Abstract
Background
Elevated lipid profiles and impaired glucose homeostasis are risk factors for several cardiovascular diseases (CVDs), which, subsequently, represent a leading cause of early mortality, worldwide. The aim of the current study was to conduct a systematic review and meta-analysis of the effect of apple cider vinegar (ACV) on lipid profiles and glycemic parameters in adults.
Methods
A systematic search was conducted in electronic databases, including Medline, Scopus, Cochrane Library, and Web of Knowledge, from database inception to January 2020. All clinical trials which investigated the effect of ACV on lipid profiles and glycemic indicators were included. Studies were excluded if ACV was used in combination with other interventions or when the duration of intervention was < 2 weeks. To account for between-study heterogeneity, we performed meta-analysis using a random-effects model.
Results
Overall, nine studies, including 10 study arms, were included in this meta-analysis. We found that ACV consumption significantly decreased serum total cholesterol (− 6.06 mg/dL; 95% CI: − 10.95, − 1.17; I2: 39%), fasting plasma glucose (− 7.97 mg/dL; 95% CI: − 13.74, − 2.21; I2: 75%), and HbA1C concentrations (− 0.50; 95% CI: − 0.90, − 0.09; I2: 91%). No significant effect of ACV consumption was found on serum LDL-C, HDL-C, fasting insulin concentrations, or HOMA-IR. The stratified analysis revealed a significant reduction of serum TC and TG in a subgroup of patients with type 2 diabetes, those who took ≤15 mL/day of ACV, and those who consumed ACV for > 8-weeks, respectively. Furthermore, ACV consumption significantly decreased FPG levels in a subgroup of studies that administered ACV for > 8-weeks. Further, ACV intake appeared to elicit an increase in FPG and HDL-C concentrations in apparently healthy participants.
Conclusion
We found a significant favorable effect of ACV consumption on FPG and blood lipid levels.
Similar content being viewed by others
Background
Cardiovascular diseases (CVDs), collectively, are regarded as the number one cause of early mortality, worldwide [30]. According to the World Health Organization (WHO), approximately 17.7 million deaths were attributable to CVD in 2015, and it is projected to account for more than 23.6 million by 2030 [2]. Indeed, given the high economic costs of CVDs on healthcare systems, especially in developing countries, prevention and management of CVDs is of high priority.
Dyslipidemia, in particular hypercholesterolemia, and hyperglycemia are regarded as the most important contributors to CVD events [24, 25]. Lifestyle modifications (dietary changes and physical activity), along with pharmacological interventions, such as statins, fibrates, and insulin sensitizers, are routinely used to manage these metabolic disorders [1, 14, 19, 26]. However, low adherence to lifestyle recommendations and reported adverse reactions of synthetic agents [38, 41] highlights the necessity of discerning novel and efficacious approaches. In this line, the benefical effect of nutraceutics and fuctional foods on human health have been well-documented [8, 36]. In contemporary research and practice, plants and their derivatives have attracted a lot of interest for their beneficial effects in controlling lipid profile and glycemic status [5, 15, 37]. Indeed, one of the most popular plant derivatives in this regard is vinegar.
Apple cider vinegar (ACV) is one of the three most common types of vinegar, produced by fermenting apples [3]. This acidic solution is consumed throughout the world as a flavoring and preservative agent in foods [22]. ACV contains a variety of flavonoids, such as gallic acid, catechin, caffeic acid, and ferulic acid [11, 31]. Animal experiments have reported that ACV has a variety of pharmacological functions, including anti-oxidant, anti-inflammatory, anti-diabetic, anti-hypertensive, and anti-hyperlipidemic properties [7, 18, 32, 39]. The effects of ACV on serum lipid parameters and glycemic markers have been investigated in several randomized clinical trials [4, 12, 16, 22, 23, 27, 33]; however, the results are equivocal. Indeed, some investigations have reported beneficial effects following ACV consumption on the aforementioned parameters [16, 22, 23, 27], although others failed to detect any effects [4, 12, 33]. It is conceivable that such contradictory findings might be due to the differences in study design and/or characteristics of participants (age, sex, clinical condition).
To the best of our knowledge, there has been no systematic compilation of the previously reported effects of ACV on lipid profiles and glycemic status. Therefore, in the current study, we performed a systematic review and meta-analysis of all published clinical trials to provide a more precise estimation of the effects of ACV on serum lipid parameters and glycemic markers in adults.
Methods
The present systematic review and meta-analysis was planned, conducted, and reported according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta- Analysis (PRISMA) statement [29].
Search strategy
A comprehensive literature search was performed to identify and appraise investigations that had assessed the effects of ACV supplementation on lipid profiles and glycemic parameters. Electronic databases, including Medline, Scopus, Cochrane Library, and Web of Knowledge, were searched from database inception to January 2020. The relevant keywords were used in combination with the Medical Subject Heading (MeSH) terms, search tag, and boolean operators (AND, OR, NOT) (Supplemental Table 1). The search keywords were seldom based on vinegar and related phrases to minimize the chance of missing studies which reported lipids or glycemic related markers as secondary outcomes. Additionally, the reference lists of related review articles and the retrieved studies were also hand-searched to detect eligible trials that might have been missed.
Study selection
After excluding duplicate studies, two authors (AH and MP) independently reviewed articles based on titles, abstracts, or full-texts to identify relevant studies. Eventually, original studies were included in the present meta-analysis if they: 1) were randomized clinical trials; 2) administered ACV as the intervention; 3) enrolled adult participants (aged ≥18 years); 4) reported lipid and glycemic parameters as the outcomes of interest. Studies that met the following criteria were excluded: 1) ACV was used in combination with other interventions; 2) studies with an intervention duration of fewer than 2 weeks; 3) studies that did not report relevant effect sizes. Table 1 shows the PICOS (participants, intervention/exposure, comparisons, outcomes, and study design) criteria which was used to define the research question.
Data extraction and risk of bias assessment
The main information from eligible studies were extracted by two researchers independently and the following data were abstracted: first author’s last name, years of publication, study location, sample size, participant’s health condition, mean age of subjects, design and duration of intervention, comparison groups, type of intervention, and main outcome. Any disagreement was settled by face-to-face discussion. Furthermore, in instances of unclear information in included studies, clarification was soughtby emailing the relevant studies’ corresponding authors.
The Cochrane Risk of Bias Tool was applied to assess potential risks of bias in included RCTs [17]. This scale is based on several items to assess the adequacy of random sequence generation, allocation concealment, blinding as well as detection of incomplete outcome data, selective outcome reporting, and other potential sources of bias. Based on recommendations of the Cochrane Handbook, judgment of each item appears by “Low”, “High”, and “Unclear” risk of bias.
Statistical analysis
All statistical analyses were conducted using the Cochrane Program Review Manager Version 5.3 (The Cochrane Collaboration, 2011, The Nordic Cochrane Centre, Copenhagen) and STATA version 11 software. To estimate the overall effect size, the mean differences (MD) and standard deviations (SDs) of all outcomes of interest, including fasting plasma glucose (FPG), fasting insulin, HbA1C, homeostasis model assessment of insulin resistance (HOMA-IR), triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density protein cholesterol (HDL-C), were collected. Net changes in these variables in intervention and control groups were computed by the subtraction of the post-measurement data from pre-intervention values if they were not already available in the original study. As SD of mean change was provided in only 2 studies [4, 22], we calculated missing SDs of change for other studies according to the following formula: [SD = square root [(SD pre-treatment)2 + (SD post-treatment)2 - (2 r × SD pre-treatment × SD post-treatment)] [17]; where the correlation coefficient (r) was examined using the following equation: [r = (SD2Baseline + SD2Final-SDChange) / (2× SD Baseline ×SD Final)] [17], in which required data were obtained from the study of Bashiri et al. [4]. Based on this, r for TG was considered as 0.73, for TC as 0.76, for LDL-C as 0.68, and for HDL-C as 0.66. The corresponding r for FPG, HbA1C, HOMA-IR and fasting insulin was assumed to be 0.66 for all [17]. To account for probable between-study heterogeneity, we applied a random-effects model in our analyses to estimate the overall effect size [10]. Inter-study heterogeneity was assessed by Cochran’s Q and I2 statistics, where P < 0.10 or I2 > 50% was regarded as possessing potential statistical heterogeneity (J. Higgins & Green). The source of heterogeneity was then explored through subgroup analysis. Effect sizes were presented as weighted mean differences with 95% confidence intervals (CI). The sensitivity analysis was applied to assess the influence of individual studies on the overall findings. Egger’s regression asymmetry test and Begg’s rank-correlation test were performed to explore potential publication bias [13, 40]. A random-effects meta-regression was conducted to identify the potential impact of putative moderators, including baseline measures, amount, and duration of apple vinegar administration on estimated net changes of outcome [6]. P-values < 0.05 were, a priori, considered as statistically significant.
Results
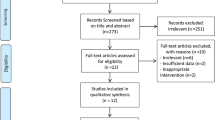
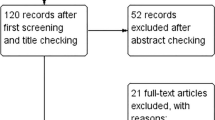
The study selection process, including the number and the reason for excluded studies are illustrated in Fig. 1. In brief, the primary electronic search yielded 4643 unduplicated records, where 4628 articles were removed following title/abstract screening, and thus, 15 studies were selected for further assessment. Six studies were excluded due to duplicated data (n = 2), duration of intervention < 1 week (n = 1), and the use of non-apple vinegar (n = 3). One selected study [23] had used 2 different doses of ACV; therefore, it was considered as 2 interdependent active arms. Therefore, 9 studies, including 10 study arms, were included in the final meta-analysis.
Study characteristics
The main characteristics of included clinical trials are provided in Table 2. Nine studies [4, 12, 16, 20, 22, 23, 27, 28, 33], comprising 686 total participants, with a mean age of 49.5 y, met the eligibility criteria and were selected for qualitative and quantitative analysis. These studies were published between 2008 and 2019, and had been conducted in Iran [4, 12, 22, 27, 28], USA [33], Japan [23], Pakistan [20], and Tunisia [16], respectively. Aside from one study [16], which did not report the gender of participants, all included studies had recruited both genders. Participants’ clinical conditions were different across the included studies; where five trials had enrolled diabetic patients [12, 16, 20, 27, 28], 2 studies had included obese and/or overweight participants [22, 23], one study had recruited type 2 diabetic patients with dyslipidemia [4], and one study did not report the condition of subjects [33]. All trials were of parallel design and the duration of intervention ranged between 30 and 90 days. The dose of ACV varied from 15 to 770 mL/day. Participants in control groups were prescribed water/beverage [16, 20, 23, 27, 28, 33] or a restricted-calorie diet [22]. Two studies [4, 12] had no administration in control groups.
Risk of bias assessment
The author’s judgment on each criterion of the risk of bias assessment is presented in Table 3. In summary, 8 trials [4, 12, 16, 20, 22, 23, 28, 33] were randomized, however, only 3 studies [20, 22, 33] had provided enough information regarding allocation concealment. Three studies [20, 23, 33] were blinded. Seven studies [4, 12, 20, 22, 23, 28, 33] had low attrition bias and/or described the reason of participants’ withdrawal. Three trials had reported the controlling of other factors that may influence outcomes [4, 20, 22].
Meta-analysis
The effect of ACV administration on lipid profile
Findings from 8 studies, with 9 effect sizes, revealed that ACV consumption significantly decreased serum TC concentrations (− 6.06 mg/dL; 95% CI: − 10.95, − 1.17, P = 0.02; I2: 39%). In addition, a trend toward a significant reduction in serum TG levels was also seen following ACV consumption (− 33.66 mg/dL; 95% CI: − 67.87, 0.54, P = 0.05; I2: 95%). No significant effect of ACV consumption on serum LDL-C (− 2.12 mg/dL; 95% CI: − 10.09, 5.85, P = 0.60; I2: 81%) and HDL-C concentrations was found after ACV consumption (0.92 mg/dL; 95% CI: − 0.42, 2.27, P = 0.18; I2: 22%) (Fig. 2).
To discern the source of heterogeneity, we performed subgroup analyses based on participant’s condition, dose of ACV consumption, and the duration of intervention. In these analyses, we found a notable decrease in both TG and TC concentrations in studies conducted on type 2 diabetic patients (TG: − 22.46 mg/dL; 95% CI: − 40.27, − 4.65; I2: 0%; TC: − 11.51 mg/dL; 95% CI: − 18.16, − 4.86; I2: 0%), as well as in studies with an ACV dose of ≤15 mL/day (TG: − 21.91 mg/dL; 95% CI: − 35.23, − 8.60; I2: 0%; TC: − 10.22 mg/dL; 95% CI: − 16.46, − 3.98; I2: 0%) and studies with > 8-weeks of intervention (TG: − 48.22 mg/dL; 95% CI: − 92.83, − 3.60; I2: 96%; TC: − 7.61 mg/dL; 95% CI: − 14.29, − 0.94; I2: 49%). However, no significant reduction in these variables was found in studies conducted on non-diabetics, studies that administered > 15 mL/day, and those with a duration of intervention of ≤8-weeks. In addition, a significant increase in serum HDL-C levels was observed in studies that recruited non-diabetics (HDL-C: 1.73 mg/dL; 95% CI: 0.28, 3.18; I2: 0%) (Table 4).
The effect of ACV administration on glycemic profile
Pooled effect size from 6 studies, with 7 effect sizes, revealed a significant reduction in FPG (− 7.97 mg/dL; 95% CI: − 13.74, − 2.21, P = 0.007; I2: 75%) after ACV consumption. The same findings were obtained for HbA1C, when we combined 6 effect sizes from 5 studies (− 0.50 mg/dL; 95% CI: − 0.90, − 0.09, P = 0.02; I2: 91%). No significant effect of ACV consumption was found on serum insulin levels (− 0.85 mg/dL; 95% CI: − 2.73, 1.02, P = 0.37; I2: 0%) and HOMA-IR (− 0.31 mg/dL; 95% CI: − 0.80, 0.17, P = 0.21; I2: 18%) (Fig. 3).
Subgroup analysis revealed a significant reduction in FPG in studies recruited non-diabetics (− 3.53 mg/dL; 95% CI: − 6.70, − 0.37; I2: 0%). Such an effect was not observed in studies that enrolled diabetic patients (− 16.28 mg/dL; 95% CI: − 33.02, − 0.47; I2: 83%). Furthermore, when studies were stratified based on duration of intervention, the lowering effect of ACV on FPG was found in studies with an intervention of > 8-weeks follow-up (− 3.78 mg/dL; 95% CI: − 6.90, − 0.66; I2: 0%). No significant effect of ACV consumption on FPG was seen in studies with a low or high dose of ACV. When we excluded the study of Mohammadpourhodki et al. (36), between-study heterogeneity became non-significant (− 3.09 mg/dL; 95% CI: − 5.83, − 0.35; I2:23%). In addition, the lowering effect of ACV on HbA1C was non-significant in all subgroups (Table 4). Due to the low number of trials in each subgroup, stratified analysis was not conducted for serum insulin levels and HOMA-IR.
Meta-regression
Findings from the meta-regression indicated an inverse association between change in FPG and HbA1C levels and baseline levels of these indicators (FPG: coefficient: -0.24; 95% CI: − 0.40, − 0.07; HbA1C: coefficient: -0.21; 95% CI: − 0.39, − 0.03). However, the effect of ACV intake on FPG and HbA1C was independent of the dose of ACV and duration of the study. Furthermore, no association was observed between change in other outcomes of interest following ACV intake and baseline measures, the dose of intervention, and duration of follow-up (Supplemental Table 2).
Sensitivity analysis
Sensitivity analysis was performed by excluding individual studies from the meta-analysis. We found that our findings about serum TG was influenced by 3 studies, and when we removed each of these studies from the analysis, the findings did change [excluding Panetta et al. [33] WMD: − 39.38 mg/dL; 95% CI: − 73.18, − 5.59; excluding Mahmoodi et al. [27] WMD: − 39.46 mg/dL; 95% CI: − 75.37, − 3.55; and excluding Khezri et al. [22] WMD: − 20.76 mg/dL; 95% CI: − 36.86, − 4.65]. This was also the case when we removed the study of Bashiri et al. [4] in our analysis on serum HDL-C levels (WMD: 1.68 mg/dL; 95% CI: 0.44, 2.92); such that serum HDL-C levels were significantly increased by ACV consumption. With regards to HbA1C, we found that the study by Mohammadpourhodki et al. [28] had a significant effect on the overall finding; such that after removing that study from the analysis, no significant effect on HbA1C was seen following ACV consumption (− 0.04%; 95% CI -0.18, 0.10).
In addition, when we excluded studies with a high-risk of bias [12, 16, 27, 28], no alterations in findings occurred. Except for the findings of HbA1C, which became non-significant (− 0.03%; 95% CI: − 0.16, 0.10) (Supplemental Figure 1).
Publication bias
Funnel plot showed a slight to moderate asymmetry in some variables (Supplemental Figure 2). Based on Egger’s regression asymmetry and Begg’s rank correlation test, we found no evidence of publication bias in studies on TG, TC, HDL-C, and HbA1C. However, a significant asymmetry was found in studies on serum LDL-C (P = 0.005) and FPG (P = 0.04) according to Egger’s regression test, although, such results were not confirmed by Begg’s rank-correlation test (LDL-C: P = 0.40; FPG: P = 0.17).
Discussion
The present systematic review and meta-analysis suggested that ACV consumption yielded beneficial effects on serum TC and FPG levels. In addition, a trend toward a significant favorable effect was also observed in serum TG concentrations.
Hyperlipidemia and hyperglycemia are common metabolic disorders that affect many people around the world [34, 36]. In spite of several strategies to manage these abnormalities, lifestyle modifications are the first line of therapy in these conditions. Indeed, findings from the present meta-analysis highlight the application of ACV, as a dietary agent, may be helpful in controlling these metabolic abnormalities [9].
The present study showed that ACV consumption improved serum levels of FPG. With regard to HbA1C, despite the significant overall effect of ACV, we found that the exclusion of one study resulted in a non-significant finding, indicating that the overall findings were study-dependent. The mechanism of the ACV effect on lipid profiles and glycemic related markers has not been well defined; however, empirical studies have suggested several potential mechanisms. Indeed, ACV can improve glycemic status by delaying gastric emptying, enhancing cellular glucose utilization and lipolysis, suppressing hepatic glucose production and lipogenesis, and facilitating insulin secretion [21, 35]. Furthermore, in our study, the beneficial effect of ACV on FPG levels was more pronounced when the duration of studies lasted > 8 weeks. Subgroup analysis revealed that the FPG lowering effect of ACV was not significant in non-diabetic patients. On the other hand, the meta-regression results indicated a negative association between changes in both HbA1C and FPG levels, and their baseline measures. These findings suggest that higher baseline values of FPG and HbA1C might contribute to a greater reduction in these markers following ACV intake.
This study revealed that ACV consumption might reduce serum TC concentrations; where the effect of ACV on lipid profiles might be attributed to its stimulation of acid bile excretion, increasing lipolysis and decreasing lipogenesis [21, 35]. Subgroup analysis indicated a greater beneficial effect on both TC and TG levels among type 2 diabetics patients. In addition, the effect on TG and TC was more notable when interventions lasted > 8 weeks. The results from subgroup analysis also showed a greater lowering effect of ACV on TG and TC levels in doses of ≤15 ml/day. Therefore, 15 ml/day might represent the optimum effective dose of ACV. However, our findings from meta-regression analysis did not indicate an association between the dosage of ACV intake and serum alterations of these parameters. Nevertheless, the null results from meta-regression analysis might be due to the paucity of data in terms of ACV doses in published studies. We also found a significant improvement in HDL-C among non-diabetic participants. Given that diabetic patients are susceptible to higher levels of oxidative stress and the reductions of the expression and/or activity of HDL-C’s anti-oxidative enzymes, such as paraoxonase, in these patients, it is reasonable to expect a higher dosage of ACV would be more effective in these individuals. Therefore, further investigations would be required to shed light on this issue in diabetic patients.
ACV appears to be a safe natural supplement with a functional role in controlling glycemic and lipid profiles. Only two studies [4, 20] had reported some side effects (such as stomach burning and ACV intolerance) following consumption of this supplement. The present systematic review and meta-analysis has some limitations which must be taken into account. The number of included studies, in particular in terms of insulin and HOMA-IR, was relatively low, thereby precluding reliable conclusions to be drawn. Between-study heterogeneity was high for some outcomes, and although we endeavoured to find the source of heterogeneity in subgroup analysis, the heterogeneity remained significant in some subgroups. Most studies did not control the participant’s dietary intake, which might influence study outcomes. Finally, some trials were potentially high risk of bias in methodology, especially in blinding. However, except for HbA1C, the findings did not change by excluding studies with a high risk of bias.
Conclusion
Following systematic review and meta-analysis, we found that ACV consumption might beneficially affect glycemic status and lipid parameters in adults; however, due to some limitations, the findings should be interpreted with caution. Considering that ACV is a safe food, it could be considered as a functional food and adjuvant therapy in the management of metabolic abnormalities. However, further studies are needed to clarify all possible beneficial effects of ACV on glycemic markers and lipid profiles.
Availability of data and materials
Not applicable.
Abbreviations
- CVDs:
-
Cardiovascular diseases
- ACV:
-
Apple cider vinegar
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta- Analysis
- MeSH:
-
Medical Subject Heading
- MD:
-
Mean differences
- SDs:
-
Standard deviations
- FPG:
-
Fasting plasma glucose
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- TG:
-
Triacylglycerol
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density protein cholesterol
References
Association A. D. 7. Approaches to glycemic treatment. Diabetes Care. 2016a;39(Supplement 1):S52–9.
Association A. D. 8. Cardiovascular disease and risk management. Diabetes Care. 2016b;39(Supplement 1):S60–71.
Banna AA, Kawar NS. Behavior of parathion in apple juice processed into cider and vinegar. J Environ Sci Health B. 1982;17(5):505–14. https://doi.org/10.1080/03601238209372337.
Bashiri R, Ghadiri-Anari A, Hekmatimoghadam H, Dehghani A, Najarzadeh A. The effect of apple vinegar on lipid profiles and anthropometric indices in type 2 diabetes patients with dyslipidemia: a randomized clinical trial. SSU J. 2014;22(5):1543–53.
Baumgartner S, Mensink RP, Plat J. Plant sterols and stanols in the treatment of dyslipidemia: new insights into targets and mechanisms related to cardiovascular risk. Curr Pharm Des. 2011;17(9):922–32. https://doi.org/10.2174/138161211795428795.
Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14(2):134–43. https://doi.org/10.1007/s11121-013-0377-7.
Bounihi A, Bitam A, Bouazza A, Yargui L, Koceir EA. Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharm Biol. 2017;55(1):43–52. https://doi.org/10.1080/13880209.2016.1226369.
Cicero AF, Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016;23(11):1134–44. https://doi.org/10.1016/j.phymed.2015.11.009.
Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Nutr Rev. 2017;75(9):731–67. https://doi.org/10.1093/nutrit/nux047.
Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
DuPont MS, Bennett RN, Mellon FA, Williamson G. Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. J Nutr. 2002;132(2):172–5. https://doi.org/10.1093/jn/132.2.172.
Ebrahimi-Mamaghani M, Arefhosseini S, Golzarand M, Aliasgarzadeh A, Vahed-Jabbary M. Long-term effects of processed Berberis vulgaris on some metabolic syndrome components. Iran J Endocrinol Metab. 2009;11(1):41–7.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Farmer JA, Gotto AM Jr. Current and future therapeutic approaches to hyperlipidemia. Adv Pharmacol. 1996;35:79–114 Elsevier.
Guo M, Liu Y, Gao Z-Y, Shi, D.-z. Chinese herbal medicine on dyslipidemia: progress and perspective. Evid Based Complement Alternat Med. 2014;2014. https://doi.org/10.1155/2014/163036.
Halima BH, Sarra K, Mohamed S, Louay T, Fethi BS, Houda BJ, et al. Apple cider vinegar ameliorates hyperglycemia and hyperlipidemia in Tunisian type 2 diabetic patients. Int J Multidisciplinary Curr Res. 2017;5:1453–9.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. 2021. Available from www.training.cochrane.org/handbook.
Iman M, Moallem SA, Barahoyee A. Effect of apple cider vinegar on blood glucose level in diabetic mice. Pharm Sci. 2015;20(4):163.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2015;38(1):140–9. https://doi.org/10.2337/dc14-2441.
Kausar S, Abbas MA, Hajra Ahmad NY, Ahmed Z, Humayun N, Ashfaq H, et al. Effect of apple cider vinegar in type 2 diabetic patients with poor glycemic control: a randomized placebo controlled design®. Health Sci. 2019a;8(2):149–59.
Kausar S, Humayun A, Ahmed Z, Abbas MA, Tahir A. Effect of apple cider vinegar on glycemic control, hyperlipidemia and control on body weight in type 2 diabetes patients. Health Sci. 2019b;8(5):59–74.
Khezri SS, Saidpour A, Hosseinzadeh N, Amiri Z. Beneficial effects of apple cider vinegar on weight management, visceral adiposity index and lipid profile in overweight or obese subjects receiving restricted calorie diet: a randomized clinical trial. J Funct Foods. 2018;43:95–102. https://doi.org/10.1016/j.jff.2018.02.003.
Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem. 2009;73(8):1837–43. https://doi.org/10.1271/bbb.90231.
Lehto S, Rönnemaa T, Haffher SM, Pyörälä K, Kallio V, Laakso M. Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes. 1997;46(8):1354–9. https://doi.org/10.2337/diab.46.8.1354.
Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease?: a meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–55. https://doi.org/10.1001/archinte.164.19.2147.
Mahamuni SP, Khose RD, Menaa F, Badole SL. Therapeutic approaches to drug targets in hyperlipidemia. BioMedicine. 2012;2(4):137–46. https://doi.org/10.1016/j.biomed.2012.08.002.
Mahmoodi M, Hosseini-zijoud S-M, Nabati S, Modarresi M, Mehrabian M, Sayyadi A, et al. The effect of white vinegar on some blood biochemical factors in type 2 diabetic patients. J Diabetes Endocrinol. 2013;4(1):1–5.
Mohammadpourhodki R, Sargolzaei MS. The effects of apple vinegar on fasting blood sugar (FBS) and glycosylated hemoglobin in patients with type 2 diabetes. Prensa Medica Argent. 2019;104:1–4.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):434–41. https://doi.org/10.1161/CIR.0000000000000157.
Natera R, Castro R, de Valme García-Moreno M, Hernández MJ, García-Barroso C. Chemometric studies of vinegars from different raw materials and processes of production. J Agric Food Chem. 2003;51(11):3345–51. https://doi.org/10.1021/jf021180u.
Nazıroğlu M, Güler M, Özgül C, Saydam G, Küçükayaz M, Sözbir E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J Membr Biol. 2014;247(8):667–73. https://doi.org/10.1007/s00232-014-9685-5.
Panetta CJ, Jonk YC, Shapiro AC. Prospective randomized clinical trial evaluating the impact of vinegar on lipids in non-diabetics. World J Cardiovasc Dis. 2013;3(02):191–6. https://doi.org/10.4236/wjcd.2013.32027.
Patti AM, Al-Rasadi K, Giglio RV, Nikolic D, Mannina C, Castellino G, et al. Natural approaches in metabolic syndrome management. Arch Med Sci. 2018;14(2):422–41. https://doi.org/10.5114/aoms.2017.68717.
Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr Rev. 2014;72(10):651–61. https://doi.org/10.1111/nure.12125.
Sahebkar A, Serban M-C, Gluba-Brzózka A, Mikhailidis DP, Cicero AF, Rysz J, et al. Lipid-modifying effects of nutraceuticals: an evidence-based approach. Nutrition. 2016;32(11):1179–92. https://doi.org/10.1016/j.nut.2016.04.007.
Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. J Altern Complement Med. 2004;10(2):369–78. https://doi.org/10.1089/107555304323062365.
Sharma ST, Nestler JE. Prevention of diabetes and cardiovascular disease in women with PCOS: treatment with insulin sensitizers. Best Pract Res Clin Endocrinol Metab. 2006;20(2):245–60. https://doi.org/10.1016/j.beem.2006.02.003.
Shishehbor F, Mansoori A, Sarkaki A, Jalali M, Latifi S. Apple cider vinegar attenuates lipid profile in normal and diabetic rats. Pak J Biol Sci. 2008;11(23):2634–8. https://doi.org/10.3923/pjbs.2008.2634.2638.
Sterne J, Bradburn M. Meta-analysis in Stata. In: Egger M, Smith G, Altman D, editors. Systematic Reviews in Health Care. London: BMJ Publishing Group; 2001. p. 347–72.
Thompson PD, Panza G, Zaleski A, Taylor B. Statin-associated side effects. J Am Coll Cardiol. 2016;67(20):2395–410. https://doi.org/10.1016/j.jacc.2016.02.071.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
M.P., and A.H. carried out the concept, design and drafting of this study. A.N., A.H. and M.P. searched databases, screened articles and extracted data. A. E performed the acquisition, analysis, and interpretation of data. A.E., and C.C. critically revised the manuscript. All authors approved the final version of the manuscript. A.E. are the guarantors of this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1.
Sensitivity analysis. Abbreviations: TG: Triacylglycerol; TC: Total-Cholesterol; LDL-C: Low-density Lipoprotein Cholesterol; HDL-C: High-density Lipoprotein Cholesterol; FBS: Fasting Blood Glucose.
Additional file 2: Supplemental Figure 2.
Funnel plot illustrating publication bias in the studies reporting effect of apple cider vinegar intake on the lipid profiles and glycemic related markers. Abbreviations: TG: Triacylglycerol; TC: Total-Cholesterol; LDL-C: Low-density Lipoprotein Cholesterol; HDL-C: High-density Lipoprotein Cholesterol; FBS: Fasting Blood Glucose; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance.
Additional file 3: Supplemental Table 1.
The search strategy used for each database.
Additional file 4: Supplemental Table 2.
Meta-regression of the association between the change in outcomes of interest response to ACV intake and potential moderator.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hadi, A., Pourmasoumi, M., Najafgholizadeh, A. et al. The effect of apple cider vinegar on lipid profiles and glycemic parameters: a systematic review and meta-analysis of randomized clinical trials. BMC Complement Med Ther 21, 179 (2021). https://doi.org/10.1186/s12906-021-03351-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03351-w