Abstract
Objective
The association between segmental body composition and bone mineral density (BMD) remains uncertain. The primary aim of this cross-sectional investigation was to elucidate the connection between segmental body composition and BMD within the United States adult population.
Methods
We selected a cohort of 10,096 individuals from the National Health and Nutrition Examination Survey (NHANES) database, with a mean age of 39 years and a mean BMI of 28.5 kg/m². The parameter of segmental body composition was achieved by quantifying body fat and lean mass percentages across various anatomical regions, including the torso, Android, Gynoid, arms and legs. We conducted a weighted multivariate linear regression analysis to investigate the association between segmental body composition and total BMD. Additionally, subgroup analysis was performed based on age and gender.
Results
We found an inverse association between fat proportion in each anatomical region and total BMD, with the arm and leg regions demonstrating the most significant negative correlation. Conversely, a positive correlation was observed between lean mass and BMD across all anatomical regions. These associations remained consistent in subgroup analyses.
Conclusion
Our investigation revealed a negative association between adipose levels in various anatomical regions and BMD among Americans aged 20 to 59. Importantly, higher fat proportion in the extremities exerted the most deleterious impact on BMD. Furthermore, an increase in lean mass within each anatomical region was ascertained to confer a positive effect on bone health. Consequently, the evaluation of segmental body composition is well-positioned to predict bone health status.
Similar content being viewed by others
Introduction
Osteoporosis is a metabolic skeletal disorder characterized by a reduction in bone mass and deterioration of bone microstructure, leading to weakened bone strength and significantly heightened susceptibility to low-energy or fragile fractures [1]. Osteopenia and osteoporosis collectively afflict 53.4 million elderly individuals in the United States, with their prevalence expected to rise as the population ages [2]. Osteoporosis-induced fractures in the United States occur approximately 1.5 million times each year [3]. The economic burden of treating fractures arising from osteoporosis is projected to reach nearly 50 billion by 2040, imposing a substantial strain on the American economy and society [4]. A decrease in bone mineral density (BMD), a reliable indicator of osteoporosis, is intrinsically associated with an elevated fracture risk [5, 6]. Consequently, the identification of risk factors correlated with BMD decline assumes paramount importance in the prediction and prevention of osteoporosis.
Obesity is a chronic metabolic condition influenced by an interplay of environmental and genetic variables, characterized by an abnormal or excessive accumulation of adipose tissue [7]. Over the last three decades, the prevalence of overweight and obesity in the United States has surged to alarming proportions. This widespread epidemic has given rise to a multitude of comorbidities, including an elevated susceptibility to metabolic disorders, cardiovascular disease, and mortality [8]. Despite an expanding body of evidence that highlights the connection between obesity and various health outcomes, a considerable debate lingers concerning the association between obesity and bone health [9]. Traditionally, it was believed that obesity provided protection against osteoporosis [10, 11], but an increasing body of research has demonstrated that adipose tissue does not confer benefits to bone health [12]. Body mass index (BMI) is commonly utilized to gauge obesity status, but its inability to accurately reflect body composition and predict metabolic disease risk has been questioned [13, 14]. Therefore, numerous studies have proposed that the assessment of body composition, a cornerstone of human metabolism and physiology [15], holds the potential to significantly enhance our understanding of obesity, metabolic health, chronic diseases, aging, and the intricate relationships between metabolic disorders and fat distribution [16,17,18].
The synthesis and metabolism of adipose cytokines exhibit a remarkable degree of variability depending on the specific location of the adipose tissue [19]. Consequently, a more effective approach for assessing and predicting metabolic disorders involves the meticulous examination of segmental body composition [20, 21]. Recent research has illuminated distinct relationships between the accumulation of adipose tissue in the upper and lower body about the risk of obesity-related metabolic disorders and comorbidities [22]. However, a lack of comprehensive studies centered on segmental body composition and its connection to BMD has left the association between segmental body composition and BMD shrouded in uncertainty. In a cross-sectional study conducted in southern Sri Lanka, a significant positive correlation emerged between lean and fat mass in different body areas and BMD among adults aged 30–54 years [23]. However, a prospective community-based cohort investigation explored the relationship between bone strength and fat mass in various areas of the body regions, revealing a negative correlation with central fat, such as Android fat, while simultaneously demonstrating a positive correlation with fat mass in the leg and Gynoid region [24]. Therefore, our endeavor aims to assess the proportions of fat and lean mass in various body regions to foster a deeper understanding of the association between segmental body composition and BMD.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a sophisticated and comprehensive research effort known for its multifaceted, multi-stage probabilistic sampling design, which enables the collection of diverse samples representing the U.S. population accurately. By conducting in-person interviews and standardized physical examinations within cutting-edge mobile screening facilities, NHANES surpasses the limitations of conventional survey methodologies, resulting in a rich database covering nutrition, health, and a myriad of other parameters. This invaluable dataset supports the estimation of disease prevalence and incidence rates, making NHANES a potent tool for policymakers in developing well-informed and effective public health policies for the broader population [25, 26].
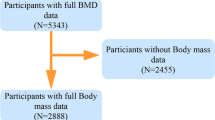
Dual-energy X-ray Absorptiometry (DXA) represents a medical imaging technique typically restricted to individuals aged 8 to 59 years for various reasons. In our investigation, we focused our attention exclusively on individuals aged 20 to 59 years who met the criteria for undergoing DXA examinations from 2011 to 2018. Specifically, pregnant women, individuals exceeding 450 pounds in weight, those taller than 6 feet 5 inches, and individuals who had recently undergone radiation contrast agent (barium) treatments were ineligible for DXA examinations. Furthermore, certain DXA results were considered invalid due to issues like excessive X-ray noise, problems with positioning resulting from excessive scanning areas, overlapping limbs, and pathological obesity. Participants without data on total BMD, segmental body composition, or those taking anti-osteoporosis medications were systematically excluded. As a result, the final cohort comprised 10,096 participants for the study. (Fig. 1)
Exposure-segmental body composition
DXA examination was utilized to assess the segmental body compositions conducted by a team of rigorously trained and certified radiology technologists using Hologic QDR-4500 A fan-beam densitometers (Hologic; Bedford, MA, USA). All DXA examination data were meticulously analyzed with Hologic APEX software(version 4.0), which was proficient in quantifying multiple regional components, including fat and lean soft tissue. Fat and lean mass were measured in various anatomical regions, encompassing the torso, Android, Gynoid, legs, and arms.
The torso area is delineated as the area from the lower edge of the chin to the lower perimeter of the diagonal line extending through the femoral neck and converging below the pubic symphysis, with a vertical boundary outside the ribs. The area below the lower edge of the torso is designated as the leg area [27]. The Android area is the lower torso area surrounded by two lines: the horizontal line positioned beneath the pelvis and the line automatically situated above the pelvic line. The Gynoid area is defined by the upper and lower lines. The upper line measures 1.5 times the height of the Android area below the pelvic line, and the lower line measures twice the height of the Android area [28] (Fig. 2). The fat mass percentage (FM%) and lean mass percentage (LM%) are calculated by dividing the respective fat or lean mass by the total mass of the corresponding body segment weight. For instance, Torso FM% is derived by dividing the fat mass within the torso by the total mass of the torso.
Outcome-BMD
The participants’ BMD was measured using the same DXA technology employed for evaluating segmental body compositions. DXA scans provided comprehensive bone assessments, including data on total BMD. For further details on inspection protocols and quality control, please refer to the NHANES website(www.cdc.gov/nchs/nhanes/index.htm).
Covariates
We collected demographic variables, including age, gender (male and female), race(Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, other race), education level(less than high school, high school, more than high school), and family income to poverty ratio. Furthermore, comprehensive physical assessments were conducted, which included the measurements of weight(kg), height(cm), body mass index(BMI)(kg/m2), arm circumference(cm), and waist circumference(cm). The BMI was calculated through the division of an individual’s weight(kg) by the square of their height(m2). In addition, participants were categorized into three distinct groups predicated upon smoking status: never smoked, formerly smoked, and current smoking. Alcohol consumption was quantified in terms of the frequency of alcohol use over a year. Physical activity levels were stratified by Physical Activity Guidelines, with recommendations of ≥ 75 min per week of vigorous or ≥ 150 min per week of moderate physical activity: active (meeting or exceeding the level of recommended activity), less active (below the recommended activity level), and inactive (no engagement in physical activity) [29]. Moreover, dietary intake of protein, calcium, and phosphorus was assessed through two separate 24-hour food recall interviews. Hypertension was defined as individuals with an average systolic or diastolic blood pressure exceeding 140/90 mm/Hg, as measured on three separate occasions, or those prescribed antihypertensive medications. Participants with glycosylated hemoglobin (HbA1c) levels ≥ 6.5%, and those under medication for diabetes management were classified as having diabetes.
Statistical analysis
The data underwent rigorous processing following the stipulated protocols of the NHANES database. All subsequent analyses incorporated the sample weights and multi-period combination weights. Continuous variables were presented as mean values with corresponding standard deviations, and P-values were calculated using a weighted linear regression model. Categorical variables were expressed as percentages, and the corresponding P-values were calculated via the weighted chi-square test.
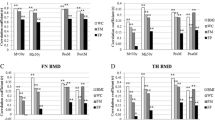
Our preliminary analysis revealed a strong correlation between the FM% and LM% in both the left and right arms and legs (Fig. 3). Therefore, the FM% and LM% values for the overall arms and legs were represented by averaging the values from both the left and the right sides. Given the non-normal distribution of data concerning body composition measurements, FM% and LM% values for the arms, legs, Android area, Gynoid region, and torso, as well as the total FM% and LM%, were expressed in quartiles for this study. The first quartile served as a reference point to elucidate the relationship between body composition and BMD.
A weighted multiple linear regression model was employed to evaluate the association between segmental body composition quartiles and total BMD. The results were presented as β, 95% confidence interval (CI), and P-values. In Model 1, no covariate adjustments were made to comprehensively examine the association. Model 2 incorporated adjustments for age and gender to account for potential confounding effects. Building upon Model 2, Model 3 introduced additional adjustments that included variables such as race, education level, family income to poverty ratio, smoking status, alcohol consumption, physical activity, protein intake, calcium intake, phosphorus intake, hypertension, diabetes, height, weight, BMI, arm circumference, and waist circumference.
Subsequent analyses were stratified based on age and gender. All statistical analyses were conducted utilizing EmPower Stats (https://www.empowerstats.com) and R software (version 3.6.3). A p-value less than 0.05 was considered statistically significant.
Results
Characteristics of the selected participants
The characteristics of the subjects, stratified by quartiles of total BMD (Q1:0.697-1.039 g/ cm²; Q2:1.039–1.108 g/ cm²; Q3: 1.108-1.18 g/ cm²; Q4: 1.18-1.88 g/ cm²), were summarized in Table 1. The study cohort comprised a total of 10,096 participants with an average age of 39 years and an average BMI of 28.5 kg/m². Notably, discernible disparities were observed among the BMD quartile groups in terms of personal habits, demographic characteristics, comorbidities, and physical measurements, with the exception of smoking status. Participants in the lowest BMD quartile were more likely to be older Caucasian women of shorter stature, lower body weight, reduced BMI, diminished arm and waist circumference, and had achieved a lower level of educational attainment. Additionally, these participants demonstrated lower daily consumption of alcohol, protein, calcium, and phosphorus, coupled with higher rates of smoking and physical inactivity.
The association between body composition parameters and total BMD
The association between body composition parameters and BMD was detailed in Table 2. In the unadjusted model, it was observed that the FM% of each body segment (Arm, Leg, Android region, Gynoid region, Torso) exhibited a negative correlation with BMD, while the LM% of each body segment showed a positive correlation with BMD. Importantly, even after adjusting the confounding variables in Model 2 and Model 3, this relationship remained robust. Furthermore, in Model 3, it was found that the highest quartile of Arm FM% [-0.0882,(-0.0983,-0.0780)] and leg FM% in the highest quartile [-0.0858,(− 0.0945,-0.0771)] had a more significant impact on BMD compared to other body regions.
The association between segmental body composition and total BMD by age and gender
We categorized our study participants into two age groups: 20–39 years old and 40–59 years old, and further stratified them by gender to conduct a subgroup analysis based on Model 3(Table 3). Our findings discovered the correlation between segmental body composition and BMD remained consistent across gender and age groups.
Discussion
In this study, we investigated the relationship between segmental body composition and total BMD. Our analysis unveiled that FM% and LM% in various anatomical regions exhibited a significant association with total BMD. Remarkably, this association remained robust after adjusting for relevant confounding variables and proved consistent across different gender and age groups. FM% displayed an inverse correlation with total BMD, with the most significant impact originating from FM% in both the upper and lower extremities. Conversely, our exploration uncovered a positive relationship between LM% and BMD across all anatomical regions. These findings underscored the significance of incorporating segmental body composition as a critical factor in the assessment of the variables influencing BMD.
The distribution of adipose tissue exerts a profound impact on systemic metabolism, increasing susceptibility to metabolic disorders. The pivotal determinant in this interplay lies in the diverse capability of adipose tissue to generate bioactive molecules that can impact various bodily tissues [30, 31]. Studies have demonstrated inherent genetic and developmental differences in adipocytes across distinct anatomical regions, with varying associations with BMD [32, 33]. For instance, previous research by Kuwahata et al. [34] suggested that trunk fat mass, owing to its non-weight-bearing effect, might exert a more significant impact on BMD augmentation than peripheral fat mass. Moreover, Matsuo et al. [35] found that in premenopausal women, fat distribution in the upper body had a stronger association with regional bone density than that in the lower extremities. Furthermore, studies on Korean adolescents indicated that fat mass in the torso area might enhance bone density more than in the extremities [36]. Douchi et al. [37] observed a greater impact of upper-body fat on BMD in premenopausal Japanese women compared to overall adiposity. However, it is essential to note that our findings differ from these prior investigations. These disparities may be attributed to the relatively modest sample sizes in most studies, typically involving only dozens or hundreds of subjects, as well as differences in the age, gender, and ethnicity of the cohorts under examination. Discrepancies also arise from the covariates considered in the correlation analyses within the respective models. In our study, we found that the distribution of adipose tissue across all anatomical regions, whether central or peripheral, upper body or lower body, demonstrated an inverse correlation with BMD. Surprisingly, the most pronounced impact on BMD was observed in the legs and arms, despite these regions not having the highest proportion of adipose tissue.
The adipose depots in the arms and legs primarily consist of subcutaneous fat, while the central region contains a combination of visceral and subcutaneous fat. A study [34] suggested that visceral fat might have a greater positive influence on BMD compared to subcutaneous fat, attributed to the biochemical components generated by adipocytes. On the contrary, trunk adipose tissue, due to its non-weight-bearing effect, was inherently predisposed to more effectively enhance BMD than peripheral fat. Furthermore, investigations had illuminated the adverse effects of excess visceral adipose tissue on sex hormone-binding globulin (SHBG) levels, leading to an excess of free sex hormones circulating in the body. Studies revealed a robust correlation between SHBG and fat distribution in the Android region, resulting in elevated levels of free estrogen and testosterone, which can play pivotal roles in promoting higher BMD [35, 38]. An observational study suggested that estrogen regulation might elucidate the favorable link between adipose tissue in the Gynoid region and BMD [39]. Additionally, Omentin, a specific adipose factor synthesized and secreted in visceral adipose tissue [40], had been demonstrated to play an essential role in modulating the activities of osteoblasts and osteoclasts, providing a promising avenue for improving osteoporosis and reducing fracture risk [41]. Hence, our hypothesis revolved around the idea that, despite the negative correlation between visceral fat and BMD, there may be an underlying mechanism within visceral fat that promotes bone health. This suggested that subcutaneous fat might pose a greater risk to bone health when compared to visceral fat.
Recent research had substantiated the divergent implications of adipose accumulation in the upper and lower body in relation to the susceptibility to obesity-related metabolic disorders and complications [42]. Interestingly, adipose tissue in the lower body appeared to provide protection against these health risks [43, 44]. Remarkably, our investigation unveiled a negative correlation between adipose tissue in the legs and BMD. One plausible explanation lies in the influence of sex hormones, recognized for their critical role in bone growth and maintenance [45, 46]. Studies had observed an inverse correlation between androgenic activity and lower extremity adipose tissue [47] and reduced androgenic activity in lower body adipose depots [48]. Moreover, the association between leg fat and BMD may be intricately intertwined with physical activity. Physical activity and exercise routines are steadfast defenders of musculoskeletal integrity, facilitating adipose reduction and guarding against osteoporosis [49, 50]. Lower extremity adipose tissue proportion may serve as an indicator of physical activity. An insightful study involving stroke patients shed light on the detrimental consequences of physical inactivity, emphasizing its contribution to the accumulation of adipose deposits in the lower limbs [51]. Concurrently, resistance-based exercise routines have been demonstrated to reduce lower limb adiposity [52]. Additionally, intramuscular adipose tissue in the femoral compartment may exert an adverse influence on motor function and physical activity levels [53]. Therefore, a vicious cycle between inactivity and the accumulation of adipose tissue may develop. Consequently, a lack of exercise contributes to augmented adiposity within the lower limbs and a concurrent decrease in bone mass.
Our investigation revealed a significant association between body composition across various anatomical regions and BMD, particularly focusing on the adipose parameters in the arms and legs, indicating an increased risk of BMD reduction. To mitigate this risk, we emphasized the importance of gaining lean mass, which had been substantiated to wield a significant protective effect on BMD. These findings hold significant relevance in light of the global concerns surrounding obesity and osteoporosis. Both conditions have been linked to suboptimal dietary patterns, excessive calorie intake, and insufficient physical activity [54, 55]. As such, we advocate a multifaceted approach to address these issues, including high-intensity resistance exercise combined with the supplementation of calcium and protein. A calcium-rich diet assumes a critical role in regulating energy metabolism and reducing fat accumulation during periods of excessive consumption [56]. Inadequate protein intake has been associated with declines in lean mass [57]. Maintaining optimal daily protein intake is essential for preserving lean mass and preventing bone loss [58]. Furthermore, exercise intervention emerges as a dual-faceted solution, preserving lean mass while effectively managing the loss of adipose tissue [59]. Studies have shown that exercise intensity is significantly correlated with increases in lean mass and BMD, simultaneously resulting in reductions in adipose weight [60]. Consequently, the effective management of dietary habits and regular exercise is of paramount importance in addressing the intricate interplay between body composition and BMD.
While our study benefits from substantial sample size and the use of contemporary DXA data, it is essential to acknowledge several inherent limitations. Firstly, our research adopted a cross-sectional study design, which inevitably constrains our ability to establish causal relationships. Secondly, our study data primarily comprises non-institutionalized individuals in the United States, specifically within the age range of 20 to 59 years, limiting the generalization of our findings to various age groups, races, and ethnicities. Additionally, despite our best efforts, it is plausible that the association between segmental fat distribution and BMD in American adults may still be influenced by unaccounted-for confounding variables. Lastly, our study centered on total BMD for analysis, even though it acknowledges its positive correlation with regional BMD. It is conceivable that variations in the correlation between segmental body composition and regional BMD may exist, a facet that our study did not explore.
Conclusions
In this population-based study conducted in the United States, we discovered that an elevated proportion of adipose tissue across various anatomical regions poses a risk factor for BMD reduction. Furthermore, the protective role of lean mass in the preservation of bone health had been validated by our research. These associations showed consistency across gender and age groups. Consequently, the inclusion of segmental body composition analysis in clinical assessments, with specific attention to the fat ratio of legs and arms, should be considered highly relevant for both predictive and preventive measures against the deleterious effects of osteoporosis. However, to further strengthen and substantiate these findings, additional prospective cohort studies should be required.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/NHANES/index.htm. The datasets generated and analyzed during the current study are available in the ZENODO repository, https://doi.org/10.5281/zenodo.7661039.
Abbreviations
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- DXA:
-
Dual-energy X-ray absorptiometry
- FM%:
-
Fat mass percentage
- LM%:
-
Lean mass percentage
- NHANES:
-
National Health and Nutrition Examination Survey
- SHBG:
-
Sex hormone-binding globulin
References
Anam AK, Insogna K. Update on osteoporosis screening and management. Med Clin North Am. 2021;105:1117–34.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2014;29:2520–6.
Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016;374:2096–7.
Miller PD. Management of osteoporosis. Dis Mon. 1999;45:21–54.
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2005;20:1185–94.
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70.
Qiao D, Li Y, Liu X, Zhang X, Qian X, Zhang H, et al. Association of obesity with bone mineral density and osteoporosis in adults: a systematic review and meta-analysis. Public Health. 2020;180:22–8.
Wu LW, Lin YY, Kao TW, Lin CM, Wang CC, Wang GC, et al. Mid-arm circumference and All-Cause, Cardiovascular, and Cancer Mortality among obese and non-obese US adults: the National Health and Nutrition Examination Survey III. Sci Rep. 2017;7:2302.
Kotsis V, Jordan J, Micic D, Finer N, Leitner DR, Toplak H, et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the high-risk patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced Hypertension, Diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. 2018;36:1427–40.
Reid IR. Fat and bone. Arch Biochem Biophys. 2010;503:20–7.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Piñar-Gutierrez A, García-Fontana C, García-Fontana B, Muñoz-Torres M. Obesity and bone health: a complex relationship. Int J Mol Sci 2022;23.
Prentice AM, Jebb SA. Beyond body mass index. Obes Reviews: Official J Int Association Study Obes. 2001;2:141–7.
Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int J Obes. 2016;40:883–6.
Heymsfield SB, Smith B, Wong M, Bennett J, Ebbeling C, Wong JMW, et al. Multicomponent density models for body composition: review of the dual energy X-ray absorptiometry volume approach. Obes Reviews: Official J Int Association Study Obes. 2021;22:e13274.
Müller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Reviews: Official J Int Association Study Obes. 2012;13(Suppl 2):6–13.
Bosello O, Vanzo A. Obesity paradox and aging. Eat Weight Disord. 2021;26:27–35.
Linge J, Borga M, West J, Tuthill T, Miller MR, Dumitriu A, et al. Body composition profiling in the UK Biobank Imaging Study. Obes (Silver Spring Md). 2018;26:1785–95.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3.
Tatsukawa Y, Misumi M, Kim YM, Yamada M, Ohishi W, Fujiwara S, et al. Body composition and development of Diabetes: a 15-year follow-up study in a Japanese population. Eur J Clin Nutr. 2018;72:374–80.
Chi JH, Shin MS, Lee BJ. Association of type 2 Diabetes with anthropometrics, bone mineral density, and body composition in a large-scale screening study of Korean adults. PLoS ONE. 2019;14:e0220077.
Piché ME, Vasan SK, Hodson L, Karpe F. Relevance of human fat distribution on lipid and lipoprotein metabolism and Cardiovascular Disease risk. Curr Opin Lipidol. 2018;29:285–92.
Lekamwasam S, Weerarathna T, Rodrigo M, Arachchi WK, Munidasa D. Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab. 2009;27:83–8.
Kim JH, Choi HJ, Ku EJ, Hong AR, Kim KM, Kim SW, et al. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos International: J Established as Result Cooperation between Eur Foundation Osteoporos Natl Osteoporos Foundation USA. 2016;27:1161–8.
Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation procedures. Vital and Health Statistics Series 2 Data Evaluation and Methods Research. 2020;1:1–35.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital and Health Statistics Series 2 Data Evaluation and Methods Research 2013:1–24.
Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442–54.
Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2012;27:2208–16.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for americans. JAMA. 2018;320:2020–8.
Chan GC, Divers J, Russell GB, Langefeld CD, Wagenknecht LE, Xu J, et al. Adipose tissue depot volume relationships with spinal trabecular bone mineral density in African americans with Diabetes. PLoS ONE. 2018;13:e0191674.
Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity Complications. Mol Aspects Med. 2013;34:1–11.
Yerges-Armstrong LM, Miljkovic I, Cauley JA, Sheu Y, Gordon CL, Wheeler VW, et al. Adipose tissue and volumetric bone mineral density of older afro-caribbean men. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2010;25:2221–8.
Zhang J, Jin Y, Xu S, Zheng J, Zhang Q, Chen J, et al. Associations of fat mass and fat distribution with bone mineral density in Chinese obese population. J Clin Densitom. 2015;18:44–9.
Kuwahata A, Kawamura Y, Yonehara Y, Matsuo T, Iwamoto I, Douchi T. Non-weight-bearing effect of trunk and peripheral fat mass on bone mineral density in pre- and post-menopausal women. Maturitas. 2008;60:244–7.
Matsuo T, Douchi T, Nakae M, Uto H, Oki T, Nagata Y. Relationship of upper body fat distribution to higher regional lean mass and bone mineral density. J Bone Miner Metab. 2003;21:179–83.
Jeon HC, Lee K, Kim J, Park TJ, Kang DW, Park DJ. The relationship between body fat percent and bone Mineral Density in Korean adolescents: the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1), 2010. Korean J Family Med. 2014;35:303–8.
Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Nagata Y. Relationship between body fat distribution and bone mineral density in premenopausal Japanese women. Obstet Gynecol. 2000;95:722–5.
Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70:473–9.
Aedo S, Blümel JE, Carrillo-Larco RM, Vallejo MS, Aedo G, Gómez GG, et al. Association between high levels of gynoid fat and the increase of bone mineral density in women. Climacteric: The Journal of the International Menopause Society. 2020;23:206–10.
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metabolism. 2006;290:E1253–61.
Zhao A, Xiao H, Zhu Y, Liu S, Zhang S, Yang Z, et al. Omentin-1: a newly discovered warrior against metabolic related Diseases. Expert Opin Ther Targets. 2022;26:275–89.
Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Reviews Endocrinol. 2015;11:90–100.
Zhang X, Hu EA, Wu H, Malik V, Sun Q. Associations of leg fat accumulation with adiposity-related biological factors and risk of metabolic syndrome. Obes (Silver Spring Md). 2013;21:824–30.
Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, Frayn KN, et al. Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic Complications. Diabetes. 2014;63:3785–97.
Narla RR, Ott SM. Bones and the sex hormones. Kidney Int. 2018;94:239–42.
Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–47.
Hetland ML, Haarbo J, Christiansen C. Regional body composition determined by dual-energy X-ray absorptiometry. Relation to training, sex hormones, and serum lipids in male long-distance runners. Scand J Med Sci Sports. 1998;8:102–8.
Heiss CJ, Sanborn CF, Nichols DL, Bonnick SL, Alford BB. Associations of body fat distribution, circulating sex hormones, and bone density in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1591–6.
Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ: Can Med Association Journal = Journal de l’Association medicale canadienne. 2006;174:801–9.
Winett RA, Carpinelli RN. Potential health-related benefits of resistance training. Prev Med. 2001;33:503–13.
Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic Stroke. Osteoporos International: J Established as Result Cooperation between Eur Foundation Osteoporos Natl Osteoporos Foundation USA. 2005;16:1769–79.
Nickols-Richardson SM, Miller LE, Wootten DF, Ramp WK, Herbert WG. Concentric and eccentric isokinetic resistance training similarly increases muscular strength, fat-free soft tissue mass, and specific bone mineral measurements in young women. Osteoporos International: J Established as Result Cooperation between Eur Foundation Osteoporos Natl Osteoporos Foundation USA. 2007;18:789–96.
Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, Sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637.
Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, Afanador-Restrepo DF, Carcelén-Fraile MDC, López-Ruiz E. Current status of the diagnosis and management of osteoporosis. Int J Mol Sci 2022;23.
Olateju IV, Opaleye-Enakhimion T, Udeogu JE, Asuquo J, Olaleye KT, Osa E, et al. A systematic review on the effectiveness of diet and exercise in the management of obesity. Diabetes & Metabolic Syndrome. 2023;17:102759.
Zemel MB. Role of calcium and dairy products in energy partitioning and weight management. Am J Clin Nutr. 2004;79:907s–12s.
Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M, et al. Association of protein intake with the change of lean mass among elderly women: the osteoporosis risk factor and Prevention - Fracture Prevention Study (OSTPRE-FPS). J Nutritional Sci. 2015;4:e41.
Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham osteoporosis study. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2000;15:2504–12.
Dolan E, Swinton PA, Sale C, Healy A, O’Reilly J. Influence of adipose tissue mass on bone mass in an overweight or obese population: systematic review and meta-analysis. Nutr Rev. 2017;75:858–70.
Goh VH, Tong TY. Association of age and physical exercise with bodyweight and body composition in Asian Chinese men. The Aging male: The Official Journal of the International Society for the Study of the Aging Male. 2010;13:265–74.
Acknowledgements
We appreciate the NHANES Project’s unrestricted distribution of the data, as well as the anonymous volunteers and all NHANES Project employees.
Funding
This research was supported in part by Zhejiang Traditional Chinese Medicine Science and Technology Program under grant number 2023ZL256.
Author information
Authors and Affiliations
Contributions
Fabo Feng and Yanze Lin took involved in the study design. Ruiji Wu and Xun Wang conducted clinical assessments. Ruiji Wu and Jinlei Zhou were responsible for data collection. Yanze Lin and Ruiji Wu analyzed the data. The text was written by Yanze Lin and was reviewed by additional writers. All the authors contributed to this article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by NCHS IRB/ERB. The participants provided their written informed consent to participate in this study. Furthermore, all methods were performed following relevant guidelines and regulations. For detailed information, see the following URL: https://www.cdc.gov/nchs/nhanes/irba98.htm.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Y., Wang, X., Wu, R. et al. Association between segmental body composition and bone mineral density in US adults: results from the NHANES (2011–2018). BMC Endocr Disord 23, 246 (2023). https://doi.org/10.1186/s12902-023-01506-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01506-z