Abstract
Background
Different methods of acetabular reconstruction with total hip arthroplasty (THA) for Crowe II and III of adult developmental dysplasia of the hip (DDH) acetabular bone defect have been implemented clinically. However, the biomechanical effect of different augmented materials for acetabular reconstruction in THA on shell stability has never been discussed.
Methods
In the present study, autologous bone graft (BG)and metal (Ti6Al4V) augment (MA) were simulated with several acetabular bone defect models of DDH in THA. The contact pressure and micromotion between the shell and host bone were measured for evaluating the shell stability using a finite element method.
Results
The peak contact stress between shell and host bone was higher in the MA situation (12.45 vs 8.71 MPa). And the load transfer path was different, for BG models, the high local contact stresses were found at the junction of bone graft and host bone while for MA models the concentrated contact stresses were at the surface of MA. The peak relative micromotion between shell and host bone was higher in the MA situation (12.61 vs 11.13 µm). However, the peak micromotion decreased in the contact interface of MA and cup compared to the BG models.
Conclusions
The higher micromotion was found in MA models, however, enough for bone ingrowth, and direct stronger fixation was achieved in the MA-cup interface. Thus, we recommended the MA can be used as an option, even for Crowe III, however, the decision should be made from clinical follow-up results.
Similar content being viewed by others
Introduction
Acetabular reconstruction with total hip arthroplasty (THA) for Crowe II and III of adult developmental dysplasia of the hip (DDH) is a challenge [1]. Because you have to premeditate the position of the arthroplasty cup, compared to Crowe I and IV, which the position of arthroplasty cup is the original true acetabular position in most cases, although acetabular reconstruction with THA for Crowe IV is much more difficult [2], the degree of acetabular bone defect [3], and the techniques of reconstruction [4, 5], In this study, augmentations for acetabular reconstruction of DDH with THA by restoring the original center of femoral head rotation in the situation of Crowe II and III bone defect were discussed [6].
Acetabular bone defect in DDH can be regarded as an inherent existence [7]. To what extent acetabular cup uncoverage affect the stability after THA matters. It has been suggested this uncoverage should not exceed 30% of the cup generally [8]. For obtain adequate bone coverage in the bone defect more than 30%, the use of a small cup size with medialization or high hip center positioning of cup for stable fixation of the acetabular component is an option [9]. However, the hip center of rotation (COR) was changed [10]. To restore the HCOR and establishing normal biomechanics of the hip, autologous bone graft was a traditional material for roof acetabular reconstruction, and the long-term outcomes was obtained [11], however, the complications such as the resorption of bone graft resulted in instability of acetabular component [12]. Recently, the metal augments were developed for acetabular reconstruction in primary and revision THA [13], and the short-term results was promising [14, 15]. Although the biomechanical behavior of different augment materials (Ti6Al4V vs Trabecular Metal) has been compared in stress level using the finite element method [16]. However, the biomechanical comparison of bone graft and metal augments on cup stability of the interface between acetabular component and host bone is missing, more information is needed. We hypothesized that the metal augment could provide a stable cup stability as equally as the bone graft provided.
The purpose of the study was to establish several acetabular bone defect models of DDH reconstructed with Ti6Al4V augment and autologous bone graft in THA and compare the influence of the two materials on biomechanical behavior (cup stability) of the interface between acetabular component and host bone using a finite element method.
Materials and methods
Construction of acetabular bone defect models of DDH
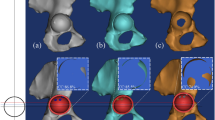
A healthy volunteer (Sex: male, age: 27 years, Height: 164 cm, Body weight: 66 kg) without any musculoskeletal disease or history of hip joint operations was recruited. quantitative computed tomography (QCT) was scanned in combination with a calibration phantom (B-MAS200, Kyoto-kagaku, Kyoto, Japan) for calibrating the bone mineral density [17]. The resolution of each CT image was 512 by 512 pixels with a slice thickness of 1.0 mm, and the pixel size was 0.782 mm/pixel under 120 kV and 102.50 mA conditions. We used the commercial software MIMICS (v 22, Materialise, Belgium) to reconstruct the intact right iliac and femoral bone, and methods can be found in our previous study [18].
According to Crowe’s classification for adult developmental dysplasia of the hip (DDH) [19], there are two methods to evaluate the degree of DDH, one is subluxation of femoral head that can be measured by the elevation of femoral head center. Another one is the ratio of the distance of proximal dislocation to the pelvic height [20]. The acetabular bone defect models of Crowe II and III in THA were made by elevating the femoral head center or center of rotation of femoral head (COR) [21] from original COR (black solid line) to 55% (Green dotted line), 65% (purple dotted line), (Crowe II); 75% (yellow dotted line), 85% (red dotted line), (Crowe III) of the femoral head radius, which can be regarded as the COR dislocation percentage (Fig. 1a). When COR reached the top range of femoral head, the COR dislocation percentage was recorded as 100% [22].
Reconstruction of acetabular bone defect models of DDH in THA
The acetabular bone defect models were simulated with Boolen operation using a CAD system (SolidWorks 2016, SolidWorks Corp, USA). The lost part of acetabular by Boolen operation was preserved as the metal augment (MA) (Ti6Al4V) and structural autologous bone graft (BG) geometrical shape (Fig. 1b) [23]. Two screws with the diameter of 6.5 mm were inserted to fix the MA or BG [16]. Acetabular cup (shell, linear) and femoral (head) prostheses were made in SolidWorks according to the size of the subject’s acetabulum and femoral head. The acetabular cup was a 52 mm PINNACLE cup without porous coating (Depuy, America) [24]. Cup inclination of 40 degrees and anteversion of 20 degrees were preset using anterior pelvic plane (APP) [25]. The ceramic liner (Depuy, America) with 32 mm femoral ceramic head (Link, Germany) were implanted [26]. The solid models were assembled for Reconstruction of acetabular bone defect models in THA.
Material properties of finite element modeling
Mesh size of the models were approximately 1 mm with four-node tetrahedral elements, which has been validated from the study [27]. In this study, each element of iliac bone was assigned with isotropic heterogeneous Young’s modulus based on QCT data form our previous study [18] (Fig. 1c). the parameters used for converting Hounsfield Units (HU) to radiographic CT density (\({\rho }_{QCT}(\mathrm{g}/{\mathrm{cm}}^{3})\) (Eq. (1)) were calculated from the B-MAS200 phantom [17], and from \({\rho }_{QCT}\) to Ash density (\({\rho }_{ash}(\mathrm{g}/{\mathrm{cm}}^{3})\) (Eq. (2)) [28], then then the apparent density that was calculated from the ash density with a ratio of 0.6 [29] was converted to the elastic modulus (Eq. (3)) [30].
Hip protheses (shell, linear, and head), two augmental materials and the screws were assigned with isotropic homogenneous elastic properties from literature (Table 1) [16, 22].
Loading and boundary conditions
Hip contact force of single-legged stance without taking account of muscles [24] was performed at the femoral head center (Fig. 1d). The pubic symphysis and sacroiliac joint were fully fixed to prevent translation and rotation. The interface of screws and bone was tied contact, the friction coefficient between bone and augment materials interface, the bone and cup interface were set as 0.8 [16, 22], which was press-fit contact pattern without screw implantation [31, 32], the head and linear interface was 0.06 [33]. The FE analysis was performed using a general-purpose FEA software program (ABAQUS 2019, Dassault Systems, Providence, RI).
Evaluation of the simulation
The effect of different materials of augment on stability of acetabular cup was evaluated by the contact pressure with CPRESS [34, 35] and relative micromotion [21, 24, 36] in each of the DeLee and Charnley Zones [37]. The micromotion of the shell in the surrounding bone stock was evaluated using the relative tangential node displacements in the contact surface. The postprocessor ABAQUS enables the prediction of tangential displacements (CSLIP) in the two perpendicular directions t1 und t2 throughout the whole surface of the implant bed. The maximum amounts of micromotion were calculated in each finite element \(n\) by calculation (Eq. (4)) [39]
Results
The peak contact stress between shell and host bone (including BG/MA contact area) was higher in the MA situation (12.45 vs 8.71 MPa) (Fig. 2a). For BG situation, the higher contact stress was in zone 3 (8.71 MPa), for MA situation, the higher contact stress was in zone 1 (12.45 MPa) (Fig. 3a). The concentration of contact stress for the shell was present in the superoposterior corner of zone 1 and inferoanterior corner of zone 3 (Fig. 4), for the host bone (including BG/MA contact area), the concentration of contact stress was in the junction area of BG situation and over the MA surface of MA situation (Fig. 5).
The peak relative micromotion between shell and host bone (including BG/MA contact area) was higher in the MA situation (12.61 vs 11.13 µm) (Fig. 2b). The higher relative micromotion was in zone 3, for either BG (11.13 µm) or MA (12.61 µm) situation (Fig. 3b). The concentration of relative micromotion for the shell was present in the inferoposterior corner of zone 3 for either BG or MA situation (Fig. 6).
The relationship of contact stress and micromotion was compared between the two reconstructed materials (Fig. 7). Three regions (3 × 3 mm squares) inside each of the DeLee and Charnley Zones were harvested. Averaged contact pressure and micromotion in each square of every model were used to represent the contact pressure and micromotion in that region. From the results of linear regression analysis, the contact pressure and micromotion had a negative relationship. However, the acetabular reconstruction with BG had a poor fitness with the R2 value of 0.001 (Fig. 7A) compared to the MA situation with the R2 value of 0.947 (Fig. 7B).
Discussion
The primary purpose of the study was to quantitatively compare the influence of two materials (Ti6Al4V augment and autologous bone graft) for acetabular reconstruction of DDH on acetabular stability after THA using a finite element method. Crown II and III FE models were simulated by elevating the femoral head center. The mechanical parameters of contact pressure predicted with CPRESS, relative micromotion calculated with CSLIP in the interface between shell and host bone were utilized to evaluate the acetabular stability.
The overall peak contact pressure was slightly higher in the MA situation, indicating that acetabular reconstruction with MA such as Titanium alloy had more contact pressure compared to BG such as structural bone graft (Fig. 2a). However, the magnitude was higher than the normal hip peak contact pressure, even the dysplasia hip peak contact pressure in the single-leg standing condition [40,41,42]. The reason may be the biomechanics of hip had been changed by THA compared to the normal configuration [43, 44], including the effect of fraction, material properties and porosity between the interface of implant-bone [45]. It has been confirmed that acetabular component with porosity was able to reduce the maximum contact stress on the bone surface [46].
The peak contact pressure decreased in Zone 2 of the DeLee and Charnley Zones either for the BG models or the MA models compared with Zone 1, 3 (Fig. 3a), which was corresponded with the contact stress distribution (Figs. 4 and 5). The interface stress transmission from acetabular component to surrounding bone featured in the superior dome of Zone 1, and inferioanterior area of pubic branch. However, the load transfer path was different in Zone 1, for BG models, the high local contact stresses were found at the junction of BG and host bone while for MA models the concentrated contact stresses were at the surface of MA. The results were similar with the biomechanical study [47] about segmental acetabular rim defects reconstructed with bone graft and reinforcement ring, that the peak stress concentration was located in the superior-posterior of the acetabulum. This suggested that the full fixation postoperatively in the superior-posterior dome should be needed for the initial stability.
The peak micromotion was slightly higher for the MA models (11.92–12.61 µm) (Fig. 2b), lower than the relative displacement (20–40 µm) for adequate bone ingrowth from reports [48, 49], indicating that acetabular reconstruction with MA and BG could provide enough initial stability for cup bone ingrowth to guarantee good long-term results. The predicted relative micromotion between the interface of cup and host bone was consistent with the previous biomechanical study [50]. However, the acetabular reconstruction with GB has the disadvantage with graft resorption and collapse at the early postoperative stage [51,52,53]. Alternatively, the metal augments such as Tritanium acetabular wedge augments can be used with less micromotion for adequate bone ingrowth and stable clinical follow-up results [54, 55].
Compared to BG models in DeLee and Charnley zones, the peak micromotion decreased in zone 1 of MA models without zone 2 and 3 (Fig. 3b), indicating that acetabular reconstruction with MA had an excessive direct fixation with the cup (zone1), but the MA and BG materials had little influence on the host bone (zone 2, 3). The results were corresponded with the interface micromotion distribution of cup and host bone (Fig. 6), the micromotion distribution was similar in zone 2 and 3. High micromotion was located in the inferioposterior corner of ischial branch and inferioanterior corner of pubic branch. Numerical results indicated that support from superior dome, ischial branch and pubic branch was necessary to obtain the initial stability in case of DDH or revision THA [56, 57].
The micromotion and contact stress had a negative relationship in bone-implant surface including the implant-augment contact area. For MA models, a good fitness with R2 = 0.947 was present, because the less micromotion was, the more contact stress displaced in Square 1 of implant-MA surface. In contrast, for BG models, the more micromotion was, the higher contact stress in Square 1 of implant-BG surface compared to the implant-MA surface, however, the good fitness with R2 = 0.964 was present only considering the implant-host bone surface (Fig. 7A black solid line). This indicated that the MA was able to provide stronger direct fixation with cup connection.
Limitations were: (1) The present study was performed with a computational simulation method, though it was validated [24, 58, 59]. The biomechanical test should be added to enrich the results more convincingly [60, 61]. (2) There are many factors that influence the secondary bone fixation or the cup stability [62], the main factor was the type of implant surface coated design [48]. The cup and MA used in the study was not the porous coated design, this may influence the predicted results, however, the fraction parameters between contact surface were defined as porous coated situation [16, 22]. (3) The acetabular cup fixation method was not the press-fit technique used in clinical [31], but a press-fit contact pattern between cup and bone was decided by simulating an equivalent friction coefficient from literature [16], and the cup-bone relative micromotion may be changed [32]. (4) The present study was only focus on acetabular component-host bone interface to evaluate the stability of cup, the augment-bone interface should be investigated further to study the biomechanical behavior of MA and BG directly. (5) There was only one example of the FEA model, which may affect the universality of the study, and it was tested mechanically without muscle force, just with the contact hip joint force instead, which should be considered in the future research. (6) theoretically, the BG should be priority, because of its synostosis with host bone [63], while the MA with host bone was an osteointegration [64], however, the biological factors was not considered in present study, just the mechanical properties addressed.
Conclusions
Acetabular reconstruction of DDH with MA in THA is an emerging technique compared to BG with a long history. From the predicted results, the load transfer path was different in the implant-bone interface with the two augment materials. And a higher micromotion was found in the MA models, however, the micromotions of the both in the implant-bone interface were lower than measurements for adequate bone ingrowth, especially, MA -implant interface had a less micromotion than the GB-implant interface. Thus, we recommended the MA can be used as an option, even for Crowe III, however, the decision should be made from clinical follow-up results.
Availability of data and materials
The data that support the findings of this study are available from CP, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of CP.
Abbreviations
- THA:
-
Total Hip Arthroplasty
- DDH:
-
Adult Developmental Dysplasia of the Hip
- HCOR:
-
Hip Center of Rotation (COR)
- QCT:
-
Quantitative Computed Tomography
- CAD:
-
Computer Assisted Design
- MA:
-
Metal Augment
- BG:
-
Bone Graft
- APP:
-
Anterior Pelvic Plane
- HU:
-
Hounsfield Unit
- FEA:
-
Finite Element Analysis
References
Greber EM, Pelt CE, Gililland JM, Anderson MB, Erickson JA, Peters CL. Challenges in Total Hip Arthroplasty in the Setting of Developmental Dysplasia of the Hip. J Arthroplasty. 2017;32(9S):S38–44.
Atilla B, Ali H, Aksoy MC, Caglar O, Tokgozoglu AM, Alpaslan M. Position of the acetabular component determines the fate of femoral head autografts in total hip replacement for acetabular dysplasia. J Bone Joint Surg Br. 2007;89(7):874–8.
Sakellariou VI, Christodoulou M, Sasalos G, Babis GC. Reconstruction of the Acetabulum in Developmental Dysplasia of the Hip in total hip replacement. Arch Bone Jt Surg. 2014;2(3):130–6.
Bicanic G, Barbaric K, Bohacek I, Aljinovic A, Delimar D. Current concept in dysplastic hip arthroplasty: Techniques for acetabular and femoral reconstruction. World J Orthop. 2014;5(4):412–24.
Wen X, Zuo J, Liu T, Gao Z, Xiao J. Bone defect map of the true acetabulum in hip dysplasia (Crowe type II and III) based on three-dimensional image reconstruction analysis. Sci Rep. 2021;11(1):22955.
Sanchez-Sotelo J, Berry DJ, Trousdale RT, Cabanela ME. Surgical treatment of developmental dysplasia of the hip in adults: II. Arthroplasty options. J Am Acad Orthop Surg. 2002;10(5):334–44.
Dapuzzo MR, Sierra RJ. Acetabular considerations during total hip arthroplasty for hip dysplasia. Orthop Clin North Am. 2012;43(3):369–75.
Li H, Mao Y, Oni JK, Dai K, Zhu Z. Total hip replacement for developmental dysplasia of the hip with more than 30% lateral uncoverage of uncemented acetabular components. Bone Joint J. 2013;95-B(9):1178–83.
Zhang L, Lu X. Acetabular Cup Positioning during Total Hip Replacement in Osteoarthritis Secondary to Developmental Dysplasia of the Hip – a Review of the Literature. Acta Chir Orthop Traumatol Cech. 2019;86(2):93–100.
Shen J, Sun J, Ma H, Du Y, Li T, Zhou Y. High Hip Center Technique in Total Hip Arthroplasty for Crowe Type II–III Developmental Dysplasia: Results of Midterm Follow-up. Orthop Surg. 2020;12:1245–52.
Kim M, Kadowaki T. High long-term survival of bulk femoral head autograft for acetabular reconstruction in cementless THA for developmental hip dysplasia. Clin Orthop Relat Res. 2010;468(6):1611–20.
Oommen AT, Krishnamoorthy VP, Poonnoose PM, Korula RJ. Fate of bone grafting for acetabular defects in total hip replacement. Indian J Orthop. 2015;49(2):181–6.
Abolghasemian M, Tangsataporn S, Sternheim A, Backstein DJ, Safir OA, Gross AE. Porous metal augments: big hopes for big holes. Bone Joint J. 2013;95-B(11 Suppl A):103–8.
Restrepo C, Heller S, Chen AF. Tritanium acetabular wedge augments: short-term results. Ann Transl Med. 2016;4(12):235.
Ohashi H, Yo H, Ikawa T, Minami Y, Teraoka T. Acetabular reconstruction with porous metal augments for primary and revision total hip arthroplasty. Ortho Pro. 2018;100-B:Suppl_11.
Fu J, Ni M, Chen J, Li X, Chai W, Hao L, Zhang G, Zhou Y. Reconstruction of Severe Acetabular Bone Defect with 3D Printed Ti6Al4V Augment: A Finite Element Study. Biomed Res Int. 2018;2018(14):6367203.
Knowles NK, Reeves JM, Ferreira LM. Quantitative Computed Tomography (QCT) derived Bone Mineral Density (BMD) in finite element studies: a review of the literature. J Exp Orthop. 2016;3(1):36.
Wang Y, Yamako G, Okada T, Arakawa H, Nakamura Y, Chosa E. Biomechanical effect of intertrochanteric curved varus osteotomy on stress reduction in femoral head osteonecrosis: a finite element analysis. J Orthop Surg Res. 2021;16(1):465.
Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61(1):15–23.
Jawad MU, Scully SP. In brief: Crowe’s classification: arthroplasty in developmental dysplasia of the hip. Clin Orthop Relat Res. 2011;469(1):306–8.
Abolghasemian M, Samiezadeh S, Jafari D, Bougherara H, Gross AE, Ghazavi MT. Displacement of the hip center of rotation after arthroplasty of Crowe III and IV dysplasia: a radiological and biomechanical study. J Arthroplasty. 2013;28(6):1031–5.
Wang C, Ouyang Y, Liu H, Xu C, Xiao H, Hu Y, Li Y, Zhong D. Surgery simulation teaching based on real reconstruction aid versus traditional surgical live teaching in the acquisition of an adult total hip arthroplasty surgical technique for developmental dysplasia of the hip: a randomized comparative study. BMC Med Educ. 2020;20(1):228.
Zhao X, Chosa E, Yamako G, Watanabe S, Deng G, Totoribe K. Effect of acetabular reinforcement ring with hook for acetabular dysplasia clarified by three-dimensional finite element analysis. J Arthroplasty. 2013;28(10):1765–9.
Du Y, Fu J, Sun J, Zhang G, Chen J, Ni M, Zhou Y. Acetabular Bone Defect in Total Hip Arthroplasty for Crowe II or III Developmental Dysplasia of the Hip: A Finite Element Study. Biomed Res Int. 2020;2020(25):4809013.
Bhaskar D, Rajpura A, Board T. Current Concepts in Acetabular Positioning in Total Hip Arthroplasty. Indian J Orthop. 2017;51(4):386–96.
Hsu JT, Tsai MT, Chang CH, Fuh LJ, Lai KA, Liu ZL, Tu MG, Huang HL. Finite Element Analysis of the Effects of Sizes of Acetabular Components on the Initial Stability of the Acetabular Cup. J Med Bio Eng. 2008;28(2):59–63.
Dutt A. Effect of Mesh Size on Finite Element Analysis of Beam. Inter J Mech Eng. 2015;2(12):8–10.
Schileo E, Dall’ara E, Taddei F, Malandrino A, Schotkamp T, Baleani M, Viceconti M. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. J Biomech. 2008;41(11):2483–91.
Ali AA, Cristofolini L, Schileo E, Hu H, Taddei F, Kim RH, Rullkoetter PJ, Laz PJ. Specimen-specific modeling of hip fracture pattern and repair. J Biomech. 2014;47(2):536–43.
Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36(7):897–904.
Takao M, Nakamura N, Ohzono K, Sakai T, Nishii T, Sugano N. The results of a press-fit-only technique for acetabular fixation in hip dysplasia. J Arthroplasty. 2011;26(4):562–8.
Spears IR, Pfleiderer M, Schneider E, Hille E, Morlock MM. The effect of interfacial parameters on cup-bone relative micromotions. A finite element investigation J Biomech. 2001;34(1):113–20.
Ma L, Rainforth WM. The effect of lubrication on the friction and wear of Biolox®delta. Acta Biomater. 2012;8(6):2348–59.
Schüller HM, Dalstra M, Huiskes R, Marti RK. Total hip reconstruction in acetabular dysplasia. A finite element study. J Bone Joint Surg Br. 1993;75(3):468–74.
Rapperport DJ, Carter DR, Schurman DJ. Contact finite element stress analysis of porous ingrowth acetabular cup implantation, ingrowth, and loosening. J Orthop Res. 1987;5(4):548–61.
Clarke SG, Phillips AT, Bull AM. Validation of FE micromotions and strains around a press-fit cup: introducing a new micromotion measuring technique. Ann Biomed Eng. 2012;40(7):1586–96.
Miyagawa T, Matsumoto K, Komura S, Akiyama H. Total hip arthroplasty using a three-dimensional porous titanium acetabular cup: an examination of micromotion using subject-specific finite element analysis. BMC Musculoskelet Disord. 2021;22(1):308.
DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;1:20–32.
Kluess D, Wieding J, Souffrant R, Mittelmeier W, Bader R. Finite Element Analysis in Orthopaedic Biomechanics. In: Moratal D, editor. Finite Element Analysis. London: IntechOpen; 2010. p. 151–70.
Genda E, Iwasaki N, Li G, MacWilliams BA, Barrance PJ, Chao EY. Normal hip joint contact pressure distribution in single-leg standing–effect of gender and anatomic parameters. J Biomech. 2001;34(7):895–905.
Wang X, Peng J, Li D, Zhang L, Wang H, Jiang L, Chen X. Does the optimal position of the acetabular fragment should be within the radiological normal range for all developmental dysplasia of the hip? A patient-specific finite element analysis. J Orthop Surg Res. 2016;11(1):109.
Zou Z, Chávez-Arreola A, Mandal P, Board TN, Alonso-Rasgado T. Optimization of the position of the acetabulum in a ganz periacetabular osteotomy by finite element analysis. J Orthop Res. 2013;31(3):472–9.
Sorbie C, Zdero R, Bryant JT. Normal and Prosthesic Hip Biomechanics. In: Poitout DG, editor. Biomechanics and Biomaterials in Orthopedics. London: Springer; 2004. p. 528–49.
Lunn DE, Lampropoulos A, Stewart TD. Basic biomechanics of the hip. Orthopaedics and Trauma. 2016;30(2):239–46.
Gao X, Fraulob M, Haïat G. Biomechanical behaviours of the bone–implant interface: a review. J R Soc Interface. 2019;16:20190259.
Moussa A, Rahman S, Xu M, Tanzer M, Pasini D. Topology optimization of 3D-printed structurally porous cage for acetabular reinforcement in total hip arthroplasty. J Mech Behav Biomed Mater. 2020;105.
Xiao J, Zhao X, Wang Y, Yang Y, Zhao J, Gao Z, Zuo J. Application of Acetabular Reinforcement Ring with Hook for Correction of Segmental Acetabular Rim Defects during Total Hip Arthroplasty Revision. J Bionic Eng. 2018;15:154–9.
Mukherjee K, Gupta S. Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol. 2016;15(2):389–403.
Liu X, Niebur GL. Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech Model Mechanobiol. 2008;7(4):335–44.
Zuo J, Xu M, Zhao X, Shen X, Gao Z, Xiao J. Effects of the depth of the acetabular component during simulated acetabulum reaming in total hip arthroplasty. Sci Rep. 2021;11(1):9836.
Gerber SD, Harris TW. Femoral head autografting to augment acetabular deficiency in patients requiring total hip replacement: a minimum five-year and an average seven-year follow-up study. J Bone Joint Surg Am. 1986;68:1241–8.
Gross AE, Catre MG. The use of femoral head autograft shelf reconstruction and cemented acetabular components in the dysplastic hip. Clin Orthop Relat Res. 1994;298:60–6.
Hintermann B, Morscher EW. Total hip replacement with solid autologous femoral head graft for hip dysplasia. Arch Orthop Trauma Surg. 1995;114:137–44.
Jeong M, Kim HJ, Lim SJ, Moon YW, Park YS. Revision Total Hip Arthroplasty Using Tantalum Augment in Patients with Paprosky III or IV Acetabular Bone Defects: A Minimum 2-year Follow Up Study. Hip Pelvis. 2016;28(2):98–103.
Löchel J, Janz V, Hipfl C, Perka C, Wassilew GI. Reconstruction of acetabular defects with porous tantalum shells and augments in revision total hip arthroplasty at ten-year follow-up. Bone Joint J. 2019;101-B(3):311–6.
Ghanem M, Zajonz D, Heyde CE, Roth A. Acetabular defect classification and management: Revision arthroplasty of the acetabular cup based on 3-point fixation. Orthopade. 2020;49(5):432–42.
Von Hertzberg-Boelch SP, Wagenbrenner M, Arnholdt J, Frenzel S, Holzapfel BM, Rudert M. Custom Made Monoflange Acetabular Components for the Treatment of Paprosky Type III Defects. J Pers Med. 2021;11(4):283.
Anderson AE, Peters CL, Tuttle BD, Weiss JA. Subject-specific finite element model of the pelvis: development, validation and sensitivity studies. J Biomech Eng. 2005;127(3):364–73.
Hicks JL, Uchida TK, Seth A, Rajagopal A, Delp SL. Is my model good enough? Best practices for verification and validation of musculoskeletal models and simulations of movement. J Biomech Eng. 2015;137(2).
Beckmann NA, Bitsch RG, Gondan M, Schonhoff M, Jaeger S. Comparison of the stability of three fixation techniques between porous metal acetabular components and augments. Bone Joint Res. 2018;7(4):282–8.
Morosato F, Traina F, Schierjott RA, Hettich G, Grupp TM, Cristofolini L. Primary Stability of Revision Acetabular Reconstructions Using an Innovative Bone Graft Substitute: A Comparative Biomechanical Study on Cadaveric Pelvises. Materials (Basel). 2020;13(19):4312.
Amirouche F, Solitro G, Broviak S, Gonzalez M, Goldstein W, Barmada R. Factors influencing initial cup stability in total hip arthroplasty. Clin Biomech (Bristol, Avon). 2014;29(10):1177–85.
Liu S, Tao S, Tan J, Hu X, Liu H, Li Z. Long-term follow-up of fibular graft for the reconstruction of bone defects. Medicine (Baltimore). 2018;97(40).
Macák D, Džupa V, Krbec M. Individuální titanová acetabulární komponenta vyrobená 3D tiskem: výhody a limity použití [Custom-Made 3D Printed Titanium Acetabular Component: Advantages and Limits of Use]. Acta Chir Orthop Traumatol Cech. 2021;88(1):69–74.
Acknowledgements
I would like to thank Mincong Wang for using much of his time to revise the manuscript and provide a lot of useful suggestions.
Funding
This work was supported by JSPS KAKENHI Grant Number JP17K01362. The funder had no role in design of the study, the collection, analysis, and interpretation of the data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YW and MW contributed to the work equally as the first co-authors. YW collected the images and conducted the finite element simulation, YW and MW played a major role in writing the manuscript. LD analyzed the images. GY provided the devices and technical support, checked the results. CL, YN, GY, EC and CP checked and revised the manuscript for publication. All authors agreed for the final submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Review Committee of Miyazaki University (NO.0–0672) and informed consent was obtained from the subject. All methods were performed in accordance with the relevant guidelines and regulations of ethics committee of Miyazaki University.
Consent for publication
Written informed consent was obtained from the subject for publication of this article and any personal information or images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Wang, M., Li, C. et al. Biomechanical effect of metal augment and bone graft on cup stability for acetabular reconstruction of total hip arthroplasty in hip dysplasia: a finite element analysis. BMC Musculoskelet Disord 23, 277 (2022). https://doi.org/10.1186/s12891-022-05168-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05168-1