Abstract
Background
In this meta-analysis, findings from recent studies on the preventive properties of anthocyanins (ACN) against cardiovascular disease are summarized.
Methods
MEDLINE, PubMed, Embase, Cochrane Library, and Google Scholar were searched and 2512 studies were found in a preliminary search. After screening of titles and abstracts, 47 studies met the inclusion criteria (randomized clinical trial design and sufficient data of outcomes). Studies were excluded based on the following criteria: incomplete data; obscurely reported outcomes, or lack of control groups; and animal studies.
Results
The results showed that intervention with ACNs resulted in a significant decrease in body mass index ((MD),− 0.21; 95% CI, − 0.38, − 0.04; P < 0.001) and body fat mass (MD: − 0.3%, 95% CI − 0.42 to − 0.18%, p < 0.001). Pooled data comparing ACN with control showed statistically significant effect on fasting blood sugar and HbA1c. However, the reductions were significantly more in the subjects with type 2 diabetes and in those who used ACN as supplement/extract. The subgroup analysis test showed that there was a significant effect of ACN on triglyceride concentrations, total cholesterol, LDL-C and HDL-C concentrations in all subgroups of participants (with vs. without dyslipidemia at baseline) and intervention type (supplement/extract vs. food). However, we did not observe any significant effect on apo A and apo B concentrations.
Conclusions
ACN intake in the forms of natural foods and supplements can induce healthy changes in body fat mass, glycemic and lipidemic status and these effects are more prominent in the subjects with above-normal values. This meta-analysis was registered at http://www.crd.york.ac.uk/Prospero (Registration no. CRD42021286466).
Similar content being viewed by others
Background
Cardiovascular diseases (CVD), comprising ischemic heart disease, stroke, heart failure, peripheral arterial disease, and a number of other cardiac and vascular conditions are still the leading contributors to disease burden worldwide. According to the World Health Organization (WHO) by 2025, over 28 million individuals will die from CVD annually [1, 2]. There is, therefore, an urgent need to develop and implement safe and cost-effective interventions and policies [3].
Over the past decade, there has been an increasing interest in lifestyle and dietary interventions to lessen chronic disease risks. A huge body of evidence shows that fruits and vegetables intake is inversely associated with the risk of the most common chronic diseases including CVD [4]. These effects are attributed to the abundance of bioactive compounds in fruits and vegetables [5].
Anthocyanins (ACN) seem to be the major subgroup of flavonoids (pigments occurring in fruits and vegetables) having exclusive metabolic and biological behaviors [6, 7]. There are more than 700 structurally distinct ACN derivatives that naturally occur in plants as anthocyanidin (aglycone) attached to sugar moieties [8]. Important sources of ACN in a typical American diet include berries, currants, grapes, wine and vegetable sources include (red) cabbages, radishes, onions, grains, roots and tubers [9].
A growing body of evidence indicates that consumption of ACN-rich products can reduce the risk of CVD, mainly in the subjects at risk [10, 11]. For example, a clinical research demonstrated that consumption of pomegranate juice, which contains ACN and tannins, lowers blood pressure and carotid intima media thickness [12]. Also, meta-analyses of prospective cohort studies suggest that high intakes of ACN may be associated with a decreased risk of CVD [13, 14] and type 2 diabetes (T2D) [15]. Though a meta-analytical study reported the beneficial impacts of ACN on cardiometabolic health some five years ago [16], current data are still conflicting [17, 18]. Furthermore, the possible effects of ACN on some important variables including waist circumference and body fat mass have not already been summarized and analyzed. In this meta-analysis, findings from recent studies on the preventive properties of ACN against CVD are summarized and current knowledge of the field is updated. To do this, we included 41 clinical trial studies and performed subgroup analysis and meta-regression for the cardiometabolic risk factors including body mass index, waist circumference and body fat mass, that have not been examined in the previous meta-analysis.
Methods
This systematic review and meta-analysis was planned, conducted, and reported according to the widely accepted quality standards [19] and was registered at http://www.crd.york.ac.uk/Prospero (Registration no. CRD42021286466).
Types of interventions and outcome measures
The interventions investigated in this review were giving ACN either as supplement or food in various doses and frequencies as compared with a control group. No limit was placed on the dose, type of vehicle or frequency at which supplements and/or fortified foods were taken.
Outcome measures were cardiometabolic risk factors including:
Anthropometric measures: weight (kg), body mass index (BMI) (kg/m2), waist circumference (WC) (cm), body fat mass (FM)(%).
Glycemic indicators: fasting blood glucose (FBG) (mg/dL), fasting serum insulin (FSI) (µIU/mL), two-hour glucose tolerance test (mg/dL), homeostasis model assessment-estimated insulin resistance (HOMA-IR), glycated hemoglobin (HbA1c) (%).
Lipid profile: serum triglyceride (TG) (mg/dL), total cholesterol (TC) (mg/dL), low density lipoprotein-cholesterol (LDL-C) (mg/dL), high density lipoprotein-cholesterol (HDL-C) (mg/dL), apolipoprotein A (Apo A) (mg/dL), apolipoprotein B (Apo B) (mg/dL).
Search strategy
A comprehensive search of electronic databases was conducted for eligible trials up to September 2021. The following databases were covered: National Library of Medicine (PubMed), Scopus, Web of Science (WoS), and Cochrane Database of Systematic Reviews (Cochrane Library, CDSR) using the following search terms in titles and abstracts: 1- (anthocyanin) AND (Anthropometric measures: weight, body mass index (BMI), waist circumference (WC), fat mass (FM)) 2- (anthocyanin) AND (Glycemic indicators: fasting blood glucose (FBG), fasting serum insulin (FSI), two-hour glucose tolerance test, homeostasis model assessment-estimated insulin resistance (HOMA-IR), HbA1c), 3- (anthocyanin) AND (lipid profile: serum triglyceride (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), apolipoprotein A (apo A), apolipoprotein B (apo B)).
All of the studies were limited to English language and those in humans. All the references in the papers of the included studies were scanned for further relevant reports.
Types of studies: inclusion and exclusion criteria
Two investigators independently searched and assessed articles for eligibility according to the following inclusion criteria:
-
(1)
all studies with a randomized clinical trial design;
-
(2)
data description as mean ± SD and sufficient data of outcomes at both baseline and the end of intervention in each group or the net change values provided
Studies were excluded based on the following criteria:
-
(1)
incomplete data;
-
(2)
duplicate publication of articles;
-
(3)
obscurely reported outcomes, or lack of control groups; and
-
(4)
animal as well as non-interventional studies
Data extraction and management
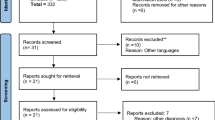
Two reviewing authors independently screened titles or abstracts to exclude studies that failed to meet the inclusion criteria and then obtained the full-text reports for further evaluations. Discrepancies were resolved through consensus. Detailed data of study design, context, participants’ information, interventions and outcomes were extracted. For multi-armed studies, pairs of arms relevant to the review were compared. Data of the control group were used for each intervention group comparison. The weight assigned to the control group was reduced by dividing the control group number (N) by the number of intervention groups. The selection process in sufficient detail to complete a PRISMA flow diagram was recorded in the supplementary data.
Assessment of risk of bias in the included studies
In order to assess the risk of bias for each of the included studies, the two reviewing authors independently assessed the quality of the included studies according to the Cochrane risk of bias tool [20] and following criteria: (i) random sequence generation, (ii) allocation concealment, (iii) blinding of participants, (iv) blinding of personnel and outcome assessment, (v) incomplete outcome data, and (iv) selective outcome reporting and other bias (bias due to problems not covered elsewhere, e.g. industry funding).
Assessment of reporting biases
Funnel plots were used to assess the potential existence of bias (were not shown). We performed a regression asymmetry test for detection of bias [21]. In this study, p < 0.05 was considered significant.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity and there were sufficient data, we would investigate it using subgroup as well as sensitivity analyses. The following subgroup analyses were carried out:
-
participants’ health status ( having diabetes, dyslipidemia, …)
-
type of intervention (supplements/extract vs. food)
Sensitivity analysis
The aim of the sensitivity analysis was to examine the effect size when some studies had less stringent inclusion criteria. If there were sufficient data to allow for sensitivity analysis, this analysis would be performed for examining the effects on results by excluding:
-
(i) Trials at high risk of bias, as specified in the Assessment of risk of bias in included studies section.
-
(ii) Trials with small sample sizes (less than 15 participants in each group);
Moreover, the leave-one-out analysis was performed to assess the key studies with substantial impact on between-study heterogeneity.
Meta-regression
Random‐effects model meta‐regression analyses were performed to assess sources of inter-trial heterogeneity. The follow-up time, ACN dose and variables at baseline (according to model and dependent variable) were considered as covariates.
Statistical analyses
Meta-analysis was performed using STATA version 17.0 (StataCorp, College Station, TX). For continuous outcomes, a mean difference (MD) and 95% confidence interval (CI) calculated for each study (i.e. intervention group minus control group differences).
The data reported as median were converted to mean and SD using the formula recommended by the Cochrane Collaboration Handbook [20].
In addition, heterogeneity was assessed using Q and I2 tests. The fixed effect model was used as pooling method when there was no statistically significant heterogeneity (I2 < 50%), whereas the random-effects model was employed on the contrary (I2 > 50%).
The Egger’s test was used to assess risk of publication bias.
Results
Initially, 2,512 studies were found in a preliminary search from which 2,444 studies were excluded after removing duplicates and primary screening of titles and abstracts, another 21 studies were excluded due to following reasons:
-
1) There was no control group;
-
2) The studies provided no available data.
Finally, a total of 47 studies were selected for the analysis. A flow diagram showing the selection process is presented in Fig. 1.
Characteristics and bias risk assessment of included studies
The basic characteristics of the 47 randomized clinical trials (RCTs) are listed in Table 1. There were eight studies in healthy participants [22,23,24,25,26,27,28,29], four in prediabetic adults [30,31,32,33], eight in subjects with metabolic syndrome [34,35,36,37,38,39,40,41], one in elderly with mild to moderate dementia Alzheimer [42], four in overweight/obese subjects [43,44,45,46], one in adults with cardiovascular risk factors [47], seven in patients with T2D [48,49,50,51,52,53,54], eight in dyslipidemia [55,56,57,58,59,60,61,62], one in subjects with non-alcoholic fatty liver disease [63] and three in patients with ischemic coronary heart disease [64,65,66]. Trial intervention duration ranged from 3 weeks [46] to 24 months [50]. The sample size from all the trials ranged between 20 [29, 54, 67] and 160 participants [32, 33].
A total of 23 trials compared provision of ACN supplement/extract in comparison with placebo or no intervention [23, 25, 28, 31,32,33, 43, 44, 46, 48,49,50,51,52, 54,55,56,57,58,59,60,61,62,63, 65].
Risk of bias in included studies
All trials provided data on losses to follow-up; eleven studies reported losses of > 10% [28, 30, 31, 34, 36, 40, 42, 44, 48, 55, 65] (Additional file 1: Figures S1 and S2). In thirteen trials, the information on the methods used for allocation concealment was unclear [23, 24, 30, 34,35,36,37,38, 42, 43, 47, 67, 68]. Three trials reported small sample size (< 15 subjects in each arm) [27, 67, 68].
Effect of anthocyanin on body mass index
Changes of BMI (kg/m2) after intervention with ACN were evaluated in twenty studies. From two studies that had multi-arm intervention, two pairs of arms were included in the analysis [30, 37]. Four studies included participants who were overweight or obese at baseline [27, 43,44,45].
The results of twenty trials showed that intervention with ACN resulted in a significant decrease in BMI (1,280 participants, mean difference (MD),− 0.21; 95% CI, − 0.38, − 0.04; P < 0.001) (Additional file 1: Figure S3).
The meta-regression analysis (random effects and maximum likelihood) with the difference of means as the dependent variable and the follow-up time (β = 0.01; 95% CI − 0.02, 0.042; p = 0.484), ACN dose (β = 0.00; 95% CI − 0.001, 0.00; p = 0.466) and BMI at baseline (β = 0.23; 95% CI − 0.04, 0.09; p = 0.490) as covariates showed no significant effects.
After conducting stratified analyses according to the type of ACN (supplement/extract vs. food), we found a statistically significant effect in both subgroups (Fig. 2). However, unlike supplement (MD: − 0.35; 95% CI − 0.47, − 0.23; P < 0.001), using ACN as powdered or food was accompanied by an increase in BMI in the intervention group as compared with control (MD: 0.1, 95% CI 0.04, 0.16; P = 0.001).
Sensitivity analyses were performed and made no remarkable change in overall results (MD: − 0.20, 95% CI − 0.39, − 0.04, P = 0.01). The results of leave-one-out analysis were shown in the (Additional file 1: Figure S4).
The Egger’s tests indicated no significant publication bias (P = 0.605).
Effect of anthocyanin on waist circumference
Eight trials compared the effect of ACN intake, as supplement/foods, versus control on waist circumference (WC). From one trial, two paired-arms were included in the analysis. Pooled data showed no statistically significant effect on WC (614 participants, MD: 0.78 cm, 95% CI − 0.43, 1.98 cm; P = 0.21) (Additional file 1: Figure S5).
Sensitivity analysis was performed for examining the effects of excluding trials with small sample sizes. The results were shown no significant effect (MD: − 0.09 cm, 95% CI − 1.25, 1.07; P = 0.88).
The results of leave-one-out analysis were shown in the (Additional file 1: Figure S6).
Effect of anthocyanin on body fat mass
Four trials compared the effect of ACN intervention with control on body fat mass (FM). Pooled data showed statistically significant effect on FM changes (150 participants, MD: − 0.3%, 95% CI − 0.42 to − 0.18%, p < 0.001) (Additional file 1: Figure S7).
The meta-regression analysis (random effects and maximum likelihood) with the difference of means as the dependent variable and the follow-up time (β = 0.01; 95% CI − 0.24, 0.27, p = 0.916) and used dose of ACN (β = 0.001; 95% CI − 0.01, 0.014, p = 0.853) as covariates showed the insignificant effects.
There were no data to allow for sensitivity analysis. The Egger’s tests indicated no significant publication bias (P = 0.881).
The results of leave-one-out analysis were shown in the (Additional file 1: Figure S8).
Effect of anthocyanin on fasting blood glucose
We pooled and analyzed data from 28 RCTs evaluating the effects of ACN intake on FBG. From three studies that had multi-arm intervention, two pairs of arms were included in the analysis [30, 37]. The forest plots for the effects of ACN intake on FBG are shown in the Additional file 1: Figure S9. The pooled MD was − 2.55 mg/dL (95%CI − 4.23, − 0.87), indicating a significantly greater reduction in FBG concentrations in the ACN intervention group than in the control (placebo) group.
The subgroup analyses were carried out to explore the impact of type of intervention (supplement/extract vs. food) and health status of participants (with diabetes vs. without diabetes). Significant reductions were observed in both subgroups of T2D patients (434 participants, MD: − 15.33 mg/dL; 95% CI − 25.8, − 7.86 mg/dL; P < 0.001) and participants without diabetes (1124 participants, MD: − 1.55 mg/dL; 95% CI − 2.38, − 0.72 mg/dL, P = 0.001). However, reduction of serum glucose concentrations was significantly more in the subjects with T2D (p < 0.001).
After a subgroup analysis, we found that FBG decreased significantly only in those who used ACN as supplement/extract (MD: − 3.13 mg/dL, 95% CI − 4.9, − 1.35 mg/dL; P = 0.004) (Fig. 3).
The univariate meta-regression suggested that a higher baseline FBG was associated with a better effect of ACN intake on FBG changes (β = − 0.25; 95% CI − 0.34, − 0.15; P < 0.001) and this association remained significant after adjusting for duration of intervention, age of participants, dose of ACN and baseline BMI (β = − 0.25; 95% CI − 0.37, − 0.12; P < 0.001).
Sensitivity analyses were performed to test the robustness of the results and made no remarkable change in overall results (MD: − 3.0 mg/dL, 95%CI − 5.6, − 0.4 mg/dL, P = 0.02).
The results of leave-one-out analysis were shown in the (Additional file 1: Figure S10).
The Egger’s tests suggested that no significant publication bias (P = 0.089).
Effect of anthocyanin on fasting serum insulin
Eleven randomized controlled trials (two paired-arms from two studies) (n = 653) had measured fasting insulin. Results showed no significant effect of ACN intake on fasting insulin (MD: 0.64 µIU/mL; 95% CI − 0.33, 1.61 µIU/mL; P = 0.19) (Additional file 1: Figure S11).
A subgroup analysis was conducted to investigate heterogeneity using known factors that might influence circulating insulin, including participants (with vs. without diabetes) and intervention type (supplement/extract vs. food). Analyses did not show any significant influence (Additional file 1: Figure S12).
Results of sensitivity analysis showed no significant effect of ACN intake on fasting insulin (MD: 0.064 µIU/mL; 95% CI − 92, 1.09 µIU/mL; P = 0.87). The regression asymmetry test was not significant (p = 0.413).
The results of leave-one-out analysis were shown in the supplementary file (Additional file 1: Figure S13).
Effect of anthocyanin on HbA1c
There were ten studies (three paired-arms from two studies) that had examined the effects of ACN intake on HbA1c. Meta-analysis results indicated that ACN consumption decreased HbA1c (MD: − 0.14%; 95% CI − 0.26, − 0.02%; P = 0.03). However, subgroup analysis showed this effect only in the participants with T2D (MD: − 0.29%; 95% CI − 0.47, − 0.10%; P = 0.003) and in those trials that used ACN as supplement/extract (MD: − 0.17%; 95% CI − 0.27, − 0.07; P = 0.001) (Fig. 4, and Additional file 1: Figure S14).
The univariate meta-regression suggested that a higher baseline FBG was associated with a better effect of ACN intake on HbA1c changes (β = − 0.13; 95% CI − 0.24, − 0.03; p = 0.011) and this association remained significant after adjusting for ACN dose and baseline BMI (β = − 0.16; 95% CI − 0.31, − 0.019; p = 0.026).
Sensitivity analyses were performed to test the robustness of the results and revealed no remarkable change in overall results (MD: − 0.19%, 95%CI − 0.4, − 0.02%, P = 0.08). The Egger’s tests indicated no significant publication bias (P = 0.532).
The results of leave-one-out analysis were shown in the (Additional file 1: Figure S15).
Effect of anthocyanin on serum triglycerides concentrations
We pooled and analyzed data from 38 RCTs evaluating the effects of ACN intake on serum TG concentration. From Four studies [30, 32, 37, 59] that had multi-arm intervention, two pairs of arms were included in the analysis (Additional file 1: Figure S16). The pooled MD was − 9.46 mg/dL (95%CI, − 14.6, − 4.31 mg/dL; P < 0.001, indicating a significantly greater reduction in TG concentrations in participants who received ACN than those used placebo.
The subgroup analysis test showed that there was a significant effect of ACN on TG concentrations in all subgroups of participants (with vs. without dyslipidemia at baseline) and intervention type (supplement/extract vs. food). Furthermore, there were no significant differences between subgroups (p = 0.27 and p = 0.13, respectively) (Additional file 1: Figure S17).
In meta-regression, changes in serum TG concentrations following ACN intervention were found to be independent of treatment duration (β = − 0.49; 95% CI − 2.17, 1.18; p = 0.566), baseline BMI (β = − 0.56; 95% CI − 2.3, 1.18; p = 528) and age of subjects (β = 0.59; 95% CI − 0.39, 1.58; p = 0.239). Nevertheless, changes in TG were significantly associated with the baseline TG concentrations (β = − 0.21; 95% CI − 0.29, − 0.12; p < 0.001).
Results of sensitivity analysis showed significant effect of ACN intake on serum TG similar to the overall effect (MD: − 11.68 mg/dL; 95% CI − 18.04, − 5.32 mg/dL; P < 0.001). The regression asymmetry test was not significant (p = 0.971).
The results of leave-one-out analysis were shown in the (Additional file 1: Figure S18).
Effect of anthocyanin on total cholesterol concentrations
Data from 40 trials (four trials with multi-arm interventions) involving 2433 subjects showed that consumption of ACN, as compared with placebo, reduces serum TC concentration (MD: − 6.98%; 95% CI − 11.97, − 2.0 mg/dL; P = 0.01) (Additional file 1: Figure S19).
Subgroup analyses were carried out for participants (with vs. without dyslipidemia at baseline) and intervention (supplement/extract vs. food). The mean change in TC concentration in trials using supplement/extract (MD: − 9.76 mg/dL, 95% CI − 17.33 to − 2.2 mg/dL; P = 0.011) was greater than from daily foods (MD: − 3.10 mg/dL; 95% CI − 8.24, 2.05 mg/dL; P = 0.238). Also, studies with baseline dyslipidemia showed greater decreases in TC concentrations (MD: − 13.41 mg/dL; 95% CI − 22.95, − 3.88 mg/dL; P = 0.006) than those without baseline dyslipidemia (MD: − 2.30 mg/dL; 95% CI − 6.78, 2.18 mg/dL; P = 0.315) (Additional file 1: Figure S20).
The univariate meta-regression suggested that a higher baseline TC was associated with a better effect of ACN intake on TC changes (β = − 0.36; 95% CI − 0.60, − 0.15; P = 0.002). Adjustments for ACN dose (β = 0.017; 95% CI − 0.02, 0.052; P = 0.327), duration of intervention (β = 1.12; 95% CI − 0.19, 1.44; P = 0.853) and baseline BMI (β = − 0.36; 95% CI − 1.71, 0.98; P = 0.594) revealed no significant effect of these variables.
Sensitivity analyses were performed to test the robustness of the results and showed effects similar to the overall effects (MD: − 8.63 mg/dL, 95%CI − 14.6, − 2.61, P < 0.001). The regression asymmetry tests suggested that no significant publication bias (P = 0.200).
The results of leave-one-out analysis were shown in supplementary file (Additional file 1: Figure S21).
Effect of anthocyanin on LDL-C concentrations
Thirty nine trials compared the effect of ACN intervention on serum LDL-C concentrations with control. Pooled data showed statistically significant effect on LDL-C changes (MD: − 6.91 mg/dL; 95% CI − 9.99, − 3.83 mg/dL; P < 0.001) (Additional file 1: Figure S22).
The effect of ACN was similar in all types of intervention (supplement/extract vs. food) (Additional file 1: Figure S23). Nevertheless, ACN intake could decrease serum LDL-C concentrations only in dyslipidemic subjects (MD: − 10.68 mg/dL; 95% CI − 14.85, − 6.52 mg/dL; P < 0.001) but not in those with normol blood lipids (MD: − 3.77 mg/dL; 95% CI − 7.6, 0.06: P = 0.054).
Our meta-regression suggested the association between ACN intake and LDL-C was not affected by duration of intrvention (β = − 0.25; 95% CI − 1.03, 0.53; P = 0.531), dose of intervention (β = − 0.003; 95% CI − 0.028, 0.021; P = 0.786), BMI at baseline (β = − 0.56; 95% CI − 1.54, 0.41; P = 0.257) and LDL-C concentrations at baseline (β = − 0.12; 95% CI − 0.32, 0.07; P = 0.216).
Results of sensitivity analysis showed significant effect of ACN intake on serum LDL-C MD:—7.72 mg/dL; 95% CI − 11.23, − 4.21 mg/dL; P < 0.001). The regression asymmetry test was not significant (p = 0.365).
The results of leave-one-out analysis were shown in (Additional file 1: Figure S24).
Effect of anthocyanin on serum HDL-C concentrations
Serum HDL-C concentration was evaluated in 39 studies (four studies with multiple-arms). Meta-analysis revealed positive effect of ACN intake on HDL-C concentrations (MD: 1.71 mg/dL; 95% CI 0.43, 2.08 mg/dL; p = 0.01) (Additional file 1: Figure S25).
Subgroup analyses were carried out for participants (with vs. without dyslipidemia at baseline) and intervention (supplement/extract vs. food). The mean change in serum HDL-C concentration in those trials on subjects with dyslipidemia (MD: 2.35 mg/dL; 95% CI 0.26, 4.45 mg/dL; P = 0.028) was greater than the trials conducted on participants normal blood lipids (MD: 0.73 mg/dL; 95% CI 0.03, 1.43 mg/dL; P = 0.041) (Additional file 1: Figure S26).
Findings from meta-regression suggested the association between ACN intake and serum HDL-C was not affected by duration of intervention (β = 0.26; 95% CI − 0.06, 0.58; P = 0.114), dose of intervention (β = − 0.002; 95% CI − 0.01, 0.005; P = 0.494), BMI at baseline (β = − 0.0008; 95% CI − 0.34, 0.34; P = 0.996) and serum HDL-C concentrations at baseline (β = − 0.04; 95% CI − 0.09, 0.012; P = 0.130).
Sensitivity analyses were performed to test the robustness of the results and showed positive effect of ACN intake on HDL-C concentrations similar to the overall effects (MD: − 2.16 mg/dL, 95% CI 0.71, 3.6 mg/dL, P < 0.001). The Egger’s tests indicated no significant publication bias (P = 0.182).
The results of leave-one-out analysis were shown in (Additional file 1: Figure S27).
Effect of anthocyanin on serum apolipoprotein A (Apo A) concentrations
Meta-analysis of RCTs yielded a weighted estimate for the overall effect of ACN intake of MD: 3.94 mg/dL (95% CI 0.77 to 7.11) on Apo A concentrations (Additional file 1: Fig. S28). Apo A estimates were not significantly different among subgroups (without dyslipidemia vs. with dyslipidemia and supplement/extract vs. food) (Additional file 1: Figure S29).
The meta-regression analysis suggested that effect of the ACN intake on Apo A concentrations was not affected by duration of intrvention (β = − 0.49; 95% CI − 2.5, 1.51; P = 0.631), dose of intervention (β = 0.0005; 95% CI − 0.02, 0.021; P = 0.962), BMI at baseline (β = − 0.71; 95% CI − 2.76, 1.32; P = 0.490) and Apo A concentrations at baseline (β = 0.18; 95% CI − 0.06, 0.42; P = 0.155).
Results of sensitivity analysis showed similar significant effect of ACN intake on serum apo A, (MD: 3.91 mg/dL; 95% CI 0.16, 7.65 mg/dL; P = 0.04). The regression asymmetry test was not significant (p = 0.301).
The results of leave-one-out analysis were shown in (Additional file 1: Figure S30).
Effect of anthocyanin on serum apolipoprotein B (Apo B) concentrations
The meta-analysis showed that ACN intake was not associated with significant changes in serum apo B concentrations (MD: − 2.36 mg/dL; 95% CI − 5.55, 0.83 mg/dL; P = 0.15). There was no statistically significant changes in circulating apo B in subgroup analysis, either (Additional file 1: Figures S31 and S32).
The meta-regression analysis suggested that effect of ACN intake on serum apo B concentrations was not affected by duration of intervention (β = − 1.13; 95% CI − 3.64, 1.38; P = 0.378), dose of intervention (β = − 0.015; 95% CI − 0.04, 0.009; P = 0.223), BMI at baseline (β = 3.6; 95% CI − 3.02, 10.22; P = 0.287) and apo B concentrations at baseline (β = 0.39; 95% CI − 0.26, 1.05; P = 0.241).
Results of sensitivity analysis showed no significant effect of ACN intake on serum apo B, (MD: − 1.9 mg/dL; 95% CI − 5.27, 1.47 mg/dL; P = 0.27). The regression asymmetry test was not significant, either (p = 0.128).
The results of leave-one-out analysis were shown in (Additional file 1: Figure S33).
Discussion
The findings of this meta-analysis confirmed the positive effects of ACN intake on certain cardiometabolic risk factors including BMI and more importantly FM, glycemic and lipidemic profiles. A combination of these factors occur in metabolic syndrome (MetS) that is characterized by central obesity, dysglycemia, above normal blood pressure, low serum HDL-C and high TG concentrations [69]. There is a remarkable higher risk of developing various diseases including CVD and diabetes in the subjects with MetS and that is why prevention and treatment of MetS can actually reduce CVD risk [70].
Anthocyanins and body mass index
We found the positive effect of ACN consumption on reduction of BMI which further confirms previous reports [71, 72]. Nevertheless, our finding on the supplement form-dependent effect of ACN consumption on BMI is quite noticeable, i.e. ACN supplement (like tablet) causes decrease whereas powdered ACN results in increase in BMI. On re-analysis of the data, however, we found out that this controversial finding was mostly due to one study [41]. Notwithstanding, in that study the increase in body weight and hence BMI was mostly due to lean body mass and not fat mass [41]. When we excluded this study from analysis, this effect was removed (MD − 0.14 (− 0.66, 0.9), P = 0.610).
Sensitivity analysis using the leave-one-out approach indicated that the direction of the combined estimates did not vary markedly with the removal of each study in turn. However, removal of Stull et al. [41] that used yogurt and skim milk-based smoothie as intervention, changed the effect size from − 0.21 to − 0.35.
Anthocyanins and body fat mass
Some evidence indicates that truncal adiposity is the core constituent of the metabolic derrangements observed in MetS [73, 74]. Our data showed the positive effect of ACN intake on total FM and not on central obesity, as judged by WC. However, we were unable to perform subgroub analysis due to the limited eligible RCTs.
The association between body FM variability and CVD risk has already been reported [75]. Nonetheless, strong evidence supports the contribution of truncal fat distribution, even in the context of normal BMI, to the increased risk of CVD [76, 77]. One possible explanation for this dicrepancy in results could be the difference in precision of the methods of two assessements as FM is assessed using an instrument like dual-energy X-ray absorptiometry (DEXA) whereas WC is measured using a measuring tape that is less sensitive to detect changes. This is especially the case when changes occur in visceral fat [78] that is more related to CVD risk [79].
The exact mechanism of anti-adipogenesis function of ACN has not been fully elucidated yet. Some evidence suggests an inhibitory effect of ACN against adipocyte differentiation that might be exerted through 5' adenosine monophosphate-activated protein kinase (AMPK) signaling pathway activation [80, 81].
Anthocyanins and glycemic status
The findings of this meta-analysis confirmed the alleviative effect of ACN on glycemic status including FBS and HbA1c. Notwithstanding, the effect on both variables was more prominent in those subjects with above-normal values, notably in the subjects with T2D. It has been documented that ACN has the potential to upregulate and also downregulate certain glucose metabolic pathways including AMPK whereby exerts its modulating effect on glucose homeostasis [82]. Other proposed mechanisms for anti-diabetic actions of ACN include inhibition of α-amylase, α-glucosidase and modulating effect on glucose transporters, inhibition of glycogenolysis and modulation of gut microbiota [83].
We found better effect on HbA1c of ACN when consumed as a supplement. It has been shown that ACN in foods may interact with other biomolecules including proteins and polysaccharides that may affect ACN activity [83]. Evaluation of the efficacy of ACN in natural foods versus supplements need further investigations.
Anthocyanins and lipidemic status
Our findings documented the effects of ACN on healthy changes of blood lipids including a decrement of TG, TC and LDL-C and an increment of HDL-C. Again here, better effects were achieved in the subjects with deranged blood lipid profile. Nevertheless, data from this meta-analysis failed to show any effect of ACN on serum concentrations of apo A and apo B. The mechanism of action of ACN on healthy modification of blood lipids is under investigation. Some studies indicate that ACN can reduce TC possibly via increased fecal excretion of neutral and acidic sterols together with down-regulation of hepatic β-hydroxy β-methyl glutaryl coenzyme A (HMGCoA) reductase, the rate-limiting enzyme of cholesterol biosynthesis [84]. The mechanism of TG-lowering effect of ACN remains to be elucidated. This effect has been attributed to the reduction of apo B and CIII [85]. Notwithstanding, our meta-analysis failed to show any significant effect of ACN on serum apo B concentrations. ACN-induced decrement of circulating LDL-C may be partly due to downregulation of cholesteryl ester transfer protein [86] and upregulation of LDL-receptor [87]. It has also been proposed that ACN can affect both liver and blood lipids contents via activation of AMPK. Activated AMPK in turn induces acetyl coenzyme A carboxylase phosphorylation and upregulates hepatic peroxisome proliferator-activated receptor-alpha (PPAR-α), acylCoA oxidase and palmitoyltrasferase-1 A [88].
Limitations
This study has some limitations. We did not evaluate the antioxidant effect of ACN that can have a major contribution to prevention of CVD [89, 90] and other diseases [91, 92]. Nonetheless, the efficacy of ACN as an antioxidant has been recently confirmed in a meta-analysis [93]. Limited studies on effects of ACN intake on waist circumference, fat mass, apo A and apo B concentrations (n < 10) could effect on generalizability of results of meta-regression tests. Furthermore, we did not evaluate the effect of ACN on biomarkers of systemic and vascular inflammation, which has an axial role in both development and progression of CVD [94, 95].
The other limitation of our study is scanty available RCTs that evaluated the long-term effects of ACN intake. Though some RCTs had treatment durations of up to two years, the question of the effectiveness of long-term ACN intake remains unanswered due to the lack of experimental evidence.
In some RCTs, especially those that used ACN-containing foods, the exact amount of ACN consumption was not reported. Tt was not, therefore, possible to perform dose–response analysis. Besides, in most RCTs, ACN intake from dietary sources was not adjusted and this could affect the results.
The intervention strategy across the RCTs was diverse with respect to the dosage of ACN, the frequency of intake and type of intervention (food/supplement), which inevitably contributed to the high levels of heterogeneity. We attempted to reduce the heterogeneity by choosing appropriate statistical models and using sensitivity analyses. Notwithstanding, this point should be considered when interpreting and generalizing the results.
Finally, the present meta-analysis employed just the papers published in English. Thus, the papers in other languages or unpublished data might potentially increase the heterogeneity in the present results.
Conclusion
The findings of this meta-analysis indicate that ACN intake in the forms of natural foods and supplements can induce healthy changes in body FM, glycemic and lipidemic status and these effects are more prominent in the subjects with above-normal values. Further well-designed studies are needed to evaluate and compare the efficacy of ACN consumption as natural food sources versus supplements. Meanwhile, fortification of foods and drinks with ACN can also be considered to increase ACN intake in the general population as a preventive strategy against many diseases notably CVD.
Availability of data and materials
All data generated or analyzed during this study were presented in this article.
Abbreviations
- DEXA:
-
Dual-energy X-ray absorptiometry
- FBG:
-
Fasting blood glucose
- FM:
-
Fat mass
- FSI:
-
Fasting serum insulin
- HbA1c:
-
Glycated hemoglobin
- HDL-C:
-
High density lipoprotein-cholesterol
- HMGCoA:
-
β-Hydroxy β-methyl glutaryl coenzyme A (HMGCoA)
- HOMA-IR:
-
Homeostasis model assessment-estimated insulin resistance
- LDL-C:
-
Low density lipoprotein-cholesterol (LDL-C)
- MD:
-
Mean difference
- MetS:
-
Metabolic syndrome
- PPAR-α:
-
Peroxisome proliferator-activated receptor-alpha
- RCTs:
-
Randomized clinical trials
- TC:
-
Total cholesterol
- T2D:
-
Type 2 diabetes
- TG:
-
Triglyceride
- WC:
-
Waist circumference
- WHO:
-
World Health Organization
- WoS:
-
Web of Science
References
World Health Organization. Cardiovascular diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en/index.html 2009. https://ci.nii.ac.jp/naid/10025942218/en/. Accessed 28 Aug 2022.
Dugani S, Gaziano TA. 25 by 25: Achieving global reduction in cardiovascular mortality. Curr Cardiol Rep. 2016;18(1):10.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56.
González S. Dietary bioactive compounds and human health and disease. MDPI. 2020;12:348.
Lila MA, Burton-Freeman B, Grace M, Kalt W. Unraveling anthocyanin bioavailability for human health. Annu Rev Food Sci Technol. 2016;7:375–93.
Mendoza J, Basílio N. Rationalizing the color in heavenly blue anthocyanin: a complete kinetic and thermodynamic study. Food Nutr Res. 2018;122(19):4982–92.
Wallace TC, Giusti MM. Anthocyanins. Adv Nutr. 2015;6(5):620–2.
Khoo HE, Azlan A. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61(1):1361779.
Kimble R, Keane KM, Lodge JK, Howatson G. Dietary intake of anthocyanins and risk of cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2019;59(18):3032–43.
Gaiz A, Mosawy S, BusAdmin G, Colson N, Singh I, Gaiz AA. Potential of anthocyanin to prevent cardiovascular disease in diabetes. Altern Ther Health Med. 2018;24(3):40.
Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23(3):423–33.
Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111(1):1–11.
Kimble R, Keane KM, Lodge JK, Howatson G. Dietary intake of anthocyanins and risk of cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2019;59(18):3032–43.
Guo X, Yang B, Tan J, Jiang J, Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70(12):1360–7.
Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2017;8(5):684–93.
Moosavian SP, Maharat M, Chambari M, Moradi F, Rahimlou M. Effects of tart cherry juice consumption on cardio-metabolic risk factors: a systematic review and meta-analysis of randomized-controlled trials. Complement Ther Med. 2022;71:102883.
Ou JLS, Yang D, Liu MH. Effects of anthocyanins in composite meals on cardiometabolic outcomes—a systematic review of randomized controlled feeding trials. Nutrients. 2020;12(12):3781.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:142.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr. 2014;69(2):122–7.
Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. 2009;139(12):2266–71.
Bakuradze T, Tausend A, Galan J, Groh IAM, Berry D, Tur JA, et al. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radical Res. 2019;53(sup1):1045–55.
Murkovic M, Abuja P, Bergmann A, Zirngast A, Adam U, Winklhofer-Roob B, et al. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr. 2004;58(2):244–9.
Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. 2015;145(6):1185–93.
Wright OR, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the QUENCH trial. Can J Physiol Pharmacol. 2013;91(6):480–8.
Yarahmadi M, Askari G, Kargarfard M, Ghiasvand R, Hoseini M, Mohamadi H, et al. The effect of anthocyanin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int J Prev Med. 2014;5(12):1594.
Riso P, Klimis-Zacas D, Del Bo’ C, Martini D, Campolo J, Vendrame S, et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013;52:949–61.
An JH, Kim DL, Lee TB, Kim KJ, Kim SH, Kim NH, et al. Effect of Rubus Occidentalis extract on metabolic parameters in subjects with prediabetes: a proof-of-concept, randomized, double-blind Placebo-controlled clinical trial. Phytother Res. 2016;30(10):1634–40.
Yang L, Ling W, Yang Y, Chen Y, Tian Z, Du Z, et al. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes—a randomized controlled trial. Nutrients. 2017;9(10):1104.
Yang L, Ling W, Qiu Y, Liu Y, Wang L, Yang J, et al. Anthocyanins increase serum adiponectin in newly diagnosed diabetes but not in prediabetes: a randomized controlled trial. Nutr Metab. 2020;17:1–8.
Yang L, Qiu Y, Ling W, Liu Z, Yang L, Wang C, et al. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: a randomized controlled trial. Eur J Nutr. 2021;60:1935–44.
Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–7.
Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, et al. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res. 2010;30(7):462–9.
Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res. 2011;31(3):190–6.
Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr. 2014;144(6):830–7.
Jeong HS, Hong SJ, Lee TB, Kwon JW, Jeong JT, Joo HJ, et al. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res. 2014;28(10):1492–8.
Kim H, Simbo SY, Fang C, McAlister L, Roque A, Banerjee N, et al. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose-or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018;9(6):3097–103.
Puupponen-Pimiä R, Seppänen-Laakso T, Kankainen M, Maukonen J, Törrönen R, Kolehmainen M, et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol Nutr Food Res. 2013;57(12):2258–63.
Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, et al. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2015;7(6):4107–23.
Kent K, Charlton K, Roodenrys S, Batterham M, Potter J, Traynor V, et al. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur J Nutr. 2017;56(1):333–41.
Davinelli S, Bertoglio JC, Zarrelli A, Pina R, Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J Am Coll Nutr. 2015;34(sup1):28–33.
Lee M, Sorn SR, Park Y, Park H-K. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: a randomized controlled trial. J Med Food. 2016;19(11):995–1003.
Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–8.
Alnajjar M, Barik SK, Bestwick C, Campbell F, Cruickshank M, Farquharson F, et al. Anthocyanin-enriched bilberry extract attenuates glycaemic response in overweight volunteers without changes in insulin. J Funct Foods. 2020;64:103597.
Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87(2):323–31.
Kianbakht S, Abasi B, Dabaghian FH. Anti-hyperglycemic effect of Vaccinium arctostaphylos in type 2 diabetic patients: a randomized controlled trial. Complement Med Res. 2013;20(1):17–22.
Lee I, Chan Y, Lin C, Lee W, Sheu WHH. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med. 2008;25(12):1473–7.
Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145(4):742–8.
Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp (a), and paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci. 2012;17(4):355.
Soltani R, Gorji A, Asgary S, Sarrafzadegan N, Siavash M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: a randomized double-blind placebo-controlled clinical trial. Evid Based Complement Altern Med. 2015;2015:1.
Stote KS, Wilson MM, Hallenbeck D, Thomas K, Rourke JM, Sweeney MI, et al. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: an 8-week, double-blind, randomized, placebo-controlled trial. Curr Dev Nutr. 2020;4(4):nzaa030.
Chan SW, Chu TT, Choi SW, Benzie IF, Tomlinson B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytother Res. 2021;35(6):3236–45.
Kianbakht S, Abasi B, Hashem DF. Improved lipid profile in hyperlipidemic patients taking Vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2014;28(3):432–6.
Naruszewicz M, Łaniewska I, Millo B, Dłużniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis. 2007;194(2):e179–84.
Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90(3):485–92.
Soltani R, Hakimi M, Asgary S, Ghanadian SM, Keshvari M, Sarrafzadegan N. Evaluation of the effects of Vaccinium arctostaphylos L Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evid Based Complemet Altern Med. 2014;2014:6.
Xu Z, Xie J, Zhang H, Pang J, Li Q, Wang X, et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—a randomized controlled trial. Eur J Clin Nutr. 2021;75(2):345–54.
Zhang X, Zhu Y, Song F, Yao Y, Ya F, Li D, et al. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutr Metab. 2016;13(1):1–12.
Zhao Y, Xu H, Tian Z, Wang X, Xu L, Li K, et al. Dose-dependent reductions in plasma ceramides after anthocyanin supplementation are associated with improvements in plasma lipids and cholesterol efflux capacity in dyslipidemia: a randomized controlled trial. Clin Nutr. 2021;40(4):1871–8.
Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013;23(9):843–9.
Zhang P-W, Chen F-X, Di Li W-HL, Guo H-H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine. 2015;94(20):e758.
Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, et al. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96(6):810–4.
Gurrola-Díaz CM, García-López PM, Sánchez-Enríquez S, Troyo-Sanromán R, Andrade-Gonzalez I, Gómez-Leyva J. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine. 2010;17(7):500–5.
Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93(5):934–40.
Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45(2):113–22.
Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28(2):193–9.
Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786.
Li X, Zhai Y, Zhao J, He H, Li Y, Liu Y, et al. Impact of metabolic syndrome and it’s components on prognosis in patients With cardiovascular diseases: a meta-analysis. Front Cardiovasc Med. 2021;8: 704145.
Tucakovic L, Colson N, Santhakumar AB, Kundur AR, Shuttleworth M, Singh I. The effects of anthocyanins on body weight and expression of adipocyte’s hormones: Leptin and adiponectin. J Funct Foods. 2018;45:173–80.
Park S, Choi M, Lee M. Effects of anthocyanin supplementation on reduction of obesity criteria: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13(6):2121.
Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7.
Bonikowske AR, Barillas Lara MI, Koepp KE. Fat mass index better identifies metabolic syndrome: insights from patients in early outpatient cardiac rehabilitation. J Clin Med. 2019;8(12):2147.
Saito Y, Takahashi O, Arioka H, Kobayashi D. Associations between body fat variability and later onset of cardiovascular disease risk factors. PLoS ONE. 2017;12(4): e0175057.
Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40(34):2849–55.
Blüher M, Laufs U. New concepts for body shape-related cardiovascular risk: role of fat distribution and adipose tissue function. Eur Heart J. 2019;40(34):2856–8.
Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity. 2011;19(2):402–8.
Ruiz-Castell M, Samouda H, Bocquet V, Fagherazzi G, Stranges S, Huiart L. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population based study. Sci Rep. 2021;11(1):9121.
Han MH, Kim HJ, Jeong JW, Park C, Kim BW, Choi YH. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of vitis coignetiae pulliat is associated with the activation of AMPK signaling pathway. Toxicol Res. 2018;34(1):13–21.
Lee B, Lee M, Lefevre M, Kim HR. Anthocyanins inhibit lipogenesis during adipocyte differentiation of 3T3-L1 preadipocytes. Plant Foods Hum Nutr. 2014;69(2):137–41.
Les F, Cásedas G, Gómez C, Moliner C, Valero MS, López V. The role of anthocyanins as antidiabetic agents: from molecular mechanisms to in vivo and human studies. J Physiol Biochem. 2021;77(1):109–31.
Oliveira H, Fernandes A, Brás F, NMateus, de Freitas NV, Fernandes I. Anthocyanins as antidiabetic agents—in vitro and in silico approaches of preventive and therapeutic effects. Molecules. 2020;25(17):3813.
Liang Y, Chen J, Zuo Y, Ma KY, Jiang Y, Huang Y, et al. Blueberry anthocyanins at doses of 0.5 and 1 % lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur J Nutr. 2013;52(3):869–75.
Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145(4):742–8.
Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90(3):485–92.
de Souza MO, Souza ESL, de Brito Magalhães CL, de Figueiredo BB, Costa DC, Silva ME, et al. The hypocholesterolemic activity of açaí (Euterpe oleracea Mart.) is mediated by the enhanced expression of the ATP-binding cassette, subfamily G transporters 5 and 8 and low-density lipoprotein receptor genes in the rat. Nutr Res. 2012;32(12):976–84.
Li D, Wang P, Luo Y, Zhao M, Chen F. Health benefits of anthocyanins and molecular mechanisms: update from recent decade. Crit Rev Food Sci Nutr. 2017;57(8):1729–41.
Reis JF, Monteiro VVS, de Souza GR, do Carmo MM, da Costa GV, Ribera PC, et al. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med. 2016;14(1):1–16.
Garcia C, Blesso CN. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radical Biol Med. 2021;172:152–66.
Tena N, Martín J, Asuero AG. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants. 2020;9(5):451.
Speer H, D’Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health—a focus on oxidative stress, inflammation and disease. Antioxidants. 2020;9(5):366.
Fallah AA, Sarmast E, Jafari T. Effect of dietary anthocyanins on biomarkers of oxidative stress and antioxidative capacity: a systematic review and meta-analysis of randomized controlled trials. Journal of Functional Foods. 2020;68: 103912.
Ruparelia N, Choudhury R. Inflammation and atherosclerosis: what is on the horizon? Heart. 2020;106(1):80–5.
Ministrini S, Carbone F, Montecucco F. Updating concepts on atherosclerotic inflammation: from pathophysiology to treatment. Eur J Clin Invest. 2021;51(5): e13467.
Acknowledgements
Not applicable.
Funding
This work has received no funding.
Author information
Authors and Affiliations
Contributions
The study was designed by BN with the intellectual assistance of TN. The comprehensive search of databases and reviewing of the manuscripts were performed by ZY and HR. All statistical analyses were done by BN. The preliminary manuscript was written by BN with the assistance of ZY and HR and was finalized by TN. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure 1S. Risk of bias summary: Reviewer authors’ judgements about each “risk of bias” item for every single included study; Figure 2S. Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies; Figure 3S. Forest plot of the effect of anthocyanins compared with control on body mass index (kg/m2); Figure 4S. The result of leave-one-out analysis of the effect of anthocyanin on body mass index (kg/m2); Figure 5S. Forest plot of the effect of anthocyanins compared with control on waist circumference (cm); Figure 6S. The result of leave-one-out analysis of the effect of anthocyanin on waist circumference (cm); Figure 7S. Forest plot of the effect of anthocyanins compared with control on body fat mass (%); Figure 8S. The result of leave-one-out analysis of the effect of anthocyanin on body fat mass (%); Figure 9S. Forest plot of the effect of anthocyanins compared with control on fasting blood glucose (mg/dL); Figure 10S. The result of leave-one-out analysis of the effect of anthocyanin on fasting blood glucose (mg/dL); Figure 11S. Forest plot of the effect of anthocyanins compared with control on fasting serum insulin (µIU/mL); Figure 12S. Forest plot of subgroup analysis of the effect of anthocyanins compared with control on fasting serum insulin (µIU/mL); Figure 13S. The result of leave-one-out analysis of the effect of anthocyanin on fasting serum insulin (µIU/mL); Figure 14S. Result of subgroup analysis of the effect of anthocyanins compared with control on HbA1c (%); Figure 15S. The result of leave-one-out analysis of the effect of anthocyanin on HbA1c (%); Figure 16S. Forest plot of the effect of anthocyanins compared with control on serum triglycerides concentrations (mg/dL); Figure 17S. Result of subgroup analysis of the effect of anthocyanins compared with control on serum triglyceride concentrations (mg/dL); Figure 18S. The result of leave-one-out analysis of the effect of anthocyanin on serum triglyceride concentrations (mg/dL); Figure 19S. Forest plot of the effect of anthocyanins compared with control on serum total cholesterol (mg/dL); Figure 20S. Result of subgroup analysis of the effect of anthocyanins compared with control on serum total cholesterol (mg/dL); Figure 21S. The result of leave-one-out analysis of the effect of anthocyanin on serum total cholesterol concentrations (mg/dL); Figure 22S. Forest plot of the effect of anthocyanins compared with control on serum LDL-C concentrations (mg/dL); Figure 23S. The result of subgroup analysis of the effect of anthocyanins compared with control on serum LDL-C concentrations (mg/dL); Figure 24S. The result of leave-one-out analysis of the effect of anthocyanin on serum LDL-C concentrations (mg/dL); Figure 25S. Forest plot of the effect of anthocyanins compared with control on serum HDL-C concentrations (mg/dL); Figure 26S. Result of subgroup analysis of the effect of anthocyanins compared with control on serum HDL-C concentrations (mg/dL); Figure 27S. The result of leave-one-out analysis of the effect of anthocyanin on serum HDL-C concentrations (mg/dL); Figure 28S. Forest plot of the effect of anthocyanins compared with control on serum Apo A concentrations (mg/dL); Figure 29S. Result of subgroup analysis of the effect of anthocyanins intake compared with control on serum Apo A concentrations (mg/dL); Figure 30S. The result of leave-one-out analysis of the effect of anthocyanin on serum Apo A concentrations (mg/dL); Figure 31S. Forest plot of the effect of anthocyanins compared with control on serum Apo B concentrations (mg/dL); Figure 32S. Result of subgroup analysis of the effect of anthocyanins intake compared with control on serum Apo B concentrations (mg/dL); Figure 33S. The result of leave-one-out analysis of the effect of anthocyanin on serum Apo B concentrations (mg/dL).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Neyestani, T.R., Yari, Z., Rasekhi, H. et al. How effective are anthocyanins on healthy modification of cardiometabolic risk factors: a systematic review and meta-analysis. Diabetol Metab Syndr 15, 106 (2023). https://doi.org/10.1186/s13098-023-01075-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01075-0