Abstract
Although stress and adversity are largely universal experiences, people exposed to greater hardship are at increased risk for negative health consequences. Recent studies identify accelerated biological aging as a mechanism that could explain how trauma and adversity gives rise to poor health, and advances in this area of study coincide with technological innovations in the measurement of biological aging, particularly epigenetic profiles consistent with accelerated aging derived from DNA methylation. In this review, we provide an overview of the current literature examining how adversity might accelerate biological aging, with a specific focus on social and health behaviors. The most extensive evidence in this area suggests that health-compromising behaviors, particularly smoking, may partially explain the association between adversity and accelerated aging. Although there is relatively less published support for the role of social behaviors, emerging evidence points to the importance of social connection as a mechanism for future study. Our review highlights the need to determine the extent to which the associations from adversity to accelerated aging are consistent with causal processes. As we consider these questions, the review emphasizes methodological approaches from the causal inference literature that can help deepen our understanding of how stress and trauma might result in poor health. The use of these methodologies will help provide evidence as to which behavioral interventions might slow aging and improve health, particularly among populations that more often experience adversity and trauma.
Similar content being viewed by others
Introduction
In the 87 years since Hans Selye first integrated the concept of stress into the study of health [1,2,3], a wealth of empirical research now demonstrates that people who experience stress and adversity are at greater risk of illness and premature death [4,5,6,7]. Findings in this area link a wide array of stress and adversity exposures with numerous health outcomes that span disease states, organ systems, and the human lifespan [8, 9]. For example, compared to people who report less stress, people under greater stress are more likely to develop respiratory illnesses when exposed to the same viruses [10]. Similarly, people with greater stress show slower healing than less stressed people with the same wounds [11,12,13]. At a population health level, widowed adults are twice as likely to die during the week following their spouse’s death and almost three times as likely to develop heart disease [14, 15]. Despite convincing evidence that these associations are robust, identifying the biological processes that explain how adversity impacts the body has proven challenging. What psychological and behavioral processes activate or maintain disease-relevant physiology among people exposed to high levels of stress? Over the last decade, emerging empirical evidence points to biological aging as a multisystem physiological mechanism that could help explain how adversity translates to ill health, and the latest work in this area builds on critical stress-health associations observed over the last century.
Advances in the study of biological aging have coincided with a developing area of research focused on the study of health and aging, termed geroscience [16,17,18]. A key theme within geroscience is that aging is the strongest correlate of chronic disease and premature death [19]. In this framework, slowing aging—specifically the aging of the body, termed biological aging—has the potential to reduce the risk for disease and death across multiple organ systems, reducing disability and improving longevity as people grow older chronologically [19,20,21,22,23]. The value of biological aging as a mechanism linking stress to health is, in part, due its disease-agnostic nature, which better matches myriad published associations between stress and disease. This review summarizes the current literature linking stress, trauma, and adversity to biological aging, with a specific focus on behaviors that might explain this association, specifically social and health behaviors. We conclude by highlighting the limitations of the current literature linking adversity to biological aging and opportunities to move this area of research toward stronger causal inference that can ultimately support future theoretical and intervention advances.
The many metrics of biological aging
Biological aging refers to the progressive deterioration of physiological systems and functions that are required for survival [24]. Chronological age—that is, chronological time since birth—is not the same as biological age, and a major theme in modern geroscience is in identifying and studying heterogeneity in aging profiles and health decline [25,26,27]. Because many chronic diseases—e.g., cardiovascular disease, metabolic diseases, neoplastic diseases, and Alzheimer’s disease—are more common as people age chronologically, the search for markers of accelerated biological aging has garnered considerable scientific attention [28]. Work in this area includes but is not limited to, assessing changes in genomic stability and DNA methylation (DNAm), cell senescence, and mitochondrial functionality [28]. Telomeres, proteins protecting chromosome ends [29, 30], were among the earliest biomarkers of aging, and telomere length plays an important role in cellular senescence [31], with the shortening of telomeres reflecting accelerated biological aging. Leukocyte telomere length is associated with all-cause mortality [32], risk for cardiovascular disease [33], and risk for Alzheimer’s disease [34]. Importantly, psychosocial stressors are believed to play a role in accelerating telomere shortening via sympathoadrenal, neuroendocrine, and/or immune-inflammatory mechanisms that heighten oxidative stress and damage telomere ends [35]. Other metrics have proven useful in operationalizing biological aging, including the development of the pace of aging [36] and other equations [37, 38] that use biomarkers spanning multiple organ systems to characterize the coordinated deterioration of physiological function. For example, Belsky et al. [36] developed, refined [39], and validated a pace of aging metric in the Dunedin Study birth cohort across 19 biomarkers. They showed that the pace of aging for individuals in this cohort—all born in the same year and thus the chronological age since birth—was associated with cognitive and physical health decline in midlife.
As geroscience became increasingly interested in the dissociation between chronological and biological aging, technical advances in molecular sequencing technologies [27], especially the quantification of epigenetic DNAm, have led to the emergence of a series of methylation clocks [40, 41]. In brief, the extant clocks and methylation-based pace of aging metrics are based on DNAm of cytosines at CpG dinucleotides, which has direct relevance for RNA gene expression [28, 42]. Changes in epigenetic mechanisms are considered a hallmark of aging biology [17], though given the use of machine learning approaches to derive methylation measure algorithms, it is unclear whether epigenetic measures of aging are directly accessing biological aging or indirect, but useful, biological correlates [43].
Although it is beyond the scope of this review to detail all of the available epigenetic clocks (interested readers can see recent reviews, [41, 44]), we can summarize key trends in the literature as follows: (1) Following a series of seminal papers in 2013 [45, 46], several DNAm clocks emerged in the literature, many of which were trained to predict chronological age [27]; (2) Although many of the different clock metrics are correlated with each other and other aspects of biological aging, these correlations are often low and suggest that each clock measures different aspects of biological aging [44]. The first generation metrics, born from machine learning algorithms designed to predict chronological age, do not have clear links to the biology that underpins aging, which led to the development of second-generation clocks designed to more fully capture the functional changes associated with biological aging [47], some of which are specifically designed to predict mortality risk [48,49,50]. Most recently, epigenetic measures of aging have emerged that were directly trained on longitudinally assessed measures of biological aging, such as DunedinPACE [42], resulting in a third-generation of clocks—or perhaps a new category of DNAm measure altogether. These DNAm measures are increasingly used to predict mortality risk and healthspan [42, 51]. The ability to assess biological aging at a single point in time using DNAm, which would typically require costly and time-consuming longitudinal assessment, has allowed for a host of new social and behavioral research linking biopsychosocial predictors to health via accelerated aging.
The many dimensions of adversity and associations with accelerated aging
Adversity can be conceptualized a number of ways, spanning the type, timing, and duration of a stressful experience. The measurement of stress and adversity can include the assessment of exposure to stressful events or situations, as well as people’s subjective experiences of adversity, typically by measuring their perceptions of stress. Stressful events are measured in a variety of ways. One common method is counting the number of stressful experiences an individual has experienced, such as the number of adverse childhood experiences (ACEs; [52, 53]) or stressful life events [54,55,56]. More recently, there have been calls for integrative and detailed assessment of stressful events [57] to better capture the range and timing of stressors beyond counts [58, 59]. Despite measurement challenges, ACEs (abuse, neglect, and household dysfunction experienced by children) and stressful life event counts are linked to accelerated aging [55, 60, 61]. For example, experiencing common stressful life events is associated with changes in biological aging among adolescents [62]. However, stress constructs that assess specific events should not be considered exhaustive. Other common adversities linked to biological aging include growing up in socioeconomic disadvantage [63, 64], membership in a racial or ethnic minority group [65, 66], and discrimination [67]. For example, African American adolescents who had higher levels of perceived discrimination showered faster biological aging if they did not have a supportive family environment [68]. These broader measures of chronic adversity almost certainly play an important role in modulating the experiences of stress and aging across the lifespan.
A subset of stressful events—i.e., traumas, criterion A traumas, or traumatic events—are defined as particularly harmful and have the potential to cause posttraumatic stress disorder, a collection of symptoms related to re-experiencing traumatic events, avoiding reminders of trauma, and experiencing changes in cognition, mood, and arousal. The hyperarousal symptoms that contribute to the diagnosis of PTSD correlated with dysregulations in physiology, including changes to cardiovascular physiology and inflammation [69,70,71,72] and poor health. More recent work finds that the experience of trauma [73,74,75], as well as the development of PTSD [75, 76], are linked to accelerated aging. For example, in a sample of 339 trauma-exposed veterans, higher levels of PTSD hyperarousal symptoms were associated with high DNAm biological age [77]. Notably, associations of trauma and PTSD with accelerated aging span multiple methods of assessment. People with PTSD show accelerated aging as assessed by functional tests [78], telomeres [79], pace of aging [55], and epigenetic measures of aging (i.e., DNAm; [71, 73]).
The experience of stress and trauma can be contrasted with measures of adversity that seek to capture people’s subjective experiences of stress. The same event experienced by different people can have a different impact on health depending on individual characteristics, including genetic vulnerability to stress, personality, or availability of psychological services. Widely used measures like the Perceived Stress Scale [80] seek to capture people’s subjective feeling of stress and show consistent associations with biological aging [55, 81,82,83,84]. Stress perceptions are typically self-reported and have the advantage of being cost-effective and simple to administer. Notably, both stressful event counts and subjective perceptions of stress can vary across developmental periods with different relevance to aging. There is a necessary component of timing and duration implicit within the experience of adversity or stressful events, both in terms of acuity versus chronicity [85], and the specific timing within human development (e.g., spanning infancy, childhood, adulthood, midlife, and older age). Different rates of aging and epigenetic outcomes have been linked to adversity across many periods of the human lifespan [86,87,88], including slowed prenatal development of the central nervous system [89, 90], accelerated aging in childhood [86], and different rates of biological aging among older adults [91].
Behavioral mechanisms linking adversity to accelerated aging
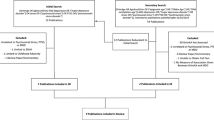
It is widely known that early adversity and childhood stress are associated with poor distal health outcomes [92, 93], as is the experience of chronic psychological stress [8]. Although it is certainly the case that the experience of stress and trauma can have direct, dysregulatory effect on physiological systems in a manner that accelerates biological aging (cf. [94,95,96,97,98,99,100,101,102,103,104]), it is also likely that intermediate behaviors play a role in the causal chain from adversity to accelerated aging and poor health. Figure 1 provides a process model framework for understanding the ways in which stress, adversity, and trauma may portend behavioral changes that accelerate biological aging. Although it is possible to prevent some trauma and adversity from occurring or to reduce stress responses through treatment, it is impossible to prevent all stress and adversity. Identifying potentially health-relevant sequelae of adversity that could accelerate aging would present additional mechanistic targets for mitigating the adverse consequences of the stressful experiences. Here we present current evidence for two broad classes of potential mechanisms of action: social connectedness and health behaviors. Following Fig. 1, our review of the extant evidence on these topics is framed in terms of what we know about (a) the associations between adversity, stress, trauma and behavioral mechanisms, and (b) the associations between social/health behaviors and accelerated biological aging. We posit that changes in these potential mediating processes play a role in sculpting physiological responses that accelerate biological aging. In short, stress, adversity, and PTSD often alter our social relationships and health behaviors. High stress may have causal effects on social withdrawal and/or portend relationship conflict; it may disrupt sleep, and people may smoke to manage the adverse emotional experiences of the stress itself. In turn, these behaviors organize changes in physiological functioning that may alter genomic signaling in a way that contributions to illness and disease progression.
A Conceptual pathway linking adversity to health through changes in health behaviors, social behaviors, and subsequent changes in biological aging. B Illustration of how adversity could influence the rate at which a person ages biologically by increasing the likelihood of unhealthy behavior change, which accelerates biological aging and hastens progressions towards, disease, disability, and death. C Specific illustrative examples of molecular mechanisms shown to link health behaviors to biological aging in the brain, heart, and lungs.
Social connection and disconnection
Social connectedness is a multifactorial umbrella term that represents the degree to which people are and perceive themselves to be embedded in nurturing social relationships [105]. Included under the social connectedness umbrella are structural elements of social network properties (e.g., isolation or living alone) and social roles (e.g., marital status), functional components (including perceived social isolation, which is the definition of loneliness), and the quality of these close relationships (see [106]). The available evidence indicates that adversity, stress, and trauma are highly correlated with disrupted social relationship functioning in adulthood. Early social adversity plays a critical role in shaping infant and adult attachment orientations, which organize the mental models people hold about the reliability and trustworthiness of others in close relationships [107, 108]. Childhood maltreatment is a significant risk factor for the perpetration of intimate partner violence [109]. Socioeconomic adversity in childhood is a strong predictor of relationship dissolution in young adulthood, with evidence indicating early socioeconomic adversity predicts relationship dissolution directly as well as indirectly through interpersonal conflict and low levels of future orientation [110]. The available evidence also suggests that PTSD is highly associated with poor relationship functioning and increased risk for divorce [111,112,113], and this appears to be especially true for combat-exposed veteran populations [114]. In addition, meta-analytic and experimental evidence has indicated that PTSD both predicts and is predicted by low levels of social support [115, 116]. For example, Bourassa and colleagues found that exposure therapy increased perceived social support among active duty solders with PTSD in an experimental trial [116]. Finally, recent evidence suggests that, at the level of the US population, loneliness increased during the COVID-19 pandemic in relation to pre-pandemic levels [117], and these disturbances in social functioning are hypothesized to play a role in shaping pandemic-related increases in mental health disorder [118].
Although the associations between adversity, stress, trauma and social relationship functioning are well established, the literature on social relationship functioning and accelerated biological aging is relatively nascent. When compared to married adults, those who are separated or divorced evidence shorter salivary and leukocyte telomere length [119, 120]. Interestingly, telomere shortening does not appear to mediate the elevated risk between marital status and cardiovascular disease mortality [119]. Relatively new research from the Health and Retirement Study (HRS) suggests that perceived social support from and contact with close others, especially close friends, were prospectively associated with slower pace of aging (assessed via DunedinPoAm38) and lower GrimAge, two indices of epigenetic (DNAm) age acceleration [121]. Independent analyses with the HRS data provide a conceptual replication of these findings and find that the absence of marital and friend relationships are related to an older GrimAge and faster pace of aging [122]. The inability to establish peer relationships characterized by autonomy and relatedness in adolescence is also associated with older GrimAge at age 30 [123], and these variables accounted for nearly 8% of the variance in epigenetic age acceleration over and above variance accounted for by cigarette smoking and demographic factors. In a unique, but small (N = 40), study of work-related trauma exposures among paramedicine students, GrimAge acceleration was significantly lower among participants who reported increased social support at baseline and follow-up [124]. In a longitudinal study of African American adolescents in rural Georgia, Brody and colleagues [66] found that high family support moderated the relationship between experiencing racial discrimination and epigenetic age acceleration (at ages 20 and 22 years). Adolescents from high support families who experienced high and stable discrimination showed comparable age acceleration to people not experiencing discrimination, whereas those adolescents experiencing discrimination and having a low support family showed up to 2 years of epigenetic age acceleration on a first-generation DNAm clock. A family-based intervention research with the same cohort showed reductions in harsh parenting in the treatment condition was associated with reduced epigenetic age acceleration [67]. This finding, in particular, supports the hypothesis that social relationship functioning can serve as a critical mechanism linking life stress and biological age acceleration.
Health behaviors
Health behaviors present some of the most plausible mechanisms that could explain how adversity accelerates biological aging, with extensive empirical support for the associations between adversity, unhealthy behaviors, and accelerated aging. Evidence for these associations exist across an array different adversities, unhealthy behaviors, and measures of aging across the lifespan. For example, childhood adversity is linked to unhealthy behaviors in midlife [125], as well as to smoking, physical inactivity, risky sexual behavior, problematic alcohol use, and problematic drug use in a meta-analytic framework [126]. The association between adversity and unhealthy behavior extends to later periods of life as well. Adults who report experiencing more stressful life events or higher levels of perceived stress are more likely to also smoke [127,128,129], have poor diet [130, 131], or be physically inactive [132, 133]. Older adults who experience interpersonal stressors, such as divorce or widowhood, are also more likely to be physically inactive or smoke [134,135,136]. Adversity is associated with less engagement with health protective behaviors as well—people exposed to more stress and adversity are more likely to endorse vaccine hesitancy [137] and may delay preventative healthcare [138, 139]. The broad associations between adversity and unhealthy behaviors also extend to trauma and PTSD. People with PTSD are at greater risk of smoking, physical inactivity, poor diet [140], and engaging in risky behaviors [140,141,142], though there is some evidence that these associations are explained by depressive symptoms [143]. The health behavior of sleep is notable as a possible link between PTSD and accelerated aging. Sleep disturbance is one of the central features of PTSD [144] and is associated with both PTSD [145,146,147] and stress, broadly speaking [148]. Notably, PTSD treatment improves sleep outcomes [149], suggesting an element of reversibility of this risk with efficacious PTSD treatment.
Health behaviors are perhaps the most robust predictors of biological aging, with the expectation that unhealthy behavior leads to poor health through accelerated aging, at least in part. Accelerated aging is associated with heavy alcohol use [150, 151], cannabis use [152], diet [153,154,155,156,157], physical activity [153, 158], and disturbed sleep [159]. Summarizing the extensive literature linking biological aging and health behaviors—even if limited to epigenetic measures of biological aging—is beyond the scope of this review. However, two points are notable. First, the associations between health behaviors and epigenetic measures of aging have initial evidence of reversibility. For example, Waziry and colleagues [156] found that caloric restriction was experimentally associated with slowed epigenetic aging in the CALERIE study, though the degree to which these changes are associated with improved long-term health is unknown. Evidence that changes in health behavior result in changes in aging measures would represent gold-standard evidence as to the most promising targets for future interventions attempting to slow aging among people experiencing adversity. Second, there is an extensive body of empirical evidence linking health behaviors to accelerated aging focused on smoking [50, 102,103,104, 160,161,162,163]. This includes evidence of broad associations [103, 160, 161], as well as changes in tissue specific to the lungs [102, 162]. For example, a recent study using the Health and Retirement Study found that lifetime history of exposure to cigarette smoke was associated with multiple epigenetic measures of aging, which helped explain the link between smoking and the development of chronic diseases and premature mortality [163]. Indeed, the association between smoking and biological aging is so well established that it is common for researchers to control for smoking status in studies or even in the creation of methylation clocks themselves. For example, methylation-derived pack years are included in the GrimAge clock’s calculation [50], due to the clear association between smoking and mortality risk. This creates a conundrum when studying stress, adversity, and accelerated aging; namely, smoking is likely both on the causal pathway linking adversity to aging [129], directly associated with changes in methylation (Fig. 1), and in some cases, included as part of the outcome being assessed [50]. Figure 2 illustrates how biological aging could change over time based on changes in smoking behavior related to adversity experienced across the lifespan. It is notable, however, that several studies find that indicators of stress and adversity, including PTSD [74, 76], remain associated with accelerated aging when controlling for smoking, suggesting that while smoking might help explain the association between adversity and aging, there are likely other mechanisms of action that require future study.
Incorporating the science of causal inference into the study of adversity and accelerated aging
Although the associations between early adversity, psychological stress, trauma, and accelerated biological aging are well established, much of the work in this area is correlational; indeed, the same can be said for most of the research on the putative social and health behavior “mechanisms” that link these constructs. One of the main limitations—and areas of opportunity—in the study of adversity and aging is the lack of more rigorous studies that can speak to causal processes. Experimental studies and randomized controlled trials, of course, remain the gold standard for assessing the causality of adversity and associations with age acceleration (or any other health, health-relevant, or disease biomarker). As we discuss below, interventions can target the psychological consequences of adversity, or they can directly target the mechanisms that link life stress exposures to accelerated biological aging (see Fig. 1). With relatively few exceptions [67], however, this research has yet to be conducted in humans. Nevertheless, there exists a promising suite of tools for making better correlational inferences when relying on observational data [164,165,166,167,168,169,170], and our contention is that research on adversity and accelerated biological aging will benefit enormously by using these tools (cf., [171]). Table 1 includes several examples of the methods and potential applications to questions of causal inference in the study of adversity and accelerated biological aging.
Two core themes in this literature are relevant to the current review. First, confounding is endemic in observational research and cannot be (fully) addressed by covariate adjustment alone [172,173,174]. Central to this theme is the idea that life event exposures are non-random, and any effort to understand the putative consequences of early adversity, mid-life discrimination, or combat-related trauma must contend with the individual differences and demographic variables. These individual characteristics almost certainly play a role in shaping who is exposed and how often they are exposed to stressful events, the impact of these stressors on behavior and functioning, and the potential biological age acceleration alleged to result from adversity. Second, all statistical methods make certain assumptions and these assumptions limit causal inference conclusions that can be drawn in any area of study. Consequently, researchers are increasingly calling for the triangulation of methodological and statistical approaches when dealing with questions of causal inference [175, 176]. For example, recent research on the effects of smoking on suicidal ideation and attempts compared the results of between-family analyses to those following Mendelian randomization (MR) analyses, which is an effective method for evaluating residual confounding and potential reverse causation [177]; importantly, the observational/correlational analyses showed a strong positive association between the constructs, but the quasi-causal MR analyses showed no evidence for a causal effect. (It is important to keep in mind that any interpretation of these findings depends on the quality of the MR analysis. As a general conceptual framework, triangulation depends on the strength of the data that is informing the causal inference strategy. In other words, efforts toward triangulation depend not only on the assumptions of all the triangulating approaches (a broad feature of this work), but also the quality of the data available in a particular study (a specific limitation in the application of the method).
It is common for different methodological approaches to yield dissociated findings (and this is especially true when research incorporates analyses from between- and within-family models, see [168, 178, 179]) and triangulating across different approaches is a key element of scientific falsification [180, 181], the powerful idea that scientific advances unfold not through the slow accumulation of evidence in favor of an association of interest, but instead the inability to rule-out the plausibility of the association. Statistical methods that make different assumptions can provide increased opportunities for falsification, which can increase confidence in the robustness of a result. Alternatively, a lack of consensus across methods and the accumulation of discrepant findings during triangulation can provide the initial evidence that a single scientific paradigm is insufficient for solving the scientific question at hand [182], leading to new discoveries that challenge the status quo. When combined with modern causal inference analytic procedures (see Table 1), triangulation is a powerful conceptual tool for identifying causal effects. Importantly, the methods outlined in Table 1 are complementary—alone, none have the power to rule-out a causal effect and each method has a set of biases and assumptions; but when used together via triangulating approaches, especially in cases where RCTs are not possible, these methods are powerful.
What methods might be most helpful in moving the study of adversity and accelerated biological aging toward greater confidence in causal processes? We detail three approaches with the potential to help advance our understanding of this topic: RCTs targeting mechanisms of action, longitudinal mediational analyses, and twin studies.
Randomized controlled trials (RCTs) targeting mechanisms of action
The NIH Science of Behavior Change (SOBC; https://commonfund.nih.gov/behaviorchange) initiative was designed to move intervention science from a broad implementation of treatment packages (for health behavior change) to a focus on targeted mechanisms of action [183, 184]. Key components of this work involve showing that (a) interventions can engage those targets (referred to as target engagement), (b) that changes in these mechanistic targets yield changes in the outcomes of interest (referred to as target validation)—terms with origins from pharmaceutical development and the broad field of experimental medicine (see [185]). Related to the topic of adversity, trauma and accelerated aging, the goal of experimental research would be showing that targeting social relationship functioning or health behaviors could then slow biological aging. For example, among combat-exposed veterans with diagnosed PTSD, can cognitive behavioral therapy for insomnia (CBTi) improve sleep and are improvements in sleep quality or duration associated with differences in accelerated aging? These interventions might be bundled with other treatment strategies, including those that focus on social functioning and/or medication, within the context of factorial experimental designs [186]. As the gold standard, increased use of RCTs seems a promising next step in the use of epigenetic aging measures.
Despite the promise of RCTs, it is important to note that using epigenetic aging outcomes in geroprotective trials has limitations, including questions about the reliability of measurement and periods required to observe meaningful changes in biological aging. Current epigenetic measures show variability in reliability of measurement that would make RCTs more challenging. For example, Higgins-Chen and colleagues reported that using principal-component (PC) adjusted clocks results in around 1.5 years of deviations between replicates [187]. Although an improvement over the 9 years found in non-PC adjusted clocks, this level of variability remains a barrier to detecting therapeutic effects. Similarly, the ideal timeline to detect clinically- or statistically-relevant change in epigenetic measures of aging is unclear, and any measurement strategy must be timed to capture the precise resolution of the causal change process [188]. For clock metrics trained on mortality, multiple years might be necessary to detect reliable change, limiting the value of RCTs that follow participants for under a year. Measures trained on physiological aging (e.g., DunedinPACE), might produce reliable change over a shorter period, however, this remains to be tested directly. These challenges are not unique to RCTs, but would make these studies challenging to conduct successfully given the cost and time involved in clinical trials. However, there is the potential that new clocks or PC approaches might produce measures that are more reliable in the future. If RCTs routinely collect methylation data, it would allow for future reanalysis of trials. Regardless of the ultimate method, linking changes in social and/or health behavior to changes in biological aging through RCTs or some other form of experimental intervention study would be critical to support theory and future intervention research aiming to slow biological aging.
Pursue longitudinal mediational studies
Longitudinal mediation refers to models that help interrogate the extent to which intervening variables, termed mediators, explain the observed association between a predictor and outcome [189]. The most rigorous mediation models use multiple assessments of the relevant variables and can provide evidence as to the causal pathways that might link a broad risk factor, such as adversity, with aging or health outcomes via putative mechanisms of action. For example, a longitudinal cohort that assessed PTSD, perceived social support, and epigenetic aging at multiple occasions would allow researchers test whether people who develop PTSD evidence decreases in their perceived social support, which in turn predict accelerations in epigenetic measures of aging. Such approaches are particularly valuable in modeling change in mediators and outcomes that help overcome issues of temporal precedence and the directionality of associations. Although some studies are beginning to examine changes in aging at multiple occasions, much of the recent biological aging research has been limited to cross-sectional associations. As more longitudinal epigenetic data becomes available in longitudinal cohorts and laboratory studies, conducting more mediation analysis become feasible. For example, Wolf and colleagues recently found that GrimAge mediated the association between externalizing psychopathology and inflammatory phenotypes in a sample of 214 trauma-exposed military veterans [190]. Mediation analyses that examine whether a putative casual factor that explains the associations between exposure and outcome can serve as a valuable first step in determining future intervention targets. The use of longitudinal mediation models, particularly with multiple assessments of aging, health behavior, and social connection, would provide more stringent evidence as to how adversity might affect aging, albeit with limited ability to make specific causal claims.
Take advantage of cotwin control designs
RCTs and longitudinal mediation are highly time and resource intensive study designs. As we note above, one of the challenges that emerges outside of RCTs is confounding; for example, it is quite possible that neuroticism is associated with both stress, adversity, and trauma exposures, as well as poor sleep and increased likelihood of smoking, all of which are associated with accelerated biological aging. Cotwin control designs [191, 192] are uniquely suited to examine genetic confounding. Applied to the example above, genetic confounding would operate when the genetics of smoking, for example, account for the phenotypic association between smoking and accelerated aging. Cotwin designs leverage the genetic relatedness of twins to create a counterfactual situation [193]. Because monozygotic twins share a genotype, a biometric decomposition that compares twins who are and are not exposed to a specific life event (or, who are differ quantitatively in their responses to life event exposures) allows research to control for genetic and shared environmental influences that may confound the exposure/outcome association. In this sense, cotwin designs provide a powerful falsification methodology that operates in many ways akin to a hierarchical regression analysis: After accounting for shared genetic and environmental influences between a putative exposure and outcome, do within-pair differences in twins’ exposure remain significantly associated with the outcome in question [191]? Figure 3 provides an illustration of a biometric cotwin model examining whether (within-pair) differences in stress exposure or life adversity are associated with metrics of accelerated biological aging (see Table 1 as well). This model specification allows us to examine whether an intrapair difference in a predictor variable (including, for example, discordance in PTSD diagnoses or quantitative differences in perceived stress) with an intrapair difference in a metric of epigenetic age acceleration. If the observed (i.e., phenotypic) association between the intrapair differences in X and Y remains significant after accounting for genetic and shared environmental confounds, the findings are consistent with a causal relationship and the estimated effect size of the association will be less biased because two major sources of confounding are attenuated/removed [191].
Using causal inference to advance theory and treatment
How would the use of casual inference advance our knowledge of how stress, trauma, and adversity might accelerate biological aging? The value of causal inference is tied to the aims of the research program in question. Most studies of biological aging and adversity present statistical associations without the ability to make causal claims, however the objective of these findings are generally focused on one of two long-term targets— (1) better understanding the causal pathway that might link adversity to accelerated aging and poor health, i.e., theory, or (2) clinical interventions that are attempting to slow aging and improve health for people affected by high levels of stress, adversity, or trauma, i.e., prevention and treatment. Said differently, some programs of research aim to “carve nature at its joints,” whereas others are more agnostic about the reasons for causality and instead are focused on slowing aging in clinical settings. In the case of explicating the potential causal pathway from adversity to poor health, studies that are limited to associations, and particularly cross-sectional associations, cannot move the field towards causal inference. As stated above, statistical controls and temporal ordering can help limit alternative explanations for observed associations, but there are limits to how close observation studies can cleave to causal inference. Similarly, animal studies have an important role to play in the study of adversity and aging [193, 194], though drawing inferences from animal models comes with its own challenges [194, 195]. As a result, it is critical to move beyond findings that show broad associations between adversity and biological aging in humans if we wish to determine how adversity might cause more rapid aging and downstream health outcomes. Without using more sophisticated methods, describing the causal pathways from stress to health will remain challenging.
In the case of supporting interventions aiming to slow aging to improve health, the use of causal inference might appear less critical. Any association between stress and aging, or putative mediators, presents a plausible intervention target that might then be tested using RCTs as the gold standard methods of causality. The translational pathway—from observing broad risk, to evidence of mediation for plausible mechanisms, to testing interventions in an RCT framework—is defensible in the abstract and might achieve the stated goal of determining how to slow aging. However, it is notable that relatively few prospective RCTs have examined whether interventions slow aging generally (for an example, see the CALERIE trial, [156]), and fewer still among people who have experienced adversity or trauma. Although this may reflect the nascent nature of the area of research in this area, which might change as epigenetic measures of aging present a more realistic surrogate endpoint for use in RCTs, the cost and difficulty with running RCTs will remain, particularly given concerns with reliability and timeline when assessing epigenetic measures of aging [187]. Mechanisms of slowing aging that have evidence more closely approaching causal inference would presumably have a higher likelihood of a successful trial. Such evidence could be more convincing to the stakeholders necessary to fund and conduct aging interventions, including granting agencies, medical system administrators, and the clinicians delivering the interventions.
Conclusions and future directions
In this review, we have briefly summarized the state of the literature linking adversity to accelerated aging, including two behavioral mechanisms that might explain this association, social connection and health behaviors. Given the existing literature, there is a need to use rigorous methodologies to move observational studies stress and aging closer towards causal inference. The use of RCTS, longitudinal mediation models, and cotwin control designs, for example, would help provide more confidence that putative mechanisms—such as social and health behaviors—explain how adversity is linked to accelerated aging. This evidence would provide a stronger theoretical understanding of the causal pathways from adversity to accelerated aging and poor health, which would support future intervention efforts to slow aging and improve health among people who experience stress, trauma, and PTSD.
References
Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. https://doi.org/10.1038/138032a0
Selye H. History and general outline of the stress concept. In: Stress in health and disease. Boston, Massachusetts: Butterworths, Inc; 1976.
Selye H, Fortier C. Adaptive reaction to stress. Psychosom Med. 1950;12:149–57.
Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110:1711–6. https://doi.org/10.1016/j.amjcard.2012.08.004
Vahedian-Azimi A, Moayed MS. Updating the meta-analysis of perceived stress and its association with the incidence of coronary heart disease. Int J Med Rev. 2019;6:146–53.
Schutte NS, Malouff JM. The relationship between perceived stress and telomere length: a meta-analysis. Stress Health. 2016;32:313–9. https://doi.org/10.1002/smi.2607
Prior A, Fenger-Grøn M, Larsen KK, Larsen FB, Robinson KM, Nielsen MG, et al. The association between perceived stress and mortality among people with multimorbidity: a prospective population-based cohort study. Am J Epidemiol. 2016;184:199–210. https://doi.org/10.1093/aje/kwv324
Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. https://doi.org/10.1001/jama.298.14.1685
Thoits PA. Stress and health: major findings and policy implications. J Health Soc Behav. 2010;51:S41–S53. https://doi.org/10.1177/0022146510383499
Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–12. https://doi.org/10.1056/NEJM199108293250903
Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. https://doi.org/10.1016/s0140-6736(95)92899-5
Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–46. https://doi.org/10.1159/000104862
Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Crit Care Nurs Clin N Am. 2012;24:201–13. https://doi.org/10.1016/j.ccell.2012.03.006
Kaprio J, Koskenvuo M, Rita H. Mortality after bereavement: a prospective study of 95,647 widowed persons. Am J Public Health. 1987;77:283–7. https://doi.org/10.2105/ajph.77.3.283
Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. https://doi.org/10.1161/01.cir.99.16.2192
Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. https://doi.org/10.12703/P5-5
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. https://doi.org/10.1016/j.cell.2013.05.039
Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet. 2005;366:1578–82.
Barzilai N, Cuervo AM, Austad S. Aging as a biological target for prevention and therapy. JAMA. 2018;320:1321–2.
Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–92. https://doi.org/10.1038/s41586-019-1365-2
Justice J, Miller JD, Newman JC, et al. Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci. 2016;71:1415–23. https://doi.org/10.1093/gerona/glw126
Kennedy BK. NIH conference on advances in geroscience: impact on healthspan and chronic disease. Bethesda, MD: National Institutes of Health; 2013.
Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J Gerontol A Biol Sci Med Sci. 2017;72:210–5. https://doi.org/10.1093/gerona/glw191
Gilbert SF. Developmental biology. In: Aging: the biology of senescence. 6th ed. Sunderland (MA): Sinauer Associates; 2000. https://www.ncbi.nlm.nih.gov/books/NBK10041
Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–9. https://doi.org/10.1093/gerona/glt162
Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13. https://doi.org/10.1016/j.cell.2014.10.039
Rutledge J, Oh H, Wyss-Coray T. Measuring biological age using omics data. Nat Rev Genet. 2022;23:715–27. https://doi.org/10.1038/s41576-022-00511-7
Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. https://doi.org/10.1016/j.ebiom.2017.03.046
Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73.
Aubert G, Lansdorp PM. Telomeres and aging. Physiological Rev. 2008;88:557–79.
Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184:306–22. https://doi.org/10.1016/j.cell.2020.12.028
Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. https://doi.org/10.1016/j.arr.2018.09.002
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. https://doi.org/10.1136/bmj.g4227.
Forero DA, González-Giraldo Y, López-Quintero C, Castro-Vega LJ, Barreto GE, Perry G. Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2016;71:1069–73. https://doi.org/10.1093/gerona/glw053
Rentscher KE, Carroll JE, Mitchell C. Psychosocial stressors and telomere length: a current review of the science. Annu Rev Public Health. 2020;41:223–45. https://doi.org/10.1146/annurev-publhealth-040119-094239
Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. https://doi.org/10.1073/pnas.1506264112
Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–8. https://doi.org/10.1016/j.mad.2005.10.004
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age. J Gerontol A Biol Sci Med Sci. 2013;68:667–74. https://doi.org/10.1093/gerona/gls233
Elliott ML, Caspi A, Houts RM, Ambler A, Broadbent JM, Hancox RJ, et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1:295–308.
Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–84. https://doi.org/10.1038/s41576-018-0004-3
Bergsma T, Rogaeva E. DNA methylation clocks and their predictive capacity for aging phenotypes and healthspan. Neurosci Insights. 2020;15:2633105520942221. https://doi.org/10.1177/2633105520942221
Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton P, Arsenault L, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife. 2022;11:e73420. https://doi.org/10.7554/eLife.73420
Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. https://doi.org/10.1016/j.arr.2021.101348
Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187:1220–30. https://doi.org/10.1093/aje/kwx346
Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–67. https://doi.org/10.1016/j.molcel.2012.10.016
Horvath S. DNA methylation age of human tissues and cell types [published correction appears in Genome Biol. 2015;16:96]. Genome Biol. 2013;14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Yang Z, Wong A, Kuh D, et al. Correlation of an epigenetic mitotic clock with cancer risk. Genome Biol. 2016;17:205. https://doi.org/10.1186/s13059-016-1064-3
Zhang Y, Wilson R, Heiss J, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. https://doi.org/10.1038/ncomms14617.
Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–91. https://doi.org/10.18632/aging.101414
Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11:303–27. https://doi.org/10.18632/aging.101684
McCrory C, Fiorito G, Hernandez B, et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J Gerontol A Biol Sci Med Sci. 2021;76:741–9. https://doi.org/10.1093/gerona/glaa286
Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. https://doi.org/10.1016/s0749-3797(98)00017-8
Felitti VJ. The relation between adverse childhood experiences and adult health: turning gold into lead. Perm J. 2002;6:44–47. https://doi.org/10.7812/TPP/02.994
Bourassa KJ, Rasmussen LJH, Danese A, et al. Linking stressful life events and chronic inflammation using suPAR (soluble urokinase plasminogen activator receptor. Brain Behav Immun. 2021;97:79–88. https://doi.org/10.1016/j.bbi.2021.06.018.
Bourassa KJ, Caspi A, Brennan GM, et al. Which types of stress are associated with accelerated biological aging? Comparing perceived stress, stressful life events, childhood adversity, and posttraumatic stress disorder. Psychosom Med. 2023;85:389–96. https://doi.org/10.1097/PSY.0000000000001197
Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol. 2007;40:313–32. https://doi.org/10.1007/s10464-007-9134-z
Slavich GM. Stressnology: the primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain Behav Immun. 2019;75:3–5.
Shields GS, Slavich GM. Lifetime stress exposure and health: a review of contemporary assessment methods and biological mechanisms. Soc Personal Psychol Compass. 2017;11:e12335. https://doi.org/10.1111/spc3.12335
Epel ES, Crosswell AD, Mayer SE, et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol. 2018;49:146–69. https://doi.org/10.1016/j.yfrne.2018.03.001
Belsky DW, Caspi A, Cohen HJ, et al. Impact of early personal-history characteristics on the Pace of Aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell. 2017;16:644–51. https://doi.org/10.1111/acel.12591
Kim K, Yaffe K, Rehkopf DH, et al. Association of adverse childhood experiences with accelerated epigenetic aging in midlife. JAMA Netw Open. 2023;6:e2317987. https://doi.org/10.1001/jamanetworkopen.2023.17987
Sumner JA, Gao X, Gambazza S, et al. Stressful life events and accelerated biological aging over time in youths. Psychoneuroendocrinology. 2023;151:106058. https://doi.org/10.1016/j.psyneuen.2023.106058
Raffington L, Belsky DW, Kothari M, Malanchini M, Tucker-Drob EM, Harden KP. Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics. 2021;147:e2020024406. https://doi.org/10.1542/peds.2020-024406
Steptoe A, Zaninotto P. Lower socioeconomic status and the acceleration of aging: an outcome-wide analysis. Proc Natl Acad Sci USA. 2020;117:14911–7. https://doi.org/10.1073/pnas.1915741117
Shen B, Mode NA, Noren Hooten N, et al. Association of race and poverty status with DNA methylation-based age. JAMA Netw Open. 2023;6:e236340. https://doi.org/10.1001/jamanetworkopen.2023.6340
Bourassa KJ, Halverson TF, Garrett ME, Hair L, Dennis M, VA Mid Atlantic MIRECC Workgroup. Demographic characteristics and epigenetic biological aging among post-9/11 veterans: associations of DunedinPACE with sex, race, and age. Psychiatry Res. 2024;336:115908. https://doi.org/10.1016/j.psychres.2024.115908
Brody GH, Miller GE, Yu T, Beach SR, Chen E. Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: a replication across two longitudinal cohorts. Psychol Sci. 2016;27:530–41. https://doi.org/10.1177/0956797615626703
Brody GH, Yu T, Chen E, Beach SR, Miller GE. Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. J Child Psychol Psychiatry. 2016;57:566–74. https://doi.org/10.1111/jcpp.12495
Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63:585–94. https://doi.org/10.1097/00006842-200107000-00011
Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–46. https://doi.org/10.1037/0033-2909.133.5.725
Bourassa KJ, Hendrickson RC, Reger GM, Norr AM. Posttraumatic stress disorder treatment effects on cardiovascular physiology: a systematic review and agenda for future research. J Trauma Stress. 2021;34:384–93. https://doi.org/10.1002/jts.22637
Fonkoue IT, Marvar PJ, Norrholm S, et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain Behav Immun. 2020;83:260–9. https://doi.org/10.1016/j.bbi.2019.10.021.
Wolf EJ, Maniates H, Nugent N, et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology. 2018;92:123–34. https://doi.org/10.1016/j.psyneuen.2017.12.007
Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–62. https://doi.org/10.1038/mp.2014.111
Bourassa KJ, Garrett ME, Caspi A, Dennis M, Hall KS, Moffitt TE, Taylor GA; VA Mid Atlantic MIRECC Workgroup; Ashley-Koch AE, Beckham JC, Kimbrel NA. Posttraumatic stress disorder, trauma, and accelerated biological aging among post-9/11 veterans. Transl Psychiatry. 2024;14:4. https://doi.org/10.1038/s41398-023-02704-y.
Lohr JB, Palmer BW, Eidt CA, et al. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23:709–25. https://doi.org/10.1016/j.jagp.2015.04.001
Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with posttraumatic stress disorder and mortality. Psychosom Med. 2018;80:42–48. https://doi.org/10.1097/PSY.0000000000000506
Hall KS, Beckham JC, Bosworth HB, Sloane R, Pieper CF, Morey MC. PTSD is negatively associated with physical performance and physical function in older overweight military Veterans. J Rehabil Res Dev. 2014;51:285–95. https://doi.org/10.1682/JRRD.2013.04.0091
Li X, Wang J, Zhou J, Huang P, Li J. The association between post-traumatic stress disorder and shorter telomere length: A systematic review and meta-analysis. J Affect Disord. 2017;218:322–6. https://doi.org/10.1016/j.jad.2017.03.048
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Casaletto KB, Staffaroni AM, Elahi F, et al. Perceived stress is associated with accelerated monocyte/macrophage aging trajectories in clinically normal adults. Am J Geriatr Psychiatry. 2018;26:952–63. https://doi.org/10.1016/j.jagp.2018.05.004
Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–5. https://doi.org/10.1073/pnas.0407162101
Reed RG. Stress and immunological aging. Curr Opin Behav Sci. 2019;28:38–43. https://doi.org/10.1016/j.cobeha.2019.01.012
Mathur MB, Epel E, Kind S, et al. Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun. 2016;54:158–69. https://doi.org/10.1016/j.bbi.2016.02.002
Cohen S, Murphy MLM, Prather AA. Ten surprising facts about stressful life events and disease risk. Annu Rev Psychol. 2019;70:577–97. https://doi.org/10.1146/annurev-psych-010418-102857
O’Donovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. https://doi.org/10.1016/j.biopsych.2011.01.035
Jovanovic T, Vance LA, Cross D, et al. Exposure to violence accelerates epigenetic aging in children. Sci Rep. 2017;7:8962. https://doi.org/10.1038/s41598-017-09235-9. Published 2017 Aug 21
McLaughlin KA, Colich NL, Rodman AM, Weissman DG. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med. 2020;18:96. https://doi.org/10.1186/s12916-020-01561-6
Walsh K, McCormack CA, Webster R, Pinto A, Lee S, Feng T, et al. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc Natl Acad Sci USA. 2019;116:23996–4005. https://doi.org/10.1073/pnas.1905890116
Kotsakis Ruehlmann A, Sammallahti S, Cortés Hidalgo AP, et al. Epigenome-wide meta-analysis of prenatal maternal stressful life events and newborn DNA methylation. Mol Psychiatry. 2023;10:1038/s41380–023-02010-5. https://doi.org/10.1038/s41380-023-02010-5
Nwanaji-Enwerem JC, Cardenas A, Gao X, et al. Psychological stress and epigenetic aging in older men: the VA normative aging study. Transl Med Aging. 2023;7:66–74. https://doi.org/10.1016/j.tma.2023.06.003
Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–97. https://doi.org/10.1037/a0024768
Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Dev Psychopathol. 2011;23:939–54. https://doi.org/10.1017/S0954579411000411
Ryder AL, Azcarate PM, Cohen BE. PTSD and physical health. Curr Psychiatry Rep. 2018;20:116. https://doi.org/10.1007/s11920-018-0977-9
Lupo G, Gaetani S, Cacci E, Biagioni S, Negri R. Molecular signatures of the aging brain: finding the links between genes and phenotypes. Neurotherapeutics. 2019;16:543–53. https://doi.org/10.1007/s13311-019-00743-2
Xiong Y, Hong H, Liu C, Zhang YQ. Social isolation and the brain: effects and mechanisms. Mol Psychiatry. 2023;28:191–201. https://doi.org/10.1038/s41380-022-01835-w
Sikora E, Bielak-Zmijewska A, Dudkowska M, Krzystyniak A, Mosieniak G, Wesierska M, et al. Cellular senescence in brain aging. Front Aging Neurosci. 2021;13:646924. https://doi.org/10.3389/fnagi.2021.646924
Guarnieri T, Filburn CR, Zitnik G, Roth GS, Lakatta EG. Contractile and biochemical correlates of beta-adrenergic stimulation of the aged heart. Am J Physiol. 1980;239:H501–H508. https://doi.org/10.1152/ajpheart.1980.239.4.H501
Vujic A, Lerchenmüller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. https://doi.org/10.1038/s41467-018-04083-1.
Moreira JBN, Wohlwend M, Wisløff U. Exercise and cardiac health: physiological and molecular insights. Nat Metab. 2020;2:829–39. https://doi.org/10.1038/s42255-020-0262-1
Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, et al. DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1124–L1137. https://doi.org/10.1152/ajplung.00293.2015
Walters MS, De BP, Salit J, Buro-Auriemma LJ, Wilson T, Rogalski AM, et al. Smoking accelerates aging of the small airway epithelium. Respir Res. 2014;15:94. https://doi.org/10.1186/s12931-014-0094-1.
Gao X, Gào X, Zhang Y, Breitling LP, Schöttker B, Brenner H. Associations of self-reported smoking, cotinine levels and epigenetic smoking indicators with oxidative stress among older adults: a population-based study. Eur J Epidemiol. 2017;32:443–56. https://doi.org/10.1007/s10654-017-0248-9
Iakovou E, Kourti M. A comprehensive overview of the complex role of oxidative stress in aging, the contributing environmental stressors and emerging antioxidant therapeutic interventions. Front Aging Neurosci. 2022;14:827900. https://doi.org/10.3389/fnagi.2022.827900
Holt-Lunstad J. Social connection as a public health issue: the evidence and a systemic framework for prioritizing the “Social” in social determinants of health. Annu Rev Public Health. 2022;43:193–213. https://doi.org/10.1146/annurev-publhealth-052020-110732
Holt-Lunstad J. The major health implications of social connection. Curr Dir Psychol Sci. 2021;30:251–9.
Howe D, Betts L. Attachment across the lifecourse: a brief introduction. London, UK: Bloomsbury Publishing; 2023.
Ciccheti D, Toth SL. Child maltreatment and attachment organization. In: Attachment theory: social, developmental, and clinical perspectives. 2013. p. 279.
Li S, Zhao F, Yu G. A meta-analysis of childhood maltreatment and intimate partner violence perpetration. Aggression Violent Behav. 2020;50:101362.
Bae D, Wickrama KAS. Pathways linking early socioeconomic adversity to diverging profiles of romantic relationship dissolution in young adulthood. J Fam Psychol. 2019;33:23–33. https://doi.org/10.1037/fam0000465
Taft CT, Watkins LE, Stafford J, Street AE, Monson CM. Posttraumatic stress disorder and intimate relationship problems: a meta-analysis. J Consult Clin Psychol. 2011;79:22–33. https://doi.org/10.1037/a0022196
Miller MW, Wolf EJ, Reardon AF, et al. PTSD and conflict behavior between veterans and their intimate partners. J Anxiety Disord. 2013;27:240–51. https://doi.org/10.1016/j.janxdis.2013.02.005
Whisman MA. Marital dissatisfaction and psychiatric disorders: results from the National Comorbidity Survey. J Abnormal Psychol. 1999;108:701–6.
Jordan BK, Marmar CR, Fairbank JA, et al. Problems in families of male Vietnam veterans with posttraumatic stress disorder. J Consult Clin Psychol. 1992;60:916–26. https://doi.org/10.1037/0022-006x.60.6.916
Wang Y, Chung MC, Wang N, Yu X, Kenardy J. Social support and posttraumatic stress disorder: a meta-analysis of longitudinal studies. Clin Psychol Rev. 2021;85:101998. https://doi.org/10.1016/j.cpr.2021.101998
Bourassa KJ, Smolenski DJ, Edwards-Stewart A, Campbell SB, Reger GM, Norr AM. The impact of prolonged exposure therapy on social support and PTSD symptoms. J Affect Disord. 2020;260:410–7. https://doi.org/10.1016/j.jad.2019.09.036
Ernst M, Niederer D, Werner AM, et al. Loneliness before and during the COVID-19 pandemic: a systematic review with meta-analysis. Am Psychol. 2022;77:660–77. https://doi.org/10.1037/amp0001005
Saltzman LY, Hansel TC, Bordnick PS. Loneliness, isolation, and social support factors in post-COVID-19 mental health. Psychol Trauma. 2020;12:S55–S57. https://doi.org/10.1037/tra0000703
Chen R, Zhan Y, Pedersen N, et al. Marital status, telomere length and cardiovascular disease risk in a Swedish prospective cohort. Heart. 2020;106:267–72. https://doi.org/10.1136/heartjnl-2019-315629
Whisman MA, Robustelli BL, Sbarra DA. Marital disruption is associated with shorter salivary telomere length in a probability sample of older adults. Soc Sci Med. 2016;157:60–67. https://doi.org/10.1016/j.socscimed.2016.03.029
Hillmann AR, Dhingra R, Reed RG. Positive social factors prospectively predict younger epigenetic age: findings from the Health and Retirement Study. Psychoneuroendocrinology. 2023;148:105988. https://doi.org/10.1016/j.psyneuen.2022.105988
Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE. Accelerated epigenetic aging mediates link between adverse childhood experiences and depressive symptoms in older adults: Results from the Health and Retirement Study. SSM Popul Health. 2022;17:101071. https://doi.org/10.1016/j.ssmph.2022.101071.
Allen JP, Danoff JS, Costello MA, Loeb EL, Davis AA, Hunt GL, et al. Adolescent peer struggles predict accelerated epigenetic aging in midlife. Dev Psychopathol. 2023;35:912–25. https://doi.org/10.1017/S0954579422000153
Mehta D, Bruenig D, Pierce J, Sathyanarayanan A, Stringfellow R, Miller O, et al. Recalibrating the epigenetic clock after exposure to trauma: The role of risk and protective psychosocial factors. J Psychiatr Res. 2022;149:374–81. https://doi.org/10.1016/j.jpsychires.2021.11.026
Bourassa KJ, Moffitt TE, Harrington H, Houts R, Poulton R, Ramrakha S, et al. Childhood adversity and midlife health: shining a light on the black box of psychosocial mechanisms. Prev Sci. 2023;24:817–28. https://doi.org/10.1007/s11121-022-01431-y
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. https://doi.org/10.1016/S2468-2667(17)30118-4
Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9:466–78. https://doi.org/10.1037/0278-6133.9.4.466
McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–55. https://doi.org/10.1046/j.1360-0443.2003.00408.x
Osler M, McGue M, Lund R, Christensen K. Marital status and twins’ health and behavior: an analysis of middle-aged Danish twins. Psychosom Med. 2008;70:482–7. https://doi.org/10.1097/PSY.0b013e31816f857b
Gavrieli A, Farr OM, Davis CR, Crowell JA, Mantzoros CS. Early life adversity and/or posttraumatic stress disorder severity are associated with poor diet quality, including consumption of trans fatty acids, and fewer hours of resting or sleeping in a US middle-aged population: a cross-sectional and prospective study. Metabolism. 2015;64:1597–610. https://doi.org/10.1016/j.metabol.2015.08.017
Schweren LJS, Larsson H, Vinke PC, et al. Diet quality, stress and common mental health problems: a cohort study of 121,008 adults. Clin Nutr. 2021;40:901–6. https://doi.org/10.1016/j.clnu.2020.06.016
Aldana SG, Sutton LD, Jacobson BH, Quirk MG. Relationships between leisure time physical activity and perceived stress. Percept Mot Skills. 1996;82:315–21. https://doi.org/10.2466/pms.1996.82.1.315
Yoshiuchi K, Inada S, Nakahara R, Akabayashi A, Park H, Park S, et al. Stressful life events and habitual physical activity in older adults: 1-year accelerometer data from the Nakanojo Study. Ment Health Phys Act. 2010;3:23–5.
Bourassa KJ, Ruiz JM, Sbarra DA. Smoking and physical activity explain the increased mortality risk following marital separation and divorce: evidence from the english longitudinal study of ageing. Ann Behav Med. 2019;53:255–66. https://doi.org/10.1093/abm/kay038
Ding D, Gale J, Bauman A, Phongsavan P, Nguyen B. Effects of divorce and widowhood on subsequent health behaviours and outcomes in a sample of middle-aged and older Australian adults. Sci Rep. 2021;11:15237. https://doi.org/10.1038/s41598-021-93210-y
Rueggeberg R, Wrosch C, Miller GE. The different roles of perceived stress in the association between older adults’ physical activity and physical health. Health Psychol. 2012;31:164–71. https://doi.org/10.1037/a0025242
Acar-Burkay S, Cristian DC. Cognitive underpinnings of COVID-19 vaccine hesitancy. Soc Sci Med. 2022;301:114911. https://doi.org/10.1016/j.socscimed.2022.114911
Leiferman JA, Pheley AM. The effect of mental distress on women’s preventive health behaviors. Am J Health Promot. 2006;20:196–9. https://doi.org/10.4278/0890-1171-20.3.196
Buckley TC, Mozley SL, Bedard MA, Dewulf AC, Greif J. Preventive health behaviors, health-risk behaviors, physical morbidity, and health-related role functioning impairment in veterans with post-traumatic stress disorder. Mil Med. 2004;169:536–40. https://doi.org/10.7205/milmed.169.7.536
van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: a systematic review and meta-analysis. Health Psychol. 2018;37:407–16. https://doi.org/10.1037/hea0000593
Pericot-Valverde I, Elliott RJ, Miller ME, Tidey JW, Gaalema DE. Posttraumatic stress disorder and tobacco use: a systematic review and meta-analysis. Addict Behav. 2018;84:238–47. https://doi.org/10.1016/j.addbeh.2018.04.024
Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol. 2012;31:194–201. https://doi.org/10.1037/a0025989
Hoerster KD, Campbell S, Dolan M, Stappenbeck CA, Yard S, Simpson T, et al. PTSD is associated with poor health behavior and greater Body Mass Index through depression, increasing cardiovascular disease and diabetes risk among U.S. veterans. Prev Med Rep. 2019;15:100930. https://doi.org/10.1016/j.pmedr.2019.100930
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.). 2022. https://doi.org/10.1176/appi.books.9780890425787
Lam L, Ho FY, Wong VW, Chan KW, Poon CY, Yeung WF, et al. Actigraphic sleep monitoring in patients with post-traumatic stress disorder (PTSD): A meta-analysis. J Affect Disord. 2023;320:450–60. https://doi.org/10.1016/j.jad.2022.09.045
Lewis C, Lewis K, Kitchiner N, Isaac S, Jones I, Bisson JI. Sleep disturbance in post-traumatic stress disorder (PTSD): a systematic review and meta-analysis of actigraphy studies. Eur J Psychotraumatol. 2020;11:1767349. https://doi.org/10.1080/20008198.2020.1767349
Zhang Y, Ren R, Sanford LD, Yang L, Zhou J, Zhang J, et al. Sleep in posttraumatic stress disorder: a systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev. 2019;48:101210. https://doi.org/10.1016/j.smrv.2019.08.004
Baddam SKR, Olvera RL, Canapari CA, Crowley MJ, Williamson DE. Childhood trauma and stressful life events are independently associated with sleep disturbances in adolescents. Behav Sci. 2019;9:108. https://doi.org/10.3390/bs9100108
Ho FY, Chan CS, Tang KN. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016;43:90–102. https://doi.org/10.1016/j.cpr.2015.09.005
Carter A, Bares C, Lin L, et al. Sex-specific and generational effects of alcohol and tobacco use on epigenetic age acceleration in the Michigan longitudinal study. Drug Alcohol Depend Rep. 2022;4:100077. https://doi.org/10.1016/j.dadr.2022.100077
Rosen AD, Robertson KD, Hlady RA, et al. DNA methylation age is accelerated in alcohol dependence. Transl Psychiatry. 2018;8:182. https://doi.org/10.1038/s41398-018-0233-4
Allen JP, Danoff JS, Costello MA, Hunt GL, Hellwig AF, Krol KM, et al. Lifetime marijuana use and epigenetic age acceleration: A 17-year prospective examination. Drug Alcohol Depend. 2022;233:109363. https://doi.org/10.1016/j.drugalcdep.2022.109363
Galkin F, Kovalchuk O, Koldasbayeva D, Zhavoronkov A, Bischof E. Stress, diet, exercise: common environmental factors and their impact on epigenetic age. Ageing Res Rev. 2023;88:101956. https://doi.org/10.1016/j.arr.2023.101956
Kim Y, Huan T, Joehanes R, McKeown NM, Horvath S, Levy D, et al. Higher diet quality relates to decelerated epigenetic aging. Am J Clin Nutr. 2022;115:163–70. https://doi.org/10.1093/ajcn/nqab201
Sae-Lee C, Corsi S, Barrow TM, Kuhnle GGC, Bollati V, Mathers JC, et al. Dietary intervention modifies DNA methylation age assessed by the epigenetic clock. Mol Nutr Food Res. 2018;62:e1800092. https://doi.org/10.1002/mnfr.201800092
Waziry R, Ryan CP, Corcoran DL, Huffman KM, Kobor MS, Kothari M, et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial [published correction appears in Nat Aging. 2023 Jun;3(6):753]. Nat Aging. 2023;3:248–57. https://doi.org/10.1038/s43587-022-00357-y
Phang M, Ross J, Raythatha JH, Dissanayake HU, McMullan RL, Kong Y, et al. Epigenetic aging in newborns: role of maternal diet. Am J Clin Nutr. 2020;111:555–61. https://doi.org/10.1093/ajcn/nqz326
Xu M, Zhu J, Liu XD, Luo MY, Xu NJ. Roles of physical exercise in neurodegeneration: reversal of epigenetic clock. Transl Neurodegener. 2021;10:30. https://doi.org/10.1186/s40035-021-00254-1.
Carroll JE, Prather AA. Sleep and biological aging: a short review. Curr Opin Endocr Metab Res. 2021;18:159–64. https://doi.org/10.1016/j.coemr.2021.03.021
Beach SR, Dogan MV, Lei MK, Cutrona CE, Gerrard M, Gibbons FX, et al. Methylomic aging as a window onto the influence of lifestyle: tobacco and alcohol use alter the rate of biological aging. J Am Geriatr Soc. 2015;63:2519–25. https://doi.org/10.1111/jgs.13830
Cardenas A, Ecker S, Fadadu RP, Huen K, Orozco A, McEwen LM, et al. Epigenome-wide association study and epigenetic age acceleration associated with cigarette smoking among Costa Rican adults. Sci Rep. 2022;12:4277. https://doi.org/10.1038/s41598-022-08160-w
Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–80. https://doi.org/10.1378/chest.08-1419
Klopack ET, Carroll JE, Cole SW, Seeman TE, Crimmins EM. Lifetime exposure to smoking, epigenetic aging, and morbidity and mortality in older adults. Clin Epigenetics. 2022;14:72. https://doi.org/10.1186/s13148-022-01286-8. Published 2022 May 28
Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95:S144–S150. https://doi.org/10.2105/AJPH.2004.059204. Suppl 1
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. https://doi.org/10.1093/hmg/ddu328
Grosz MP, Rohrer JM, Thoemmes F. The taboo against explicit causal inference in nonexperimental psychology. Perspect Psychol Sci. 2020;15:1243–55. https://doi.org/10.1177/1745691620921521
Hammerton G, Munafò MR. Causal inference with observational data: the need for triangulation of evidence [published correction appears in Psychol Med. 2021 Jul;51(9):1591]. Psychol Med. 2021;51:563–78. https://doi.org/10.1017/S0033291720005127
D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103:S46–S55. https://doi.org/10.2105/AJPH.2013.301252
Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci. 2007;2:377–95. https://doi.org/10.1111/j.1745-6916.2007.00050.x
Chatton A, Rohrer JM. The Causal Cookbook: Recipes for Propensity Scores, G-Computation, and Doubly Robust Standardization. Advances in Methods and Practices in Psychological Science. 2024;7(1). https://doi.org/10.1177/25152459241236149
Moriarity DP, Mengelkoch S, Slavich GM. Incorporating causal inference perspectives into psychoneuroimmunology: a simulation study highlighting concerns about controlling for adiposity in immunopsychiatry. Brain Behav Immun. 2023;113:259–66. https://doi.org/10.1016/j.bbi.2023.06.022
Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. https://doi.org/10.1097/00001648-200009000-00011
Rohrer JM. Thinking clearly about correlations and causation: graphical causal models for observational data. Adv Methods Practices Psychol Sci. 2018;1:27–42.
McNamee R. Confounding and confounders. Occup Environ Med. 2003;60:227–34. https://doi.org/10.1136/oem.60.3.227
Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45:1866–86. https://doi.org/10.1093/ije/dyw314
Whisman MA, Sbarra DA, Beach SRH. Intimate relationships and depression: searching for causation in the sea of association. Annu Rev Clin Psychol. 2021;17:233–58. https://doi.org/10.1146/annurev-clinpsy-081219-103323
Harrison R, Munafò MR, Davey Smith G, Wootton RE. Examining the effect of smoking on suicidal ideation and attempts: triangulation of epidemiological approaches. Br J Psychiatry. 2020;217:701–7. https://doi.org/10.1192/bjp.2020.68
Sbarra D, Trejo S, Harden KP, Oliver JC, Klimentidis Y. Genotypic and socioeconomic risks for depressive symptoms in two US cohorts spanning early to older adulthood. OSF. PsyArXiv. https://doi.org/10.31234/osf.io/g5vk4
Howe LJ, Nivard MG, Morris TT, Hansen AF, Rasheed H, Cho Y, et al. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat Genet. 2022;54:581–92. https://doi.org/10.1038/s41588-022-01062-7
Popper KR. The logic of scientific discovery. New York: Basic Books; 1943.
Popper KR. Science as falsification. Conjectures and refutations. 1963;1:33–9.
Kuhn T. The structure of scientific revolution. Chicago: University of Chicago Press; 1962.
Nielsen L, Riddle M, King JW, NIH Science of Behavior Change Implementation Team, Aklin WM, Chen W, et al. The NIH Science of Behavior Change Program: transforming the science through a focus on mechanisms of change. Behav Res Ther. 2018;101:3–11. https://doi.org/10.1016/j.brat.2017.07.002
Stoeckel LE, Hunter C, Onken L, Green P, Nielsen L, Aklin WM, et al. The NIH science of behavior change program: looking toward the future. Behav Ther. 2023;54:714–8. https://doi.org/10.1016/j.beth.2023.03.006
Insel TR. The NIMH experimental medicine initiative. World Psychiatry. 2015;14:151–3. https://doi.org/10.1002/wps.20227
Farrell AK, Stanton SCE, Sbarra DA. Good theories in need of better data: combining clinical and social psychological approaches to study the mechanisms linking relationships and health. Perspect Psychol Sci. 2022;17:863–83. https://doi.org/10.1177/17456916211027563
Higgins-Chen AT, Thrush KL, Wang Y, Minteer CJ, Kuo PL, Wang M, et al. A computational solution for bolstering reliability of epigenetic clocks: Implications for clinical trials and longitudinal tracking. Nat Aging. 2022;2:644–61. https://doi.org/10.1038/s43587-022-00248-2
Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558–77. https://doi.org/10.1037/0021-843X.112.4.558.
Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Res Human Dev. 2009;6:144–64.
Wolf EJ, Miller MW, Hawn SE, et al. Longitudinal study of traumatic-stress related cellular and cognitive aging. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2023.11.009
Turkheimer E, Harden KP. Behavior genetic research methods. In: Handbook of research methods in social and personality psychology. Cambridge Publishing: New York; 2014. pp. 159–87.
McGue M, Osler M, Christensen K. Causal Inference and observational research: the utility of twins. Perspect Psychol Sci. 2010;5:546–56. https://doi.org/10.1177/1745691610383511
Pingault JB, O’reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet. 2018;19:566–80. https://doi.org/10.1038/s41576-018-0020-3
Patterson SK, Petersen RM, Brent LJN, Snyder-Mackler N, Lea AJ, Higham JP. Natural animal populations as model systems for understanding early life adversity effects on aging. Integr Comp Biol. 2023;63:681–92. https://doi.org/10.1093/icb/icad058
Polsky LR, Rentscher KE, Carroll JE. Stress-induced biological aging: a review and guide for research priorities. Brain Behav Immun. 2022;104:97–109. https://doi.org/10.1016/j.bbi.2022.05.016
Pearl J, Glymour M, Jewell NP. Causal inference in statistics: a primer. John Wiley & Sons; 2016.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference [published correction appears in Stat Med. 2014 Nov 30;33(27):4859-60] [published correction appears in Stat Med. 2019 Sep 10;38(20):3960] [published correction appears in Stat Med. 2020 Sep 10;39(20):2693]. Stat Med. 2014;33:2297–340. https://doi.org/10.1002/sim.6128.
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6. https://doi.org/10.1038/s43586-021-00092-5
Grosz M, Ayaita A, Arslan RC, Buecker S, Ebert T, Hünermund P, et al. Natural experiments: missed opportunities for causal inference in psychology. PsyArXiv https://doi.org/10.31234/osf.io/dah3q
Angrist JD. Lifetime earnings and the Vietnam era draft lottery: evidence from social security administrative records. Am Econ Rev. 1990: 313–36.
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–64. https://doi.org/10.1093/aje/kwv254
Hernán MA, Wang W, Leaf DE. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328:2446–7. https://doi.org/10.1001/jama.2022.21383
Lahey BB, D’Onofrio BM. All in the family: comparing siblings to test causal hypotheses regarding environmental influences on behavior. Curr Dir Psychol Sci. 2010;19:319–23. https://doi.org/10.1177/0963721410383977
Loh WW, Ren D. A tutorial on causal inference in longitudinal data with time-varying confounding using g-estimation. PsyArXiv. https://doi.org/10.31234/osf.io/f23gj
Acknowledgements
This research was supported by Award #IK2CX002694 to KJB from the Clinical Science and Research and Development (CSR&D) Service of VA ORD. DAS was supported in part by grants R01MH125414-01 and R01AG078361-01 from the NIH. The first author also received support from the VA Mid-Atlantic Mental Illness Research, Education and Clinical Center (MIRECC), the Mental Health and Research Services of the Durham VA Healthcare System, and the Durham VA Geriatrics Research, Education, and Clinical Center (GRECC). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA, the U.S. government, or any other affiliated institution. The authors do not have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Contributions
KJB and DAS conceptualized the review and organized the initial literature review, KJB drafted the manuscript with assistance from DAS and prepared the figures, DAS provided revisions and both KJB and DAS provided final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bourassa, K.J., Sbarra, D.A. Trauma, adversity, and biological aging: behavioral mechanisms relevant to treatment and theory. Transl Psychiatry 14, 285 (2024). https://doi.org/10.1038/s41398-024-03004-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03004-9
- Springer Nature Limited