factors related to history of psychiatric disorders, current psychological symptoms, and traumatic experiences) with biological and facial aging acceleration. Then, for 99,180 participants with complete psychological and trauma-related data (i.e., no missing items for all exposure variables), we identified clusters of participants with distinct psycho-trauma exposure patterns using the latent class analysis (LCA) method (see the “Identification of clusters of participants with distinct psychological and trauma exposure patterns” section for details) and compared those indexes of biological and facial aging acceleration between the identified psycho-trauma clusters.
Psychological and trauma-related factors
We studied psychological and trauma-related factors related to two perspectives: psychological conditions and traumatic experiences at recruitment (see Additional file 1: Table S1).
Firstly, we assessed psychological conditions through history of psychiatric disorders and current psychological symptoms. To ascertain the history of psychiatric disorders, we retrieved information regarding the diagnoses of psychiatric disorders from the UK Biobank self-report, inpatient hospital, or primary care data at recruitment. We identified four major subtypes of psychiatric disorders, including depression [14], anxiety [15], stress-related disorders [26], and substance misuse [27], according to their corresponding International Classification of Diseases [ICD] 9th or 10th edition (ICD-9/10) codes (see Additional file 1: Table S2). Besides the dichotomous variable (yes or no), to reflect the severity level, we generated ordinal variables, which were in order labeled as “No,” “Self-report or primary care only,” and “Inpatient care” based on the data sources of identified diagnosis codes. Current psychological symptoms at recruitment were measured using four psychometric screening questions (i.e., the frequency of feeling depressed, feeling little interest in doing things, feeling tense, and feeling tired over the preceding 2 weeks) asking for core symptoms of depression [28] and generalized anxiety disorder [29]. Each response used a 4-point Likert scale, ranging from “Not at all” to “Nearly every day.”
Then, the studied traumatic experiences included ACEs and recent stressful life events (SLEs) within 2 years before the baseline. ACEs were assessed by the Childhood Trauma Screener (CTS) [30] during the online follow-up, which consisted of five questions related to physical abuse, physical neglect, emotional abuse, emotional neglect, and sexual abuse, respectively. Each item was measured on a 5-point Likert scale (“Never true,” “Rarely true,” “Sometimes true,” “Often,” and “Very often true”), and we applied previously validated thresholds [30] to determine the presence or absence of each ACE (see Additional file 1: Table S3). In addition, at recruitment, participants were asked about their experiences with six recent SLEs, including serious illness, injury or assault to themselves, serious illness, injury or assault to close relative, death of close relative, death of spouse or partner, marital separation, and financial difficulty, during the last 2 years.
Indexes of aging acceleration
In the current study, we assessed aging acceleration in two ways. The primary index was biological aging acceleration, which was defined as the presence of non-genetically determined portion of shortened LTL (i.e., LTL deviation > 0). The quantification of this index was achieved through two steps. First, we calculated the polygenic risk score (PRS) for LTL, which serves as a proxy for the genetically determined proportion of LTL. Specifically, the PRS for LTL was computed using least absolute shrinkage and selection operator (LASSO) [31, 32] regression based on publicly available summary statistics of GWAS from independent samples [31], containing 5,067,039 single-nucleotide polymorphisms (SNPs) for the 332,359 eligible UK Biobank participants. Second, we constructed a linear regression model incorporating the effects of age, sex, and PRS for LTL (i.e., observed LTL ~ genetically determined LTL + age + sex). Then, we defined LTL deviation (median [range], 1.23% [− 45.61% to 45.52%]) as the non-genetically determined portion of shortened LTL which was 100 times of LTL residual divided by predicted LTL (i.e., (predicted LTL − observed LTL)/predicted LTL × 100%). LTL deviation > 0 indicated the presence of biological aging acceleration (i.e., the participants were biologically older than expected).
Facial aging acceleration was measured using a self-reported question at baseline, asking whether participants looked older or younger than their chronological age. The response options were recoded into a binary variable (i.e., “No” = “Younger than you are” or “About your age” and “Yes” = “Older than you are”), to indicate the presence of facial aging acceleration.
Covariates
Data on socio-demographics (e.g., age and sex), socioeconomic (e.g., annual household income and educational years, see Additional file 1: Table S4), and lifestyle (e.g., alcohol drinking status, smoking status, and use of sun protection) factors were collected through questionnaires at baseline. In addition, body mass index (BMI) was calculated from height and weight measured at the initial assessment center visit. To measure area-based deprivation, the Townsend deprivation index (TDI) [33] was generated based on the postcode of the participants’ family address, with higher scores indicating greater deprivation. In addition, we used the Charlson comorbidity index (CCI) [34] to assess baseline somatic comorbidities, based on self-reported, hospital inpatient, and primary care data.
Statistical analysis
Identification of clusters of participants with distinct psycho-trauma patterns
Based on the “poLCA” package in R [35], LCA was used to identify the potential clusters of participants with distinct psycho-trauma exposure patterns, taking into account the correlations and interactions of those trauma and psychological factors. LCA is a model-based approach that utilizes maximum likelihood estimation to evaluate the probability of a participant belonging to each cluster [36]. We compared a series of LCA models in which the number of clusters varied from 2 to 10. The model with 5 clusters was considered optimal as it obtained low values of consistent Akaike information criterion and sample-size adjusted Bayesian information criterion (see Additional file 1: Table S5) but higher average latent class posterior probability and entropy [37], implying superior statistical and interpretable significance. We then applied radar charts to visualize the distinct psycho-trauma patterns of those clusters, which exhibited deviations of each factor in relation to the total population level (see details about the visualization process of the identified patterns in the Additional file 2: Supplementary method).
Associations analyses for psychological and trauma-related factors and patterns with aging acceleration
Logistic regression models were used to examine the associations of 19 individual psychological and trauma factors (i.e., factor-based analyses) and the identified exposure patterns (i.e., cluster-based analyses) with the studied aging acceleration indexes, including the presence of LTL deviation > 0 (yes or no), and facial aging acceleration (yes or no). The models were adjusted for age (as a continuous variable), sex (male or female), BMI (< 25 kg/m2, 25 to 30 kg/m2, ≥ 30 kg/m2, or unknown), CCI (as a continuous variable), smoking and alcohol drinking status (never, previous, current, or unknown), annual household income (less than £18,000, £18,000 to 30,999, £31,000 to 51,999, £52,000 to 100,000, greater than £100,000, or unknown), TDI (as a continuous variable), educational years (7, 10, 13, 15, 19, 20, or unknown), and use of sun protection (most of the time, always, sometimes, never/rarely, do not go out in the sunshine, or unknown). For the factor-based analyses, we used the Benjamini-Hochberg (BH) method to adjust p-values for multiple testing. We considered a false discovery rate (FDR) of < 0.05 as statistically significant. To test the robustness of those results across different outcome definitions, all above-mentioned analyses were repeated for LTL deviation (as a continuous variable) and an alternative definition of the presence of biological aging acceleration (i.e., LTL deviation more than 1SD), based on the linear and logistic regression models, respectively. Furthermore, to detect if the involvement of possible mediators or community-level variable (i.e., TDI that coded according to postal address) in the logistic and linear models could heavily bias our estimates, we conducted additional sensitivity analyses for the factor-based and the cluster-based analyses, including (1) partial (i.e., age, sex, education, annual household income, TDI, and BMI) and full (i.e., additionally adjusted for possible mediators) adjustment for covariates in the models and (2) construction of multi-level logistic and linear regression models to consider TDI as a cluster variable (see Additional file 2: Supplementary method for detailed methodology). For LTL deviation > 0 and facial aging acceleration, we separately calculated odds ratios (ORs) for males and females by dividing the study population into two different sex subgroups. For cluster-based analyses, we then assessed the differences of sub-grouped ORs by introducing an interaction term to the regression models. We conducted repeated cluster-based analyses after performing 10 times of multiple imputation among 107,607 participants, who fully completed the online questionnaire, to assess the influence of missingness (0.0–2.58%, see Additional file 1: Table S6) of psychological and trauma-related factors on the estimation. The overview of the analysis steps was shown in Fig. 1B. All data analysis and visualization were performed based on R 4.0.2.
Results
The mean age of the 332,359 participants that included in the factor-based association analyses (i.e., the association of 19 individual psychological and trauma-related factors with biological and facial aging) was 56.82 years, and 46.40% (n = 154,224) were male (Fig. 1), which was comparable with the 99,180 participants included in the cluster-based analysis (mean age = 56.11 years, male% = 44.86%). Also, other characteristics of those two analytic populations seemed to be similar, except for a higher socioeconomic status (i.e., longer education years and higher annual family incomes) and lower proportions of aging acceleration observed in the population of the cluster-based analyses (Table 1).
The identified clusters of participants with distinct psycho-trauma patterns
We labeled the 5 identified clusters with distinct psycho-trauma patterns according to their most featured profile (Fig. 2 and Additional file 1: Table S7). Specifically, cluster 1 was labeled as “Absence of trauma and psychopathology” (n = 67,410), which was characterized by lower possibilities of suffering all studied psychopathology and trauma than the total population level. Likewise, cluster 2 to 3 were named as “recent SLEs and mild psychopathology” (n = 18,861) and “ACEs, recent SLEs, and severe psychopathology” (n = 2256). Finally, we observed two clusters of participants who both suffered ACEs but had diverse psychological conditions, named as “ACEs without psychopathology” (cluster 4, n = 7880) and “ACEs with psychopathology” (cluster 5, n = 2773), respectively.
The identified psycho-trauma clusters. The y-axis values (ranging from 0 to 10) for each psychological and trauma-related exposure represent the relative level within identified cluster compared to the total population (for detailed methodology, please refer to Additional file 2: Supplementary method). The colors represent three distinct patterns of combined psychological and trauma-related factors. Green refers to the cluster “Absence of trauma and psychopathology” (n = 67,410), characterized by lower possibilities of suffering all studied trauma and psychopathology than the total population level. Red represents the cluster “Recent SLEs and mild psychopathology” (n = 18,861) and “ACEs, recent SLEs, and severe psychopathology” (n = 2256), which have a similar pattern with combining traumatic experiences along with a matching level of psychopathology (i.e., different levels of traumatic experiences [recent SLEs and recent SLEs plus ACEs] and psychopathology [mild and severe]). Yellow represents the cluster as “ACEs without psychopathology” (n = 7880) and “ACEs with psychopathology” (n = 2773), who both suffered ACEs but had diverse psychological conditions. ACEs, adverse childhood events. SLEs, stressful life events
The associations of psychological and trauma-related factors and clusters with aging acceleration
Of the 19 psychological and trauma-related factors, 10 (mainly factors related to the history of psychiatric disorders and ACEs) showed significant associations with biological aging acceleration (i.e., 9 for LTL deviation > 0, 8 for LTL deviation, and 4 for LTL deviation > 1 SD) (see the inner circle of Fig. 3 and Additional file 1: Tables S8 and S9), with the highest estimates observed for a previous inpatient diagnosis of stress-related disorders (OR = 1.63 [95% CI 1.25 − 2.13]) and substance misuse (OR = 1.21 [95% CI 1.12 − 1.32]). Notably, most ACEs were associated with biological aging acceleration (ORs ranged from 1.06 to 1.11). For facial aging acceleration, we identified 10 factors (mainly factors related to current psychological symptoms, see the outer circle in Fig. 3). Specifically, a previous diagnosis of depression (OR = 1.18 for self-report or primary care and OR = 1.37 for inpatient) and the presence of all current psychological symptoms (ORs ranged from 1.40 to 2.45) were associated with facial aging acceleration. Also, we observed significant associations of facial aging acceleration with emotion-related ACEs (i.e., emotional abuse and emotional neglect) and some subtypes of recent SLEs (e.g., financial difficulty and serious illness, injury or assault to themselves).
The association of the psychological and trauma-related factors with biological aging acceleration (LTL deviation > 0) and facial aging acceleration. The numbers in the circle and degree of colors of the circle in Fig. 3 indicates the magnitude of odds ratios (ORs), derived from the fully adjusted logistic regression models. ORs with BH adjusted p-value < 0.05 were considered as statistically significant and shown in bold. Estimates with 95% confidence intervals are detailed in Additional file 1: Table S8. LTL, the length of telomeres. TDI, Townsend deprivation index. CCI, Charlson comorbidity index. BMI, body mass index. “a” symbol indicates the following: the associations of the psychological and trauma-related factors with biological aging acceleration were adjusted for age, sex, education, annual household income, TDI, BMI, smoking status, alcohol drinking status, and CCI. “b” indicates the following: the associations of the psychological and trauma factors with facial aging acceleration were adjusted for age, sex, education, annual household income, TDI, BMI, smoking status, alcohol drinking status, CCI, and the use of sun protection
For the cluster-based associations, using the “Absence of trauma and psychopathology” cluster as a reference, the cluster featuring “ACEs, recent SLEs, and severe psychopathology” demonstrated the most pronounced risk excess in biological aging acceleration (OR = 1.13 [95% CI 1.03 − 1.23], Table 2). Intriguingly, while the “ACEs with psychopathology” cluster showed a strong association with biological aging acceleration (OR = 1.13 [95% CI 1.05 − 1.23]), no such association was observed for the “ACEs without psychopathology” cluster (OR = 1.04 [95% CI 0.99 − 1.09]). We observed largely similar risk patterns when using LTL deviation and the presence of LTL deviation more than 1SD as alternative measurements of biological aging acceleration (Additional file 1: Table S10). For facial aging acceleration, we found significant associations for all clusters with the presence of current psychological symptoms (irrespective of symptom severity, Table 2), particularly for the cluster “ACEs, recent SLEs, and severe psychopathology” cluster (OR = 2.20 [95% CI 1.75 − 2.76]). Also, largely similar results were obtained when performing a repeated cluster-based analyses using the 10 datasets after multiple interpolation (Additional file 1: Table S11).
After conducting the two sensitivity analyses mentioned above (i.e., partial and full adjustment for covariates, and considering TDI as a cluster variable in the logistic and linear regression models), our estimation for the factor-based and the cluster-based analyses remained largely unchanged, demonstrating the robustness of the results (Additional file 1: Tables S12-S15).
The sex-specific association of psychological and trauma-related factors and patterns with aging acceleration
Compared with those of male participants, we observed a stronger magnitude of these associations for both biological and facial aging acceleration among female participants (see Additional file 1: Fig. S1). We then observed sex-specific associations between psychological and trauma-related factors and facial aging acceleration, with some recent SLEs over the past 2 years (e.g., financial difficulty) only being associated with an increased risk of aging acceleration in men. There were no significant interactions between sex group and the identified psychological and trauma-related clusters for risk of biological aging acceleration (P for interaction = 0 0.166, Additional file 1: Table S16) and facial aging acceleration (P for interaction = 0 0.640, Additional file 1: Table S17).
Discussion
Based on 332,359 participants from the UK Biobank, our study provided robust evidence that most adverse psychological and trauma exposures were associated with an elevated risk of biological aging acceleration (10 out of 19 studied factors) measured by a newly developed biological aging acceleration index (LTL deviation > 0 or LTL deviation or LTL deviation > 1 SD) which takes into account genetic determinants of LTL shortening, and facial aging acceleration (10 out of 19), highlighting the substantial impacts of psychological and traumatic experiences on accelerating the aging process. Specifically, factors related to history of psychiatric disorders and childhood adversities were primarily linked with biological aging acceleration, whereas current psychological symptoms were noted for their pronounced associations with an excess risk of facial aging acceleration. More importantly, we identified five major psycho-trauma clusters based on clustering analyses, and further found that the cluster characterized by “ACEs with psychopathology” was associated with both biological and facial aging acceleration, in contrast to null results for the cluster featured by “ACEs without psychopathology.” Such findings imply that interventions targeting on psychological resilience to ACEs might have the potential to mitigate the detrimental effects of ACEs on the aging process.
In line with the results of previous meta-analyses investigating the association of LTL with depression [14] (n = 34,347), anxiety [15] (n = 19,424), stress-related disorders [26] (n = 3851), and substance misuse [27] (n = 7203) on LTL, our study also observed significant associations between a history of those psychiatric disorders and biological aging acceleration indexed by LTL deviation, based on a much larger sample size (n = 332,359). Likewise, significant negative associations between ACEs and biological aging acceleration (e.g., shorter LTL [17], older biological age [38], and DNA methylation-based epigenetic age acceleration [39]) have been reported, while existing data on adulthood or recent traumatic events are scarce. For facial aging acceleration, only one previous prospective cohort study of 1826 twins found that higher depression scores (i.e., emotion-related current psychological symptoms) were associated with an increased risk of self-reported facial aging acceleration [19]. Therefore, our study substantially extended the current evidence on the importance of various psycho-trauma factors, particularly current psychological symptoms and emotion-related ACEs, on facial aging acceleration. Recent major adverse experiences, such as serious illness, injury, or assault to a close relative or themselves, showed moderate impacts on facial aging acceleration but a very mild effect on biological aging acceleration. This fits findings based on a study of 3034 US residents aged 25 to 74, suggesting self-reported health condition decline would produce increases in subjective age [40]. However, financial difficulty experienced during the last 2 years seemed to be linked to facial aging acceleration among males. Sex-specific associations between certain SLEs (e.g., financial difficulty) and health adversities (e.g., cardiovascular disease and all-cause mortality [41]) were also found in prior investigations, possibly reflecting the differentiated social roles related to each sex.
The complexity of psycho-trauma adversities has been revealed [42]. For instance, individuals exposed to adverse childhood events were also prone to adversities during adulthood [43], both of which contributed to undermined physical conditions among exposed individuals [44]. Therefore, it is crucial, but challenging, to determine the patterns of psycho-trauma exposure for weighting the attributable risk to each individual factor on a specific outcome of interest. In the present study, with enriched data on psychological and trauma factors and well-developed unsupervised machine learning algorithms, we, for the first time, identified five major exposure patterns among our study population. In brief, more than two thirds (67.97%, 67,410/99,180) of the included individuals were considered to have total absence of trauma and psychopathology, while all the others suffered certain trauma and psychopathology. The most common exposure patterns are those combining traumatic experiences (mainly recent SLEs) along with a matching extent of psychopathology (e.g., patterns 2–3, including different levels of traumatic experience [recent SLEs and recent SLEs plus ACEs] and psychopathology [mild and severe]). Intriguingly, we additionally identified two clusters of individuals with similar ACEs exposure but opposite psychological condition (i.e., with and without psychopathology). The finding that an excess risk of biological and facial aging acceleration was only noted for the “ACEs with psychopathology” pattern but not “ACEs without psychopathology” pattern imply a strong mediating role of psychological distress on the effect of ACEs on the aging acceleration. Without any directly comparable data, a similar conclusion could be drawn from a Danish study of 324 male adults, which also found that 16% of the effect of ACEs on LTL shortening (i.e., biological aging acceleration) was mediated by current depressive symptoms [45]. As aging acceleration, particularly biological aging acceleration, is considered as a proxy of overall health status [46] and has been linked with many disease outcomes in later life (e.g., cancer [47] and cardiovascular diseases [48]), our findings (i.e., psychological distress might play a mediating role on the effect of ACEs on the aging acceleration) provide strongest evidence so far, prompting the use of psychological interventions for preventing adverse health consequences among individuals exposed to ACEs.
The potential mechanisms linking trauma and psychopathology to accelerated aging might include neuroendocrine responses through the hypothalamic–pituitary–adrenal (HPA) axis, oxidative stress, and inflammation [49]. The activation of the HPA axis by stress from trauma causes a surge of glucocorticoids [50] such as cortisol, leading to the generation of reactive oxygen species (ROS) by increasing the metabolic rate and mitochondrial activity [51]. Subsequently, ROS might damage telomeres and inhibit telomerase activity, both of which contribute to telomeres shortening [49]. Elevated glucocorticoids can increase the expression of pro-inflammatory genes (e.g., iNOS, IL-1β, TNF-α) and decrease the expression of anti-inflammatory genes including IL-1ra, IL-10, and MKP-1, also leading to telomeres shortening [49]. Additionally, the production of glucocorticoids, ROS, and inflammatory markers would destroy collagen and elastin in the skin, accelerating wrinkling and sagging and leading to facial aging [52]. Interestingly, it is reported that the alternations of HPA axis induced by stress actions after traumatic events [53] or psychopathology (e.g., depression [54] and anxiety [55]) are possibly reversible, which explains our findings of no accelerated aging among participants with childhood adversities but who maintained good psychological conditions.
The major merit of this study is the use of a large community-based cohort from the UK Biobank, providing data of comprehensive assessments on psychological and trauma-related factors (e.g., history of psychiatric disorders, current psychological symptoms, recent SLEs, and ACEs), social demographic factors, somatic comorbidities, and lifestyles. In addition, benefiting from the availability of individual-level genotyping data and measurement of telomeres, we proposed LTL deviation > 0 as an index of biological aging, which represented the presence of non-genetically determined proportions shortened LTL (i.e., non-genetically determined aging acceleration), thereby contributing to a more accurate measure of aging and better interpretation of results. Last, to account for the complex interactions between psychological and trauma-related factors, we used LCA to capture clusters with distinct psycho-trauma patterns. The cluster-based analyses provide clearer clues about the substantial contributing factors to the studied outcomes, as well as identifying individuals at high risk of aging acceleration who need targeted interventions.
Major limitations include its cross-sectional design. Although the studied trauma-related factors (such as ACEs and recent SLEs) ensure the chronological order (from exposure to outcomes of interest) by their definitions, the fact that data on most exposure factors and measurements of aging acceleration were collected at the same time provides a very weak basis for any causal inference. The inclusion of several covariates (e.g., smoking status and alcohol drinking status) that might mediate the impact of psycho-trauma related factors and clusters on aging acceleration resulted an underestimation of the studied association. In addition, despite the self-related facial aging acceleration have also been widely used in previous studies [56, 57], as an index for subjective aging [58] with proven associations with risk of mortality [59], the validity of such measurement remains uncertain. It is particularly notable as merely less than 2% of participants reported “looking older than the chronological age” in the present study. Furthermore, there are biases in the assessment of facial aging acceleration, which can be influenced by psychological and traumatic factors, making this measure less valid compared to biological aging acceleration in our study. Therefore, the results for facial aging acceleration analyses need to be interpreted with cautious, and studies which applied more objective or preferable approach for facial aging acceleration measurement (e.g., facial aging evaluated by nurses or other healthcare professionals [18]) are needed to verify our findings. Finally, the generalization of our findings to both the general UK population and other populations needs to be done with caution, as the UK Biobank participants were only 5.5% of the invited individuals [23].
Conclusions
In conclusion, our study demonstrates significant associations of psycho-trauma related factors and patterns with both biological and facial aging acceleration. The findings of aging acceleration among ACEs exposed individuals with psychopathology, but not those with psychopathology, underscore the potential of interventions aiming to improve individuals’ psychological resilience on preventing aging acceleration.
Availability of data and materials
Data from the UK Biobank are available to all researchers upon submitted application. All codes associated with the current submission are available and can be requested by contacting the corresponding authors.
Abbreviations
- LTL:
-
Leukocyte telomere length
- ACEs:
-
Adverse childhood experiences
- PRS:
-
Polygenic risk score
- GWAS:
-
Genome-wide association study
- LCA:
-
Latent class analysis
- ICD:
-
International Classification of Diseases
- SLEs:
-
Stressful life events
- CTS:
-
Childhood Trauma Screener
- LASSO:
-
Least absolute shrinkage and selection operator
- SNPs:
-
Single-nucleotide polymorphism
- BMI:
-
Body mass index
- TDI:
-
Townsend deprivation index
- CCI:
-
Charlson comorbidity index
- BH:
-
Benjamini-Hochberg
- FDR:
-
False discovery rate
- ORs:
-
Odds ratios
- HPA:
-
Hypothalamic-pituitary-adrenal
- ROS:
-
Reactive oxygen species
References
Collaborators GMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50.
Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, Druss B, Dudek K, Freeman M, Fujii C. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiary. 2017;16(1):30–40.
Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiary. 2014;13(2):153–60.
Wang Y-H, Li J-Q, Shi J-F, Que J-Y, Liu J-J, Lappin JM, Leung J, Ravindran AV, Chen W-Q, Qiao Y-L. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25(7):1487–99.
Van der Kooy K, Van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–26.
McManus S, Bebbington P, Jenkins R, Brugha T. Mental health and wellbeing in England: the adult psychiatric morbidity survey 2014. McManus S, Bebbington P, Jenkins R, Brugha T, editors. 2016. [cited 2024 Aug 28]. Available from: https://openaccess.city.ac.uk/id/eprint/23646/.
Bellis MA, Hughes K, Leckenby N, Perkins C, Lowey H. National household survey of adverse childhood experiences and their relationship with resilience to health-harming behaviors in England. BMC Med. 2014;12(1):1–10.
Oral R, Ramirez M, Coohey C, Nakada S, Walz A, Kuntz A, Benoit J, Peek-Asa C. Adverse childhood experiences and trauma informed care: the future of health care. Pediatr Res. 2016;79(1):227–33.
Treatment CfSA. Trauma-informed care in behavioral health services. 2014.
Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–8.
Calado RT, Young NS. Telomere diseases. New Engl J Med. 2009;361(24):2353–65.
Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H. Determinants of telomere length across human tissues. Science. 2020;369(6509):eaaz6876.
Schneider CV, Schneider KM, Teumer A, Rudolph KL, Hartmann D, Rader DJ, Strnad P. Association of telomere length with risk of disease and mortality. JAMA Intern Med. 2022;182(3):291–300.
Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: a meta-analysis. J Affect Disord. 2016;191:237–47.
Malouff JM, Schutte NS. A meta-analysis of the relationship between anxiety and telomere length. Anxiety Stress Coping. 2017;30(3):264–72.
Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol Bull. 2020;146(9):721.
Willis M, Reid SN, Calvo E, Staudinger UM, Factor-Litvak P. A scoping systematic review of social stressors and various measures of telomere length across the life course. Ageing Res Rev. 2018;47:89–104.
Christensen K, Thinggaard M, McGue M, Rexbye H, Aviv A, Gunn D, van der Ouderaa F, Vaupel JW. Perceived age as clinically useful biomarker of ageing: cohort study. Br Med J. 2009;339:b5262.
Rexbye H, Petersen I, Johansens M, Klitkou L, Jeune B, Christensen K. Influence of environmental factors on facial ageing. Age Ageing. 2006;35(2):110–5.
Agrigoroaei S, Lee-Attardo A, Lachman ME. Stress and subjective age: Those with greater financial stress look older. Res Aging. 2017;39(10):1075–99.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
UK Biobank. Health-related outcomes data. https://www.ukbiobank.ac.uk/enable-your-research/about-our-data/health-related-outcomes-data.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
UK Biobank. Data providers and dates of data availability. https://biobank.ndph.ox.ac.uk/showcase/exinfo.cgi?src=Data_providers_and_dates.
Codd V, Denniff M, Swinfield C, Warner S, Papakonstantinou M, Sheth S, Nanus D, Budgeon C, Musicha C, Bountziouka V. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging. 2022;2(2):170–9.
Li X, Wang J, Zhou J, Huang P, Li J. The association between post-traumatic stress disorder and shorter telomere length: a systematic review and meta-analysis. J Affect Disord. 2017;218:322–6.
Navarro-Mateu F, Husky M, Cayuela-Fuentes P, Alvarez F-J, Roca-Vega A, Rubio-Aparicio M, Chirlaque MD, Cayuela ML, Martinez S, Sanchez-Meca J. The association of telomere length with substance use disorders: a systematic review and meta-analysis of observational studies. Addiction. 2021;116(8):1954–72.
Manea L, Gilbody S, Hewitt C, North A, Plummer F, Richardson R, Thombs BD, Williams B, McMillan D. Identifying depression with the PHQ-2: a diagnostic meta-analysis. J Affect Disord. 2016;203:382–95.
Stein MB, Sareen J. Generalized anxiety disorder. New Engl J Med. 2015;373(21):2059–68.
Glaesmer H, Schulz A, Häuser W, Freyberger HJ, Brähler E, Grabe H-J. The childhood trauma screener (CTS)-development and validation of cut-off-scores for classificatory diagnostics. Psychiatr Prax. 2013;40(4):220–6.
Li C, Stoma S, Lotta LA, Warner S, Albrecht E, Allione A, Arp PP, Broer L, Buxton JL, Alves ADSC. Genome-wide association analysis in humans links nucleotide metabolism to leukocyte telomere length. The American Journal of Human Genetics. 2020;106(3):389–404.
Mak TSH, Porsch RM, Choi SW, Zhou X, Sham PC. Polygenic scores via penalized regression on summary statistics. Genet Epidemiol. 2017;41(6):469–80.
Townsend PPM, Beattie A. Health and deprivation. Inequality and the North. Health Policy (New York). 1988;10(207). https://doi.org/10.4324/9781003368885.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Linzer DA, Lewis JB. poLCA: An R package for polytomous variable latent class analysis. J Stat Softw. 2011;42:1–29.
Mori M, Krumholz HM, Allore HG. Using latent class analysis to identify hidden clinical phenotypes. JAMA. 2020;324(7):700–1.
Nagin D. Group-based modeling of development Harvard University press. Cambridge: Mass; 2005. https://doi.org/10.4159/9780674041318.
Mian O, Belsky DW, Cohen AA, Anderson LN, Gonzalez A, Ma J, Sloboda DM, Bowdish DM, Verschoor CP. Associations between exposure to adverse childhood experiences and biological aging: evidence from the Canadian Longitudinal Study on Aging. Psychoneuroendocrinology. 2022;142:105821.
Kim K, Yaffe K, Rehkopf DH, Zheng Y, Nannini DR, Perak AM, Nagata JM, Miller GE, Zhang K, Lloyd-Jones DM. Association of adverse childhood experiences with accelerated epigenetic aging in midlife. JAMA Netw Open. 2023;6(6):e2317987–e2317987.
Schafer MH, Shippee TP. Age identity in context: stress and the subjective side of aging. Soc Psychol Q. 2010;73(3):245–64.
Carlsson AC, Starrin B, Gigante B, Leander K, Hellenius M-L, de Faire U. Financial stress in late adulthood and diverse risks of incident cardiovascular disease and all-cause mortality in women and men. BMC Public Health. 2014;14:1–8.
Nelson CA, Bhutta ZA, Harris NB, Danese A, Samara M. Adversity in childhood is linked to mental and physical health throughout life. Br Med J. 2020;371:m3048.
Turner S, Harvey C, Hayes L, Castle D, Galletly C, Sweeney S, Shah S, Keogh L, Spittal M. Childhood adversity and clinical and psychosocial outcomes in psychosis. Epidemiol Psychiatr Sci. 2020;29:e78.
Kendall-Tackett K. Psychological trauma and physical health: a psychoneuroimmunology approach to etiology of negative health effects and possible interventions. Psychol Trauma. 2009;1(1):35.
Osler M, Bendix L, Rask L, Rod NH. Stressful life events and leucocyte telomere length: do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun Integr. 2016;58:248–53.
Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 2023;29(5):1221–31.
Dugué PA, Bassett JK, Joo JE, Jung CH, Ming Wong E, Moreno-Betancur M, Schmidt D, Makalic E, Li S, Severi G. DNA methylation-based biological aging and cancer risk and survival: Pooled analysis of seven prospective studies. Int J Cancer. 2018;142(8):1611–9.
Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk in Communities). Circ Genom Precis Med. 2018;11(3):e001937.
Lin J, Epel E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res Rev. 2022;73: 101507.
Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30(41):13690–8.
Chatelain M, Drobniak SM, Szulkin M. The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol Lett. 2020;23(2):381–98.
Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13(3):177–90.
Weiss SJ. Neurobiological alterations associated with traumatic stress. Perspect Psychiatr Care. 2007;43(3):114–22.
Tofoli SMdC, Baes CVW, Martins CMS, Juruena M. Early life stress, HPA axis, and depression. Psychol Neurosci. 2011;4:229–34.
Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Ex Med Biol. 2020;1191:141–53.
Millard LA, Munafò MR, Tilling K, Wootton RE, Davey Smith G. MR-pheWAS with stratification and interaction: searching for the causal effects of smoking heaviness identified an effect on facial aging. PLoS Genet. 2019;15(10):e1008353.
Zhan Y, Hägg S. Association between genetically predicted telomere length and facial skin aging in the UK Biobank: a Mendelian randomization study. Geroscience. 2021;43(3):1519–25.
Kleinspehn-Ammerlahn A, Kotter-Grühn D, Smith J. Self-perceptions of aging: do subjective age and satisfaction with aging change during old age? J Gerontol B Psychol Sci Soc Sci. 2008;63(6):P377–85.
Rippon I, Steptoe A. Feeling old vs being old: associations between self-perceived age and mortality. JAMA Intern Med. 2015;175(2):307–9.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application 54803. This work uses data provided by patients and collected by the NHS as part of their care and support. This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation. We thank the team members of the West China Biomedical Big Data Center for Disease Control and Prevention for their support.
Funding
This work was supported by 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant no. ZYYC21005 to HS), 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (grant no. 2022HXFH029 to CQ), the Department of Science and Technology of Sichuan provincial government (grant no. 2022YFS0345 to CQ), Sichuan Science and Technology Program (grant no. 2024NSFSC1574 to JW), and the National Natural Science Foundation of China (No. 8230051978 to XH). The funders had no role in study design, data collection, preparation of the manuscript, and decision to publish.
Author information
Authors and Affiliations
Contributions
HS, CQ, and JW were responsible for the study’s concept and design. JW and HY were responsible for data and project management. JW, XH, YY, and YZ performed the data cleaning and analysis. JW, XH, HS, and CQ interpreted the data. JW, XH, YY, YZ, YQ, HY, JS, CQ, and HS drafted and revised the manuscript. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @JoyLab14169974 (Joy Lab).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274) and the informed consent was obtained from each participant before data collection. The present study was also approved by the biomedical research ethics committee of West China Hospital (reference number: 2019–1171).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3578_MOESM1_ESM.docx
Additional file 1: Figure S1. Sex-specific odds ratio of the psychological and trauma-related factors with biological aging accelerationand facial ageing acceleration. Table S1. The coding book. Table S2. International Classification of Diseasescodes for the history of psychiatric disorders. Table S3. The cut-off values of the Childhood Trauma Screener–5 items. Table S4. Educational attainment measures. Table S5. The Fitting of the latent class analysis model with different numbers of clusters. Table S6. The missing proportion of psychological and trauma related factors among the included participants who fully completed the online questionnaire. Table S7. The description of the psychological and trauma-related factors among the identified potential mental health clusters. Table S8. The association of the psychological and trauma-related factors with biological aging accelerationand facial ageing acceleration. Table S9. Sensitivity analyses for the association of the psychological and trauma-related clusters with biological aging acceleration by using the presence of LTL deviation more than 1SD and LTL deviation as a continuous variable. Table S10. Sensitivity analyses for the association of the psychological and trauma-related clusters with biological aging acceleration using the presence of LTL deviation more than 1SD and LTL deviation as a continuous variable. Table S11. Sensitivity analyses for the association of the identified psychological and trauma-related clusters with aging acceleration by conducting 10 times of multiple imputation. Table S12. The association of the psycho-trauma related factors with biological and facial aging acceleration when adjusting for different covariates in the logistic and linear regression model. Table S13. The association of the psycho-trauma related clusters with biological and facial aging acceleration when adjusting for different covariates in the logistic and linear regression model. Table S14. The association of the psycho-trauma related factors with biological and facial aging acceleration whether or not TDI was considered as a cluster variable in the logistic and linear regression model. Table S15. The association of the psycho-trauma related clusters with biological and facial aging acceleration whether or not TDI was considered as a cluster variable in the logistic and linear regression model. Table S16. Sex-specific association of the identified psychological and trauma-related clusters with biological aging acceleration. Table S17. Sex-specific association of the identified psychological and trauma-related clusters with facial aging acceleration.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Han, X., Yang, Y. et al. The association of psychological and trauma-related factors with biological and facial aging acceleration: evidence from the UK Biobank. BMC Med 22, 359 (2024). https://doi.org/10.1186/s12916-024-03578-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03578-7