Abstract
Background
Optimization of indirect shoot regeneration protocols is one of the key prerequisites for the development of Agrobacterium-mediated genetic transformation and/or genome editing in Passiflora caerulea. Comprehensive knowledge of indirect shoot regeneration and optimized protocol can be obtained by the application of a combination of machine learning (ML) and optimization algorithms.
Materials and methods
In the present investigation, the indirect shoot regeneration responses (i.e., de novo shoot regeneration rate, the number of de novo shoots, and length of de novo shoots) of P. caerulea were predicted based on different types and concentrations of PGRs (i.e., TDZ, BAP, PUT, KIN, and IBA) as well as callus types (i.e., callus derived from different explants including leaf, node, and internode) using generalized regression neural network (GRNN) and random forest (RF). Moreover, the developed models were integrated into the genetic algorithm (GA) to optimize the concentration of PGRs and callus types for maximizing indirect shoot regeneration responses. Moreover, sensitivity analysis was conducted to assess the importance of each input variable on the studied parameters.
Results
The results showed that both algorithms (RF and GRNN) had high predictive accuracy (R2 > 0.86) in both training and testing sets for modeling all studied parameters. Based on the results of optimization process, the highest de novo shoot regeneration rate (100%) would be obtained from callus derived from nodal segments cultured in the medium supplemented with 0.77 mg/L BAP plus 2.41 mg/L PUT plus 0.06 mg/L IBA. The results of the sensitivity analysis showed the explant-dependent impact of exogenous application of PGRs on indirect de novo shoot regeneration.
Conclusions
A combination of ML (GRNN and RF) and GA can display a forward-thinking aid to optimize and predict in vitro culture systems and consequentially cope with several challenges faced currently in Passiflora tissue culture.
Similar content being viewed by others
Introduction
Passionflower (Passiflora caerulea L.) is considered to be one of the most well-known climbing, evergreen shrub species [1]. P. caerulea is most often cultivated as a fruit crop, ornamental, or medicinal plant in virtually all tropical and subtropical regions of the world [2]. Due to the unique secondary metabolite profiles and phytochemical compositions of P. caerulea oils, there remain certain unexplored applications plant that relate to different fields of research [3, 4]. Various phenols, alkaloids, glycosides, flavonoids, and saponins, represent P. caerulea compounds of high medicinal and industrial interest [5]. Improving P. caerulea with selected utility traits broadens its biotechnological applicability, which forms the basis of the passionflower industry [6].
The micropropagation procedure, as vegetative reproduction in in vitro cultures, is an excellent way to obtain clones (i.e., plants genetically identical to the parent plants) and genetic improvement through genetic engineering approaches [7]. A new plant arises from the existing meristems of the parent plant, from adventitious meristems [8], or indirectly through the formation of callus (undifferentiated mass of tissue) [9, 10]. Micropropagation represents a common method of germplasm and biodiversity conservation for cultivated, threatened, and endangered species [1]. One of the most important purposes of micropropagation is also obtaining secondary metabolites [11,12,13]. The micropropagation process is generally carried out in a laboratory setting with controlled light and temperatures, under axenic conditions [14]. Complete isolation from the external environment provides a given plant with protection against potential threats, such as the presence of parasites, viruses, bacteria, or abiotic factors that can negatively influence growth development [15]. The development of an optimal micropropagation protocol makes it possible to obtain regenerated plants with significant healing potential, which are not easily accessible due to the small area of occurrence or are exposed to dangerous factors in the natural environment [16, 17]. One of the most effective in vitro culture methods is indirect shoot regeneration, where callus is used to obtain de novo shoots [18, 19].
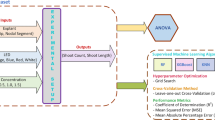
The indirect shoot regeneration protocol can be divided into three basic phases [6]. In phase I, the plant material is selected [20]. This stage is extremely important because improperly selected explants can determine the results of cultivating [21, 22]. The explants should be taken from a young, healthy plant, living in an optimal environment, developing in a favorable period of the year (in spring, plants grow most intensively and are most productive) [23]. The explant should be taken from the part of the plant that has meristematic cells, which guarantees further growth [20, 24, 25]. Sterilization of plant material to be cultured (seed or explant) is critically important to facilitate the axenic integrity of the culture [15]. Sterilization consists of rinsing the material in sodium, calcium or potassium hypochlorite or in ethanol and rinsing three times in sterile water [15]. Then, in phase II, the culture is established. The prepared explants should be transferred to a nutrient medium containing all micro- and macro-elements necessary for the in vitro plant’s growth, as well as appropriate carbohydrate sources and exogenous phytohormones determining the direction of development and influencing the physiological processes of explants [26, 27]. Explant orientation and placement within the culture vessel are also important factors impacting specimen quality. The proximity of explants to one another and proper exposure to media can dramatically influence various developmental characteristics that relate to the integrity of final products [6]. In the initial stage of growth, callus formation can be observed. Phase III consists in extending the cultivation of callus on a medium enriched with phytohormones until the formation of de novo shoots is obtained [20]. In this phase, several factors (e.g., type of callus, medium composition, plant growth regulators (PGRs), light, and temperature) are influenced indirect de novo shoot regeneration [20, 27,28,29,30] (Fig. 1). Though optimization of these factors is necessary for successful indirect shoot regeneration, conventional statistical models are often inadequate and laborious due to manual processing and sequential assessment of single factors [31]. Therefore, novel and innovative computational approaches using machine learning (ML) can be adopted to enhance the analytical and predictive measures required to optimize indirect de novo shoot regeneration [32].
Machine learning is defined as an evolving sub-branch of artificial intelligence which can be considered a reliable and promising computational method to predict and optimize a broad range of complicated biological systems [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Analyzing tissue culture datasets and predicting optimized treatments using ML algorithms represents a favorable approach to in vitro research [32, 36, 50]. Specifically, regression versions of ML algorithms (e.g., generalized regression neural network (GRNN) and random forest (RF)) are currently being applied to several areas of plant tissue culture research [32], including callogenesis [51], shoot proliferation [52], androgenesis [53], somatic embryogenesis [54], and direct shoot regeneration [55].
There is currently no study that enlists ML methods for modeling and optimizing indirect, de novo shoot regeneration. The current study represents the first. Since ML methods represent powerful approaches to glean insight about the nature of in vitro biology, this work enlists two ML algorithms (GRNN and RF) to develop a predictive model that relates callus type, PGR type, and PGR concentration to the success of indirect, de novo shoot regeneration of P. caerulea.
Materials and methods
Plant material and experimental design
Seeds of P. caerulea were purchased from the Seed and Plant Improvement Institute, Karaj, Iran. All the experiments done on P. caerulea are in compliance with relevant institutional, national, and international guidelines and legislation. The seed sterilization and germination of P. caerulea were performed based on our previous protocol [15]. In the current study, three different explants (i.e., leaf, internode, and node) with 0.5 cm lengths were selected from a four-week-old in vitro-grown seedling of P. caerulea. In order to develop callus, leaves were cultured in MS medium containing 0.6 g/L agar and 30 g/L sucrose along with 2.0 mg/ L 2,4-Dichlorophenoxyacetic acid (2,4-D) plus 0.2 mg/L indole-3-butyric acid (IBA) on the abaxial side, while internode and node explants were horizontally cultured on the mentioned medium. Cultures were maintained in a growth chamber under dark conditions at 25 °C ± 2 °C for one month, at which point the calli produced was used as explants for the indirect, de novo shoot regeneration experiment.
To study the effect of plant growth regulators and different calli (i.e., callus derived from different explants including leaf, node, and internode), MS medium containing 0.6 g/L agar and 30 g/L sucrose was used as a basal medium. The media contained various exogenous plant growth regulators at different concentrations including thidiazuron (TDZ: 0.0, 0.5, and 1.0 mg/L), 6-benzylaminopurine (BAP: 0.0, 0.5, 1.0, 1.5, 2.0 mg/L), kinetin (KIN: 0.0, 0.5, 1.0, 2.0 mg/L), putrescine (PUT: 0.0, 100, 300, 500 mg/L), and IBA (0.0, 0.05, 0.1, 0.15, 0.2 mg/L). The experiment was performed based on a completely randomized design with a total of 39 treatments in triplicate. A list of treatments is presented in Table 1. Each replicate consisted of 10 culture boxes and one callus was cultured in each box. The pH of all the media was adjusted to 5.7 before autoclaving at 121 °C at 0.1 MPa for 20 min. All the chemicals for in vitro culture were supplied by Merck (Sigma-Aldrich products, Irvine, UK). Experimental cultures were maintained in a growth chamber at 25 °C ± 2 °C, 47 ± 3 µmol m2 s− 1 irradiance for two months, at which point de novo shoot regeneration rate, number of de novo shoots, and length of de novo shoots were measured. The obtained data (Additional file 1) was used as a dataset to feed ML algorithms.
Machine learning procedures
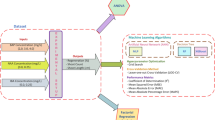
Before using ML algorithms, the data was normalized by using Box-Cox transformation. Although principal component analysis (PCA) was applied to determine outliers, no outliers were detected in the dataset. Type of callus (i.e., callus derived from different explants including leaf, node, and internode), TDZ, BAP, PUT, KIN, and IBA were considered as input variables, while de novo shoot regeneration rate, number of de novo shoots, and length of de novo shoots were fed to ML as target variables (Fig. 2a). Moreover, 80% and 20% of the dataset were randomly selected to train and test ML algorithms. In the current investigation, two supervised ML algorithms (RF and GRNN) were used to model and predict the indirect de novo shoot regeneration of P. caerulea.
The schematic representation of the step-by-step methodology of the current study including (a) dataset consists of inputs (i.e., callus type, 6-benzylaminopurine (BAP), indole-3-butyric acid (IBA), kinetin (KIN), putrescine (PUT), and thidiazuron (TDZ)) and outputs (i.e., regeneration rate, shoot number, and shoot length), (b, c), data modeling through generalized regression neural network (GRNN) and random forest (RF), respectively, and (d) optimization process through a genetic algorithm (GA).
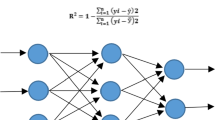
The regression version of the RF algorithm (Fig. 2b) uses different subsets of training data by randomly resampling the main dataset with the substitution for generating several T of regression trees. Moreover, the RF algorithm, during induction of tree growth, uses the best predictor among a predictor subset (p) that has been randomly selected from all input predictors. Therefore, the correlation of the different regression trees is avoided which leads to higher prediction accuracy. Finally, all T regression trees are averaged to obtain the best final prediction (Fig. 2b). GRNN as one of the sub-branches of artificial neural networks (ANNs) consisting of four layers (i.e., input, pattern, summation, and output) was used as another supervised ML algorithm in the current study (Fig. 2c). GRNN is based on a radial basis network which calculates the final prediction based on the average of all the weighted observed output data of former layers (Fig. 2c).
The accuracy and efficiency of the ML algorithms (FR and GRNN) were evaluated and compared by using three different performance criteria including coefficient of determination (R2), mean absolute error (MAE), and root mean square error (RMSE).
Optimization process
In the current study, a genetic algorithm (GA) was used to find the optimal level of TDZ, BAP, PUT, KIN, IBA, and callus type in order to maximize de novo shoot regeneration rate, number of de novo shoots, and length of de novo shoots. Hence, the developed ML models were fed to GA (Fig. 2d) where the generation number, initial population, selection function, cross-over function, crossover rate, mutation function, and mutation rate were respectively considered as 1000, 200, Roulette Wheel, two-point cross-over, 0.6, uniform, and 0.05.
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the importance degree of callus, TDZ, BAP, PUT, KIN, and IBA on de novo shoot regeneration rate, number of de novo shoots, and length of de novo shoots by calculating variable sensitivity error (VSE) and variable sensitivity ratio (VSR). VSE shows the RMSE of the developed ML model (i.e., GRNN) when the input is eliminated from the developed model. VSR equals the ratio of VSE and RMSE of the developed ML when all inputs are available. Then, the importance of input variables is ranked based on the value of VSR. All the analyses were also conducted using MATLAB® software.
Results
Effect of plant growth regulators and type of callus on indirect de novo shoot regeneration in P. caerulea
In the current study, the effect of different types and concentrations of PGRs (i.e., TDZ, BAP, PUT, KIN, and IBA) as well as callus type (i.e., callus derived from different explants including leaf, node, and internode) were evaluated on indirect shoot regeneration responses (i.e., de novo shoot regeneration rate, number of de novo shoots, and length of de novo shoots) of P. caerulea. Based on Table 1, different indirect shoot regeneration responses were obtained from different types of calli in the media containing various combinations of PGRs. The highest de novo shoot regeneration rate, the number of de novo shoots, and the length of de novo shoots were obtained from callus derived from node segment followed by calli derived from leaf and internode explants (Table 1). In relation to the combination of PGRs, the media containing 1 mg/L BAP along with 0.1 mg/L IBA led to the maximum de novo shoot regeneration rate and the number of de novo shoots, while the highest length of de novo shoots was observed in the media consisting of 0.5 mg/L TDZ along with 0.05 mg/L IBA (Table 1). Also, our results showed that there was no de novo shoot regeneration in the media without PGRs (Table 1).
In relation to the interaction between callus type and PGRs, the maximum de novo shoot regeneration rate (100 ± 0.0%) and number of de novo shoots (8.87 ± 0.233) were observed in calli derived from nodal segments cultured in the media containing 1 mg/L BAP along with 0.1 mg/L IBA (Table 1). Moreover, the highest length of de novo shoot (3 ± 0.115 cm) was observed in calli derived from nodal segments cultured in the media containing 0.5 mg/L TDZ along with 0.05 mg/L IBA (Table 1).
Evaluation of generalized regression neural network (GRNN) and random forest (RF)
In the present investigation, the indirect shoot regeneration responses (i.e., de novo shoot regeneration rate, the number of de novo shoots, and length of de novo shoots) of P. caerulea were predicted based on different types and concentrations of PGRs (i.e., TDZ, BAP, PUT, KIN, and IBA) as well as callus types (i.e., callus derived from different explants including leaf, node, and internode) using GRNN and RF algorithms. Based on the results (Table 2), the GRNN algorithm led to the development of predictive models with higher R2 in both testing and training subsets in comparison to RF for all indirect shoot regeneration responses including de novo shoot regeneration rate (R2 > 0.99 for GRNN vs. R2 > 0.96 RF), the number of de novo shoots (R2 > 0.98 for GRNN vs. R2 > 0.97 for RF), and length of de novo shoots (R2 > 0.89 for GRNN vs. R2 > 0.86 for RF). Furthermore, the observed and predicted values in all indirect shoot regeneration responses were perfectly correlated in both training and testing subsets (Fig. 3).
In addition, RMSE was used to evaluate and compare the accuracy of algorithms (i.e., GRNN and RF). The results showed that the GRNN algorithm led to higher accuracy and performance in either testing or training subsets in comparison to RF for all indirect shoot regeneration responses including de novo shoot regeneration rate (RMSE < 3.08 for GRNN vs. RMSE < 3.12 for RF), the number of de novo shoots (RMSE < 0.43 for GRNN vs. RMSE < 0.63 for RF), and length of de novo shoots (RMSE < 0.31 for GRNN vs. RMSE < 0.43 for RF) (Table 2). MAE as another performance criterion showed that the GRNN algorithm led to higher accuracy and performance in either testing or training subsets in comparison to RF for all indirect shoot regeneration responses including de novo shoot regeneration rate (MAE < 1.21 for GRNN vs. MAE < 1.45 for RF), the number of de novo shoots (MAE < 0.14 for GRNN vs. MAE < 0.25 for RF), and length of de novo shoots (MAE < 0.07 for GRNN vs. MAE < 0.12 for RF) (Table 2).
Optimization process
The developed GRNN models (the most accurate algorithm in the current investigation) were integrated into the genetic algorithm (GA) as a single-objective evolutionary optimization method to optimize the concentration of PGRs (i.e., TDZ, BAP, PUT, KIN, and IBA) and callus types (i.e., callus derived from different explants including leaf, node, and internode) for maximizing indirect shoot regeneration responses (i.e., de novo shoot regeneration rate, the number of de novo shoots, and length of de novo shoots). Based on the results of optimization using GRNN-GA (Table 3), the highest de novo shoot regeneration rate (100%) would be obtained from callus derived from nodal segments cultured in the medium supplemented with 0.77 mg/L BAP plus 2.41 mg/L PUT plus 0.06 mg/L IBA. Also, the maximum number of shoots (8.75) would be obtained from callus derived from nodal segments cultured in the medium supplemented with 0.76 mg/L BAP plus 0.005 mg/L TDZ plus 0.96 mg/L PUT plus 0.076 mg/L IBA (Table 3). Moreover, the highest length of shoot (3.1 cm) would be obtained from callus derived from nodal segments cultured in the medium supplemented with 0.002 mg/L BAP plus 0.007 mg/L KIN plus 0.5 mg/L TDZ plus 1.006 mg/L PUT plus 0.17 mg/L IBA (Table 3).
Importance degree of each input on P. caerulea indirect shoot regeneration responses
In the current study, sensitivity analysis through the calculation of variable sensitivity ratio (VSR) was conducted to assess the importance of each input variable (i.e., callus type, TDZ, BAP, PUT, KIN, and IBA) on the studied objective functions (i.e., de novo shoot regeneration rate, the number of de novo shoots, and length of de novo shoots). According to our results (Table 4), the callus type was the most important factor for indirect shoot regeneration rate followed by BAP, IBA, PUT, TDZ, and KIN respectively. Callus type > BAP > KIN > TDZ > IBA > PUT was ranked for number of shoots (Table 4). In addition, callus type > BAP > KIN > PUT > TDZ > IBA was ranked for shoot length (Table 4). VSR values for callus type are considerably higher than all PGRs (Table 4), indicating callus type to be the principal factor impacting indirect, de novo shoot regeneration. This emphasizes the explant-dependent impact of exogenous PGRs on indirect, de novo shoot regeneration.
Discussion
Indirect shoot regeneration of P. caerulea can be applied to production of secondary metabolites, clonal production, and gene bank establishment [6, 28]. The latter two of which are integral to genotype preservation, while the former has broad biotechnological and medicinal applications. However, it is necessary to optimize several factors involved in de novo soot regeneration from callus cultures [32]. PGR type and concentration, in addition to the origin of calli represent fundamental factors affecting indirect, de novo shoot regeneration [27]. Importantly, the interaction of PGRs and callus type represents a critical factor impacting success of this process, which was exemplified in our results. In fact, any given concentration of PGRs will fall within the various dose-response range according to the species and origin of the calli [6]. Therefore, the concentration of PGRs should be optimized before their application. However, constructing and optimizing tissue culture protocols represents a major challenge to the field as a whole [51]. Conventional statistical methods and large experiments involving thousands of treatments have traditionally been employed to develop tissue culture protocols [56]. Such techniques can only assess simple linear/curvilinear relationships between variables by serially assessing the influence of individual factors without accounting for dynamic, interactional effects of these factors on in vitro plant growth and development [56]. Additionally, traditional statistical methods and associated experimental systems are largely constrained by the extensive footprint of treatments and replications required for accurate data modeling [56]. Ultimately, such approaches can take insurmountable timespans and resources to develop improved, tough suboptimal tissue culture protocols [36]. Thus, due to the potential to exclude dynamic interactional effects of combined factors, optimization methods must be re-imagined using a modern approach to simultaneously optimize multiple factors for development of precision techniques [57]. For these reasons, applying new powerful approaches for analyzing and predicting in vitro culture systems is crucial [32].
Using modern computational approaches, ML offers a more simple and reliable approach to recognize and diagnose complex datasets that are commonly obtained from tissue culture experiments [32]. The powerful interoperative processes of newly developed nonlinear machine learning algorithms have recently been a focus for plant system biology [38], plant breeding [33], and plant tissue culture [32]. These methods remove uncertainties associated with dynamic tissue responses by diagnosing complex patterns and uses algorithms to predict optimal combinations of factors for desired results [36]. These patterns can then be analyzed using optimization algorithms to predict optimal combinations of factors for desired outcomes [56]. The robustness and accuracy of hybrid ML-optimization algorithms in modeling and predicting different in vitro culture systems have been previously confirmed in different species such as chrysanthemum [54, 58,59,60,61,62], passion fruit [31], Prunus rootstock [63,64,65], hazel [66], tomato [53], chickpea [52, 67], wheat [68], cannabis [56, 57, 69,70,71,72], and ajowan [73].
Therefore, in the current study, two ML algorithms (GRNN and RF) were employed to develop a predictive model for getting in-depth insight into the effect of PGRs (i.e., TDZ, BAP, PUT, KIN, and IBA) and callus types (i.e., callus derived from different explants including leaf, node, and internode) on indirect de novo shoot regeneration of P. caerulea. Our results showed that both RF and GRNN could be accurately model and predict indirect de novo shoot regeneration. In line with our results, previous studies have shown that GRNN is a powerful ML algorithm for modeling and predicting different plant biological systems such as seed germination [71], in vitro shoot regeneration [59], shoot growth and development [56], in vitro sterilization [69], secondary metabolite production [66], in vitro rooting [31], and morphological response of the aboveground parts of the plant to drought stress [74]. Moreover, the accuracy of RF has been previously demonstrated in different areas of plant science such as plant tissue culture [70], breeding [33], high-throughput phenotyping [41], and gRNA designing for CRISPR-based methods [72]. Generally, the results of the current study showed that ML is a reliable and accurate approach for predicting indirect de novo shoot regeneration.
Based on the result of sensitivity analysis, callus type was the most important factor for all the indirect regeneration parameters, followed by PGRs (i.e., BAP, IBA, PUT, TDZ, and KIN for indirect shoot regeneration rate; BAP, KIN, TDZ, IBA, and PUT for number of shoots; BAP, KIN, PUT, TDZ, and IBA for shoot length). It is well-documented that the callus type plays a key role in indirect de novo shoot regeneration [1, 6, 28]. Indeed, the various in vitro responses of each type of callus might be due to the differences in epigenetic regulation as well as endogenous sugars and phytohormones [75]. Similar to our results, previous studies demonstrate that callus type represents the most important factor influencing successful indirect, de novo shoot regeneration [6, 27, 28]. Due to the totipotent potential of callus cells, the manipulation of the concentration and ratio of PGRs leads to the differentiation of the callus cells that can ultimately result in de novo shoot regeneration [12]. Our results revealed that BAP was the second most important factor in indirect de novo shoot regeneration. In line with our results, previous studies showed that BAP led to a higher frequency of regeneration compared to other cytokinins in different Passiflora sp. such as P. trifasciata [76], P. foetida [76, 77], P. suberosa [27, 78], P. caerulea [79], P. cincinnata [80], and P. cristalina [81].
The results of the optimization process (GA) showed that the maximum de novo shoot regeneration rate would be achieved from callus derived from nodal segments cultured in the medium supplemented with 0.77 mg/L BAP plus 2.41 mg/L PUT plus 0.06 mg/L IBA. The result highlighted the importance of balances among PGRs, especially between cytokinins and auxins. In general, a low concentration of auxin and a high concentration of cytokinins induces de novo shoot regeneration [6, 12]. In line with our results, Rosa et al. [27] reported that a high concentration of cytokinin (BAP) without or with a low concentration of auxin was the best PGRs balance for indirect shoot regeneration in P. suberosa. The application of GA in optimizing plant tissue culture processes offers substantial benefits and enhances the reliability of achieving optimal outcomes [82]. GA, a robust optimization technique inspired by natural selection and genetics, proves invaluable in exploring complex solution spaces and identifying optimal configurations [63]. In the realm of plant tissue culture, GA proves particularly useful in fine-tuning critical parameters, including growth media composition, hormone concentrations, and culture conditions, to maximize desired outcomes such as callogenesis, organogenesis, rhizogenesis, and embryogenesis [29, 31, 56, 63,64,65, 68, 70]. GA can significantly reduce reliance on time-consuming and expensive trial-and-error experiments [32]. The algorithm’s ability to intelligently evolve and refine solutions based on fitness evaluations not only expedites the optimization process but also ensures more consistent and reliable results [83]. Consequently, GA empowers researchers and plant tissue culture practitioners to efficiently design and implement effective protocols, leading to enhanced plant propagation techniques and expanded biotechnological applications [36]. While previous studies have demonstrated the reliability of GA in optimizing in vitro culture processes [29, 31, 56, 63,64,65, 68, 70], it is crucial to conduct future research to validate the predicted-optimized (GRNN-GA) results obtained in the current study.
Conclusion
Optimization of indirect de novo shoot regeneration protocols is one of the key prerequisites for the development of Agrobacterium-mediated genetic transformation and/or genome editing in P. caerulea. Comprehensive knowledge related to indirect shoot regeneration leading to protocol optimization can be achieved by applying the combined ML -optimization algorithm approach. Our results showed that indirect shoot regeneration of P. caerulea could be precisely predicted and optimized using methods that link ML (i.e., GRNN and RF) to evolutionary optimization algorithms (i.e., GA). The optimized PGRs and the suitability of the developed model (GRNN-GA) in indirect shoot regeneration should be assessed by future studies in other Passiflora species. Moreover, the adaptation of a combination of ML (GRNN and RF) and GA can display a forward-thinking aid to optimize and predict in vitro culture systems and consequentially cope with several challenges faced currently in Passiflora in vitro culture.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- ANN:
-
Artificial neural network
- BAP:
-
6-benzylaminopurine
- GA:
-
Genetic algorithm
- GRNN:
-
Generalized regression neural network
- IBA:
-
Indole-3-butyric acid
- KIN:
-
Kinetin
- MAE:
-
Mean absolute error
- ML:
-
Machine learning
- PGR:
-
Plant growth regulator
- PUT:
-
Putrescine
- R2 :
-
Coefficient of determination
- RF:
-
Random forest
- RMSE:
-
Root mean square error
- TDZ:
-
Thidiazuron
- VSE:
-
Variable sensitivity error
- VSR:
-
Variable sensitivity ratio
References
Pacheco G, Simão MJ, Vianna MG, Garcia RO, Vieira MLC, Mansur E. In vitro conservation of Passiflora—A review. Sci Hort. 2016;211:305–11. https://doi.org/10.1016/j.scienta.2016.09.004.
Jafari M, Daneshvar MH, Lotfi A. In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants. BioTechnologia. 2017;98(2):113–9. https://doi.org/10.5114/bta.2017.68310.
Şesan TE, Oancea AO, Ştefan LM, Mănoiu VS, Ghiurea M, Răut I, Constantinescu-Aruxandei D, Toma A, Savin S, Bira AF, et al. Effects of Foliar Treatment with a Trichoderma Plant Biostimulant Consortium on Passiflora caerulea L. Yield and Quality Microorganisms. 2020;8(1):123. https://doi.org/10.3390/microorganisms8010123.
Jafari M, Daneshvar MH, Lotfi-Jalalabadi A. Direct organogenesis of passion flower (Passiflora caerulea L.) via leaf and petiole explants. Iran J Hortic Sci. 2018;49(2):375–82. https://doi.org/10.22059/ijhs.2017.217879.1104.
Smilin Bell Aseervatham G, Abbirami E, Sivasudha T, Ruckmani K. Passiflora caerulea L. fruit extract and its metabolites ameliorate epileptic seizure, cognitive deficit and oxidative stress in pilocarpine-induced epileptic mice. Metab Brain Dis. 2020;35(1):159–73. https://doi.org/10.1007/s11011-019-00501-5.
Mikovski AI, Silva NTd S, Machado CdS, Otoni MD, Carvalho WC, Rocha IF, Silva DI. Tissue culture and biotechnological techniques applied to passion fruit with ornamental potential: an overview. Ornam Hortic. 2019;25:189–99. https://doi.org/10.14295/oh.v25i2.2036.
Niazian M. Application of genetics and biotechnology for improving medicinal plants. Planta. 2019;249(4):953–73. https://doi.org/10.1007/s00425-019-03099-1.
Hesami M, Daneshvar MH, Lotfi A. In vitro shoot proliferation through cotyledonary node and shoot tip explants of Ficus religiosa L. Plant Tissue Culture and Biotechnology. 2017;27(1):85–8. https://doi.org/10.3329/ptcb.v27i1.35017.
Hesami M, Daneshvar MH. Indirect organogenesis through Seedling-Derived Leaf segments of Ficus Religiosa - a multipurpose Woody Medicinal Plant. J Crop Sci Biotechnol. 2018;21(2):129–36. https://doi.org/10.1007/s12892-018-0024-0.
Hesami M, Daneshvar MH. In Vitro Adventitious shoot regeneration through Direct and Indirect Organogenesis from Seedling-derived hypocotyl segments of Ficus religiosa L.: an important Medicinal Plant. HortScience. 2018;53(1):55–61. https://doi.org/10.21273/HORTSCI12637-17.
Norouzi O, Hesami M, Pepe M, Dutta A, Jones AMP. In vitro plant tissue culture as the fifth generation of bioenergy. Sci Rep. 2022;12(1):5038. https://doi.org/10.1038/s41598-022-09066-3.
Efferth T. Biotechnology applications of plant callus cultures. Engineering. 2019;5(1):50–9. https://doi.org/10.1016/j.eng.2018.11.006.
Hesami M, Pepe M, Baiton A, Jones AMP. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol Adv. 2023;62:108074. https://doi.org/10.1016/j.biotechadv.2022.108074.
Pepe M, Leonardos ED, Marie TRJG, Kyne ST, Hesami M, Jones AM, Grodzinski B, Biology. 2022, 11(5):729. doi:https://doi.org/10.3390/biology11050729.
Jafari M, Daneshvar MH, Lotfi-Jalalabadi A. Control of in vitro contamination of Passiflora caerulea by using of sodium hypocholorite. Indo-American J Agricultural Veterinary Sci. 2016;4:8–15.
Pepe M, Marie TRJG, Leonardos ED, Hesami M, Rana N, Jones AMP, Grodzinski B. Tissue culture coupled with a gas exchange system offers new perspectives on phenotyping the developmental biology of Solanum lycopersicum L. cv. ‘MicroTom’. Front Plant Sci. 2022;13:1025477. https://doi.org/10.3389/fpls.2022.1025477.
Jafari M, Shahsavar AR. Sodium nitroprusside: its beneficial role in drought stress tolerance of “Mexican lime” (Citrus aurantifolia (Christ.) Swingle) under in vitro conditions. Vitro Cell Dev Biology - Plant. 2022;58(1):155–68. https://doi.org/10.1007/s11627-021-10218-9.
Jafari M, Daneshvar MH. Effects of sodium nitroprusside on indirect shoot organogenesis and in vitro root formation of Tagetes erecta: an important medicinal plant. Pol J Appl Sci. 2020;5(3):14–9. https://doi.org/10.34668/PJAS.2019.5.3.03.
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M. Establishment of a protocol for in vitro seed germination and callus formation of Ficus religiosa L., an important Medicinal Plant. Jundishapur J Nat Pharm Prod. 2018;13(4):e62682. https://doi.org/10.5812/jjnpp.62682.
Fernando JA, Vieira MLC, Machado SR, Appezzato-da-Glória B. New insights into the in vitro organogenesis process: the case of Passiflora. Planr Cell Tissue Organ Cult. 2007;91(1):37–44. https://doi.org/10.1007/s11240-007-9275-7.
da Silva CV, de Oliveira LS, Loriato VAP, da Silva LC, de Campos JMS, Viccini LF, de Oliveira EJ, Otoni WC. Organogenesis from root explants of commercial populations of Passiflora edulis Sims and a wild passionfruit species, P. cincinnata Masters. Planr Cell Tissue Organ Cult. 2011;107(3):407–16. https://doi.org/10.1007/s11240-011-9991-x.
Hesami M, Adamek K, Pepe M, Jones AM. Effect of explant source on phenotypic changes of in vitro grown cannabis plantlets over multiple subcultures. Biology. 2023;12(3):443. https://doi.org/10.3390/biology12030443.
Hesami M, Baiton A, Alizadeh M, Pepe M, Torkamaneh D, Jones AM. Advances and perspectives in tissue culture and genetic Engineering of Cannabis. Int J Mol Sci. 2021;22(11):5671. https://doi.org/10.3390/ijms22115671.
Salmi MS, Hesami M. Time of collection, cutting ages, auxin types and concentrations influence rooting Ficus religiosa L. stem cuttings. J Appl Environ Biol Sci. 2016;6(1):124–32.
Hesami M, Jones AMP. Potential roles of epigenetic memory on the quality of clonal cannabis plants: content and profile of secondary metabolites. Med Usage Cannabis Cannabinoids. 2023;1:1–14. https://doi.org/10.1016/B978-0-323-90036-2.00028-4.
Hesami M, Tohidfar M, Alizadeh M, Daneshvar MH. Effects of sodium nitroprusside on callus browning of Ficus religiosa: an important medicinal plant. J Forestry Res. 2020;31(3):789–96. https://doi.org/10.1007/s11676-018-0860-x.
Rosa YBCJ, Monte-Bello CC, Dornelas MC. In vitro organogenesis and efficient plant regeneration from root explants of Passiflora suberosa L. (Passifloraceae). Vitro Cell Dev Biology - Plant. 2016;52(1):64–71. https://doi.org/10.1007/s11627-016-9747-8.
Pacheco G, Garcia R, Lugato D, Vianna M, Mansur E. Plant regeneration, callus induction and establishment of cell suspension cultures of Passiflora alata Curtis. Sci Hort. 2012;144:42–7. https://doi.org/10.1016/j.scienta.2012.06.022.
Hesami M, Daneshvar MH. Development of a regeneration protocol through indirect organogenesis in chenopodium quinoa willd. Indo-American J Agricultural Veterinary Sci. 2016;4(2):25–32.
Lombardi SP, Passos IRdS, Nogueira MCS, Appezzato-da-Glória B. In vitro shoot regeneration from roots and leaf discs of Passiflora cincinnata mast. Brazilian Archives of biology and technology. 2007;50:239–47. https://doi.org/10.1590/S1516-89132007000200009.
Jafari M, Daneshvar MH, Jafari S, Hesami M. Machine learning-assisted in Vitro Rooting optimization in Passiflora caerulea. Forests. 2022;13(12):2020. https://doi.org/10.3390/f13122020.
Hesami M, Jones AMP. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl Microbiol Biotechnol. 2020;104(22):9449–85. https://doi.org/10.1007/s00253-020-10888-2.
Yoosefzadeh-Najafabadi M, Rajcan I, Eskandari M. Optimizing genomic selection in soybean: an important improvement in agricultural genomics. Heliyon. 2022;8(11):e11873. https://doi.org/10.1016/j.heliyon.2022.e11873.
Yoosefzadeh-Najafabadi M, Eskandari M, Torabi S, Torkamaneh D, Tulpan D, Rajcan I. Machine-learning-based genome-wide Association Studies for uncovering QTL underlying soybean yield and its components. Int J Mol Sci. 2022;23(10):5538. https://doi.org/10.3390/ijms23105538.
Yoosefzadeh-Najafabadi M, Torabi S, Tulpan D, Rajcan I, Eskandari M. Front Plant Sci. 2021;12:777028. https://doi.org/10.3389/fpls.2021.777028. Genome-Wide Association Studies of Soybean Yield-Related Hyperspectral Reflectance Bands Using Machine Learning-Mediated Data Integration Methods.
Niazian M, Niedbała G. Machine learning for plant breeding and biotechnology. Agriculture. 2020;10(10):436. https://doi.org/10.3390/agriculture10100436.
Ramezanpour MR, Farajpour M. Application of artificial neural networks and genetic algorithm to predict and optimize greenhouse banana fruit yield through nitrogen, potassium and magnesium. PLoS ONE. 2022;17(2):e0264040. https://doi.org/10.1371/journal.pone.0264040.
Hesami M, Alizadeh M, Jones AMP, Torkamaneh D. Machine learning: its challenges and opportunities in plant system biology. Appl Microbiol Biotechnol. 2022;106(9):3507–30. https://doi.org/10.1007/s00253-022-11963-6.
Yoosefzadeh-Najafabadi M, Tulpan D, Eskandari M. Application of machine learning and genetic optimization algorithms for modeling and optimizing soybean yield using its component traits. PLoS ONE. 2021;16(4):e0250665. https://doi.org/10.1371/journal.pone.0250665.
Niedbała G, Niazian M, Sabbatini P. Modeling Agrobacterium-mediated Gene Transformation of Tobacco (Nicotiana tabacum)—A Model Plant for Gene Transformation Studies. Front Plant Sci. 2021;12:695110. https://doi.org/10.3389/fpls.2021.695110.
Yoosefzadeh-Najafabadi M, Tulpan D, Eskandari M. Using hybrid Artificial Intelligence and Evolutionary optimization algorithms for estimating soybean yield and fresh Biomass using Hyperspectral Vegetation Indices. Remote Sens. 2021;13(13):2555. https://doi.org/10.3390/rs13132555.
Yoosefzadeh Najafabadi M, Hesami M, Eskandari M. Machine learning-assisted approaches in modernized plant breeding programs. Genes. 2023;14(4):777. https://doi.org/10.3390/genes14040777.
Yoosefzadeh-Najafabadi M, Rajcan I, Vazin M. High-throughput plant breeding approaches: moving along with plant-based food demands for pet food industries. Front Veterinary Sci. 2022;9:991844. https://doi.org/10.3389/fvets.2022.991844.
Aasim M, Ayhan A, Katırcı R, Acar A, Ali SA. Computing artificial neural network and genetic algorithm for the feature optimization of basal salts and cytokinin-auxin for in vitro organogenesis of royal purple (Cotinus coggygria scop). Ind Crops Prod. 2023;199:116718. https://doi.org/10.1016/j.indcrop.2023.116718.
Aasim M, Ali SA, Altaf MT, Ali A, Nadeem MA, Baloch FS. Artificial neural network and decision tree facilitated prediction and validation of cytokinin-auxin induced in vitro organogenesis of sorghum (Sorghum bicolor L). Planr Cell Tissue Organ Cult. 2023;153(3):611–24. https://doi.org/10.1007/s11240-023-02498-3.
Aasim M, Ali SA, Aydin S, Bakhsh A, Sogukpinar C, Karatas M, Khawar KM, Aydin ME. Artificial intelligence–based approaches to evaluate and optimize phytoremediation potential of in vitro regenerated aquatic macrophyte Ceratophyllum demersum L. Environ Sci Pollut Res. 2023;30(14):40206–17. https://doi.org/10.1007/s11356-022-25081-3.
Aasim M, Ali SA, Bekiş P, Nadeem MA. Light-emitting diodes induced in vitro regeneration of Alternanthera reineckii mini and validation via machine learning algorithms. In Vitro Cellular & Developmental Biology - Plant 2022, 58(5):816–825. doi:https://doi.org/10.1007/s11627-022-10312-6.
Mirza K, Aasim M, Katırcı R, Karataş M, Ali SA. Machine learning and Artificial neural networks-based Approach to Model and optimize Ethyl Methanesulfonate and Sodium Azide Induced in Vitro Regeneration and morphogenic traits of Water Hyssops (Bacopa monnieri L). J Plant Growth Regul. 2023;42(6):3471–85. https://doi.org/10.1007/s00344-022-10808-w.
Kirtis A, Aasim M, Katırcı R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L). Planr Cell Tissue Organ Cult. 2022;150(1):141–52. https://doi.org/10.1007/s11240-022-02255-y.
Hesami M, Naderi R, Yoosefzadeh-Najafabadi M, Rahmati M. Data-Driven modeling in plant tissue culture. J Appl Environ Biol Sci. 2017;7(8):37–44.
Fallah Ziarani M, Tohidfar M, Navvabi M. Modeling and optimizing in vitro percentage and speed callus induction of carrot via Multilayer Perceptron-Single point discrete GA and radial basis function. BMC Biotechnol. 2022;22(1):34. https://doi.org/10.1186/s12896-022-00764-4.
Aasim M, Katirci R, Baloch FS, Mustafa Z, Bakhsh A, Nadeem MA, Ali SA, Hatipoğlu R, Çiftçi V, Habyarimana E, et al. Innovation in the breeding of Common Bean through a Combined Approach of in vitro regeneration and machine learning algorithms. Front Genet. 2022;13:897696. https://doi.org/10.3389/fgene.2022.897696.
Niazian M, Shariatpanahi ME, Abdipour M, Oroojloo M. Modeling callus induction and regeneration in an anther culture of tomato (Lycopersicon esculentum L.) using image processing and artificial neural network method. Protoplasma. 2019;256(5):1317–32. https://doi.org/10.1007/s00709-019-01379-x.
Hesami M, Naderi R, Tohidfar M. Introducing a hybrid artificial intelligence method for high-throughput modeling and optimizing plant tissue culture processes: the establishment of a new embryogenesis medium for chrysanthemum, as a case study. Appl Microbiol Biotechnol. 2020;104(23):10249–63. https://doi.org/10.1007/s00253-020-10978-1.
Fakhrzad F, Jowkar A, Hosseinzadeh J. Mathematical modeling and optimizing the in vitro shoot proliferation of wallflower using multilayer perceptron non-dominated sorting genetic algorithm-II (MLP-NSGAII). PLoS ONE. 2022;17(9):e0273009. https://doi.org/10.1371/journal.pone.0273009.
Pepe M, Hesami M, Small F, Jones AMP. Comparative analysis of machine learning and evolutionary optimization algorithms for Precision Micropropagation of Cannabis sativa: prediction and validation of in vitro shoot growth and development based on the optimization of light and Carbohydrate sources. Front Plant Sci. 2021;12:757869. https://doi.org/10.3389/fpls.2021.757869.
Aasim M, Katırcı R, Akgur O, Yildirim B, Mustafa Z, Nadeem MA, Baloch FS, Karakoy T, Yılmaz G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L). Ind Crops Prod. 2022;181:114801. https://doi.org/10.1016/j.indcrop.2022.114801.
Hesami M, Naderi R, Tohidfar M. Modeling and optimizing in vitro sterilization of Chrysanthemum via Multilayer Perceptron-Non-dominated sorting genetic Algorithm-II (MLP-NSGAII). Front Plant Sci. 2019;10:282. https://doi.org/10.3389/fpls.2019.00282.
Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M. Application of adaptive neuro-fuzzy inference system-non-dominated sorting genetic Algorithm-II (ANFIS-NSGAII) for modeling and optimizing somatic embryogenesis of Chrysanthemum. Front Plant Sci. 2019;10:869. https://doi.org/10.3389/fpls.2019.00869.
Hesami M, Naderi R, Tohidfar M. Modeling and optimizing medium composition for shoot regeneration of Chrysanthemum via Radial basis function-non-dominated sorting genetic Algorithm-II (RBF-NSGAII). Sci Rep. 2019;9(1):18237. https://doi.org/10.1038/s41598-019-54257-0.
Hesami M, Alizadeh M, Naderi R, Tohidfar M. Forecasting and optimizing Agrobacterium-mediated genetic transformation via ensemble model- fruit fly optimization algorithm: a data mining approach using chrysanthemum databases. PLoS ONE. 2020;15(9):e0239901. https://doi.org/10.1371/journal.pone.0239901.
Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M. Development of support vector machine-based model and comparative analysis with artificial neural network for modeling the plant tissue culture procedures: effect of plant growth regulators on somatic embryogenesis of chrysanthemum, as a case study. Plant Methods. 2020;16(1):112. https://doi.org/10.1186/s13007-020-00655-9.
Arab MM, Yadollahi A, Eftekhari M, Ahmadi H, Akbari M, Khorami SS. Modeling and optimizing a New Culture Medium for in Vitro Rooting of G×N15 Prunus Rootstock using Artificial neural network-genetic algorithm. Sci Rep. 2018;8(1):9977. https://doi.org/10.1038/s41598-018-27858-4.
Arab MM, Yadollahi A, Shojaeiyan A, Ahmadi H. Artificial neural network genetic algorithm as powerful Tool to predict and optimize in vitro proliferation Mineral Medium for G × N15 Rootstock. Front Plant Sci. 2016;7:1526. https://doi.org/10.3389/fpls.2016.01526.
Arab MM, Yadollahi A, Ahmadi H, Eftekhari M, Maleki M. Mathematical modeling and optimizing of in Vitro Hormonal Combination for G × N15 vegetative rootstock proliferation using Artificial neural network-genetic algorithm (ANN-GA). Front Plant Sci. 2017;8:1853. https://doi.org/10.3389/fpls.2017.01853.
Salehi M, Farhadi S, Moieni A, Safaie N, Hesami M. A hybrid model based on general regression neural network and fruit fly optimization algorithm for forecasting and optimizing paclitaxel biosynthesis in Corylus avellana cell culture. Plant Methods. 2021;17(1):13. https://doi.org/10.1186/s13007-021-00714-9.
Kirtis A, Aasim M, Katırcı R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L.). Plant Cell, tissue and Organ Culture (PCTOC) 2022, 150(1):141–52. doi:https://doi.org/10.1007/s11240-022-02255-y.
Hesami M, Condori-Apfata JA, Valderrama Valencia M, Mohammadi M. Application of Artificial neural network for modeling and studying in Vitro genotype-independent shoot regeneration in wheat. Appl Sci. 2020;10(15):5370. https://doi.org/10.3390/app10155370.
Pepe M, Hesami M, Jones AM. Machine learning-mediated development and optimization of Disinfection Protocol and Scarification Method for Improved in Vitro Germination of Cannabis Seeds. Plants. 2021;10(11):2397. https://doi.org/10.3390/plants10112397.
Hesami M, Jones AMP. Modeling and optimizing callus growth and development in Cannabis sativa using random forest and support vector machine in combination with a genetic algorithm. Appl Microbiol Biotechnol. 2021;105(12):5201–12. https://doi.org/10.1007/s00253-021-11375-y.
Hesami M, Pepe M, Monthony AS, Baiton A, Phineas Jones AM. Modeling and optimizing in vitro seed germination of industrial hemp (Cannabis sativa L). Ind Crops Prod. 2021;170:113753. https://doi.org/10.1016/j.indcrop.2021.113753.
Hesami M, Yoosefzadeh Najafabadi M, Adamek K, Torkamaneh D, Jones AM. Synergizing off-target predictions for in Silico Insights of CENH3 knockout in Cannabis through CRISPR/Cas. Molecules. 2021;26(7):2053. https://doi.org/10.3390/molecules26072053.
Niazian M, Sadat-Noori SA, Abdipour M, Tohidfar M, Mortazavian SMM. Image Processing and Artificial neural network-based models to measure and predict Physical Properties of Embryogenic Callus and number of somatic embryos in Ajowan (Trachyspermum ammi (L.) Sprague). Vitro Cell Dev Biology - Plant. 2018;54(1):54–68. https://doi.org/10.1007/s11627-017-9877-7.
Jafari M, Shahsavar A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE. 2020;15(10):e0240427. https://doi.org/10.1371/journal.pone.0240427.
Duta-Cornescu G, Constantin N, Pojoga D-M, Nicuta D, Simon-Gruita A. Somaclonal variation-advantage or disadvantage in Micropropagation of the Medicinal plants. Int J Mol Sci. 2023;24(1):838. https://doi.org/10.3390/ijms24010838.
Pipino L, Braglia L, Giovannini A, Fascella G, Mercuri A. In Vitro Regeneration and Multiplication of Passiflora Hybrid “Guglielmo Betto”. In: Protocols for In Vitro Propagation of Ornamental Plants Edited by Jain SM, Ochatt SJ. Totowa, NJ: Humana Press; 2010: 153–162.
Anand SP, Jayakumar E, Jeyachandran R, Nandagobalan V, Doss A. Direct organogenesis of Passiflora foetida L. through nodal explants. Plant Tissue Culture and Biotechnology. 2012;22(1):87–91. https://doi.org/10.3329/ptcb.v22i1.11266.
Garcia R, Pacheco G, Falcão E, Borges G, Mansur E. Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (Passifloraceae). Plant Cell, Tissue and Organ Culture 2011, 106(1):47–54. doi:https://doi.org/10.1007/s11240-010-9892-4.
Severin C, Bueno M, Santín F, Giubileo MG. Respuesta in vitro de diferentes biotipos y explantos de Passiflora caerulea L. Revista Colombiana de Biotecnología 2011, 13:73–9. doi:https://doi.org/10.15446/rev.colomb.biote.
da Silva CV, de Oliveira LS, Loriato VAP, da Silva LC, de Campos JMS, Viccini LF, de Oliveira EJ, Otoni WC. Organogenesis from root explants of commercial populations of Passiflora edulis Sims and a wild passionfruit species, P. cincinnata Masters. Planr Cell Tissue Organ Cult. 2011;107(3):407–16. https://doi.org/10.1007/s11240-011-9991-x.
de Faria RB, de Carvalho IF, Rossi AAB, de Matos EM, Rocha DI, Paim Pinto DL, Otoni WC, da Silva ML. High responsiveness in de novo shoot organogenesis induction of Passiflora cristalina (Passifloraceae), a wild amazonian passion fruit species. Vitro Cell Dev Biology - Plant. 2018;54(2):166–74. https://doi.org/10.1007/s11627-017-9881-y.
Sadat-Hosseini M, Arab MM, Soltani M, Eftekhari M, Soleimani A. Applicability of soft computing techniques for in vitro micropropagation media simulation and optimization: a comparative study on Salvia macrosiphon Boiss. Ind Crops Prod. 2023;199:116750. https://doi.org/10.1016/j.indcrop.2023.116750.
Rezaei H, Mirzaie-asl A, Abdollahi MR, Tohidfar M. Comparative analysis of different artificial neural networks for predicting and optimizing in vitro seed germination and sterilization of petunia. PLoS ONE. 2023;18(5):e0285657. https://doi.org/10.1371/journal.pone.0285657.
Acknowledgements
This study was conducted in Department of horticulture science, Khuzestan Agricultural Sciences and Natural Resources University, Mollasani, Khuzestan, Iran, and the equipment and materials of this laboratory was employed.
Funding
There were no external funding sources for this study.
Author information
Authors and Affiliations
Contributions
M.J. and M.HD. wrote the main manuscript text and M.J. prepared Figs. 1-3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
This work does not involve any human participation nor live animals performed by any of the listed authors. All the experiments done on P. caerulea are in compliance with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jafari, M., Daneshvar, M.H. Prediction and optimization of indirect shoot regeneration of Passiflora caerulea using machine learning and optimization algorithms. BMC Biotechnol 23, 27 (2023). https://doi.org/10.1186/s12896-023-00796-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12896-023-00796-4