Abstract
Background
There is an increasing interest in assessing paraspinal morphology and composition in relation to low back pain (LBP). However, variations in methods and segmentation protocols contribute to the inconsistent findings in the literature. We present an on-line resource, the ParaspInaL muscLe segmentAtion pRoject (PILLAR, https://projectpillar.github.io/), to provide a detailed description and visual guide of a segmentation protocol by using the publicly available ITK-SNAP software and discuss related challenges when performing paraspinal lumbar muscles segmentations from magnetic resonance imaging (MRI).
Methods
T2-weighted and corresponding fat-water IDEAL axial MRI from 3 males and 3 females (2 chronic LBP and 1 control for each sex) were used to demonstrate our segmentation protocol for each lumbar paraspinal muscle (erector spinae, lumbar multifidus, quadratus lumborum and psoas) and lumbar spinal level (L1-L5).
Results
Proper segmentation requires an understanding of the anatomy of paraspinal lumbar muscles and the variations in paraspinal muscle morphology and composition due to age, sex, and the presence of LBP or related spinal pathologies. Other challenges in segmentation includes the presence and variations of intramuscular and epimuscular fat, and side-to-side asymmetry.
Conclusion
The growing interest to assess the lumbar musculature and its role in the development and recurrence of LBP prompted the need for comprehensive and easy-to-follow resources, such as the PILLAR project to reduce inconsistencies in segmentation protocols. Standardizing manual muscle measurements from MRI will facilitate comparisons between studies while the field is progressively moving towards the automatization of paraspinal muscle measurements for large cohort studies.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a very common symptom and now the leading cause of disability worldwide [1]. While the precise mechanisms underlying LBP remain largely unknown, there is a global consensus as to its multifactorial aetiology including, but not limited to, biophysical factors, psychological factors and societal factors [2, 3]. Despite recognition of these factors, there remains limited options for effective conservative management programs [4, 5].
Increased paraspinal muscle fatty infiltration has been associated with the presence and severity of spinal pain and dysfunction [6,7,8,9]. This has lead to a growing interest in using quantitative imaging measures of muscle composition to improve phenotyping and prognosis and to provide a necessary biomarker towards informing and measuring therapeutic success. However, findings from recent reviews reporting on the association between image-based measures of paraspinal muscle morphology and composition with LBP and related spinal pathologies remain conflicting and inconclusive [10,11,12]. Important variations including inconsistent segmentation protocols for the lumbar musculature (e.g., undefined borders, inclusion or exclusion of epimuscular fat, and use of proprietary imaging software) and image perceptions by the raters likely contributed to the inconsistencies in the literature. Hodges et al. [13] recently published a review and consensus-based recommendations to address these inconsistencies and work towards the standardisation of imaging-based measures of paraspinal muscles. Future studies should implore to follow these recommendations to allow for easier comparisons between studies.
When it comes to which image navigation software to use to perform paraspinal muscle segmentation, several factors can arise, including accessibility, costs, ease-of-use, and the goals of research. ITK-SNAP (www.itksnap.org) is a user-friendly, free open-source medical image segmentation software that has been used in several domains including cardiac, dental, brain, and spinal applications for imaging analysis and to aid in diagnosis and surgery. While relatively new to the LBP community, this software allows for segmentations in simultaneous consideration of multiple co-aligned image contrast, which is useful when examining fat vs. water derived magnetic resonance images (MRI) of muscles. ITK-SNAP also provides easy labeling tools allowing for clear identification of various muscles and structures in a single image, in addition to 3D segmentation visualization and volumetric measurement.
The ParaspInaL muscLe segmentAtion pRoject (PILLAR) is a comprehensive on-line resource of protocols and tutorials designed to provide new researchers with the information and tools to perform educated manual segmentations of lumbar musculature in ITK-SNAP. Through PILLAR, our segmentation protocol, anatomy, and borders for lumbar multifidus (LM), erector spinae (ES), quadratus lumborum (QL), and psoas (PS) are clearly defined with both detailed description and visualization. In addition, videos for the segmentation of each muscle and step-by-step guide on how to use ITK-SNAP are provided, the inclusion versus exclusion of epimuscular fat in the region of interest (ROI) is discussed, and guidelines for reporting measurement information in papers are also supplied. Furthermore, this project shows side-by-side comparisons of healthy, pathological, and aged pathological conditions (e.g., chronic LBP) in both males and females across all lumbar levels.
Objectives
The primary objective of this project is to provide a detailed description of our segmentation protocol, introduce the uses of ITK-SNAP to the LBP research community for paraspinal muscle segmentation, and provide a clear visual guide to help train new raters and facilitate larger scale comparison between studies. A secondary objective is to discuss key challenges when segmenting paraspinal muscle from axial MR images and highlight differences in paraspinal muscle morphology and composition related to age, sex and spinal pathology.
Methods
Image acquisition and reconstruction
MRI images of 3 females (27-year-old control, 32-year-old with chronic LBP, and 51-year-old with chronic LBP) and 3 males (29-year-old control, 34-year-old with chronic LBP, and 60-year-old with chronic LBP) were selected from previous ongoing research projects. Ethics approval was obtained from the Central Ethics Research Committee of the Quebec Minister of Health and Social Services and all subjects provided informed consent. All methods were carried in accordance with relevant guidelines and regulations.
All subjects underwent a routine lumbosacral MRI evaluation using a 3T GE magnet (Milwaukee, WI, USA) and started phased array body coil. Axial T2-weighted and multi-echo IDEAL (Lava-flex, 2 echo sequence) were acquired from L1 to L5 using the following MR parameters; 4-mm slice thickness, 180-mm2 field of view and 512 × 512 matrix. Slices at the mid-disc from L1-L5 were selected from T2-weighted and IDEAL fat-water images. If needed, multiplanar reconstruction (3D MPR) using the HOROS software (Version 4.0.0) was used at the L4 and L5 levels to position the image slices perpendicular to the long axis of the paraspinal musculature. ITK-SNAP (Version 3.8.0) was then used to segment LM, ES, QL, and psoas. T2-weighted images were segmented separately from fat/water images. Some images were darker than others and their contrast needed to be adjusted to visualize the borders properly. Finding the borders on the fat and water images may be more difficult especially if there is little fat in the image. Muscle is dark and fat is bright in fat images, whereas muscle is a grainy gray and fat is darker in water images.
Anatomical landmarks for segmentation
The following landmarks were used for segmentation purposes. The LM muscle has borders along the spinous process, lamina, intermuscular fascial border with the erector spinae, and the LM epimysium that is distinct from the thoracolumbar fascia (TLF) and adipose tissue. The ES shares borders with LM, the tip of the zygoapophyseal joint, intermuscular fascial border between QL, and along the aponeurosis that is distinct from the TLF and subcutaneous adipose tissue. When present, epimuscular fat was included in the ES ROI. QL extends along the fascial borders with ES and psoas, 12th rib at L1, perirenal fascia from L2-L4, and iliac crest at L4. Psoas runs along the intervertebral disc, interfascial border with the viscera, kidneys, and the intermuscular fascia between QL and ES depending on the shape of the muscle. Additional information and anatomical landmarks used to identify the medial, anterior, lateral and posterior border of each muscle [14, 15], as well as further segmentation tips are presented in Table 1.
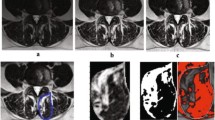
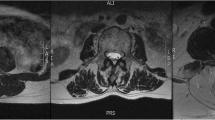
To provide researchers both new and familiar with muscular segmentation of the lumbar spine, a tutorial website for the PILLAR project was created to accompany this paper (https://projectpillar.github.io/). The website provides detailed anatomy of the lumbar musculature as well as the suggested borders for segmentation. Furthermore, several video tutorials on how to use the ITK-SNAP software, example images of manual segmentations on 3 females (Figs. 1) and 3 males (Fig. 2) and differences between the inclusion and exclusion of epimuscular fat (Fig. 3), and the standardized recommendations for manual segmentation can be found on the website. Each section of this online tutorial can be easily navigated using the menu bar on the left-hand side. More segmentations may be added in the future to show different conditions researchers may come across and will be updated accordingly as new research is published.
All images are T2-weighted images at the L3/L4 level in females. The first column (A & D) are images of the control. The middle column (B & E) are images of an age-matched individual with LBP. The third column (C & F) are images of the older individual with LBP. D, E, and F include the segmentations of the lumbar multifidus (red), erector spinae (green), quadratus lumborum (blue), and psoas (yellow) muscles
All images are T2-weighted images at the L3/L4 level in males. The first column (A & D) are images of the control. The middle column (B & E) are images of an age-matched individual with LBP. The third column (C & F) are images of the older individual with LBP. D, E, and F include the segmentations of the lumbar multifidus (red), erector spinae (green), quadratus lumborum (blue), and psoas (yellow) muscles
Discussion
Challenges with segmentation
Several challenges arise when investigating different populations and pathologies. Individuals who are older are more likely to have increased intramuscular and epimuscular fat [14]. In addition, females are also more likely to have increased epimuscular and intramuscular fat compared to males, which is likely due to females generally having a higher body fat % [16]. Lastly, those with LBP and disc degeneration typically present with increased intramuscular fat, which is believed to be related to decreased function and pain [6, 17]. The presence of intramuscular fat on MRI images may make determining muscular borders easier since a clear white line may appear on the images where the fat builds up along fascial borders. If there are large amounts of intramuscular fat, it can be difficult to determine where one muscle begins and another ends because there are multiple white lines. Epimuscular fat typically presents itself along the ES between the longissimus and iliocostalis muscles and is commonly referred to as a fat “tent”. To date, studies have both included and excluded epimuscular fat in their paraspinal muscle measurements. Researchers must clearly define in their methods whether epimuscular fat was included or excluded in the ROI as it will affect both size and muscle composition measurements [18]. In this project, our segmentation protocol included epimuscular fat if present. However, images demonstrating the difference between the inclusion and exclusion of epimusuclar fat can be found on the PILLAR Project website. Knowing the anatomy of the muscles, the shape they typically take on at certain lumbar levels, and understanding the widely accepted borders for these muscles will aid in deciding the path to follow to segment the muscle.
The paraspinal musculatures also differ between younger and older populations, sex, and individuals with the presence of LBP or other spinal pathologies. Older individuals, females, and those with LBP tend to present with increased intramuscular fat. It can be difficult to find the appropriate landmarks on MRIs during segmentation when there is little fat, especially at the borders between muscles (e.g., LM and ES, ES and QL). Males tend to have larger and thicker LM compared to females in both general and athletic populations. Individuals with LBP have presented with decreased LM cross-sectional area and thickness compared to controls.
The presence of LBP and lumbar pathologies has been associated with paraspinal muscle atrophy (e.g., reduced size) and side-to-side asymmetry [18,19,20,21]. In some cases, this can also make paraspinal muscle segmentation more difficult. Varying sizes between the right and left sides, especially with LM and QL, can lead to improper segmentation in an unconscious attempt to make both sides equal. It is important in these situations to be familiar with the muscular borders and to follow those borders for the muscle being segmented. While it is important to compare left and right sides to help with the general shape, they may not always be identical. Of note, differences in MF and ES shape between males and females have also been reported [21, 22].
Future directions
With the increased interest and greater number of researchers investigating the lumbar musculature and its role in the presence of LBP and related spinal pathologies using imaging, there are bound to be inconsistencies between measures, software, and protocols that may hinder proper comparisons between studies and populations. Hodges et al. [13] provided a clear set of recommendations with regards to physiological/pathological, confounding factors, and measurements issues that should be taken into consideration when planning an imaging study investigating lumbar musculature. The PILLAR project is adding to the current literature by providing a clear visual guide, tutorials and resourceful tool to facilitate paraspinal muscle segmentation. Not only will consistency in segmentation protocols aid in comparing studies and clinical populations, but researchers utilizing the same software to perform segmentations will also allow for easier comparisons between studies.
Segmentation is moving towards automated segmentation in many fields of research, which is segmentation completed wholly by a program. Manual segmentation is completed wholly by the researcher. In order to develop an automated segmentation program, large databases must be acquired through manual segmentation [23, 24]. As the program develops and learns from these manually segmented images, it is necessary to verify and edit boundaries, which is what is known as semi-automated segmentation (partial completion by a program, partial completion by a researcher). The PILLAR project provides the tools to aid in the creation of a large database of paraspinal muscle segmentations to move towards automated segmentation. Compared to manual segmentation, automated segmentation is faster, easier, and may allow for easier access to results, especially if it can be integrated to clinical settings.
Furthermore, segmentation is the first step towards grading any disease. While the goal of this paper was not identifying key elements to distinguishing mild, moderate, and severe LBP, utilizing the same segmentation software and protocols discussed here can provide researchers a consistent means of identifying the level of severity of LBP or other pathologies based on paraspinal musculature characteristics (i.e. intramuscular fat and muscle size). This may aid in predicting or identifying individuals at risk of experiencing mild, moderate, or severe LBP or other lumbar spine pathologies associated with paraspinal musculature characteristics.
There are several imaging software commonly used in lumbar musculature segmentation, such as 3D Slicer, ImageJ, and Horos. ITK-SNAP is a free program commonly used in brain, cardiac, and spine segmentation, and while segmentation can be done in any of the mentioned programs, ITK-SNAP provides the ability to examine musculature in a 3D view and calculate volumetric measurements, which has been rarely done to date when investigating the lumbar musculature.
Data availability
The data and resources generated from this work is available on the following website: https://projectpillar.github.io/.
Abbreviations
- LBP:
-
low back pain
- PILLAR:
-
ParaspInaL muscLe segmentAtion pRoject
- MRI:
-
Magnetic Resonance Imagin
- LM:
-
Lumbar multifidus
- ES:
-
Erector Spinae
- QL:
-
Quadratus lumborum
- PS:
-
Psoas
- ROI:
-
Region of interest
- MPR:
-
multiplanar reconstruction
- TLF:
-
Thoracolumbar fascia
References
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 Diseases and injuries, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. The Lancet. 2016;388:1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6.
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. The Lancet. 2018;391:2356–67. https://doi.org/10.1016/S0140-6736(18)30480-X.
Tagliaferri SD, Miller CT, Owen PJ, Mitchell UH, Brisby H, Fitzgibbon B, et al. Domains of chronic low back Pain and assessing treatment effectiveness: a clinical perspective. Pain Pract. 2020;20:211–25. https://doi.org/10.1111/papr.12846.
Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. The Lancet. 2018;391:2368–83. https://doi.org/10.1016/S0140-6736(18)30489-6.
Buchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, Schoene M, et al. Low back pain: a call for action. The Lancet. 2018;391:2384–8. https://doi.org/10.1016/S0140-6736(18)30488-4.
Hildebrandt M, Fankhauser G, Meichtry A, Luomajoki H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord. 2017;18:12. https://doi.org/10.1186/s12891-016-1376-1.
Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O’Sullivan R, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J. 2015;15:1593–601. https://doi.org/10.1016/j.spinee.2015.03.039.
Fortin M, Lazáry À, Varga PP, Battié MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26:2543–51. https://doi.org/10.1007/s00586-017-5228-y.
Fortin M, Gibbons LE, Videman T, Battié MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports. 2015;25:880–7.
Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural Changes of Lumbar Muscles in non-specific low back Pain: a systematic review. Pain Physician. 2016;19:E985–1000.
Kalichman L, Carmeli E, Been E. The Association between Imaging parameters of the Paraspinal Muscles, spinal degeneration, and low back Pain. BioMed Res Int. 2017;2017:1–14. https://doi.org/10.1155/2017/2562957.
Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17:1729–48. https://doi.org/10.1016/j.spinee.2017.07.002.
Hodges PW, Bailey JF, Fortin M, Battié MC. Paraspinal muscle imaging measurements for common spinal disorders: review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur Spine J. 2021;30:3428–41. https://doi.org/10.1007/s00586-021-06990-2.
Cooley JR, Hebert JJ, Zoete A de, et al. Assessing lumbar paraspinal muscle cross-sectional area and fat composition with T1 versus T2-weighted magnetic resonance imaging: Reliability and concurrent validity. PLOS ONE. 2021;16(2):e0244633. https://doi.org/10.1371/journal.pone.0244633.
Crawford RJ, Cornwall J, Abbott R, Elliott JM. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord. 2017;18:25. https://doi.org/10.1186/s12891-016-1378-z.
Rummens S, Robben E, Groef AD, Wambeke PV, Janssens L, Brumagne S, et al. Factors associated with the ultrasound characteristics of the lumbar multifidus: a systematic review. PM&R. 2020;12:82–100. https://doi.org/10.1002/pmrj.12212.
Sions JM, Elliott JM, Pohlig RT, Hicks GE. Trunk muscle characteristics of the Multifidi, Erector Spinae, Psoas, and Quadratus Lumborum in older adults with and without chronic low back Pain. J Orthop Sports Phys Ther. 2017;47:173–9. https://doi.org/10.2519/jospt.2017.7002.
Berry DB, Padwal J, Johnson S, Parra CL, Ward SR, Shahidi B. Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord. 2018. https://doi.org/10.1186/s12891-018-2059-x. 19:N.PAG-N.PAG.
Russo M, Deckers K, Eldabe S, Kiesel K, Gilligan C, Vieceli J, et al. Muscle control and non-specific chronic low back pain. Neuromodulation Technol Neural Interface. 2018;21:1–9. https://doi.org/10.1111/ner.12738.
Hodges PW, Danneels L. Changes in structure and function of the Back muscles in low back Pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther. 2019;49:464–76. https://doi.org/10.2519/jospt.2019.8827.
Xiao Y, Fortin M, Ahn J, Rivaz H, Peters TM, Battié MC. Statistical morphological analysis reveals characteristic paraspinal muscle asymmetry in unilateral lumbar disc herniation. Sci Rep. 2021;11:15576. https://doi.org/10.1038/s41598-021-95149-6.
Xiao Y, Fortin M, Battié MC, Rivaz H. Population-averaged MRI atlases for automated image processing and assessments of lumbar paraspinal muscles. Eur Spine J. 2018;27:2442–8. https://doi.org/10.1007/s00586-018-5704-z.
Xia W, Fortin M, Ahn J, Rivaz H, Battié MC, Peters TM, et al. Automatic paraspinal muscle segmentation in patients with lumbar Pathology using deep convolutional neural network. In: Shen D, Liu T, Peters TM, Staib LH, Essert C, Zhou S, et al. editors. Med. Image Comput. Comput. Assist. Interv. – MICCAI 2019. Volume 11765. Cham: Springer International Publishing; 2019. pp. 318–25. https://doi.org/10.1007/978-3-030-32245-8_36.
Roshanzamir P, Rivaz H, Ahn J, Mirza H, Naghdi N, Anstruther M, et al. Joint paraspinal muscle segmentation and inter-rater labeling variability prediction with multi-task TransUNet. In: Sudre CH, Baumgartner CF, Dalca A, Qin C, Tanno R, Van Leemput K, et al. editors. Uncertain. Safe Util. Mach. Learn. Med. Imaging. Volume 13563. Cham: Springer Nature Switzerland; 2022. pp. 125–34. https://doi.org/10.1007/978-3-031-16749-2_12.
Acknowledgements
The PILLAR project was a collaboration between the INSPIRE lab and HEALTH-X Lab at Concordia University, Montreal, QC, Canada. All authors contributed to the conceptualization of the website and manuscript.
Funding
This work was supported from a network pilot grant from The Quebec Bio-Imaging Network (FRQS-RBIQ) and a Multidisciplinary Research Grant from the PERFORM Centre. MF and YX are supported by the Fond de la Recherche en Santé du Québec (FRQS – Chercheur boursier Junior 1).
Author information
Authors and Affiliations
Contributions
Conception and Design: M.F., Y.X., M.A., B.R.; Website Conceptualization and Programming: T.Z., T.L.; Draft of Manuscript: M.A., B.R., M.F., Y.X.; All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Central Ethics Research Committee of the Quebec Minister of Health and Social Services (# CCER-19-20-09). Written informed consent was obtained from each participant. The authors confirmed that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anstruther, M., Rossini, B., Zhang, T. et al. PILLAR: ParaspInaL muscLe segmentAtion pRoject - a comprehensive online resource to guide manual segmentation of paraspinal muscles from magnetic resonance imaging. BMC Musculoskelet Disord 24, 909 (2023). https://doi.org/10.1186/s12891-023-07029-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-07029-x