Abstract
Background
Observational epidemiological studies revealed that multiple serum biomarkers can be associated with the risk of oral and oropharyngeal cancer (OC/OPC). However, the causal relationship between them remains largely unknown. This study aimed to investigate the causal relationship between potential serum biomarkers and (OC/OPC).
Methods
A two-sample Mendelian randomization (MR) approach was performed to assess the causal association of 10 serum biomarkers with the risk of OC / OPC. Summary data on OC/OPC were obtained from a GWAS meta-analysis that included 2497 cases and 2928 controls. The TwoSampleMR package in R was used to perform MR analyzes. Inverse-variance weighted (IVW), Weighted median and MR-Egger methods were used to assess causal effects.
Results
Suggestive associations with increased risk of C-reactive protein (CRP) (OR 1.52, 95% CI 1.14 to 2.02), using the IVW method. MR-Egger regression suggested that directional pleiotropy was unlikely to bias the result (P = 0.19). The findings were robust to sensitivity analyzes. The risk of OC/OPC was not associated with serum 25-hydroxyvitamin D, HDL cholesterol, LDL cholesterol, total cholesterol, triglycerides, adiponectin, leptin, HbA1C and Insulin-like growth factor 1 (IGF 1).
Conclusions
This study supports that CRP was causally associated with an increased risk of oral and oropharyngeal cancer.
Similar content being viewed by others
Background
Head and neck cancer (HNC) is a common cancer worldwide, of which 50% are oral cavity and oropharyngeal cancer (OC/OPC) [1]. More than 60% of new cases are diagnosed as advanced and the overall survival rate in advanced OC/OPC is less than 50% [2, 3]. Although human papillomavirus, chewing of betel quid, and lifestyle such as smoking and alcohol consumption are identified as risk factors for OC/OPC, its etiology is not fully known [4]. Therefore, the identification of potential risk factors is better for understanding the pathogenesis of OC/OPC and is essential to reduce the morbidity and mortality of OC/OPC. Some potential risk factors can be reflected in serum biomarkers, such as chronic inflammation, obesity, and diabetes. Each type of serum biomarker can provide different information about the state of the disease. Inflammation is an enabling characteristic of cancer [5]. C-reactive protein (CRP) is a classic biomarker of chronic inflammation, and serum CRP was correlated with various diseases [6, 7]. Several observational epidemiological studies have found an association of CRP with the incidence of OC/OPC [8, 9]. A study reported that serum lipid levels were inversely associated with the occurrence of oral cancer [10]. Furthermore, the level of adiponectin was positively associated with the risks of squamous cell carcinoma [11].However, conventional observational studies have many drawbacks, such as unmeasured confounding and reverse causation.
Mendelian randomization (MR) is a tool designed to investigate the causal relationship between exposure and disease outcome using genetic variation as instrumental variables (IVs) [12]. MR analysis using such IVs resembles randomized clinical trials and is less susceptible to confounding and reverse causation [13]. Therefore, this study used two-sample MR to investigate the causal association between serum biomarkers and the risk of OC/OPC. We used genetic variants associated with serum biomarkers as instrumental variables to improve inference for a possible influence of serum biomarkers on the risk of developing OC/OPC.
Methods
Serum biomarkers for SNP selection
Figure 1 shows a flowchart of the study design. The following search keywords were used: oral cavity and oropharyngeal cancer, risk factor. Single nucleotide polymorphisms (SNPs) associated with potential serum biomarkers were available from previously published GWAS studies of European descent. Construct genetic tools by obtaining SNPs that show robust (p < 5 × 10−8) and independent associations (r2 < 0.001). The data used in this study are available from previously published GWAS studies, which have relevant participant consent and ethical approval. The Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University approved this study.
Oral cavity and oropharyngeal cancer population
A summary of European descent GWAS statistics was used for the outcome of the risk. It contains 2497 cases and 2928 controls, and the clinical characteristics of the cases and controls can be found in a previously published GWAS study [14]. The included cases met the standards of the 10th edition of the International Classification of Diseases (ICD-10). Informed consent was obtained for all participants, and the study was approved by the relevant ethics committee.
Statistical analysis
The two-sample Mendelian randomization (MR) approach is based on three main assumptions [15]. First, genetic variants are strongly associated with serum biomarkers. Second, there are no unmeasured confounding factors related to the association of genetic variants with the outcome. Third, genetic variants affect the OC/OPC only through risk factors. Serum biomarkers with F-statistic < 10 was excluded to ensure the strength of the selected instruments (formula: F = R2×(N − 2)/(1 − R2), R2 = 2 × MAF × (1 − MAF) × beta2) [13, 14]. Power calculations in MR analysis were performed according to Brion et al. [16]. The inverse-variance weighted method (IVW) was used to explore the bidirectional causality between serum biomarkers and OC / OPC through a meta-analysis of SNP-specific Wald ratio estimates [13]. The causal effect estimates and equivalent beta coefficients were calculated, which were then converted into odds ratios (OR). The weighted median and MR-Egger methods were also implemented to assess causality. Many IVs were associated with multiple traits (pleiotropy). Sensitivity analysis was critical in MR studies to detect the underlying pleiotropy. Horizontal pleiotropy was performed using MR-Egger regression. When the MR-Egger intercept differs from zero (P < 0.05), it indicates horizontal pleiotropy or a violation of the MR assumption [24]. Leave-one-out sensitivity analysis was carried out by removing SNPs each time to explore whether the IVW analysis were biased by a single SNP. If there was no difference between the estimated MR result and the result after removing an IV, it indicates that the MR result is robust. The R version (4.0.3) with the package “TwoSampleMR” was used to perform MR analysis.
Results
Various serum biomarkers have been associated with the risk of OC/OPC in observational studies, which are susceptible to confounding and reverse causation. MR analysis can overcome these limitations by using SNPs as an instrumental variable for assumed risk factors. This study investigated 10 potential serum biomarkers for the risk of OC / OPC, including C-reactive protein (CRP) [17], serum 25-Hydroxyvitamin D [18], HDL cholesterol [19], LDL cholesterol [19], Total cholesterol [19], Triglycerides [19], Adiponectin [20], Leptin [21], HbA1C [22] and insulin-like growth factor 1 (IGF-1) [23]. The sample size, the number of SNPs, the proportion of variance explained (R2) by the SNPs, and the F statistics for the instrument are shown in Table 1. There was a statistically significant association between CRP and OC/OPC risk (OR 1.52, 95% CI 1.14 to 2.02) using the IVW method (Table 2). Additionally, the results using the weighted median method were statistically significant (OR 1.84, 95% CI 1.24 to 2.71). Similar results of risk estimation were obtained using the MR-Egger method (OR 2.07, 95% CI 1.36 to 3.16). This study provided 98% power to detect the causal effect of CRP (OR = 1.52). Figure 2 illustrates the scatter plot and the forest plot. The P-values for the CRP heterogeneity tests using the MR-Egger and IVW methods were 0.19 and 0.12, respectively, indicating no heterogeneity. Furthermore, there was no significant intercept (intercept = -0.02; SE = 0.01, P = 0.06), which indicated no horizontal pleiotropy. The CRP funnel plot was symmetrical, indicating no pleiotropic effects (Fig. 3a). In the leave-one-out sensitivity analysis, regardless of which SNP was removed, there was no fundamental effect on OC / OPC, which means that the MR result was robust (Fig. 3b).
Scatter plot and Forest plot. a Scatter plot of the possible effects of C-reactive protein and the risk of oral cavity and pharyngeal cancer, with the slope of each line corresponding to the estimated MR effect per method; b Forest plot of the MR-based effect sizes of C-reactive protein exposure instruments on oral cavity and pharyngeal cancer. The horizontal red line represents the result of the MR-egger or IVW method. Note: SNP single-nucleotide polymorphism; IVW inverse variance weighted method
Funnel plot and Leave-one-out plot. a: Funnel plot of SNPs associated with C-reactive protein and their risk of oral cavity and pharyngeal cancer. b: Leave-one-out of SNPs associated with C-reactive protein and their risk of oral cavity and pharyngeal cancer. The horizontal red line represents the result of all SNPs using the IVW method
No association was predicted between serum 25-hydroxyvitamin D, HDL cholesterol, LDL cholesterol, total cholesterol, Triglycerides, Adiponectin, Leptin, HbA1C and IGF-1, and the risk of OC/OPC was predicted (Table 2).
Discussion
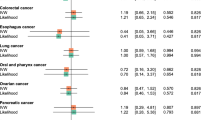
In the current study, a two-sample MR method was used to explore the correlation between 10 serum biomarkers and OC/OPC in a European population. MR analysis revealed that serum CRP levels were associated with an increased risk of OC/OPC, which may provide some of the strongest evidence to assess the causal role of CRP in OC/OPC. This study indicated an inflammatory mechanism for development and could have clinical utility in the identification of high-risk individuals. These findings reflect a recently published MR study of colorectal, breast, and gallbladder that supports the causal role of CRP in the risk of these cancers [24,25,26].
Chronic inflammation leads to cell hyperproliferation, activating a variety of cell functions, leading to malignant changes in DNA [27]. Inflammatory mediators such as nuclear factor-kappa (NF-κB) and cytokines are the main mediators involved in the pathogenesis of cancer [28]. NF-κB plays an important role in innate immunity/inflammation. Moreover, NF-κB is recognized as a crucial player in the initiation and progression [29]. CRP could be a biomarker of inflammation in the tumor microenvironment (TME), and chronic inflammation plays an active role in cell proliferation and tumorigenesis [30]. Chronic inflammation can contribute to epithelial cell mutations and epigenetic changes and can also serve as direct growth factors for tumor growth [31]. CRP could potentially promote CEA, MMP1 and MMP2 by stimulating LOX-1 receptors in colorectal cancer [32]. Chronic inflammation in the oral cavity was associated with an increased risk of HNC [33, 34]. Furthermore, smoking and heavy drinking can also cause chronic inflammation of the oral mucosa. Serum CRP level was significantly associated with the prognosis of HNC patients, and can be recommended for prognostic evaluation in clinical work [35]. Furthermore, high levels of CRP can predict lymph node metastasis, advanced tumor stage, and recurrence in OC [36]. Mechanically, CRP has the potential to solve the inflammatory environment by directly binding fibronectin to modulate fibronectin-mediated monocyte adhesion [37]. Fibronectin can regulate cell adhesion and signaling to promote oral cancer cell migration [38]. Similarly, it has the ability to stimulate white blood cells to produce IL-8 [39]. Previous studies have shown that the level of IL8 was associated with a worse overall survival in HNC patients [40]. Furthermore, a recent study found that consistent long-term use of non-steroidal anti-inflammatory drugs can be associated with a reduced risk of HNC [41]. One researcher found that anti-inflammatory drugs can reduce serum CRP [42]. Therefore, anti-inflammatory drugs can reduce the risk of HNC. Furthermore, future research could delve deeper into the specifics of these drugs and how they interact with CRP and impact the risk of OC / OPC. Taking into account this diversity of physiological activities, we can better understand the relationship between inflammation and HNC. In the future, more studies are needed to focus on the mechanism of the relationship between chronic inflammation and HNC. In addition, serum CRP should be used in OC/OPC screening tests, and patients with chronic high CRP levels need further oral and oropharyngeal examination for early detection of OC/OPC. Furthermore, the use of CRP in patient assessment can improve clinical evaluation of HNC progression.
The strength of this analysis was that an MR method was used to assess the causal relationship between CRP and OC/OPC risk, which can reduce the confounding and reverse causal bias inherent in observational studies. This MR study had sufficient power to detect moderate correlation and was unlikely to be affected by weak instrument bias. This study also has some limitations. First, this study only evaluated European ancestry to reduce the risk of population stratification, which was a well-known source of confounders in genomic data [43]. However, consistent associations have been demonstrated between CRP and oral cancer risk in Asian population [8]. The study by Zhu et al. indicated that CRP was a biomarker to assess cancer risks, which included the European, Asian, and African population [44]. Second, HPV data were not available in these summary results. HPV may contribute to the development of chronic inflammation [45, 46]. HPV-positive OC/OPC are unique from other OC/OPC and the incidence of HPV-positive OC/OPC has increased in recent years [47]. A prospective cohort study has reported that serum CRP was higher in HPV-positive than HPV-negative OPC [9]. Thus, HPV may influence the association between CRP and OC/OPC. In the future, a broader range of studies should be considered, including the OC/OPC subgroup, diverse populations, and the positive / negative HPV subgroup. Furthermore, future studies should also look at other potential biomarkers in addition to CRP that might correlate with OC/OPC.
Conclusion
In summary, this study supports a causal relationship between CRP levels and an increased risk of oral and oropharyngeal cancers.
Data Availability
The data sets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRP:
-
C-reactive protein
- HNC:
-
Head and Neck cancer
- IV:
-
instrumental variable
- IVW:
-
inverse variance weighted
- MB:
-
mega base
- MR:
-
Mendelian randomization
- OC/OPC:
-
oral cavity and oropharyngeal cancer
- OR:
-
odds ratio
- SNP:
-
single nucleotide polymorphism
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Marur S, Forastiere AA. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc. 2016;91:386–96.
O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International collaboration on oropharyngeal cancer network for staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–51.
Chan KKW, Glenny A-M, Weldon JC, Furness S, Worthington HV, Wakeford H. Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy. Cochrane Database Syst Rev. 2015;:CD010341.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Peres LC, Mallen AR, Townsend MK, Poole EM, Trabert B, Allen NE, et al. High levels of C-Reactive protein are Associated with an increased risk of Ovarian Cancer: results from the Ovarian Cancer Cohort Consortium. Cancer Res. 2019;79:5442–51.
Park S, Miller BJ. Meta-analysis of cytokine and C-reactive protein levels in high-risk psychosis. Schizophr Res. 2020;226:5–12.
Vankadara S, K P, Balmuri PK. Evaluation of serum C-Reactive protein levels in oral premalignancies and malignancies: a comparative study. J Dent (Tehran). 2018;15:358–64.
Johnson-Obaseki S, Caulley L, Corsten M, Liu G, Dimitroulakos J, Goldstein D, et al. C-reactive protein in HPV-Positive and HPV-Negative Oropharyngeal Cancer. Otolaryngol neck Surg off J Am Acad Otolaryngol Neck Surg. 2019;160:494–501.
Chawda JG, Jain SS, Patel HR, Chaduvula N, Patel K. The relationship between serum lipid levels and the risk of Oral cancer. Indian J Med Paediatr Oncol off J Indian Soc Med Paediatr Oncol. 2011;32:34–7.
Guo X-H, Wang J-Y, Gao Y, Gao M, Yu G-Y, Xiang R-L, et al. Decreased adiponectin level is associated with aggressive phenotype of tongue squamous cell carcinoma. Cancer Sci. 2013;104:206–13.
Pagoni P, Dimou NL, Murphy N, Stergiakouli E. Using mendelian randomisation to assess causality in observational studies. Evid Based Ment Health. 2019;22:67–71.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65.
Lesseur C, Diergaarde B, Olshan AF, Wünsch-Filho V, Ness AR, Liu G, et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat Genet. 2016;48:1544–50.
Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501.
Ligthart S, Vaez A, Võsa U, Stathopoulou MG, de Vries PS, Prins BP, et al. Genome analyses of > 200,000 individuals identify 58 Loci for Chronic Inflammation and highlight pathways that Link inflammation and Complex disorders. Am J Hum Genet. 2018;103:691–706.
Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. 2020;11:1647.
Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–83.
Dastani Z, Hivert M-F, Timpson N, Perry JRB, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 Diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607.
Yaghootkar H, Zhang Y, Spracklen CN, Karaderi T, Huang LO, Bradfield J, et al. Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes. 2020;69:2806–18.
Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–39.
Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin-like growth factor-1 and site-specific cancers: a mendelian randomization study. Cancer Med. 2020;9:6836–42.
Wang X, Dai JY, Albanes D, Arndt V, Berndt SI, Bézieau S, et al. Mendelian randomization analysis of C-reactive protein on Colorectal cancer risk. Int J Epidemiol. 2019;48:767–80.
Robinson T, Martin RM, Yarmolinsky J. Mendelian randomisation analysis of circulating adipokines and C-reactive protein on Breast cancer risk. Int J cancer. 2020;147:1597–603.
Barahona Ponce C, Scherer D, Brinster R, Boekstegers F, Marcelain K, Gárate-Calderón V, et al. Gallstones, body Mass Index, C-Reactive protein, and Gallbladder Cancer: mendelian randomization analysis of Chilean and European Genotype Data. Hepatology. 2021;73:1783–96.
Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81.
Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41.
Ghazi-Khanloosani M, Bandegi AR, Kokhaei P, Barati M, Pakdel A. CRP and LOX-1: a mechanism for increasing the tumorigenic potential of Colorectal Cancer Carcinoma Cell line. Pathol Oncol Res. 2019;25:1467–75.
Guha N, Boffetta P, Wünsch Filho V, Eluf Neto J, Shangina O, Zaridze D, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166:1159–73.
Ahrens W, Pohlabeln H, Foraita R, Nelis M, Lagiou P, Lagiou A, et al. Oral health, dental care and mouthwash associated with upper aerodigestive tract cancer risk in Europe: the ARCAGE study. Oral Oncol. 2014;50:616–25.
Huang S-F, Wei F-C, Liao C-T, Wang H-M, Lin C-Y, Lo S, et al. Risk stratification in oral cavity squamous cell carcinoma by preoperative CRP and SCC antigen levels. Ann Surg Oncol. 2012;19:3856–64.
Chen I-H, Liao C-T, Wang H-M, Huang J-J, Kang C-J, Huang S-F. Using SCC antigen and CRP levels as prognostic biomarkers in recurrent oral cavity squamous cell carcinoma. PLoS ONE. 2014;9:e103265.
Ullah N, Ma F-R, Han J, Liu X-L, Fu Y, Liu Y-T, et al. Monomeric C-reactive protein regulates fibronectin mediated monocyte adhesion. Mol Immunol. 2020;117:122–30.
Ramos G, de O, Bernardi L, Lauxen I, Sant’Ana Filho M, Horwitz AR, Lamers ML. Fibronectin modulates cell adhesion and signaling to promote single cell Migration of highly invasive oral squamous cell carcinoma. PLoS ONE. 2016;11:e0151338.
Sproston NR, Ashworth JJ. Role of C-Reactive protein at sites of inflammation and Infection. Front Immunol. 2018;9:754.
Le Q-T, Fisher R, Oliner KS, Young RJ, Cao H, Kong C, et al. Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced Head and Neck cancer. Clin cancer Res an off J Am Assoc Cancer Res. 2012;18:1798–807.
de la Cour CD, Dehlendorff C, Aalborg GL, von Buchwald C, Friis S, Verdoodt F, et al. Use of nonaspirin nonsteroidal anti-inflammatory Drugs and risk of Head and Neck cancer: a nationwide case-control study. Int J cancer. 2020;146:2139–46.
Yan Y, Guo T-M, Zhu C. Effects of nonsteroidal anti-inflammatory Drugs on serum proinflammatory cytokines in the treatment of ankylosing spondylitis. Biochem Cell Biol. 2018;96:450–6.
Cinelli C, LaPierre N, Hill BL, Sankararaman S, Eskin E. Robust mendelian randomization in the presence of residual population stratification, batch effects and horizontal pleiotropy. Nat Commun. 2022;13:1093.
Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and mendelian randomization analysis. BMC Med. 2022;20:301.
Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and Cervical cancer: a review. Int J cancer. 2020;146:305–20.
Liu X, Ma X, Lei Z, Feng H, Wang S, Cen X, et al. Chronic inflammation-related HPV: a Driving Force Speeds Oropharyngeal Carcinogenesis. PLoS ONE. 2015;10:e0133681.
Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA Cancer J Clin. 2015;65:401–21.
Acknowledgements
Not applicable.
Funding
This work was supported by funds from the Science and Technology Program of Guangzhou (201903010024) and the Chinese National Natural Science Foundation (81902771, 81472760).
Author information
Authors and Affiliations
Contributions
WXL, YL,PL,HL, and JY conceived the idea. WXL, PL, YL, JC, and ZYW collected and assembled the data. WXL, YL, YL,HL, and JY analyzed the data and wrote the manuscript. All authors read and agreed with the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations. The present MR study was based on previously collected and published data, no ethics approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, W., Liu, Y., Li, P. et al. Causal association of serum biomarkers with oral cavity and oropharyngeal cancer: a mendelian randomization study. BMC Oral Health 23, 987 (2023). https://doi.org/10.1186/s12903-023-03729-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03729-x